Abstract
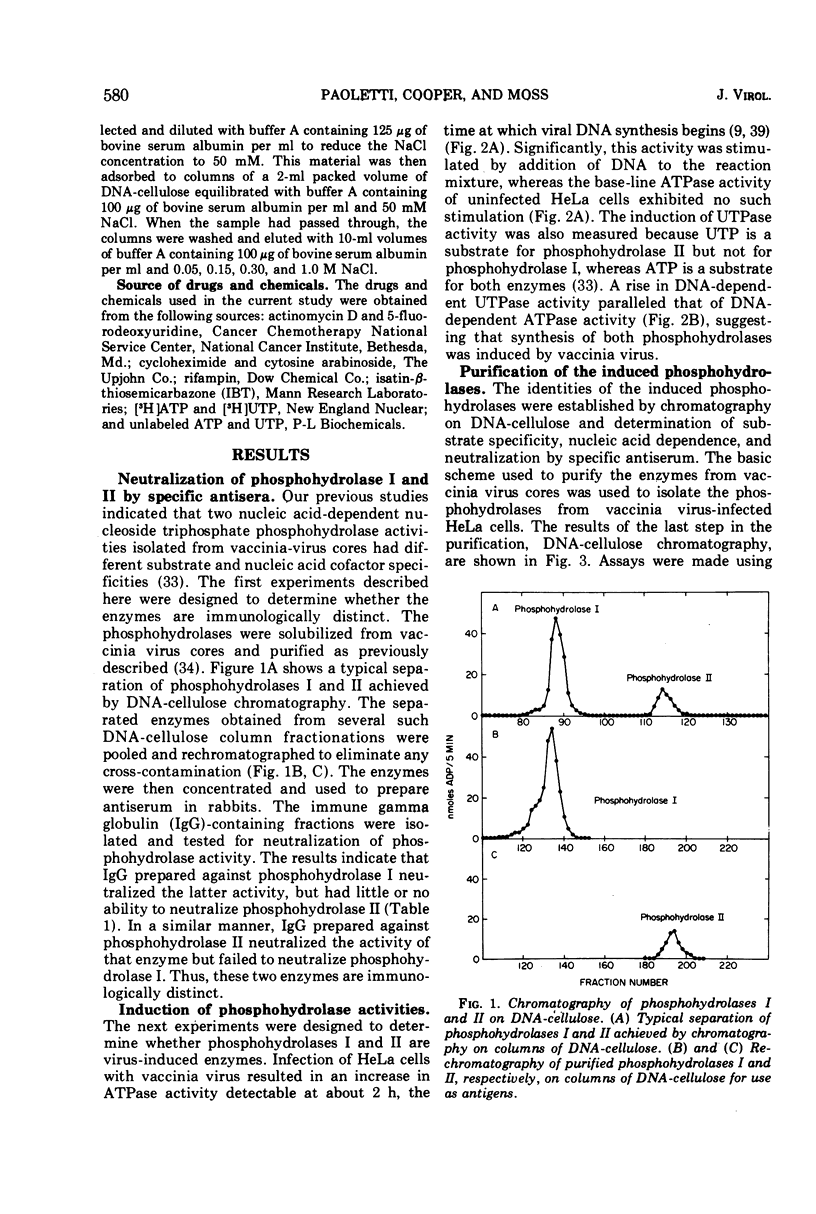
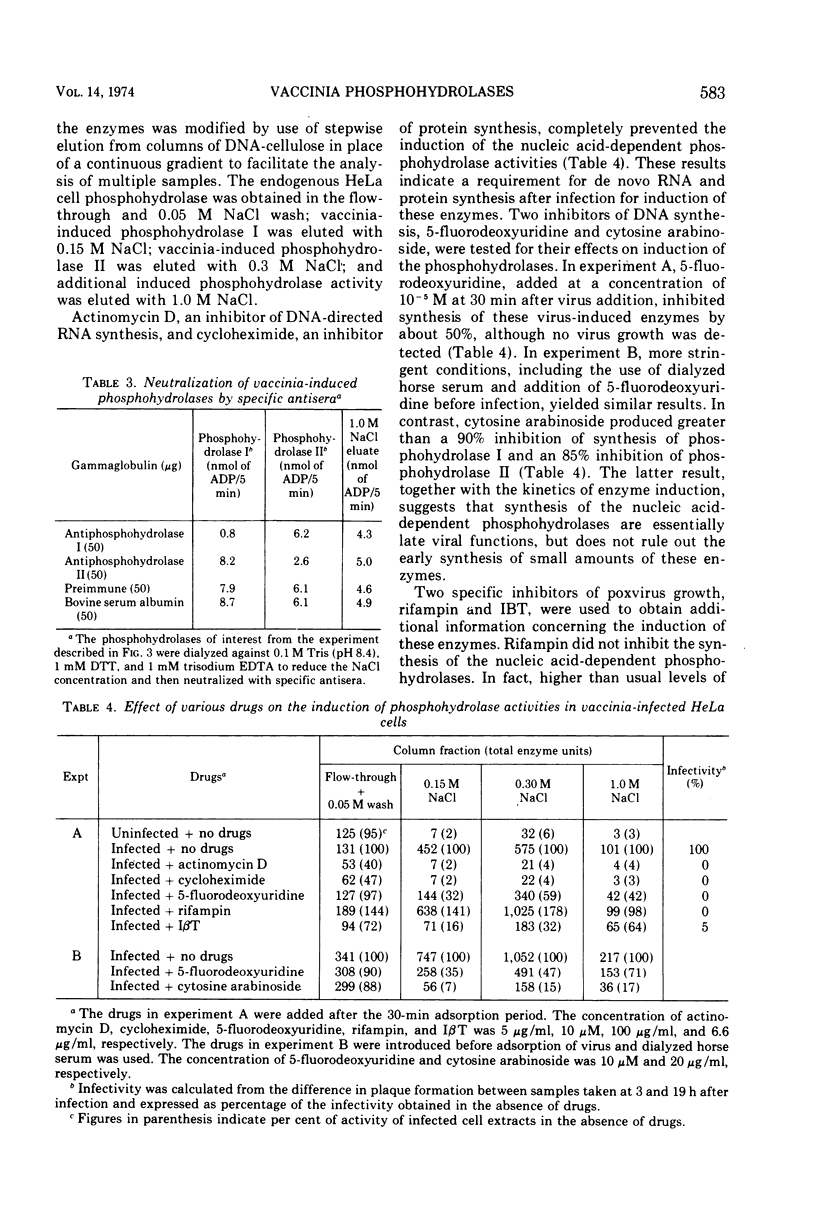
The two nucleic acid-dependent nucleoside triphosphate phosphohydrolases, previously purified from vaccinia virus cores, were shown to be immunologically distinct enzymes. Antiserum prepared against purified phosphohydrolase I and antiserum prepared against purified phosphohydrolase II only neutralized the activity of that enzyme used as antigen. Both enzymes were induced in HeLa cells after vaccinia infection. DNA-cellulose chromatography was used to purify the two phosphohydrolases from the cytoplasms of infected cells. The enzymes were identified by their different substrate specificities, nucleic acid dependence, and neutralization with specific antiserum. A third chromatographically separable nucleic acid-dependent phosphohydrolase similar to phosphohydrolase I in substrate specificity but not neutralizable by antiserum to either phosphohydrolase I or II, was also isolated from infected cells. No nucleic acid-dependent nucleoside triphosphate phosphohydrolase activity was detected by similar methods from uninfected HeLa cells. Formation of these virus-induced enzymes was prevented by actinomycin D and cycloheximide, indicating a requirement for de novo RNA and protein synthesis, respectively. The kinetics of induction and inhibition by cytosine arabinoside, an inhibitor of DNA synthesis, suggested that synthesis of the phosphohydrolases is a late viral function. Rifampin, an inhibitor of vaccinia virus growth which prevents virion assembly, had no inhibitory effect on the induction of the phosphohydrolases. This result was consistent with the finding that these enzymes exist in a soluble as well as in a particulate form in the cytoplasm of infected cells. Addition of another specific anti-poxviral drug, isatin-β-thiosemicarbazone, to vaccinia-infected cells partially inhibited induction of the phosphohydrolases.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard G., Hume V. B., Westwood J. C. The effect of thiosemicarbazones on the growth of rabbit pox virus in tissue culture. Ann N Y Acad Sci. 1965 Jul 30;130(1):92–104. doi: 10.1111/j.1749-6632.1965.tb12543.x. [DOI] [PubMed] [Google Scholar]
