Abstract
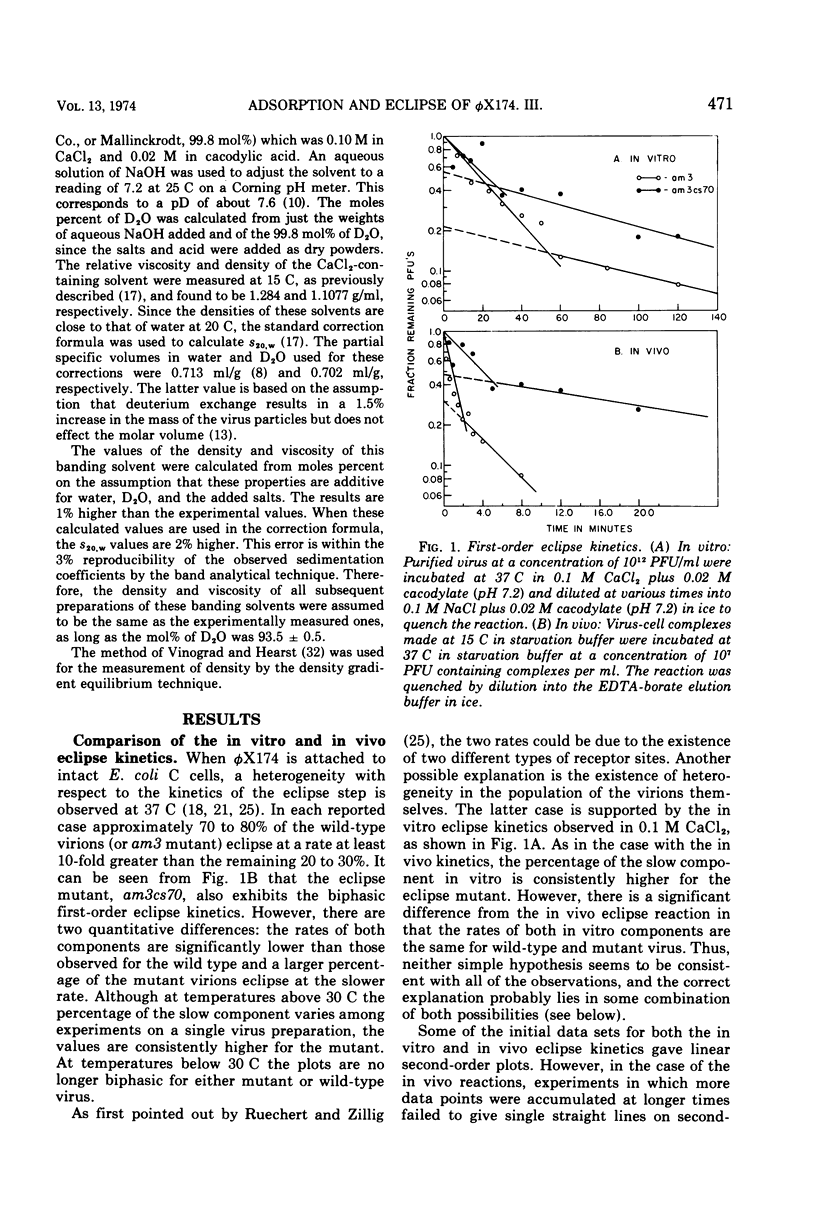
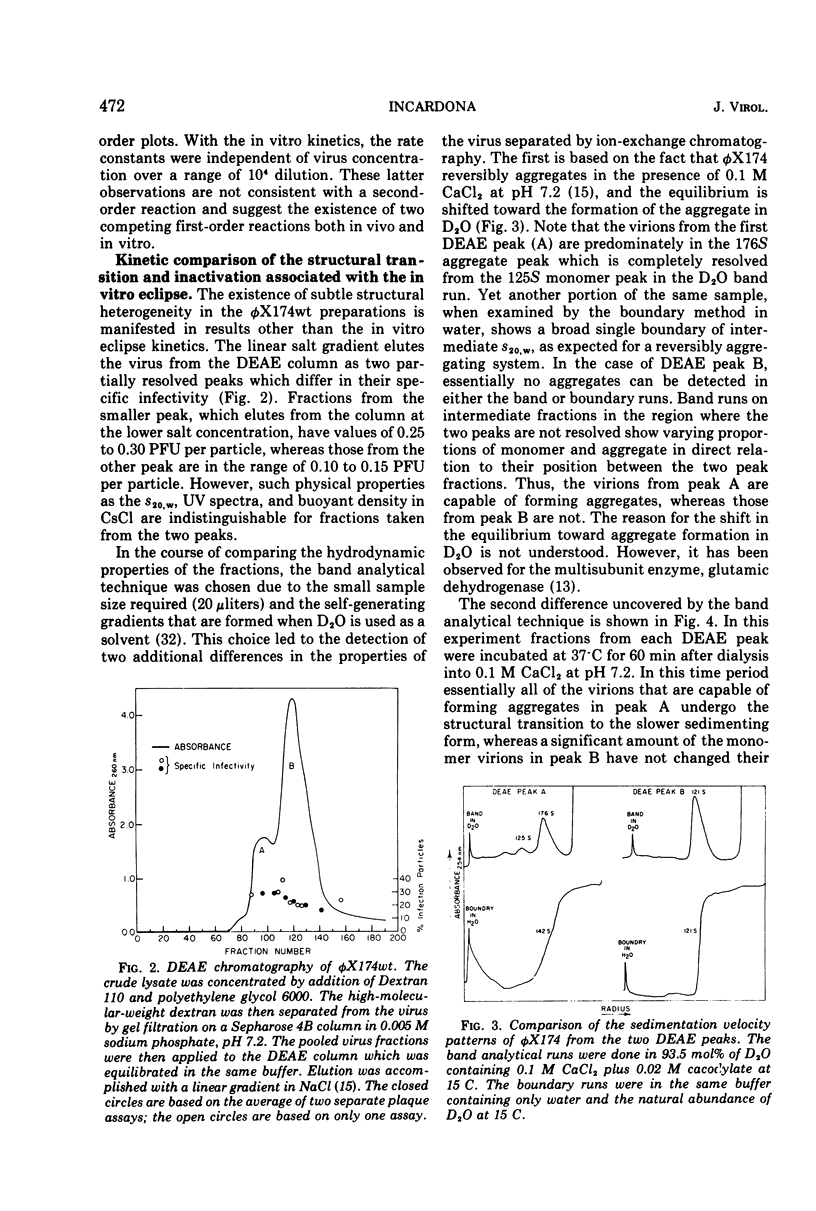
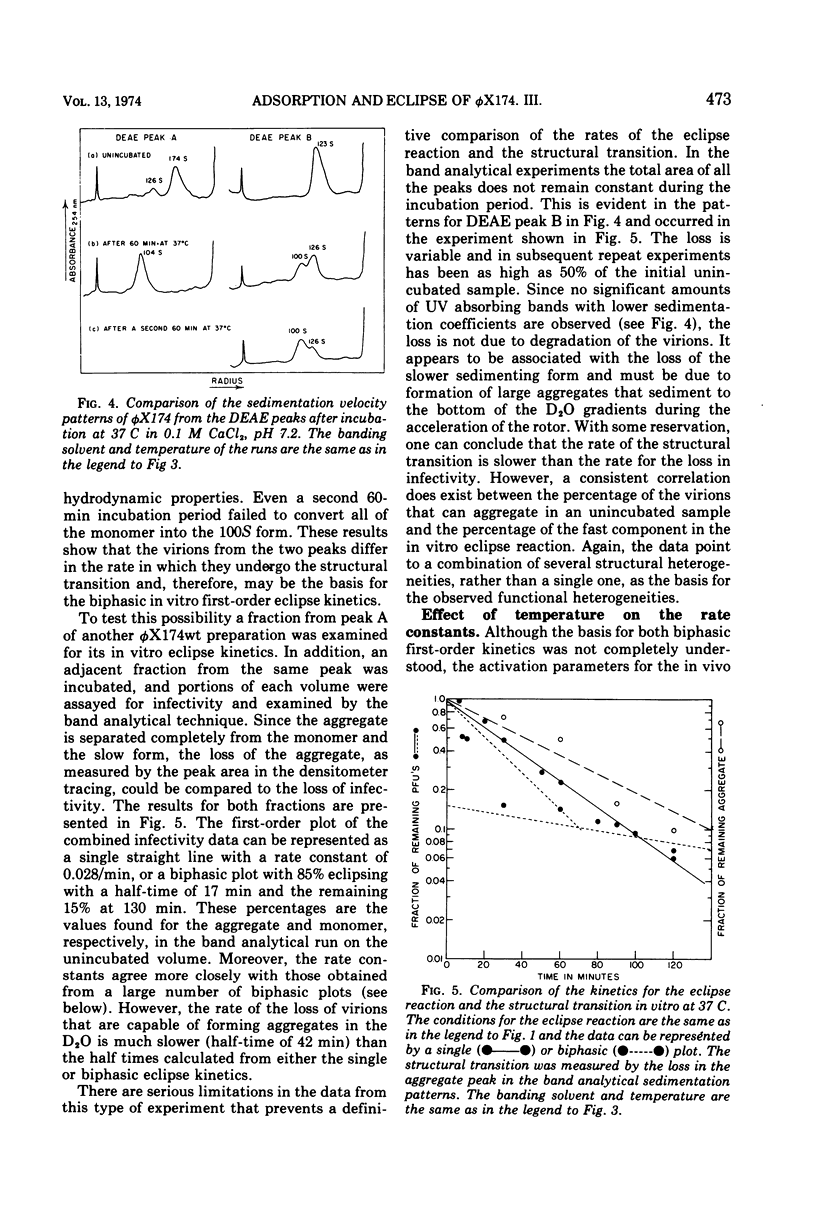
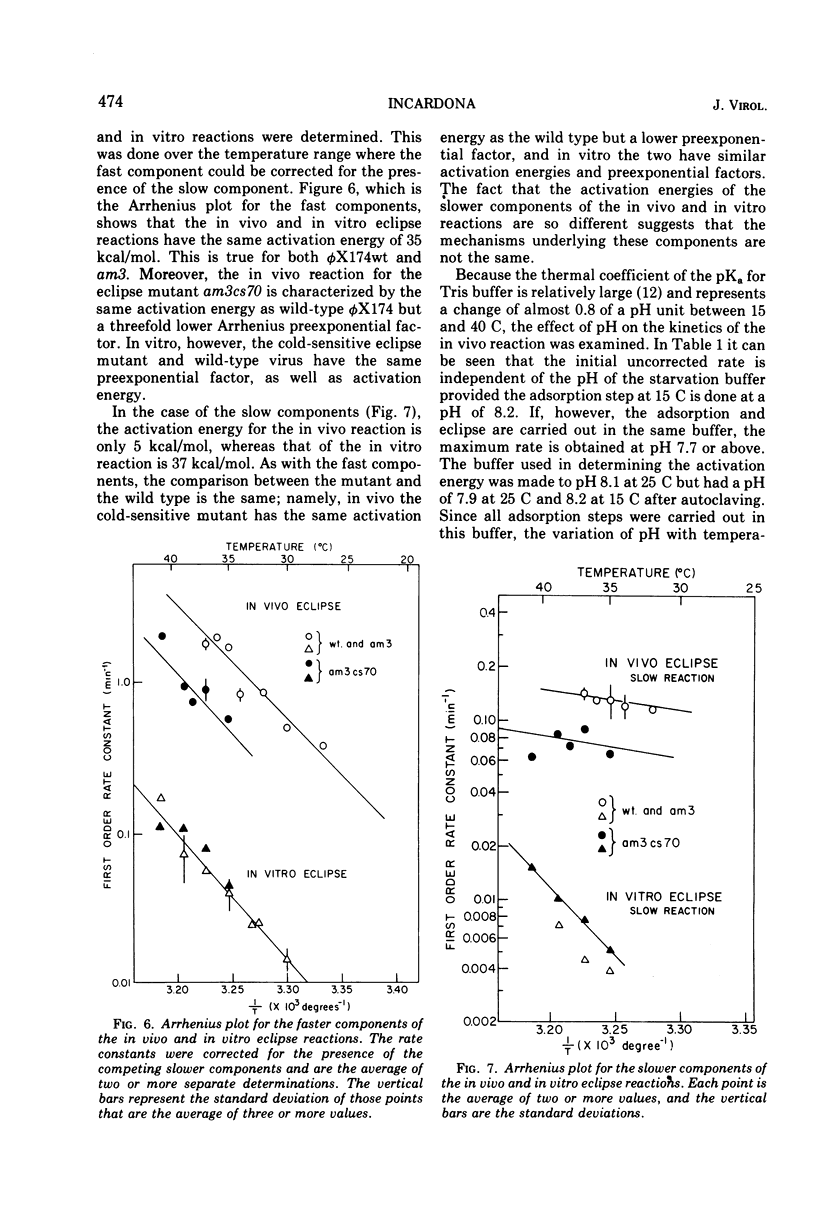
In a starvation buffer containing 10−3 M divalent cations, φX174 undergoes viral eclipse above 20 C when attached to intact host cells. An in vitro structural transition that is similar to that observed in this in vivo eclipse reaction occurs over the same temperature range in 0.1 M CaCl2 (pH 7.2). Since both reactions result in a loss of infectivity, their kinetics have been compared in this report. Both exhibit a biphasic first-order loss in PFU that is a result of two competing first-order processes. However, a single type of heterogeneity in the population of virions is not the basis for both competing slower reactions. The Arrhenius plots of the faster components show that the in vitro eclipse reaction has the same activation energy of 35 kcal/mol (ca. 1.47 × 105 J/mol) as the in vivo reaction but a 10-fold lower Arrhenius preexponential factor. This is further evidence that certain features of the in vivo mechanism are retained in the in vitro reaction. In the case of the slower components, the in vitro reaction has an activation energy of 37 kcal/mol (1.55 × 105 J/mol), whereas that of the in vivo reaction is only 5 kcal/mol (2.1 × 104 J/mol). A similar analysis has been performed on a cold-sensitive eclipse mutant of φX174. In vivo, the mutation is expressed by a two- to three-fold lower Arrhenius preexponential factor for both components of the eclipse reaction when compared to wt virus. The activation energies for both components are the same as wt virus. These results suggest that the mechanism of the eclipse reaction can be operationally divided into two aspects, each subject to mutational alteration.