Abstract
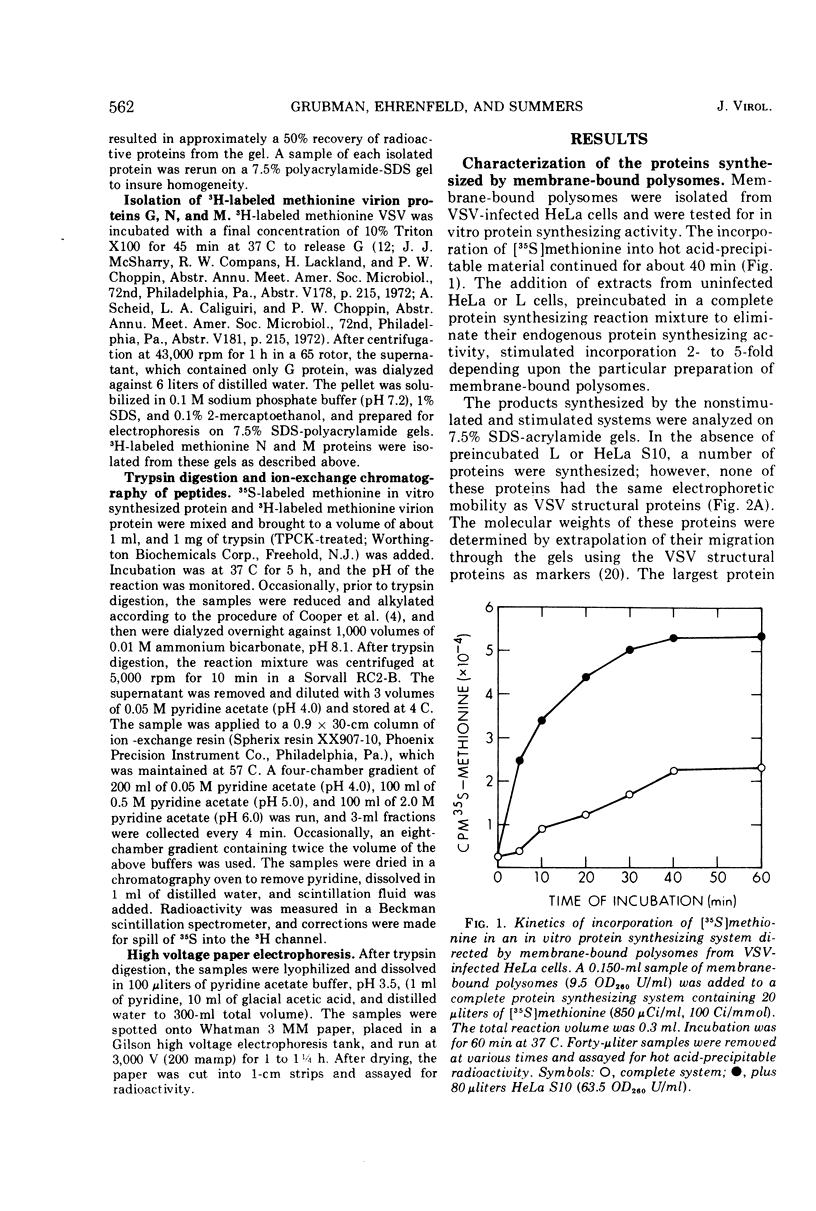
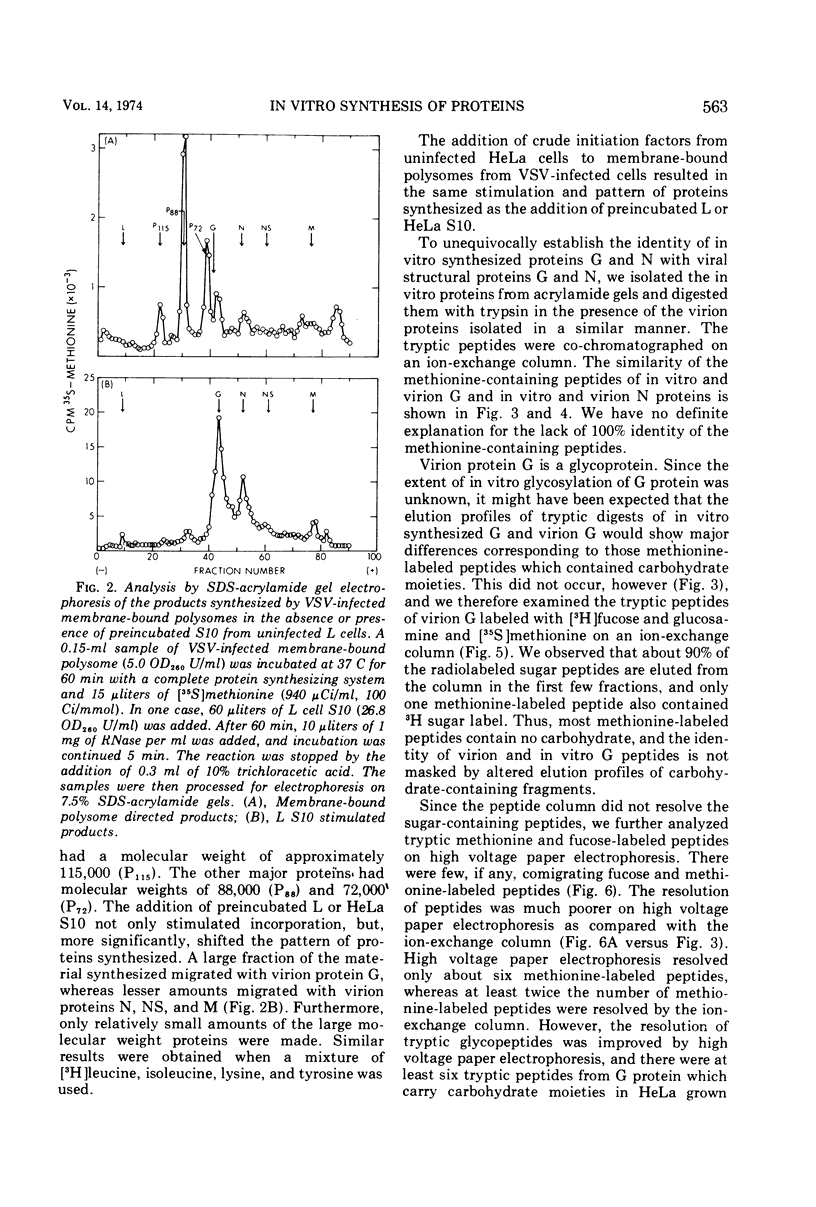
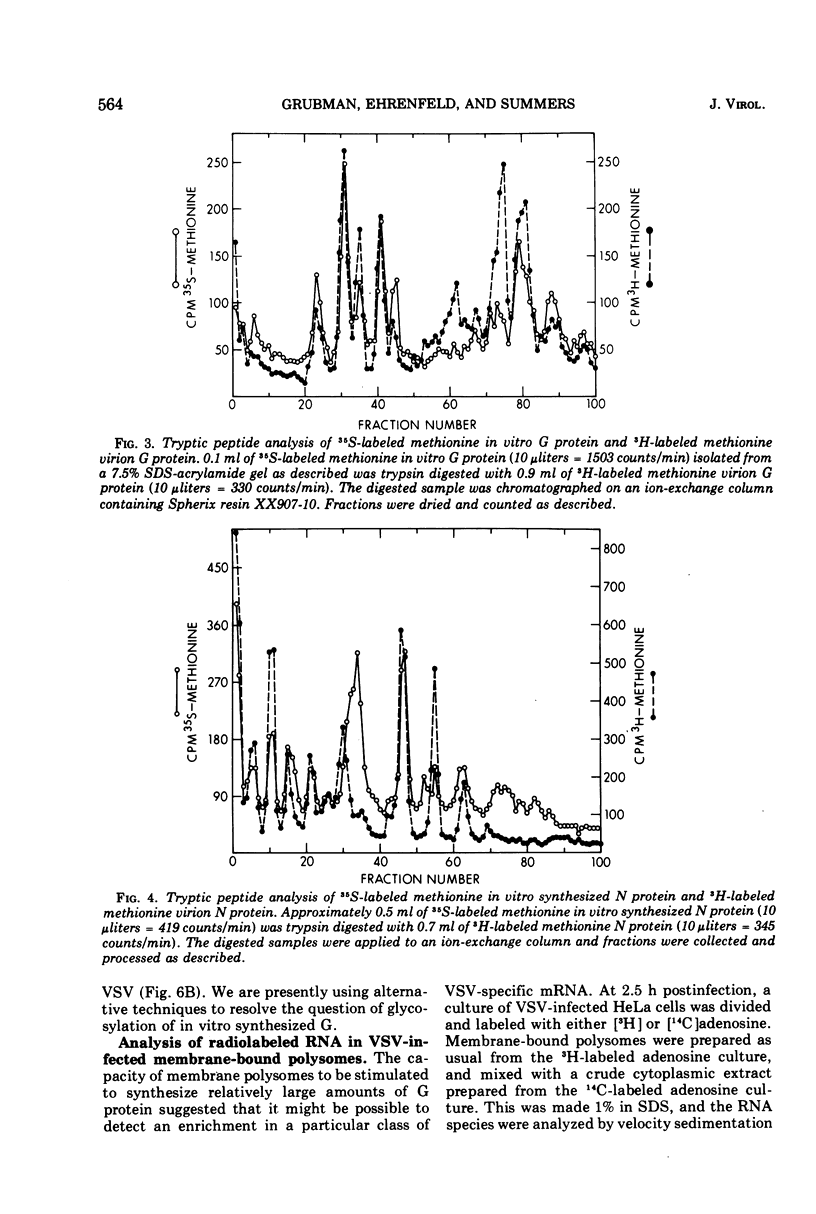
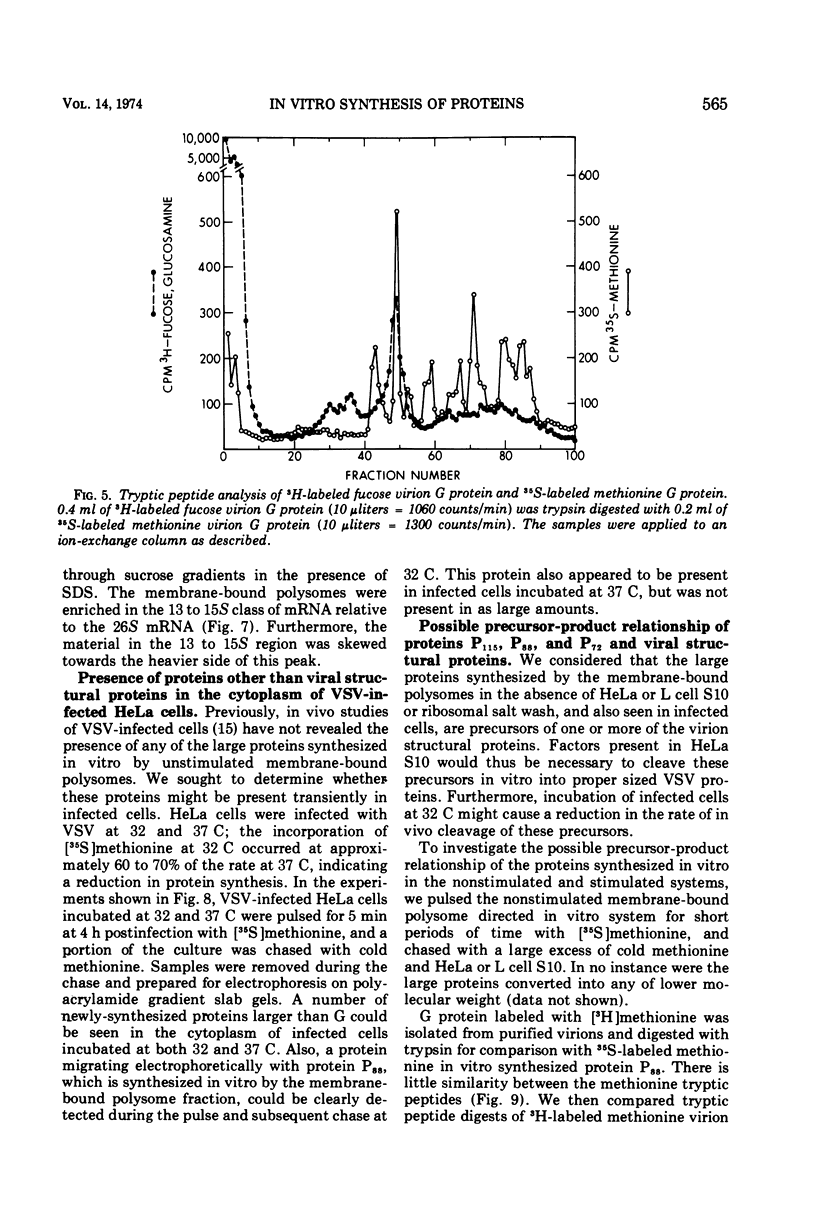
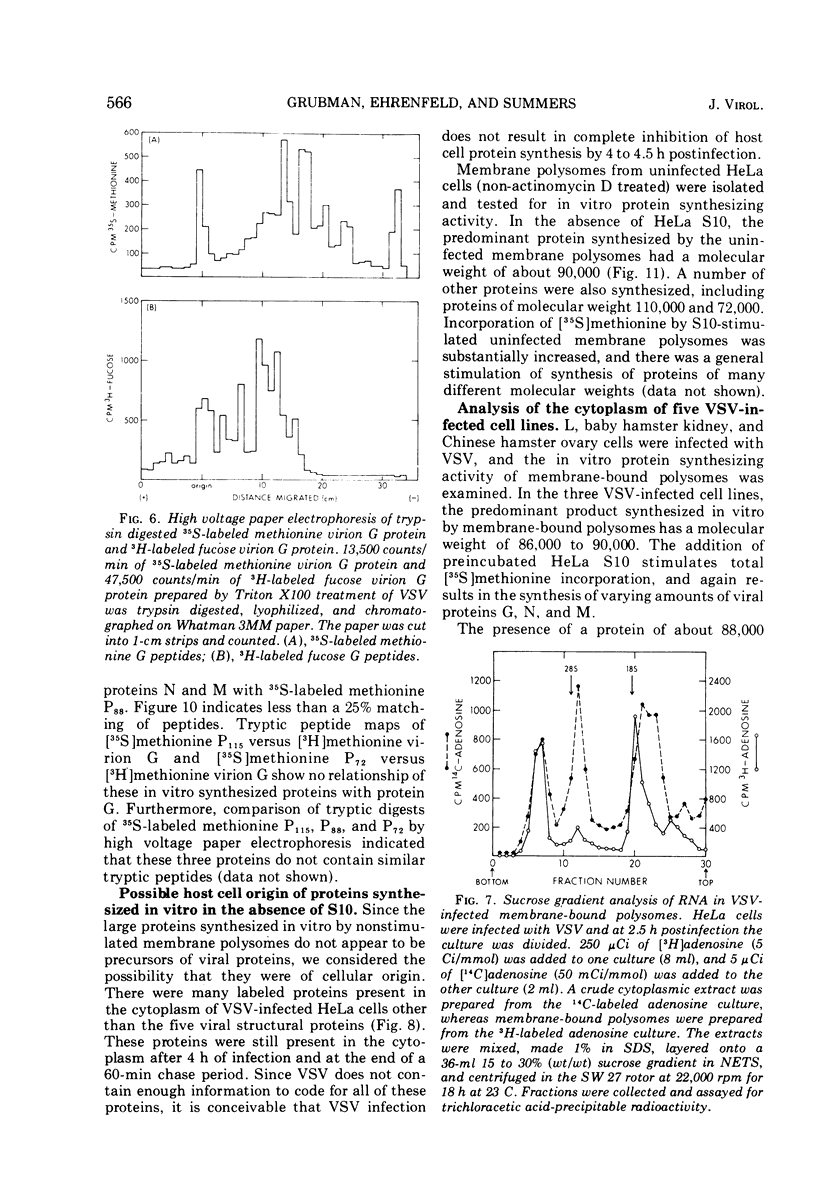
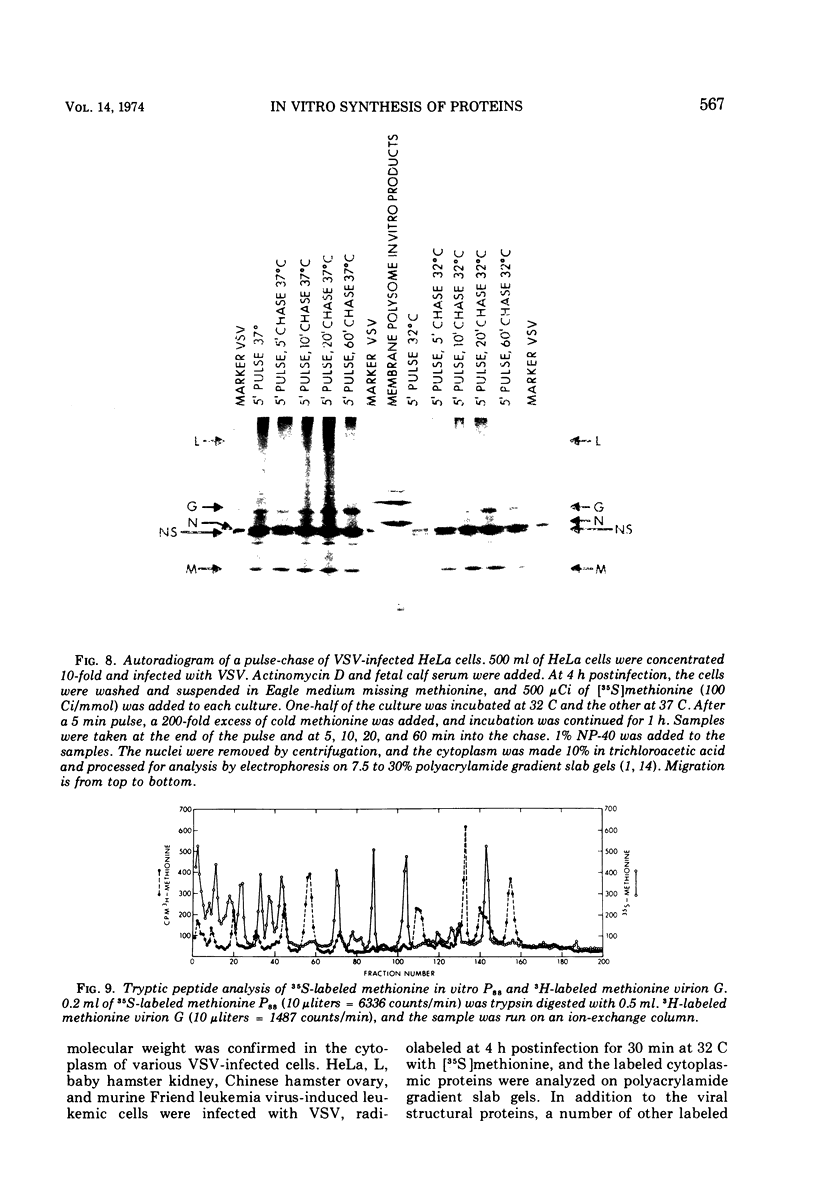
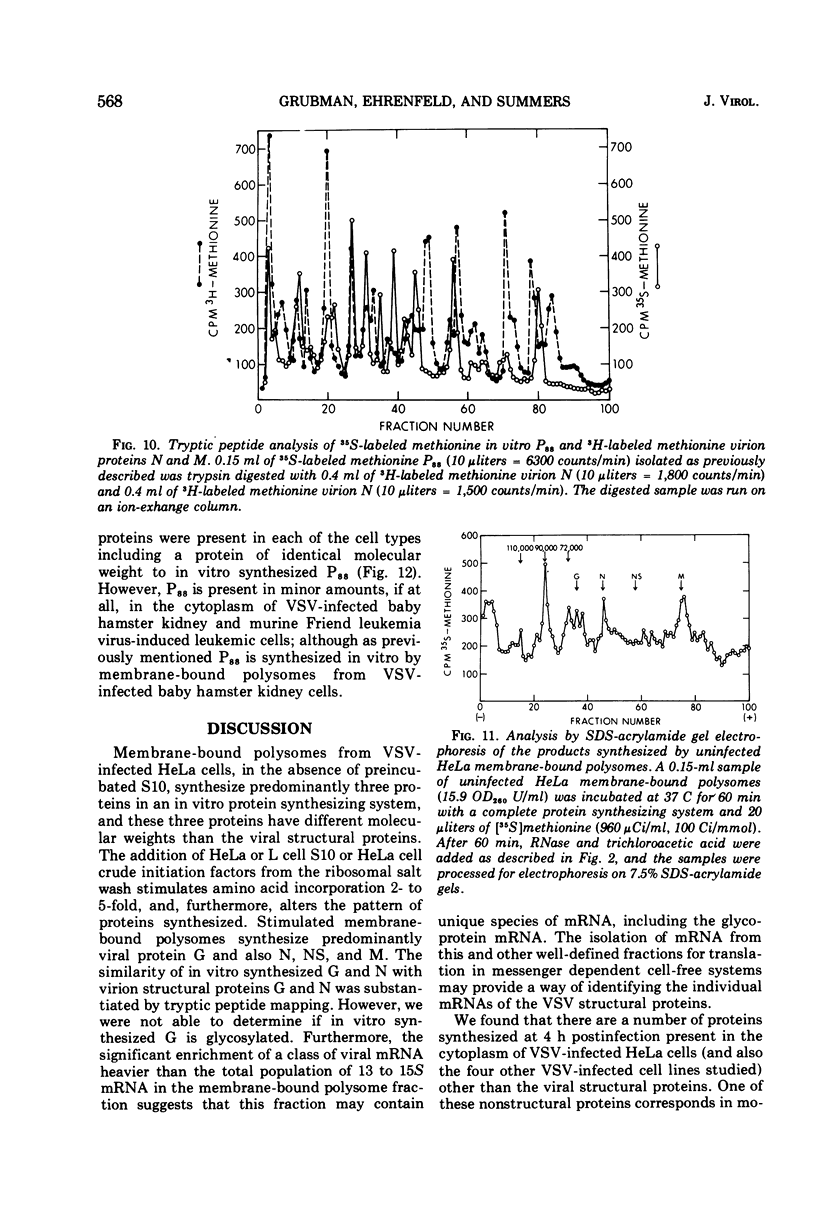
Membrane-bound polysomes from vesicular stomatitis virus (VSV)-infected HeLa cells synthesize predominantly three proteins in an in vitro protein synthesizing system. These three proteins have different molecular weights than the viral structural proteins, i.e., 115,000, 88,000, and 72,000. Addition of preincubated L or HeLa cell S10 or HeLa cell crude initiation factors stimulates amino acid incorporation and, furthermore, alters the pattern of proteins synthesized. Stimulated membrane-bound polysomes synthesize predominantly