Abstract
D-Aspartate (D-Asp) is an endogenous amino acid in the central nervous and reproductive systems of vertebrates and invertebrates. High concentrations of D-Asp are found in distinct anatomical locations, suggesting that it has specific physiological roles in animals. Many of the characteristics of D-Asp have been documented, including its tissue and cellular distribution, formation and degradation, as well as the responses elicited by D-Asp application. D-Asp performs important roles related to nervous system development and hormone regulation; in addition, it appears to act as a cell-to-cell signaling molecule. Recent studies have shown that D-Asp fulfills many, if not all, of the definitions of a classical neurotransmitter—that the molecule’s biosynthesis, degradation, uptake, and release take place within the presynaptic neuron, and that it triggers a response in the postsynaptic neuron after its release. Accumulating evidence suggests that these criteria are met by a heterogeneous distribution of enzymes for D-Asp’s biosynthesis and degradation, an appropriate uptake mechanism, localization within synaptic vesicles, and a postsynaptic response via an ionotropic receptor. Although D-Asp receptors remain to be characterized, the postsynaptic response of D-Asp has been studied and several L-glutamate receptors are known to respond to D-Asp. In this review we discuss the current status of research on D-Asp in neuronal and neuroendocrine systems, and highlight results that support D-Asp’s role as a signaling molecule.
Keywords: D-aspartate, D-amino acids, nervous system, neurotransmitter, endocrine gland
Introduction
In animals, L-amino acids are ubiquitous and used exclusively for ribosomal protein synthesis. Although less abundant, free D-amino acids have been reported in a diverse range of invertebrate and vertebrate species (Hamase et al. 2002; Fuchs et al. 2005). D-aspartic acid (D-Asp) is a D-amino acid that has received significant attention because of its presence in animal nervous and reproductive systems (D’Aniello and Giuditta 1977; Dunlop et al. 1986; Fisher et al. 1991; Hashimoto et al. 1993; D’Aniello et al. 1996; Schell et al. 1997; D’Aniello 2007; Homma 2007); however, questions about its metabolism, cellular function, biological and physiological roles, and pathological significance remain. Investigations of D-Asp in living organisms have involved various animals, including cephalopods (D’Aniello and Giuditta 1977; D’Aniello et al. 1995b; D’Aniello et al. 2011), gastropods (D’Aniello et al. 1993b; Shibata et al. 2003; Miao et al. 2006a; Spinelli et al. 2006), amphibians (Di Fiore et al. 1998; Raucci and Di Fiore 2011), reptiles (Assisi et al. 2001; Raucci and Di Fiore 2010), and mammals, including humans (Fisher et al. 1991; Hamase et al. 1999; Morikawa et al. 2007). These studies document its widespread occurrence and suggest that D-Asp plays important roles throughout the Metazoan.
In this review, we focus on the more recent reports on D-Asp in animal nervous and neuroendocrine systems, from the molecular to physiological levels, emphasizing studies that provide insight into its functions. To be considered as neurotransmitter, D-Asp needs to demonstrate the following criteria (Kandel et al. 2000): be present in the presynaptic neuron, be released into the synaptic cleft upon stimulation, induce depolarization of the postsynaptic membrane through a receptor or an ion channel, and be removed from the synaptic cleft by uptake or degradation. Hence, we highlight studies that describe its localization, transport, release and electrophysiological responses, as these suggest that D-Asp can act as a hormone, transmitter, and/or trophic factor, and accordingly, has a significant role in cell-to-cell signaling within the nervous system. Where appropriate, we also describe the analytical approaches used in these studies because chiral measurement methods are not as common as those used for neurochemical measurements. For an increasing range of cell types, single cell assays (Page et al. 2002; Rubakhin et al. 2011; Lapainis et al. 2009) now allow a selected cell’s small molecule contents to be measured, thus enabling a new range of studies on unusual neurochemical pathways, with several of these approaches well suited to examining chiral amino acids.
D-Asp distribution
D-Asp has been found in the nervous and endocrine tissues of various vertebrate animals including the frog (Di Fiore et al. 1998), lizard (Assisi et al. 2001), and chicken (Neidle and Dunlop 1990). It has also been described in mammals such as mouse (Morikawa et al. 2007), rat (Imai et al. 1995; D’Aniello et al. 1996; Hamase et al. 1999; Lee et al. 1999; Masuda et al. 2003; Han et al. 2011), and human (D’Aniello et al. 2007a; D’Aniello et al. 2005a; Fisher et al. 1991). In the nervous system, a transient increase of D-Asp occurs during development but its concentration drops to trace levels in adults, at least in the chicken (Neidle and Dunlop 1990), rat (Dunlop et al. 1986; Hashimoto et al. 1993) and human (Hashimoto et al. 1993). Dunlop may be the first to demonstrate this trend of decreasing levels of D-Asp during development by measuring higher levels of D-Asp in the cerebral hemispheres of newborn rats as compared to the levels of older animals (Dunlop et al. 1986). The development-related changes in D-Asp levels showed marked regional differences within the rat central nervous system. These regional changes were further characterized using immunohistochemistry with an anti-D-Asp antibody (Sakai et al. 1998), demonstrating that in the rat embryonic brain, D-Asp appears to emerge near the hindbrain, and then spread into the forebrain. In terms of its intracellular localization, D-Asp was first observed in the cell body of neurons in the outer layer of the neural epithelium, and then appeared in the axons once a distinct axonal layer had been established. These data support the notion that D-Asp is involved in neuronal differentiation. In an another study, D-Asp was shown to have an important role in development because intense immunoreactivity was observed within the cortical plate and subventricular zone of the early postnatal rat brain, while the immunoreactivity dropped to almost undetectable levels in adults (Wolosker et al. 2000). Moreover, the same study also reported that in all examined brain regions at all ages, D-Asp immunoreactivity was restricted to neurons but not to glia, with D-Asp staining observed in both neuronal cell bodies and neuronal fiber tracks.
Concurrently with many of the vertebrate studies, D-Asp was characterized in a number of invertebrates, often with similar results. It was reported first in the brain and optic lobes of Octopus vulgaris, with D-Asp measurements performed using liquid chromatography and the clever approach of selective degradation via the enzyme D-Asp oxidase (DAspO) (D’Aniello and Giuditta 1977). Since then, D-Asp has been reported in the nervous system of other invertebrate animals spanning a number of phyla: mollusks such as the cephalopods Sepia officinalis and Loligo vulgaris (D’Aniello and Giuditta 1978; D’Aniello et al. 1995b); opisthobranchs such as Aplysia fasciata (D’Aniello et al. 1993b), Aplysia californica (Liu et al. 1998), and Aplysia limacine (Spinelli et al. 2006); arthropods such as the crustacean Jasus lalandii (Okuma and Abe 1994); and protochordates, including the tunicate Ciona intestinalis (D’Aniello et al. 2003) and amphioxus Branchiostoma lanceolatum (D’Aniello and Garcia-Fernandez 2007). D-Asp has also been found in reproductive tissues such as the glands of O. vulgaris (D’Aniello et al. 1995a).