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbow R. M., Mayol R. F., Picchi J. C., Sinsheimer R. L. Direction of Translation and Size of Bacteriophage phiX174 Cistrons. J Virol. 1972 Jul;10(1):99–114. doi: 10.1128/jvi.10.1.99-114.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleichrodt J. F., Blok J., Berends-Van Abkoude E. R. Thermal inactivation of bacteriophage phi X174 and two of its mutants. Virology. 1968 Nov;36(3):343–355. doi: 10.1016/0042-6822(68)90160-8. [DOI] [PubMed] [Google Scholar]
- Christensen J. R. The kinetics of reversible and irreversible attachment of bacteriophage T-1. Virology. 1965 Aug;26(4):727–737. doi: 10.1016/0042-6822(65)90336-3. [DOI] [PubMed] [Google Scholar]
- Dalgarno L., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XXIV. New type of temperature-sensitive mutant. J Virol. 1968 Aug;2(8):822–829. doi: 10.1128/jvi.2.8.822-829.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger R. E., Paranchych W. Stages in phage R17 infection. 3. Energy requirements for the F-pili mediated eclipse of viral infectivity. Virology. 1970 Mar;40(3):554–564. doi: 10.1016/0042-6822(70)90199-6. [DOI] [PubMed] [Google Scholar]