viral protein G and lesser amounts of N, NS, and M. In vitro synthesized proteins G and N are very similar to virion proteins G and N based on analysis of tryptic methionine-labeled peptides. Most methionine-labeled tryptic peptides of virion G protein contain no carbohydrate moieties, since about 90% of sugar-labeled peptides co-chromatograph with only about 10% of methionine-labeled peptides. Sucrose gradient analysis of the labeled RNA present in VSV-infected membrane-bound polysomes reveals a relative enrichment in a class of viral RNA sedimenting slightly faster than the total population of the 13 to 15S mRNA, as compared to a VSV-infected crude cytoplasmic extract. A number of proteins, other than the viral structural proteins, are synthesized in the cytoplasm of five lines of VSV-infected cells. One of these proteins has the same molecular weight as the major in vitro synthesized protein, P88. In vitro synthesized protein P88 does not appear to be a precursor of viral structural proteins G, N, or M based on pulse-chase experiments and tryptic peptide mapping. Nonstimulated membrane-bound polysomes from uninfected HeLa cells synthesize the same size distribution of proteins as nonstimulated VSV-infected membrane-bound polysomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Talbot P., Brown F. The proteins of biologically active sub-units of vesicular stomatitis virus. J Gen Virol. 1970 Jun;7(3):267–272. doi: 10.1099/0022-1317-7-3-267. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Atkinson P. H., Summers D. F. Interactions of vesicular stomatitis virus structural proteins with HeLa plasma membranes. Nat New Biol. 1971 May 26;231(21):121–123. doi: 10.1038/newbio231121a0. [DOI] [PubMed] [Google Scholar]
