Abstract
The purification of coliphage MS2 dinitrophenol (DNP) conjugates provided a system for localization of the single molecule of A-protein in the capsid of the MS2 phage particle. Three A-protein preparations isolated from unconjugated MS2, overconjugated DNP-MS2, and purified 78S DNP-MS2 were tested for the presence of covalently bound DNP. The binding characteristics to Dowex 1-X8 and rabbit anti-DNP bovine serum albumin (DNP-BSA) immunoglobulin G of the 78S DNP-MS2 and overconjugated DNP-MS2 A-protein preparations indicate that the A-protein is located on the surface of the phage particle where it can be covalently conjugated with hapten. Extensive enzymatic iodination of the A-protein of intact unconjugated MS2 substantiates this conclusion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtiss L. K., Krueger R. G. Purification and characterization of coliphage MS2 hapten conjugates. J Immunol. 1973 Jan;110(1):167–174. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- EISEN H. N. PREPARATION OF PURIFIED ANTI-2,4-DINITROPHENYL ANTIBODIES. Methods Med Res. 1964;10:94–102. [PubMed] [Google Scholar]
- Heisenberg M. Formation of defective bacteriophage particles by fr amber mutants. J Mol Biol. 1966 May;17(1):136–144. doi: 10.1016/s0022-2836(66)80100-6. [DOI] [PubMed] [Google Scholar]
- Hohn T., Hohn B. Structure and assembly of simple RNA bacteriophages. Adv Virus Res. 1970;16:43–98. doi: 10.1016/s0065-3527(08)60021-4. [DOI] [PubMed] [Google Scholar]
- Hornick C. L., Karush F. The interaction of hapten-coupled bacteriophage phi-X-174 with antihapten antibody. Isr J Med Sci. 1969 Mar-Apr;5(2):163–170. [PubMed] [Google Scholar]
- Kaerner H. C. Sequential steps in the in vivo assembly of the RNA bacteriophage fr. J Mol Biol. 1970 Nov 14;53(3):515–529. doi: 10.1016/0022-2836(70)90081-1. [DOI] [PubMed] [Google Scholar]
- Karush F. Affinity and the immune response. Ann N Y Acad Sci. 1970 Feb 13;169(1):56–64. doi: 10.1111/j.1749-6632.1970.tb55970.x. [DOI] [PubMed] [Google Scholar]
- King T. P., Norman P. S., Tao N. Chemical modifications of the major allergen of ragweed pollen, antigen E. Immunochemistry. 1974 Feb;11(2):83–92. doi: 10.1016/0019-2791(74)90321-8. [DOI] [PubMed] [Google Scholar]
- Krahn P. M., O'Callaghan R. J., Paranchych W. Stages in phage R17 infection. VI. Injection of A protein and RNA into the host cell. Virology. 1972 Mar;47(3):628–637. doi: 10.1016/0042-6822(72)90552-1. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- O'Callaghan R., Bradley R., Paranchych W. Controlled alterations in the physical and biological properties of R17 bacteriophage induced by gunaidine hydrochloride. Virology. 