- Dowell C. E. Cold-sensitive mutants of bacteriophage phi-X-174. I. A mutant blocked in the eclipse function at low temperature. Proc Natl Acad Sci U S A. 1967 Sep;58(3):958–961. doi: 10.1073/pnas.58.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 28. Removal of the spike proteins from the phage capsid. J Mol Biol. 1969 Jun 28;42(3):547–557. doi: 10.1016/0022-2836(69)90242-3. [DOI] [PubMed] [Google Scholar]
- GAREN A., PUCK T. T. The first two steps of the invasion of host cells by bacterial viruses. II. J Exp Med. 1951 Sep;94(3):177–189. doi: 10.1084/jem.94.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N. Characterization and synthesis of phi X174 proteins in ultraviolet-irradiated and unirradiated cells. J Mol Biol. 1971 May 14;57(3):541–553. doi: 10.1016/0022-2836(71)90108-2. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. X. Mutations in a phi-X Lysis gene. J Mol Biol. 1966 Jul;18(3):429–447. doi: 10.1016/s0022-2836(66)80035-9. [DOI] [PubMed] [Google Scholar]
- Incardona N. L., Blonski R., Feeney W. Mechanism of adsorption and eclipse of bacteriophage phi X174. I. In vitro conformational change under conditions of eclipse. J Virol. 1972 Jan;9(1):96–101. doi: 10.1128/jvi.9.1.96-101.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona N. L., McKee S., Flanegan J. B. Noncovalent interactions in viruses: characterization of their role in the pH and thermally induced conformational changes in bromegrass mosaic virus. Virology. 1973 May;53(1):204–214. doi: 10.1016/0042-6822(73)90479-0. [DOI] [PubMed] [Google Scholar]
- Incardona N. L., Notarius H., Flanegan J. B. Measurement of temperature within the sample cell during sedimentation velocity experiments. Anal Biochem. 1971 Apr;40(2):267–280. doi: 10.1016/0003-2697(71)90385-x. [DOI] [PubMed] [Google Scholar]