- Cooper P. D., Summers D. F., Maizel J. V. Evidence for ambiguity in the posttranslational cleavage of poliovirus proteins. Virology. 1970 Jul;41(3):408–418. doi: 10.1016/0042-6822(70)90161-3. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Milstein C. Stability of cytoplasmic ribonucleic acid in a mouse myeloma: estimation of the half-life of the messenger RNA coding for an immunoglobulin light chain. J Mol Biol. 1974 Feb 5;82(4):469–481. doi: 10.1016/0022-2836(74)90242-3. [DOI] [PubMed] [Google Scholar]
- David A. E. Assembly of the vesicular stomatitis virus envelope: incorporation of viral polypeptides into the host plasma membrane. J Mol Biol. 1973 May 5;76(1):135–148. doi: 10.1016/0022-2836(73)90085-5. [DOI] [PubMed] [Google Scholar]
- Friend C., Patuleia M. C., De Harven E. Erythrocytic maturation in vitro of murine (Friend) virus-induced leukemic cells. Natl Cancer Inst Monogr. 1966 Sep;22:505–522. [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Summers D. F. In vitro protein-synthesizing activity of vesicular stomatitis virus-infected cell extracts. J Virol. 1973 Aug;12(2):265–274. doi: 10.1128/jvi.12.2.265-274.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Effect of vesicular stomatitis virus infection on the histocompatibility antigen of L cells. J Virol. 1972 Oct;10(4):578–585. doi: 10.1128/jvi.10.4.578-585.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. I. Polyacrylamide gel analysis of viral antigens. J Virol. 1969 Apr;3(4):404–413. doi: 10.1128/jvi.3.4.404-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D. Cleavage of viral precursor proteins in vivo and in vitro. J Virol. 