- Aubertin A. M., McAuslan B. R. Virus-associated nucleases: evidence for endonuclease and exonuclease activity in rabbitpox and vaccinia viruses. J Virol. 1972 Mar;9(3):554–556. doi: 10.1128/jvi.9.3.554-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Dorson J. W., Bollum F. J. Terminal riboadenylate transferase: a poly A polymerase in purified vaccinia virus. J Virol. 1973 Aug;12(2):203–208. doi: 10.1128/jvi.12.2.203-208.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer D. N., Rogers H. W., Randall C. C. Endogenous protein kinase and phosphate acceptor proteins in vaccinia virus. Virology. 1973 Mar;52(1):13–21. doi: 10.1016/0042-6822(73)90393-0. [DOI] [PubMed] [Google Scholar]
- Eron L. J., McAuslan B. R. The nature of poxvirus-induced deoxyribonucleas. Biochem Biophys Res Commun. 1966 Mar 8;22(5):518–523. doi: 10.1016/0006-291x(66)90305-6. [DOI] [PubMed] [Google Scholar]
- Gold P. H., Dales S. Localization of nucleotide phosphohydrolase activity within vaccinia. Proc Natl Acad Sci U S A. 1968 Jul;60(3):845–852. doi: 10.1073/pnas.60.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimley P. M., Rosenblum E. N., Mims S. J., Moss B. Interruption by Rifampin of an early stage in vaccinia virus morphogenesis: accumulation of membranes which are precursors of virus envelopes. J Virol. 1970 Oct;6(4):519–533. doi: 10.1128/jvi.6.4.519-533.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J. Enhanced thymidine phosphorylating activity of mouse fibroblasts (strain LM) following vaccinia infection. Biochem Biophys Res Commun. 1962 Jun 19;8:72–75. doi: 10.1016/0006-291x(62)90238-3. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. II. Synthesis of polyriboadenylic acid. J Mol Biol. 1970 May 28;50(1):19–33. doi: 10.1016/0022-2836(70)90101-4. [DOI] [PubMed] [Google Scholar]
- Katz E., Grimley P., Moss B. Reversal of anti-viral effects of rifampicin. Nature. 1970 Sep 5;227(5262):1050–1051. doi: 10.1038/2271050a0. [DOI] [PubMed] [Google Scholar]
