Abstract
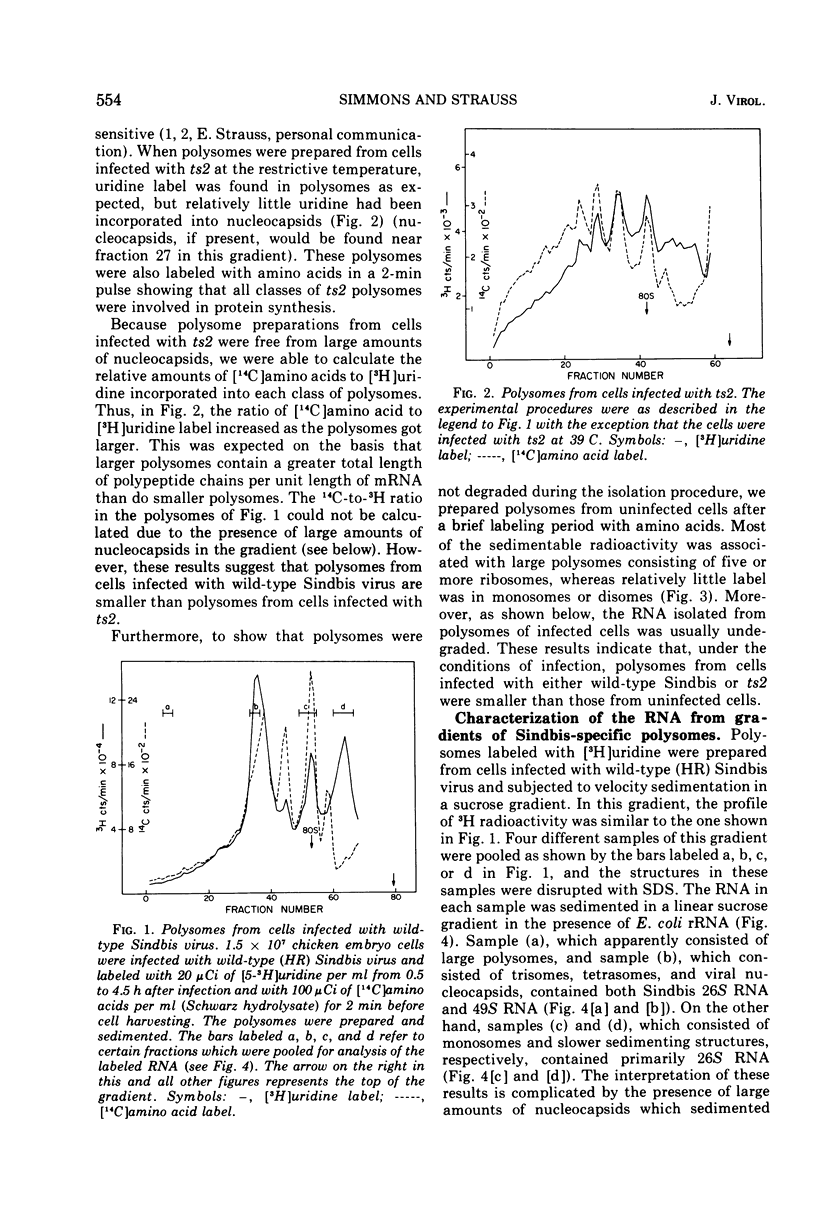
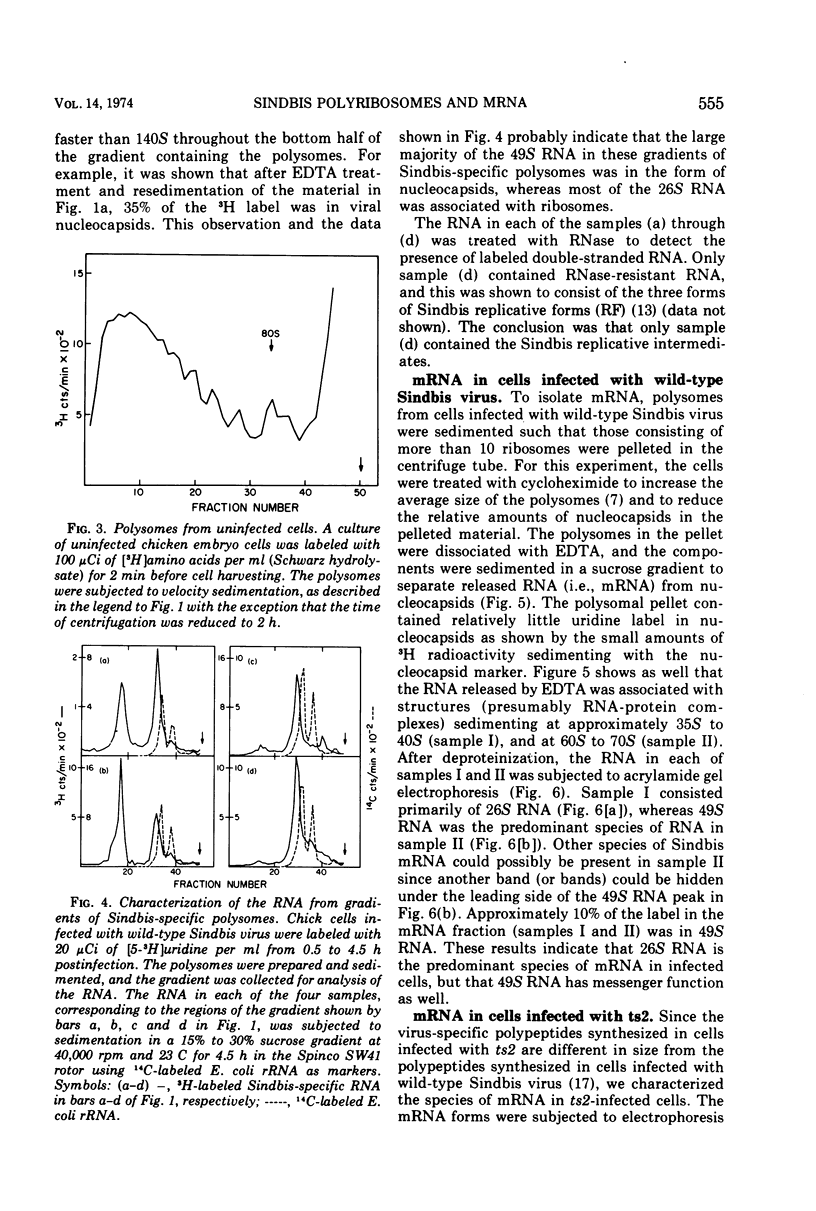
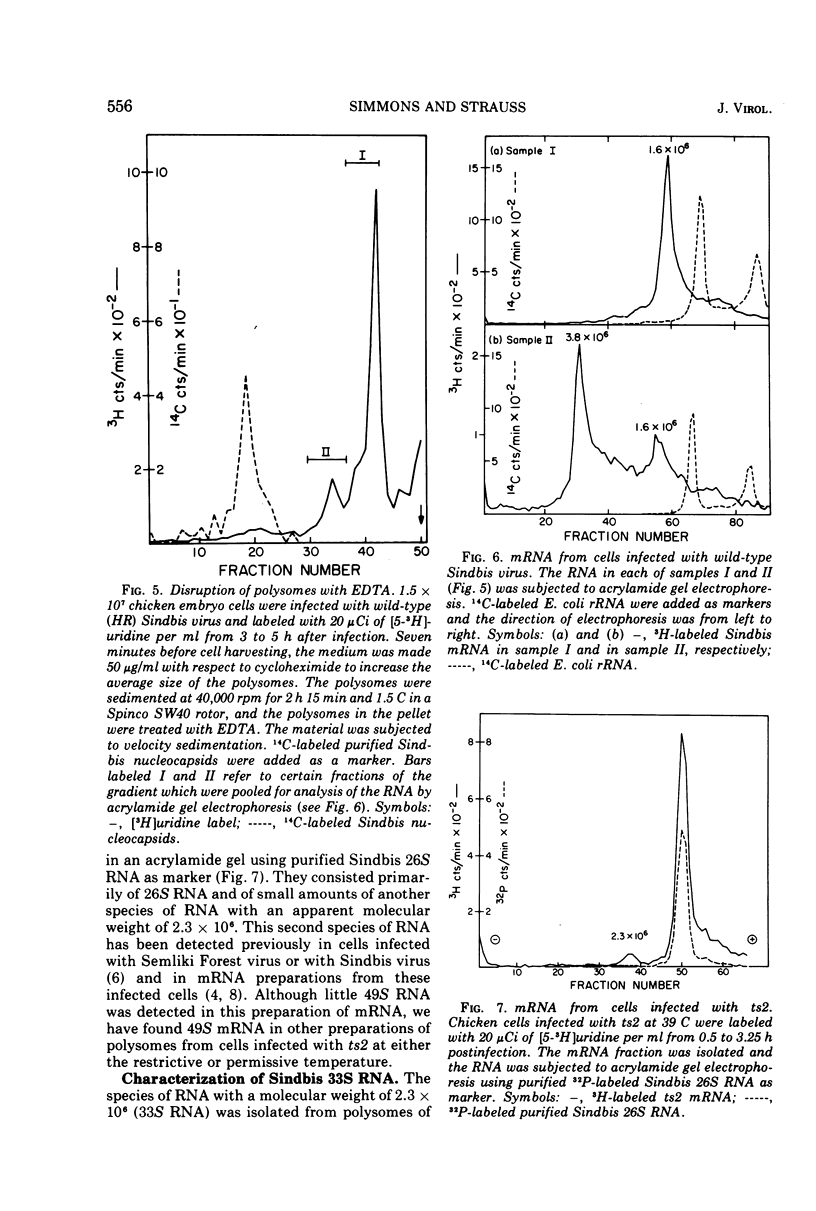
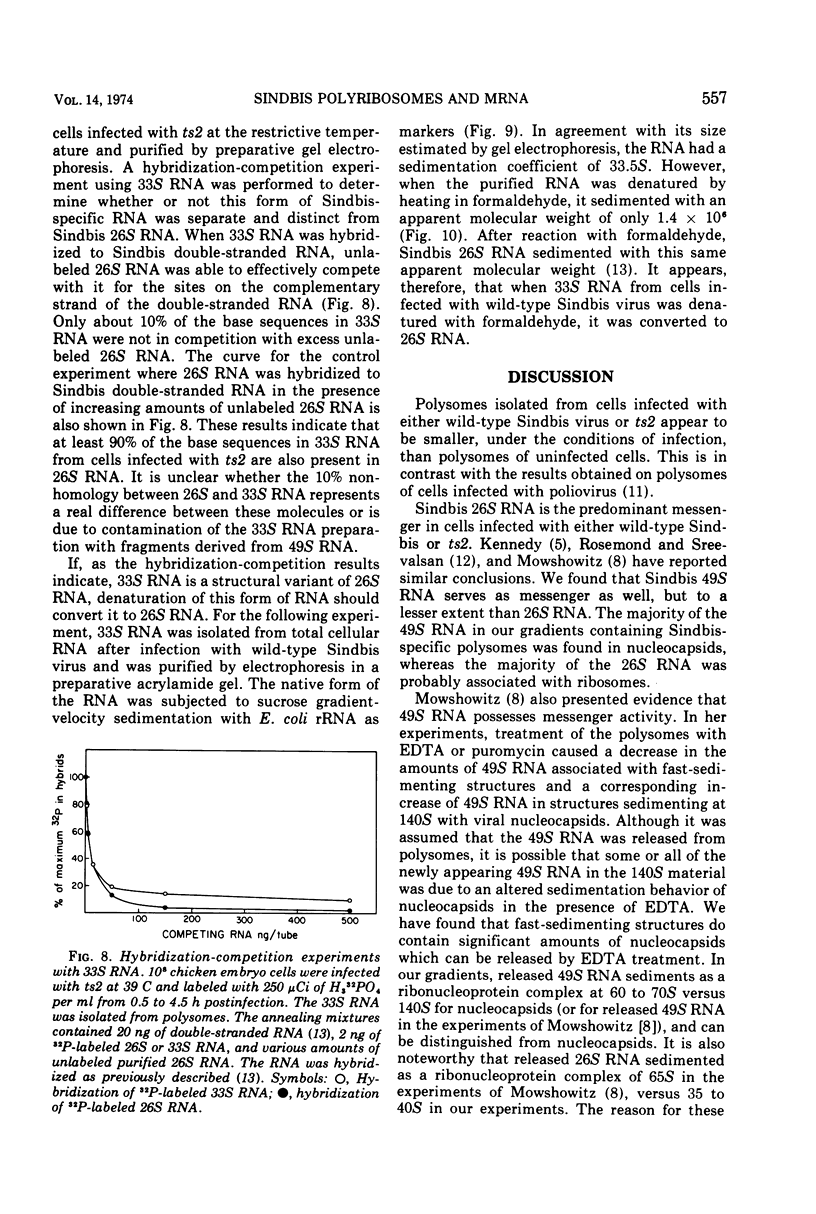
Cells infected with wild-type Sindbis virus contain at least two forms of mRNA, 26S and 49S RNA. Sindbis 26S RNA (molecular weight 1.6 × 106) constitutes 90% by weight of the mRNA in infected cells, and is thought to specify the structural proteins of the virus. Sindbis 49S RNA, the viral genome (molecular weight 4.3 × 106), constitutes approximately 10% of the mRNA in infected cells and is thought to supply the remaining viral functions. In cells infected with ts2, a temperature-sensitive mutant of Sindbis virus, the messenger forms also include a third species of RNA with a sedimentation coefficient of 33S and an apparent molecular weight of 2.3 × 106. Hybridization-competition experiments showed that 90% of the base sequences in 33S RNA from these cells are also present in 26S RNA. Sindbis 33S RNA