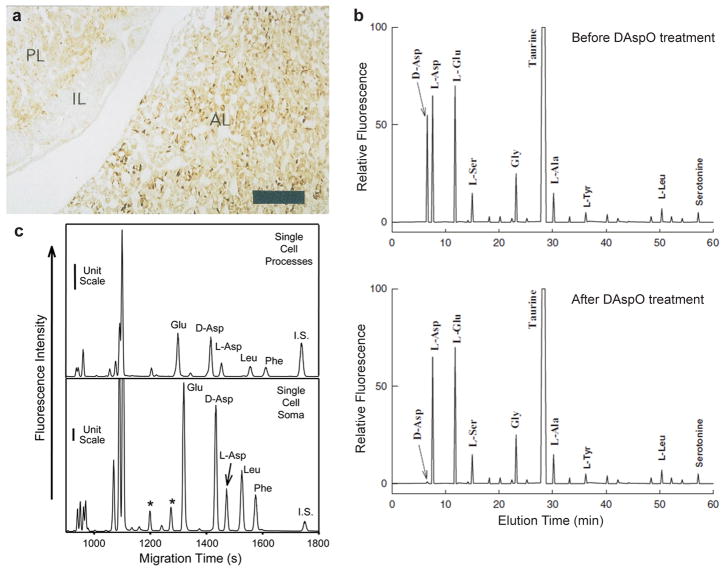
For endogenous D-Asp characterization, a range of analytical measurement approaches have been used, including chromatographic methods combined with enzymatic D-Asp digestion (Fig. 1). D-Asp localization can be examined by immunostaining with a D-Asp antibody (Fig. 1a). D-Asp quantitation has employed separations such as high performance liquid chromatography (HPLC) and capillary electrophoresis (CE) as these can provide chiral amino acid separations that enable measurements of each enantiomer (Katane and Homma 2011; Lapainis and Sweedler 2008). For enhanced confirmation of D-Asp peak identity, sample treatment by DAspO digestion (Spinelli et al. 2006) (Fig. 1b) or via immunoprecipitation (Miao et al. 2006b) can be employed.
Fig. 1. D-Asp has been characterized via multiple measurement approaches.
(a) Immunoreactivity measurement via a D-Asp antibody provides cellular localization, with the immunoreactivity against D-Asp observed in the anterior lobe (AL) and posterior lobe (PL), but not in the intermediate lobe (IL) in 6-week-old rat brain. Scale bar, 140 μm. (b) Chiral HPLC has been used for D-Asp characterization, with the D-Asp identified via its removal via D-aspartate oxidase (DAspO) digestion from Aplysia limacine cerebral ganglia neurons. Separation condition; C-18 column (0.45 cm×25 cm),1.2 mL/min flow rate with a programmed gradient consisting of solution A (5% acetonitrile in 30 mM citrate/phosphate buffer, pH 5.6) and solution B (90% acetonitrile in water). (c) Chiral capillary electrophoresis with nanoliter volume assays enables subcellular analysis, in this case from an individual Aplysia sensory neuron. *, unidentified peaks. Separation condition: 21 kV normal polarity was applied to an uncoated fused-silica capillary (65–75 cm, 50 μm i.d./360 μm o.d.) filled with separation solution consisting of 20 mM β-cyclodextrin, 50 mM sodium dodecyl sulfate in 50 mM borate buffer (pH 9.4) and 15% methanol (V/V). Panel a from (Lee et al. 1999), used with permission from Elsevier; panel b from (Spinelli et al. 2006) is adapted with permission, copyright © 2006 from John Wiley and Sons; and panel c from (Miao et al. 2005) is used with permission of the American Chemical Society, copyright 2005.
The accumulating evidence on D-Asp distribution in invertebrate animals suggests that D-Asp is involved in both neuronal and neuroendocrine systems. In neuronal cells of invertebrates, D-Asp has been found throughout the cell soma and neuronal processes (Fig. 1c). Within individually assayed sensory neurons of A. californica, D-Asp ratios (D-Asp vs. total Asp amount) in the cell soma and in morphologically different parts of neuronal processes were similar (Miao et al. 2005), although different neurons in the same ganglia contained distinct D-Asp levels (Miao et al. 2006a). Spinelli and coworkers (Spinelli et al. 2006) studied populations of cerebral ganglia neurons of A. limacine and their experiments support that D-Asp is found in both the cell soma and synaptosomes. Furthermore, they also found higher concentrations of D-Asp in the synaptic vesicles from synaptosomes than in the synaptosome preparations as a whole, and higher levels in the synaptosomes than in the cell soma, suggesting that D-Asp is indeed concentrated in synaptic vesicles. Similarly, in L. vulgaris brain neurons, D-Asp was mostly found in synaptic vesicles; D-Asp concentrations were higher in synaptic vesicles than in synaptosomes whereas D-Asp concentrations in whole brain homogenates were lower (D’Aniello et al. 2011). These studies certainly support the localization of D-Asp to both synaptic terminals and synaptic vesicles.
Biosynthesis of D-Asp
How is D-Asp formed? Even though dietary uptake of D-Asp produced by microorganisms is known to occur, the direct synthesis of D-Asp in animal cells has also been confirmed. After more than a decade of reports describing D-Asp synthesis by racemase enzymes in bacteria (Lamont et al. 1972), and D-amino acid transaminases in bacteria (Gosling and Fottrell 1978) and plants (Ogawa et al. 1973), the biosynthesis of D-Asp in higher animals was demonstrated in mammalian pheochromocytoma cells (PC12 cells), which do not spontaneously uptake extracellular D-Asp (Long et al. 1998). The study used HPLC, DAspO digestion, and immunohistochemical staining methods to show that PC12 cells contain D-Asp converted from L-Asp; D-Asp levels in these cells and the culture media increased during culturing. Since this early study, D-Asp biosynthesis has been studied in other animals and cell types: C. intestinalis (D’Aniello et al. 2003), A. limacine (Spinelli et al. 2006), A. californica (Miao et al. 2006a), S. officinalis (D’Aniello et al. 2005b), Rana esculenta (Raucci et al. 2005), and several types of mammalian cultured cells (Long et al. 2000; Long et al. 2002). Furthermore, colorimetric analyses with DAspO for Rattus norvegicus and L. vulgaris have shown that D-Asp synthesis may occur in the soma of neurons because synaptosomes and synaptic vesicles had considerably lower D-Asp production compared to that of whole brain (D’Aniello et al. 2011).
Because D-Asp appears in a D-Asp-free environment in cultured PC12 cells (Long et al. 1998), its biosynthesis has been hypothesized to occur intracellularly via an Asp racemase, transaminase, and/or the racemization of an aspartyl residue of peptides (Homma 2007; Katane and Homma 2011). Putative Asp racemases catalyze D-Asp formation using L-Asp as a substrate. Several studies using radiolabeled L-Asp support the idea that an Asp racemase is involved in D-Asp production. As examples, [14C]- L-Asp was incubated in rat embryonic neurons and [14C]-D-Asp was detected and accumulated throughout the incubation (Wolosker et al. 2000). [14C]-D-Asp synthesis from [14C]- L-Asp was also observed in cerebral ganglia neurons of A. californica (Miao et al. 2006a; Scanlan et al. 2010), and it was demonstrated that the Aplysia ganglia, which contain more neuronal cell soma, produced more [14C]-D-Asp than the sheath tissues, which predominately contain processes and terminals (Scanlan et al. 2010). The Asp racemase is expected to be pyridoxal-5’-phosphate (PLP)-dependent because aminooxyacetic acid, an inhibitor of PLP-dependent enzymes, clearly inhibited [14C]-D-Asp production in rat embryonic neurons (Wolosker et al. 2000).