1973 Aug;54(2):476–494. doi: 10.1016/0042-6822(73)90158-x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weiner A. M., Weber K. Large scale purification of A-protein from bacterior17. Eur J Biochem. 1970 Nov;17(1):63–67. doi: 10.1111/j.1432-1033.1970.tb01134.x. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. The arrangement of proteins in the human erythrocyte membrane. Biochem Biophys Res Commun. 1970 Jul 27;40(2):284–289. doi: 10.1016/0006-291x(70)91007-7. [DOI] [PubMed] [Google Scholar]
- Richelson E., Nathans D. Association of bacteriophage proteins and RNA in E. coli infected with MS2. Biochem Biophys Res Commun. 1967 Dec 29;29(6):842–849. doi: 10.1016/0006-291x(67)90296-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Steitz J. E. The reconstitution of infective bacteriophage R17. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1416–1421. doi: 10.1073/pnas.58.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G. F., Krueger R. G. Physical, biochemical, and immunological properties of coliphage MS-2 particles. J Virol. 1970 Sep;6(3):269–279. doi: 10.1128/jvi.6.3.269-279.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombauts W. A., Schroeder W. A., Morrison M. Bovine lactoperoxidase. Partial characterization of the further purified protein. Biochemistry. 1967 Oct;6(10):2965–2977. doi: 10.1021/bi00862a002. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Wickus G. G., Burge B. W. Enzymatic iodination of Sindbis virus proteins. J Virol. 1973 May;11(5):730–735. doi: 10.1128/jvi.11.5.730-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Haslam E. A. The polypeptides of influenza virus. V. Localization of polypeptides in the virion by iodination techniques. Virology. 1971 Dec;46(3):764–773. doi: 10.1016/0042-6822(71)90078-x. [DOI] [PubMed] [Google Scholar]
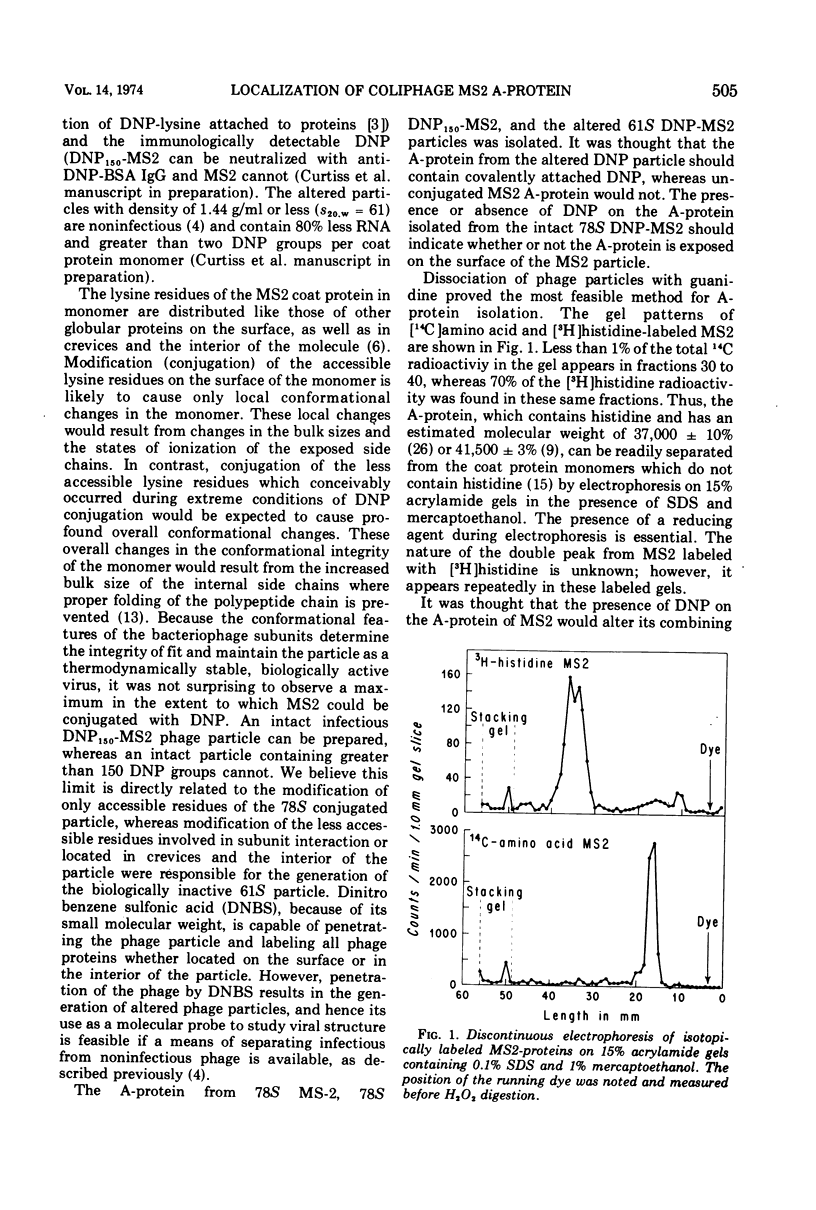
- Steitz J. A. Isolation of the A protein from bacteriphage R17. J Mol Biol. 1968 May 14;33(3):937–945. doi: 10.1016/0022-2836(68)90329-x. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., STRAND M. COMPLEXES OF F-PILI AND RNA BACTERIOPHAGE. Science. 1965 Apr 23;148(3669):511–513. doi: 10.1126/science.148.3669.511. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Ward R., Strand M. The replication cycle of RNA bacteriophages. Adv Virus Res. 1969;15:1–59. doi: 10.1016/s0065-3527(08)60873-8. [DOI] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Slobin L. I. Neutralization of haptenated bacteriophage f2 by anti-hapten antibodies: direct evidence for a critical site. J Immunol. 1972 Apr;108(4):927–936. [PubMed] [Google Scholar]