was also isolated from cells infected with wild-type virus. After reaction with formaldehyde, this species of 33S RNA appeared to be completely converted to 26S RNA. These results indicate that 33S RNA isolated from cells infected with either wild-type Sindbis or ts2 is not a unique and separate form of Sindbis RNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burge B. W., Pfefferkorn E. R. Functional defects of temperature-sensitive mutants of Sindbis virus. J Mol Biol. 1968 Jul 14;35(1):193–205. doi: 10.1016/s0022-2836(68)80047-6. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Dobos P., Faulkner P. Molecular weight of Sindbis virus ribonucleic acid as measured by polyacrylamide gel electrophoresis. J Virol. 1970 Jul;6(1):145–147. doi: 10.1128/jvi.6.1.145-147.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Levy H. B., Carter W. B. Replication of semliki forest virus: three forms of viral RNA produced during infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):440–446. doi: 10.1073/pnas.56.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I. Isolation and identification of the virus-specified RNA species found on membrane-bound polyribosomes of chick embryo cells infected with Semliki Forest virus. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1254–1258. doi: 10.1016/0006-291x(72)90846-7. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. G., Housman D., Jacobsen M. Initiation of hemoglobin synthesis. Specific inhibition by antibiotics and bacteriophage ribonucleic acid. Biochemistry. 1971 Jun 8;10(12):2348–2356. doi: 10.1021/bi00788a027. [DOI] [PubMed] [Google Scholar]
- Mowshowitz D. Identification of polysomal RNA in BHK cells infected by sindbis virus. J Virol. 1973 Apr;11(4):535–543. doi: 10.1128/jvi.11.4.535-543.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Pierce J. S., Strauss E. G., Strauss J. H. Effect of ionic strength on the binding of Sindbis virus to chick cells. J Virol. 1974 May;13(5):1030–1036. doi: 10.1128/jvi.13.5.1030-1036.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICH A., PENMAN S., BECKER Y., DARNELL J., HALL C. POLYRIBOSOMES: SIZE IN NORMAL AND POLIO- INFECTED HELA CELLS. Science. 1963 Dec 27;142(3600):1658–1663. doi: 10.1126/science.142.3600.1658. [DOI] [PubMed] [Google Scholar]
- Rosemond H., Sreevalsan T. Viral RNAs associated with ribosomes in Sindbis virus-infected HeLa cells. J Virol. 1973 Mar;11(3):399–415. doi: 10.1128/jvi.11.3.399-415.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. II. Multiple forms of double-stranded RNA isolated from infected cells. J Mol Biol. 1972 Nov 28;71(3):615–631. doi: 10.1016/s0022-2836(72)80027-5. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Lockart R. Z., Jr Heterogeneous RNA's occurring during the replication of Western equine encephalomyelitis virus. Proc Natl Acad Sci U S A. 1966 Apr;55(4):974–981. doi: 10.1073/pnas.55.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]