- Katz E., Margalith E., Winer B., Goldblum N. Synthesis of vaccinia virus polypeptides in the presence of isatin-beta-thiosemicarbazone. Antimicrob Agents Chemother. 1973 Jul;4(1):44–48. doi: 10.1128/aac.4.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman J., Moss B. Protein kinase activity from vaccinia virions: solubilization and separation into heat-labile and heat-stable components. J Virol. 1973 Oct;12(4):684–689. doi: 10.1128/jvi.12.4.684-689.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuslan B. R., Herde P., Pett D., Ross J. Nucleases of virus-infected animal cells. Biochem Biophys Res Commun. 1965 Sep 8;20(5):586–591. doi: 10.1016/0006-291x(65)90439-0. [DOI] [PubMed] [Google Scholar]
- Mcauslan B. R. Rifampicin inhibition of vaccinia replication. Biochem Biophys Res Commun. 1969 Oct 8;37(2):289–295. doi: 10.1016/0006-291x(69)90733-5. [DOI] [PubMed] [Google Scholar]
- Moss B., Katz E., Rosenblum E. N. Vaccinia virus directed RNA and protein synthesis in the presence of rifampicin. Biochem Biophys Res Commun. 1969 Aug 22;36(5):858–865. doi: 10.1016/0006-291x(69)90688-3. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Katz E., Grimley P. M. Rifampicin: a specific inhibitor of vaccinia virus assembly. Nature. 1969 Dec 27;224(5226):1280–1284. doi: 10.1038/2241280a0. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N. Letter: Protein cleavage and poxvirus morphogenesis: tryptic peptide analysis of core precursors accumulated by blocking assembly with rifampicin. J Mol Biol. 1973 Dec 5;81(2):267–269. doi: 10.1016/0022-2836(73)90195-2. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Paoletti E. Polyadenylate polymerase from vaccinia virions. Nat New Biol. 1973 Sep 12;245(141):59–63. doi: 10.1038/newbio245059a0. [DOI] [PubMed] [Google Scholar]
- Moss B., Salzman N. P. Sequential protein synthesis following vaccinia virus infection. J Virol. 1968 Oct;2(10):1016–1027. doi: 10.1128/jvi.2.10.1016-1027.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Ospina J., Grace J. T., Jr Nucleotide phosphohydrolase in purified vaccinia virus. J Virol. 1968 Mar;2(3):167–172. doi: 10.1128/jvi.2.3.167-172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya A., Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of rifampicin. Virology. 1970 Apr;40(4):1039–1051. doi: 10.1016/0042-6822(70)90150-9. [DOI] [PubMed] [Google Scholar]