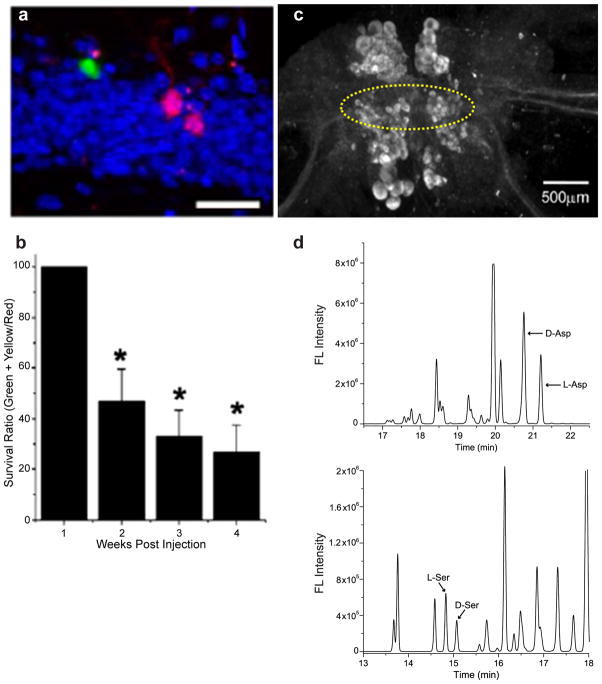
Indeed, an Asp-specific, PLP-dependent racemase has been purified from the foot muscle of the bivalve, Scapharca broughtonii (Shibata et al. 2003) and has since been cloned (Abe et al. 2006), although the presence of this racemase in the Scapharca nervous system has not been determined. Two recent studies have further clarified the enzymes present. Kim and colleagues (Kim et al. 2010) characterized an Asp racemase found in mouse brain; this was the first Asp racemase identified in an animal nervous system. This specific racemase is PLP-dependent and is expressed in brain, heart, and testes, as well as moderately expressed in the adrenal glands. This same study also demonstrated that this racemase is correlated to neurogenesis, indicating that D-Asp may work as a trophic factor (Fig. 2a, b). We recently reported a PLP-dependent racemase from the A. californica central nervous system (Wang et al. 2011); interestingly, this racemase is unique as it catalyzes both Asp and serine (Ser) racemization. The localization of this racemase corresponds to cells that we had previously shown contain both D-Asp and D-Ser, suggesting that both activities are functional within these neurons (Fig 2c, d).
Fig. 2. Asp racemase distribution and its tropic effect.
Mouse Asp racemase was expressed in adult hippocampus and (a) the racemase expression was locally suppressed by short-hairpin RNA (shRNA) against the racemase (shRNA-DR) in newborn neurons (green fluorescence) while control shRNA did not suppress the racemase expression in newborn neurons (red fluorescence); hippocampus cells (blue) were stained with 4′,6-diamidino-2-phenylindole. Scale bar, 50 μm. (b) Survival rate of newborn neurons is compared with shRNA-DR treatment vs. shRNA-control treatment. Neurons contain both shRNA-DR and control shRNA expressed yellow fluorescence. *, P <.05; n = 4 mice for each time point, ANOVA. (c) Immunohistochemical measurement of an Asp racemase in the cerebral ganglion from Aplysia capable of racemizing both D-Asp and D-Ser. The C- and F-cluster neurons are indicated by the area enclosed in the dotted line. (d) CE analysis on C- and F-cluster neurons showed high amounts of D-Asp and D-Ser. Separation condition: 27 kV normal polarity was applied to an uncoated fused-silica capillary (80 cm total length, 70 cm effective length, 75 μm i.d./360 μm o.d.) filled with separation buffer. The separation buffer for Asp enantiomers consisted of 40 mM β-cyclodextrin and 60 mM sodium deoxycholate in 200 mM borate buffer, pH 9.5. The Ser enantiomer separation buffer consisted of 10 mM γ-cyclodextrin and 50 mM sodium dodecyl sulfate in 75 mM borate buffer, pH 10.5. Panels a and b from (Kim et al. 2010) are used with permission, copyright 2010 National Academy of Sciences, U.S.A.; panels c and d from (Wang et al. 2011) are used with permission, © the American Society for Biochemistry and Molecular Biology.
When comparing the known Asp racemase sequences, those belonging to S. broughtonii and A. californica share more than a 50% sequence identity, whereas the mouse Asp racemase sequence appears distinct from the molluscan Asp racemases (Wang et al. 2011). The mouse Asp racemase sequence homology suggests it is related to glutamate-oxalacetate transaminases (Kim et al. 2010). These sequence differences may indicate that multiple Asp racemase families exist in the central nervous system of different animals.
Other possible biosynthesis pathways have been suggested for D-Asp, either via a PLP-dependent transaminase or degradation of D-Asp-containing peptides (Katane and Homma 2011); however, these may not be major D-Asp biosynthesis pathways in animals. For example, D-Asp can be produced by what is normally considered a side reaction of amino acid transaminases. Although mitochondrial transaminases from chicken and Escherichia coli convert small amounts of L-Asp into D-Asp, most of the L-Asp is converted to oxalacetate (Kochhar and Christen 1992; Vacca et al. 1995; Vacca et al. 1997). Perhaps some D-Asp may be produced in animals by a D-amino acid transaminase that accepts other D-amino acids as an amino group donor in order to convert oxaloacetic acid into D-Asp; this type of enzymatic reaction has been reported in plants (Funakoshi et al. 2008). D-aspartyl residues form due to spontaneous isomerization of L-aspartyl residues in proteins (Fujii 2002), which would result in free D-Asp release via protein degradation. It is not obvious how such a formation pathway would be cell specific and thus account for the observed cellular distributions of D-Asp. Regardless, the existence and enzymatic characterization of two distinct neuronal Asp racemases documents the ability of D-Asp to be formed under enzymatic control.
Uptake and transport of D-Asp
D-Asp uptake has been found to occur in the nervous systems of vertebrates and invertebrates, with the uptake and accumulation from the extracellular environment demonstrated in experiments using radiolabeled D-Asp. As one example, a rat hippocampal slice incubated in media containing [3H]-D-Asp showed an increase of cellular [3H]-D-Asp in a dose-dependent manner (Kuwahara et al. 1992). Similarly, the uptake of D-Asp in rat neuroendocrine tissues was shown by the accumulation of [14C]-D-Asp in pineal and pituitary glands after intravenous administration of [14C]-D-Asp (Imai et al. 1997). In A. californica, uptake and accumulation of [14C]-D-Asp was observed in both the central nervous system and in individual isolated and characterized neurons (Scanlan et al. 2010). Although its distribution was reported to be neuronal, several studies of astrocytes indicate that D-Asp occurs in glia also (Kimmich et al. 2001; Gadea et al. 2004; Lau et al. 2010). Takigawa and coworkers (Takigawa et al. 1998) showed that exogenous D-Asp taken up by rat pinealocytes dispersed into their cytoplasm but not into their nuclei; the exogenous and endogenous distribution patterns were measured and found to be the same.
D-Asp uptake may depend on a high-affinity L-glutamate (L-Glu) transporter that can take up extracellular D-Asp into cells efficiently while the transporters also uptake L-Glu and L-Asp, which compete with D-Asp. The L-Glu transporter is Na+-dependent, has a similar affinity to D-Asp, L-Asp and L-Glu, and is known to be blocked by DL-threo-beta-hydroxyaspartic acid (Kanai and Hediger 1992). The sodium- and temperature-dependent preferential uptake of D-Asp over L-Asp has been reported in Aplysia also (Scanlan et al. 2010). Experiments with cultured cells indicate that the L-Glu transporter is responsible for D-Asp uptake; PC12 cells that do not express L-Glu transporters showed no clear change in cellular D-Asp levels regardless of the extracellular D-Asp concentrations (Ramachandran et al. 1993; Long et al. 1998). On the other hand, MPT1 cells, which are PC12 variants that express L-Glu transporters, uptake D-Asp actively, whereas transporter inhibitors significantly decrease D-Asp uptake (Ramachandran et al. 1993; Adachi et al. 2004; Koyama et al. 2005). It may be that different levels of D-Asp are taken up into different tissues because of distinct populations of L-Glu transporter-expressing cells. We expect that issues concerning D-Asp uptake will be clarified by additional studies using cells with a well-defined transporter expression.