- Incardona N. L., Selvidge L. Mechanism of adsorption and eclipse of bacteriophage phi X174. II. Attachment and eclipse with isolated Escherichia coli cell wall lipopolysaccharide. J Virol. 1973 May;11(5):775–782. doi: 10.1128/jvi.11.5.775-782.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Marco R., Kornberg A. A coat protein of the bacteriophage M13 virion participates in membrane-oriented synthesis of DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):205–209. doi: 10.1073/pnas.70.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold J. E., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XXXIV. Kinetic of the attachment and eclipse steps of the infection. J Virol. 1970 Apr;5(4):427–431. doi: 10.1128/jvi.5.4.427-431.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold J. E., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXXII. Early steps in the infection process: attachment, eclipse and DNA penetration. J Mol Biol. 1970 Apr 14;49(1):49–66. doi: 10.1016/0022-2836(70)90375-x. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., GAREN A., CLINE J. The mechanism of virus attachment to host cells. I. The role of ions in the primary reaction. J Exp Med. 1951 Jan;93(1):65–88. doi: 10.1084/jem.93.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Ainsworth S. K., Dick A. J., Krahn P. M. Stages in phage R17 infection. V. Phage eclipse and the role of F pili. Virology. 1971 Sep;45(3):615–628. doi: 10.1016/0042-6822(71)90176-0. [DOI] [PubMed] [Google Scholar]