- Paolette E., Rosemond-Hornbeak H., Moss B. Two nucleid acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3273–3280. [PubMed] [Google Scholar]
- Paoletti E., Moss B. Deoxyribonucleic acid-dependent nucleotide phosphohydrolase activity in purified vaccinia virus. J Virol. 1972 Oct;10(4):866–868. doi: 10.1128/jvi.10.4.866-868.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Protein kinase and specific phosphate acceptor proteins associated with vaccinia virus cores. J Virol. 1972 Sep;10(3):417–424. doi: 10.1128/jvi.10.3.417-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Two nucleic acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Nucleotide substrate and polynucleotide cofactor specificities. J Biol Chem. 1974 May 25;249(10):3281–3286. [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus morphogenesis: a comparison of virus-induced antigens and polypeptides. J Gen Virol. 1973 Apr;19(1):65–79. doi: 10.1099/0022-1317-19-1-65. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Moss B. Single-stranded deoxyribonucleic acid-specific nuclease from vaccinia virus. Endonucleolytic and exonucleolytic activities. J Biol Chem. 1974 May 25;249(10):3292–3296. [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Paoletti E., Moss B. Single-stranded deoxyribonucleic acid-specific nuclease from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3287–3291. [PubMed] [Google Scholar]
- SALZMAN N. P. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. 1960 Jan;10:150–152. doi: 10.1016/0042-6822(60)90015-5. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Shatkin A. J. Polynucleotide ligase activity in cells infected with simian virus 40, polyoma virus, or vaccinia virus. J Virol. 1969 Nov;4(5):719–726. doi: 10.1128/jvi.4.5.719-726.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., McAuslan B. R. Effect of rifampicin on poxvirus protein synthesis. J Virol. 1970 Sep;6(3):326–332. doi: 10.1128/jvi.6.3.326-332.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson B., Joklik W. K. The inhibition of vaccinia virus multiplication by isatin-beta-thiosemicarbazone. Proc Natl Acad Sci U S A. 1965 Sep;54(3):946–953. doi: 10.1073/pnas.54.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]