Degradation of D-Asp
Besides its synthesis, the removal and degradation of a molecule is also important when determining if it is a transmitter. D-Asp degradation is likely to occur by the well-known enzyme, DAspO, found throughout the Metazoan. DAspO oxidatively degrades dicarboxylic D-amino acids (D-Asp and D-Glu, and N-methyl-D-Asp (NMDA)), while most other D-isomers of neutral and basic amino acids are degraded by the enzyme D-amino acid oxidase (DAAO) (D’Aniello et al. 1993a; D’Aniello et al. 1993c). The amino acid sequence of DAspO from beef kidney showed more than a 40% homology with that of pig kidney DAAO (Negri et al. 1992), thus it is not surprising that both enzymes require flavin adenine dinucleotide as a cofactor (Fuchs et al. 2005). The gene sequence of DAspO has been reported for bovine and mouse kidney, and human brain, with cDNA clones expressed (Setoyama and Miura 1997; Katane et al. 2007a; Simonic et al. 1997). In addition, DAspO genes have been identified in C. elegans and cDNA clones were characterized recently (Katane et al. 2007b; Katane et al. 2010). Interestingly, there are three DAspO genes in C. elegans, and each showed different levels of oxidation activity. The authors hypothesized that the C. elegans DAspOs are localized in different subcellular locations because each of these genes have different signal peptide sequences (Katane et al. 2010). One C. elegans DAspO is expected to locate in the peroxisome, which is the same cellular organelle as the DAspO localization for human and rat liver (Vanveldhoven et al. 1991; Katane et al. 2010). Perhaps this has been conserved as DAspO (as a product of its enzymatic oxidation) because it generates hydrogen peroxide, a toxic molecule that is utilized in peroxisome.
The DAspO activity correlates to the presence of D-Asp. Mouse liver DAspO has shown increased activity after the administration of D-Asp (Yamada et al. 1989; Nagasaki 1994). Similarly, yeast DAspO activity was increased, and DAspO expression induced, by increasing transcription when D-Asp was used as the sole nitrogen source in culture media (Yamada et al. 1996; Takahashi et al. 2004). Meanwhile, D-Asp and DAspO distributions in the brain and neuroendocrine system of rat appear complementary; high D-Asp levels have been found where DAspO levels were low and vice versa (Schell et al. 1997). Additionally, a magnitude higher activity of DAspO has been reported in postsynaptic membranes of R. norvegicus and L. vulgaris when compared with DAspO activity in the brain cytosol (D’Aniello et al. 2011). Thus, D-Asp appears to have a function in locations where DAspO levels are low enoughthat D-Asp is not immediately eliminated.
D-Asp release
The release of D-Asp to the extracellular environment has been measured from D-Asp-containing tissues or cells of the mammalian brain in a Ca2+-dependent manner upon chemical and electrical stimulation (Davies and Johnston 1976; Malthesorenssen et al. 1979); these stimulation methods are still widely used, not only for mammalian brain but also for neuronal cells of other species (Fig. 3a, b) (Scanlan et al. 2010). The importance of Ca2+ for triggering D-Asp release was demonstrated by introduction of ethylene glycol tetraacetic acid (EGTA), a chelating agent for Ca2+; D-Asp release after exposure to both KCl and EGTA resulted in a magnitude smaller D-Asp release than that after KCl exposure only (Wolosker et al. 2000). Preloading D-Asp with radioactive labels in the brain slice clearly demonstrated that intracellular D-Asp was released upon chemical and electrical stimulation (Palmer and Reiter 1994; Muzzolini et al. 1997; Savage et al. 2001). Besides depolarization having been induced by potassium ions or acetylcholine (which triggered D-Asp release from rat adrenal slices), nicotine application resulted in the depletion of D-Asp in the adrenal slice while L-Glu and L-Asp levels were not significantly changed (Wolosker et al. 2000). Norepinephrine is another example of a chemical stimulus for D-Asp release used in rat pinealocytes (Takigawa et al. 1998), cells in the pineal gland that contain a significant amount of D-Asp (Lee et al. 1997).
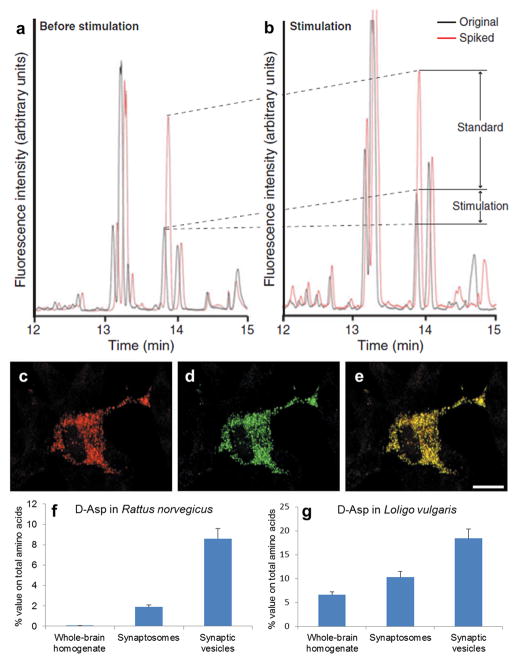
Fig. 3. D-Asp release and co-localization within synaptic vesicles.
Extracellular D-Asp concentrations (a) before and (b) after potassium ion stimulation that induced D-Asp release from cerebral ganglia of Aplysia californica. In (a) and (b), the black trace (original) is the electropherogram of the original sample and the red trace (spiked) is the electropherogram of the sample spiked with 1 μM D-Asp, arrows indicate the increase in D-Asp signal due to the addition of 1 μM of standard D-Asp and the change due to potassium ion stimulation (labeled standard and stimulation, respectively). Co-localization of D-Asp and secretory granules in PC12 cells under immunofluorescent microscopic observation of (c) chromogranin A, a marker of secretory granules and (d) D-Asp; (e) merged images of c and d. Scale bar, 10 μm. Higher D-Asp content was observed in synaptic vesicles than in other cell regions in (f) rat Rattus norvegicus and (g) squid Loligo vulgaris. Panels a and b from (Scanlan et al. 2010) are used with permission, c 2010 by John Wiley & Sons, Inc.; panels c–e from (Nakatsuka et al. 2001) are used with permission, © the American Society for Biochemistry and Molecular Biology; and panels f and g are based on the data table in (D’Aniello et al. 2011).
Savage and colleagues (Savage et al. 2001) showed electrical-stimulation-evoked D-Asp and L-Glu release from hippocampal slices. In their experiments, more than 90% of the D-Asp and L-Glu releasates were Ca2+-dependent. They also reported that D-Asp release was dramatically decreased by treatment of the hippocampal slice with either a synaptic vesicle toxin or magnesium, and moderately decreased with voltage-sensitive Ca2+ channel antagonists. Significant decrease of D-Asp release with the application of a synaptic vesicle toxin was also observed in rat cerebellar granule cells (Cousin et al. 1997) and in cultured PC12 cells (Nakatsuka et al. 2001). Studies with A. limacina and L. vulgaris also indicated that D-Asp accumulated in synaptic vesicles and was released upon stimulation (Spinelli et al. 2006; D’Aniello et al. 2011), and that vesicles were involved in the transport of D-Asp from one ganglion to another in A. californica (Miao et al. 2006a). These studies support that D-Asp is stored in synaptic vesicles and is released by exocytosis (Fig. 3c–g).