- RUECKERT R. R., ZILLIG W. Biosynthesis of virus protein in Escherichia coli C in vivo following infection with bacteriophage phi-X-174. J Mol Biol. 1962 Jul;5:1–9. doi: 10.1016/s0022-2836(62)80056-4. [DOI] [PubMed] [Google Scholar]
- Silverman P. M., Valentine R. C. The RNA injection step of bacteriophage f2 infection. J Gen Virol. 1969 Jan;4(1):111–124. doi: 10.1099/0022-1317-4-1-111. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. A slowly sedimenting, infective form of bacteriophage R17. J Mol Biol. 1968 May 14;33(3):947–951. doi: 10.1016/0022-2836(68)90330-6. [DOI] [PubMed] [Google Scholar]
- TZAGOLOFF H., PRATT D. THE INITIAL STEPS IN INFECTION WITH COLIPHAGE M13. Virology. 1964 Nov;24:372–380. doi: 10.1016/0042-6822(64)90174-6. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., STRAND M. COMPLEXES OF F-PILI AND RNA BACTERIOPHAGE. Science. 1965 Apr 23;148(3669):511–513. doi: 10.1126/science.148.3669.511. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Verbraeken E., Fiers W. Studies on the bacteriophage MS2. XX. Expansion of the virion in low salt. Virology. 1972 Dec;50(3):690–700. doi: 10.1016/0042-6822(72)90423-0. [DOI] [PubMed] [Google Scholar]
- Weisbeek P. J., van de Pol J. H., van Arkel G. A. Mapping of host range mutants of bacteriophage phiX174. Virology. 1973 Apr;52(2):408–416. doi: 10.1016/0042-6822(73)90335-8. [DOI] [PubMed] [Google Scholar]
- Woodfin B. M., Henderson R. F., Henderson T. R. Effects of D2O on the association-dissociation equilibrium in subunit proteins. J Biol Chem. 1970 Aug 10;245(15):3733–3737. [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