1972 Oct;10(4):751–759. doi: 10.1128/jvi.10.4.751-759.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Penman S. Membrane-associated protein synthesis of mammalian cells. I. The two classes of membrane-associated ribosomes. J Mol Biol. 1971 Jul 28;59(2):227–241. doi: 10.1016/0022-2836(71)90048-9. [DOI] [PubMed] [Google Scholar]
- Roumiantzeff M., Summers D. F., Maizel J. V., Jr In vitro protein synthetic activity of membrane-bound poliovirus polyribosomes. Virology. 1971 May;44(2):249–258. doi: 10.1016/0042-6822(71)90257-1. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Shaw E. N., Stewart M. L., Maizel J. V., Jr Inhibition of cleavage of large poliovirus-specific precursor proteins in infected HeLa cells by inhibitors of proteolytic enzymes. J Virol. 1972 Oct;10(4):880–884. doi: 10.1128/jvi.10.4.880-884.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Kiley M. P., Snyder R. M., Schnaitman C. A. Cytoplasmic compartmentalization of the protein and ribonucleic acid species of vesicular stomatitis virus. J Virol. 1972 Apr;9(4):672–683. doi: 10.1128/jvi.9.4.672-683.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Interferon production and inhibition of host synthesis in cells infected with vesicular stomatitis virus. J Virol. 1970 Oct;6(4):476–484. doi: 10.1128/jvi.6.4.476-484.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Wagner R. R. Action of interferon: kinetics and differential effects on viral functions. J Virol. 1970 Oct;6(4):421–429. doi: 10.1128/jvi.6.4.421-429.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]