However, spontaneous and continuous D-Asp release that is independent of exocytosis has also been proposed. This hypothesis is supported by several studies on cultured mammalian cells. PC12 cells, as discussed above in the Biosynthesis of D-Asp section, produce D-Asp within the cells but cannot uptake extracellular D-Asp. Through PC12 culturing, it was observed that intracellular and extracellular D-Asp levels increased without any specific stimulation to the cells (Long et al. 1998). Similar results were observed in MPT1 cell cultures in which D-Asp levels increased over the duration of the culturing period even though initially, no D-Asp was in the media (Adachi et al. 2004). In another study from the same group (Koyama et al. 2006), D-Asp and dopamine release was compared in PC12 cells; upon KCl stimulation, the amount of released dopamine rose after stimulation and stayed at a constant level, while D-Asp levels gradually increased independent of the extracellular KCl concentration. They also showed that D-Asp release was not sensitive to silencing SNAP-25, a protein essential for exocytosis. These results are distinct from the observed reduction of D-Asp release in PC12 cells after cleavage of SNAP-25 (Nakatsuka et al. 2001), as well as those from a hippocampal study with Ca2+ channel antagonists (Savage et al. 2001).
Another pathway for D-Asp release has been proposed via a volume-sensitive organic anion channel (VSOC); this transmembrane protein channel opens upon hypotonic stimulation. Putative VSOC inhibitors reduced D-Asp release from PC12 cells in hypotonic media (Koyama et al. 2006).
Overall, these release studies indicate that there may be multiple release pathways: via exocytosis of D-Asp-containing vesicles, spontaneous and continuous D-Asp release, and VSOC-dependent release. Furthering our understanding of these putative D-Asp release pathways obviously requires additional investigation.
Molecular and cellular functions of D-Asp
What does D-Asp do in the brain and endocrine system? We and others have hypothesized that D-Asp plays a role as a neurotransmitter or neuromodulator (Spinelli et al. 2006; Brown et al. 2007; D’Aniello 2007; Fieber et al. 2010; Scanlan et al. 2010; D’Aniello et al. 2011). As discussed in previous sections, there are many studies supporting D-Asp as a transmitter. Meanwhile, a neuromodulator is a molecule in the nervous system that does not induce a nerve impulse but may modify the depolarization profile of the postsynaptic membrane in the presence of a neurotransmitter.
One important point to keep in mind is that a selective receptor for D-Asp has yet to be identified. D-Asp is known to activate NMDA-like receptors (Verdoorn and Dingledine 1988). In addition, D-Asp may work as neuromodulator for 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid-like receptors to slow receptor activation time. For example, the activation time of the squid glutamate receptor increased three-fold with the presence of both L-Glu and D-Asp compared with the activation time induced by L-Glu alone (Brown et al. 2007). Conversely, in electrophysiological experiments on pleural and buccal ganglia cells of A. californica (Fieber et al. 2010; Carlson and Fieber 2011), some cells only responded to D-Asp, thereby suggesting that a D-Asp-specific receptor does exist. These experiments showed about half of the cells responded to both D-Asp and L-Glu to evoke an action potential, whereas the rest of the cells responded to either D-Asp or L-Glu alone. D’Aniello and coworkers (D’Aniello et al. 2011) have also performed an interesting experiment with L. vulgaris to determine whether D-Asp and L-Glu bind to the same receptor. Independent stimulations of D-Asp and L-Glu on L. vulgaris skin chromatophores indicated that the D-Asp receptor was different from the L-Glu receptor because each type of amino acid stimulated a specific color of skin chromatophore. The same study also showed an elevated level of cAMP, a putative second messenger, in rat and L. vulgaris synaptosomes induced by D-Asp (D’Aniello et al. 2011). A similar trend was found in the cerebral ganglia of A. limacine, although L-Asp did not induce increased cAMP levels (Spinelli et al. 2006). These results support the idea that D-Asp can act as an intercellular signaling molecule.
Besides acting as neurotransmitters for short-range intercellular signaling, at times cell-to-cell signaling molecules function at longer distances. Is the same functional duality true of D-Asp? For example, in the neuroendocrine system, D-Asp behaves like a hormone to regulate the reproductive system; specifically, it has been reported to be involved in steroid hormone synthesis in the rat pituitary gland, Leydig cells, and testis (D’Aniello et al. 1996; Nagata et al. 1999b; D’Aniello et al. 2000a; Nagata et al. 1999a); to increase luteinizing and growth hormone release (D’Aniello et al. 2000a) and prolactin release (D’Aniello et al. 2000b) in rat pituitary; to decrease proopiomelanocortin and α-melanocyte-stimulating hormone in mouse pituitary (Huang et al. 2006); to inhibit melatonin release (Takigawa et al. 1998); and to regulate luteinizing hormone-releasing hormone, α-melanocyte-stimulating hormone, GABA, dopamine and oxytocin in the rat hypothalamus (Pampillo et al. 2002; Wang et al. 2000). In non-mammalian tissues, D-Asp has been found to regulate hormones in the reproductive organs of the frog, lizard, and ascidian (Di Fiore et al. 1998; Assisi et al. 2001; D’Aniello et al. 2003). However, many details of the D-Asp signaling pathways remain to be elucidated. For example, D-Asp may use cAMP and cGMP as secondary messengers to regulate testosterone level in testis Leydig cells and luteinizing hormone level in pituitary of rats, respectively (Topo et al. 2009), while other studies report that D-Asp regulates mRNA transcription of hormones without affecting cAMP levels to increase testosterone in rat Leydig cells (Nagata et al. 1999a; Nagata et al. 1999b).
Another interesting possibility is that D-Asp serves as a precursor of endogenous NMDA, which may be involved in intercellular signaling (D’Aniello et al. 2000a; D’Aniello et al. 2000b; D’Aniello et al. 2003). NMDA has been detected in rat brain and neuroendocrine tissues (D’Aniello et al. 2000a; D’Aniello et al. 2000b) and in invertebrates (Sato et al. 1987; Todoroki et al. 1999; D’Aniello et al. 2003; D’Aniello et al. 2007b; Shibata et al. 2011), although many locations with substantial D-Asp have little or no detectable levels of NMDA. There are cases where NMDA is formed from D-Asp via the enzymatic activity of D-Asp methyltransferase (or the so called NMDA synthetase), which transfers the methyl group of S-adenosyl-L-methionine to D-Asp for NMDA formation (D’Aniello et al. 2000a; D’Aniello et al. 2000b; Shibata et al. 2011). Considering that DAspO also digests NMDA (D’Aniello et al. 1993a; D’Aniello et al. 1993c), and that NMDA is a well-documented agonist for NMDA receptors (Watkins and Jane 2006), perhaps NMDA is a key player in multiple signaling pathways.
While a number of the studies described above support an intercellular signaling role for D-Asp, other studies suggest non-related roles. These include the demonstration that D-Asp is localized in the nucleoli (but not in neural processes) (Wang et al. 2002), and the report that aluminum D-Asp complexes induce topological changes in supercoiled DNA (Bharathi et al. 2003). Perhaps more likely is that D-Asp, as many other small molecules, has multiple distinct roles. After all, just because a specific role for D-Asp is documented, does not preclude nor invalidate the possibility that it has a function in other systems as well.
Physiological roles of D-Asp
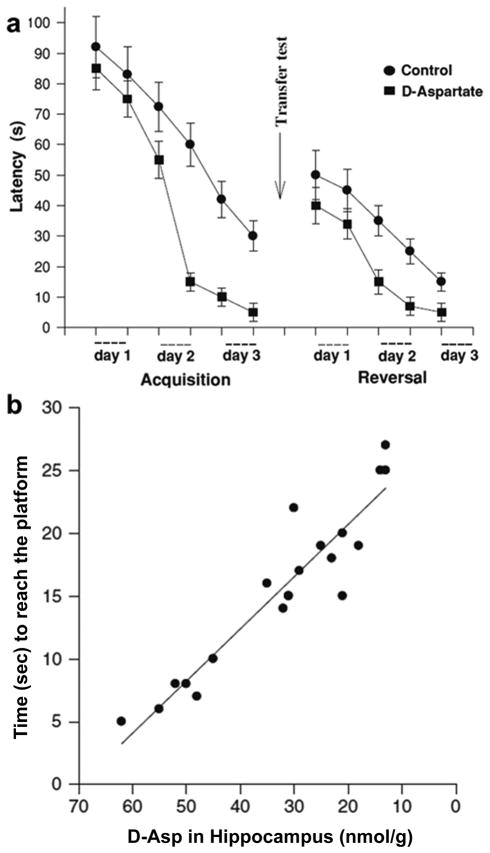
Until now, the discussion has focused on the cellular functions of D-Asp. What do these functions imply about an overall physiological function? Several lines of evidence support its role in cognitive processes. Acting as an endogenous agonist for the NMDA receptor, D-Asp stimulates the NMDA receptor by binding to its L-Glu-binding site (Fagg and Matus 1984). As a result, D-Asp, like D-Ser (Junjaud et al. 2006; Fossat et al. 2012), may be involved in learning and memory processes as well as NMDA receptor-related diseases (Katane and Homma 2011). Using both genetic and pharmacological animal models, increasing the levels of D-Asp enhanced long-term potentiation in mice hippocampal slices (Errico et al. 2008a). Chronic D-Asp elevation also reduced cortical-striatal long-term depression and attenuated pharmacologically induced schizophrenia-like symptoms (Errico et al. 2008b). In addition, oral administration of D-Asp improved the spatial learning and memory ability of treated rats when compared with control rats. Moreover, randomized rats possessing higher endogenous D-Asp levels in the hippocampus take less time to complete tasks (Fig. 4) (Topo et al. 2010). In a recent study, D-Asp was shown to rescue the hippocampal age-related synaptic plasticity deterioration in mice, implying an intriguing possibility that it can counteract the age-related reduction of NMDA receptor signaling (Errico et al. 2011b). Reduced D-Asp levels were found in human brain samples from patients with Alzheimer’s disease compared to normal brains (D’Aniello et al. 1998). Although the mechanism of how D-Asp exerts its activity at the neuronal level is still poorly understood, this set of research certainly supports the possibility that D-Asp plays a major role in the neuronal mechanisms underlying learning and memory.
Fig. 4. Improved spatial learning and memory in rats treated with D-Asp.
(a) 12 rats treated with 40 mM sodium D-Asp and 12 rats treated with 40 mM NaCl (control) were trained in the Morris water maze to measure latency in reaching the platform hidden in the maze. After the acquisition phase, the platform position was changed before starting the reversal phase, while the other settings were the same. (b) Relationship between the endogenous D-Asp in 120-day old rat hippocampus and the time to reach the platform in the Morris water maze system of the corresponding rats that were trained in the Morris water maze system. Figure is adapted from (Topo et al. 2010) and used with permission, Springer Science+Business Media.
Because abnormal functioning of the NMDA receptor has been implicated in various neuronal diseases, studies have been carried out to elucidate the relationship between endogenous D-Asp levels and the pathophysiological processes of NMDA-related diseases, such as Alzheimer’s. The differences in D-Asp concentration between normal and Alzheimer human brains were first described in 1991, where D-Asp occurred at less than half the concentration of normal levels in the white matter of Alzheimer brains (Fisher et al. 1991). In another study of Alzheimer’s disease in the human brain, D-Asp decreased in three neocortical regions, as well as the subcortical hippocampus and amygdala, but not in the cerebellum, an area which lacks the neuropathological changes of Alzheimer’s (D’Aniello et al. 1998).
Conclusions
The last decade has been an exciting time for D-Asp research. While much has been learned about its formation and degradation, additional biochemical and cellular data is certainly needed. Many of the cited studies highlight the existence, localization, formation and release of D-Asp in neurons in a surprisingly wide range of animals. Recent results include data demonstrating that D-Asp fulfills many requirements for it to be considered as a neurotransmitter, a hypothesis that has been proposed by several (Spinelli et al. 2006; D’Aniello 2007; Fieber et al. 2010; Scanlan et al. 2010; D’Aniello et al. 2011). Of course, while the studies involving mammalian tissues are exceedingly relevant and well performed, they have not completely addressed one required facet for a molecule to be considered a transmitter. The formation and/or uptake, transport to terminals, and activity-dependent release must all occur in the exact same neuron, something only demonstrated for the larger and more accessible invertebrate neurons to date. Exciting work by the Snyder group (Kim et al. 2010) has shown that D-Asp also has a trophic role (Fig. 2a, b), which is perhaps not surprising given its presence during embryogenesis. Data on how D-Asp performs one or both roles are still needed. In fact, another well-known transmitter, serotonin, is known to have both transmitter and trophic roles (Hodges and Richerson 2008). Therefore, we will not be surprised if D-Asp is also found to have multiple distinct functions within the brain. Perhaps more intriguing is the recent speculation of a correlation between D-Asp and several mental disorders such as Alzheimer’s disease and schizophrenia (D’Aniello 2007; Katane and Homma 2011). Although preliminary work has been done (D’Aniello et al. 1998; Errico et al. 2011a; Errico et al. 2008b; Weil et al. 2006), further investigations are required to confirm these reports and demonstrate whether modifying D-Asp formation, release or degradation can impact the progression of such diseases.
Acknowledgments
This work was supported by Award Number R01 NS031609 from National Institute of Neurological Disorders and Stroke, Award Number P30 DA018310 from the National Institute on Drug Abuse, and Award Number CHE-11-11705 from the National Science Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the award agencies.
References
- Abe K, Takahashi S, Muroki Y, Kera Y, Yamada RH. Cloning and expression of the pyridoxal 5′-phosphate-dependent aspartate racemase gene from the bivalve mollusk Scapharca broughtonii and characterization of the recombinant enzyme. J Biochem. 2006;139(2):235–244. doi: 10.1093/jb/mvj028. [DOI] [PubMed] [Google Scholar]
- Adachi M, Koyama H, Long ZQ, Sekine M, Furuchi T, Imai K, Nimura N, Shimamoto K, Nakajima T, Homma H. L-glutamate in the extracellular space regulates endogenous D-aspartate homeostasis in rat pheochromocytoma MPT1 cells. Arch Biochem Biophys. 2004;424(1):89–96. doi: 10.1016/j.abb.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Assisi L, Botte V, D’Aniello A, Di Fiore MM. Enhancement of aromatase activity by D-aspartic acid in the ovary of the lizard Podarcis s. sicula. Reproduction. 2001;121(5):803–808. doi: 10.1530/rep.0.1210803. [DOI] [PubMed] [Google Scholar]
- Bharathi, Rao KSJ, Stein R. First evidence on induced topological changes in supercoiled DNA by an aluminium D-aspartate complex. J Biol Inorg Chem. 2003;8(8):823–830. doi: 10.1007/s00775-003-0484-1. [DOI] [PubMed] [Google Scholar]
- Brown ER, Piscopo S, Chun JT, Francone M, Mirabile I, D’Aniello A. Modulation of an AMPA-like glutamate receptor (SqGluR) gating by L- and D-aspartic acids. Amino Acids. 2007;32(1):53–57. doi: 10.1007/s00726-006-0349-3. [DOI] [PubMed] [Google Scholar]
- Carlson SL, Fieber LA. Physiological evidence that D-aspartate activates a current distinct from ionotropic glutamate receptor currents in Aplysia californica neurons. J Neurophysiol. 2011;106(4):1629–1636. doi: 10.1152/jn.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Hurst H, Nicholls DG. Presynaptic calcium channels and field-evoked transmitter exocytosis from cultured cerebellar granule cells. Neuroscience. 1997;81(1):151–161. doi: 10.1016/S0306-4522(97)00047-X. [DOI] [PubMed] [Google Scholar]
- D’Aniello A. D-Aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res Rev. 2007;53(2):215–234. doi: 10.1016/j.brainresrev.2006.08.005. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Di Fiore MM, Fisher GH, Milone A, Seleni A, D’Aniello S, Perna AF, Ingrosso D. Occurrence of D-aspartic acid and N-methyl-D-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. FASEB J. 2000a;14 (5):699–714. doi: 10.1096/fasebj.14.5.699. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, DiCosmo A, DiCristo C, Annunziato L, Petrucelli L, Fisher G. Involvement of D-Aspartic acid in the synthesis of testosterone in rat testes. Life Sci. 1996;59(2):97–104. doi: 10.1016/0024-3205(96)00266-4. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Dicosmo A, Dicristo C, Fisher G. D-aspartate in the male and female reproductive system of Octopus vulgaris lam. Gen Comp Endocrinol. 1995a;100(1):69–72. doi: 10.1006/gcen.1995.1134. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Donofrio G, Pischetola M, Daniello G, Vetere A, Petrucelli L, Fisher GH. Biological role of D-amino acid oxidase and D-aspartate oxidase. Effects of D-amino acids. J Biol Chem. 1993a;268 (36):26941–26949. [PubMed] [Google Scholar]
- D’Aniello A, Giuditta A. Identification of D-aspartic acid in the brain of Octopus vulgaris Lam. J Neurochem. 1977;29(6):1053–1057. doi: 10.1111/j.1471-4159.1977.tb06508.x. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Giuditta A. Presence of D-aspartate in squid axoplasm and in other regions of the cephalopod nervous system. J Neurochem. 1978;31(4):1107–1108. doi: 10.1111/j.1471-4159.1978.tb00155.x. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Lee JM, Petrucelli L, Di Fiore MM. Regional decreases of free D-aspartate levels in Alzheimer’s disease. Neuroscience Lett. 1998;250(2):131–134. doi: 10.1016/s0304-3940(98)00451-0. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Nardi G, DeSantis A, Vetere A, di Cosmo A, Marchelli R, Dossena A, Fisher G. Free L-amino acids and D-aspartate content in the nervous system of Cephalopoda. A comparative study. Comp Biochem Physiol B Biochem Mol Biol. 1995b;112(4):661–666. doi: 10.1016/0305-0491(95)00227-8. [DOI] [Google Scholar]
- D’Aniello A, Nardi G, Vetere A, Ferguson GP. Occurrence of free D-aspartic acid in the circumsoesophageal ganglia of Aplysia fasciata. Life Sci. 1993b;52(8):733–736. doi: 10.1016/0024-3205(93)90235-u. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Spinelli P, De Simone A, D’Aniello S, Branno M, Aniello F, Fisher GH, Di Fiore MM, Rastogi RK. Occurrence and neuroendocrine role of D-aspartic acid and N-methyl-D-aspartic acid in Ciona intestinalis. FEBS Lett. 2003;552(2–3):193–198. doi: 10.1016/s0014-5793(03)00921-9. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Vetere A, Petrucelli L. Further study on the specificity of D-amino acid oxidase and D-aspartate oxidase and time course for complete oxidation of D-amino acid. Comp Biochem Physiol B Biochem Mol Biol. 1993c;105(3–4):731–734. doi: 10.1016/0305-0491(93)90113-J. [DOI] [PubMed] [Google Scholar]
- D’Aniello G, Grieco N, Di Filippo MA, Cappiello F, Topo E, D’Aniello E, Ronsini S. Reproductive implication of D-aspartic acid in human pre-ovulatory follicular fluid. Hum Reprod. 2007a;22(12):3178–3183. doi: 10.1093/humrep/dem328. [DOI] [PubMed] [Google Scholar]
- D’Aniello G, Ronsini S, Guida F, Spinelli P, D’Aniello A. Occurrence of D-aspartic acid in human seminal plasma and spermatozoa: Possible role in reproduction. Fertil Steril. 2005a;84(5):1444–1449. doi: 10.1016/j.fertnstert.2005.05.019. [DOI] [PubMed] [Google Scholar]
- D’Aniello G, Tolino A, D’Aniello A, Errico F, Fisher GH, Di Fiore MM. The role of D-aspartic acid and N-methyl-D-aspartic acid in the regulation of prolactin release. Endocrinology. 2000b;141(10):3862–3870. doi: 10.1210/en.141.10.3862. [DOI] [PubMed] [Google Scholar]
- D’Aniello S, Fisher GH, Topo E, Ferrandino G, Garcia-Fernandez J, D’Aniello A. N-methyl-D-aspartic acid (NMDA) in the nervous system of the amphioxus Branchiostoma lanceolatum. BMC Neurosci. 2007b;8 doi: 10.1186/1471-2202-8-109. doi:10910.1186/1471-2202-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aniello S, Garcia-Fernandez J. D-Aspartic acid and L-amino acids in the neural system of the amphioxus Branchiostoma lanceolatum. Amino Acids. 2007;32(1):21–26. doi: 10.1007/s00726-006-0347-5. [DOI] [PubMed] [Google Scholar]
- D’Aniello S, Somorjai I, Garcia-Fernandez J, Topo E, D’Aniello A. D-Aspartic acid is a novel endogenous neurotransmitter. FASEB J. 2011;25(3):1014–1027. doi: 10.1096/fj.10-168492. [DOI] [PubMed] [Google Scholar]
- D’Aniello S, Spinelli P, Ferrandino G, Peterson K, Tsesarskia M, Fisher G, D’Aniello A. Cephalopod vision involves dicarboxylic amino acids: D-aspartate, L-aspartate and L-glutamate. Biochem J. 2005b;386:331–340. doi: 10.1042/bj20041070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies LP, Johnston GAR. Uptake and release of D-aspartate and L-aspartate by rat brain slices. J Neurochem. 1976;26(5):1007–1014. doi: 10.1111/j.1471-4159.1976.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Di Fiore MM, Assisi L, Botte V, D’Aniello A. D-Aspartic acid is implicated in the control of testosterone production by the vertebrate gonad. Studies on the female green frog, Rana esculenta. J Endocrinol. 1998;157(2):199–207. doi: 10.1677/joe.0.1570199. [DOI] [PubMed] [Google Scholar]
- Dunlop DS, Neidle A, McHale D, Dunlop DM, Lajtha A. The presence of free D-aspartic acid in rodents and man. Biochem Biophys Res Commun. 1986;141(1):27–32. doi: 10.1016/s0006-291x(86)80329-1. [DOI] [PubMed] [Google Scholar]
- Errico F, Bonito-Oliva A, Bagetta V, Vitucci D, Romano R, Zianni E, Napolitano F, Marinucci S, Di Luca M, Calabresi P, Fisone G, Carta M, Picconi B, Gardoni F, Usiello A. Higher free D-aspartate and N-methyl-D-aspartate levels prevent striatal depotentiation and anticipate L-DOPA-induced dyskinesia. Exp Neurol. 2011a;232(2):240–250. doi: 10.1016/j.expneurol.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Errico F, Nistico R, Napolitano F, Mazzola C, Astone D, Pisapia T, Giustizieri M, D’Aniello A, Mercuri NB, Usiello A. Increased D-aspartate brain content rescues hippocampal age-related synaptic plasticity deterioration of mice. Neurobiol Aging. 2011b;32(12):2229–2243. doi: 10.1016/j.neurobiolaging.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Errico F, Nistico R, Palma G, Federici M, Affuso A, Brilli E, Topo E, Centonze D, Bernardi G, Bozzi Y, D’Aniello A, Di Lauro R, Mercuri NB, Usiello A. Increased levels of D-aspartate in the hippocampus enhance LTP but do not facilitate cognitive flexibility. Mol Cell Neurosci. 2008a;37(2):236–246. doi: 10.1016/j.mcn.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Errico F, Rossi S, Napolitano F, Catuogno V, Topo E, Fisone G, D’Aniello A, Centonze D, Usiello A. D-aspartate prevents corticostriatal long-term depression and attenuates schizophrenia-like symptoms induced by amphetamine and MK-801. J Neurosci. 2008b;28(41):10404–10414. doi: 10.1523/jneurosci.1618-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg GE, Matus A. Selective association of N-methyl aspartate and quisqualate types of L-glutamate receptor with brain postsynaptic densities. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences. 1984;81(21):6876–6880. doi: 10.1073/pnas.81.21.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber LA, Carlson SL, Capo TR, Schmale MC. Changes in D-aspartate ion currents in the Aplysia nervous system with aging. Brain Res. 2010;1343:28–36. doi: 10.1016/j.brainres.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GH, Daniello A, Vetere A, Padula L, Cusano GP, Man EH. Free D-aspartate and D-alanine in normal and Alzheimer brain. Brain Res Bull. 1991;26(6):983–985. doi: 10.1016/0361-9230(91)90266-m. [DOI] [PubMed] [Google Scholar]
- Fossat P, Turpin FR, Sacchi S, Dulong J, Shi T, Rivet JM, Sweedler JV, Pollegioni L, Millan MJ, Oliet SHR, Mothet JP. Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb Cortex. 2012;22(3):595–606. doi: 10.1093/cercor/bhr130. [DOI] [PubMed] [Google Scholar]
- Fuchs SA, Berger R, Klomp LWJ, de Koning TJ. D-amino acids in the central nervous system in health and disease. Mol Genet Metab. 2005;85(3):168–180. doi: 10.1016/j.ymgme.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Fujii N. D-amino acids in living higher organisms. Origins Life Evol Biosphere. 2002;32(2):103–127. doi: 10.1023/A:1016031014871. [DOI] [PubMed] [Google Scholar]
- Funakoshi M, Sekine M, Katane M, Furuchi T, Yohda M, Yoshikawa T, Homma H. Cloning and functional characterization of Arabidopsis thaliana D-amino acid aminotransferase - D-aspartate behavior during germination. FEBS J. 2008;275(6):1188–1200. doi: 10.1111/j.1742-4658.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- Gadea A, Lopez E, Lopez-Colome AM. Glutamate-induced inhibition of D-aspartate uptake in Muller glia from the retina. Neurochem Res. 2004;29(1):295–304. doi: 10.1023/B:NERE.0000010458.45085.e8. [DOI] [PubMed] [Google Scholar]
- Gosling JP, Fottrell PF. Purification and characterization of D-amino acid aminotransferase from Rhizobium japonicum. Biochim Biophys Acta, Enzymol. 1978;522:84–95. doi: 10.1016/0005-2744(78)90324-8. [DOI] [PubMed] [Google Scholar]
- Hamase K, Homma H, Takigawa Y, Imai K. Alteration in the D-amino acid content of the rat pineal gland under anesthesia. Amino Acids. 1999;17(3):277–283. doi: 10.1007/bf01366926. [DOI] [PubMed] [Google Scholar]
- Hamase K, Morikawa A, Zaitsu K. D-Amino acids in mammals and their diagnostic value. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781(1–2):73–91. doi: 10.1016/S1570-0232(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Han H, Miyoshi Y, Ueno K, Okamura C, Tojo Y, Mita M, Lindner W, Zaitsu K, Hamase K. Simultaneous determination of D-aspartic acid and D-glutamic acid in rat tissues and physiological fluids using a multi-loop two-dimensional HPLC procedure. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3196–3202. doi: 10.1016/j.jchromb.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Kumashiro S, Nishikawa T, Oka T, Takahashi K, Mito T, Takashima S, Doi N, Mizutani Y, Yamazaki T, Kaneko T, Ootomo E. Embryonic development and postnatal changes in free D-aspartate and D-serine in the human prefrontal cortex. J Neurochem. 1993;61(1):348–351. doi: 10.1111/j.1471-4159.1993.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: Neuromodulatory and trophic effects. Respir Physiol Neuro. 2008;164(1–2):222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma H. Biochemistry of D-aspartate in mammalian cells. Amino Acids. 2007;32(1):3–11. doi: 10.1007/s00726-006-0354-6. [DOI] [PubMed] [Google Scholar]
- Huang AS, Beigneux A, Weil ZM, Kim PM, Molliver ME, Blackshaw S, Nelson RJ, Young SG, Snyder SH. D-aspartate regulates melanocortin formation and function: Behavioral alterations in D-aspartate oxidase-deficient mice. J Neurosci. 2006;26(10):2814–2819. doi: 10.1523/jneurosci.5060-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Fukushima T, Hagiwara K, Santa T. Occurrence of D-aspartic acid in rat brain pineal gland of D-aspartic acid in rat-brain pineal-gland. Biomed Chromatogr. 1995;9(2):106–109. doi: 10.1002/bmc.1130090211. [DOI] [PubMed] [Google Scholar]
- Imai K, Fukushima T, Santa T, Homma H, Sugihara J, Kodama H, Yoshikawa M. Accumulation of radioactivity in rat brain and peripheral tissues including salivary gland after intravenous administration of C-14-D-aspartic acid. P Jpn Acad B-Phys. 1997;73(3):48–52. doi: 10.2183/pjab.73.48. [DOI] [Google Scholar]
- Junjaud G, Rouaud E, Turpin F, Mothet JP, Billard JM. Age-related effects of the neuromodulator D-serine on neurotransmission and synaptic potentiation in the CA1 hippocampal area of the rat. J Neurochem. 2006;98(4):1159–1166. doi: 10.1111/j.1471-4159.2006.03944.x. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360(6403):467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4. McGraw-Hill; New York: 2000. [Google Scholar]
- Katane M, Furuchi T, Sekine M, Homma H. Molecular cloning of a cDNA encoding mouse D-aspartate oxidase and functional characterization of its recombinant proteins by site-directed mutagenesis. Amino Acids. 2007a;32(1):69–78. doi: 10.1007/s00726-006-0350-x. [DOI] [PubMed] [Google Scholar]
- Katane M, Homma H. D-Aspartate-An important bioactive substance in mammals: A review from an analytical and biological point of view. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(29):3108–3121. doi: 10.1016/j.jchromb.2011.03.062. [DOI] [PubMed] [Google Scholar]
- Katane M, Saitoh Y, Seida Y, Sekine M, Furuchi T, Homma H. Comparative characterization of three D-aspartate oxidases and one D-amino acid oxidase from Caenorhabditis elegans. Chem Biodivers. 2010;7(6):1424–1434. doi: 10.1002/cbdv.200900294. [DOI] [PubMed] [Google Scholar]
- Katane M, Seida Y, Sekine M, Furuchi T, Homma H. Caenorhabditis elegans has two genes encoding functional D-aspartate oxidases. FEBS J. 2007b;274(1):137–149. doi: 10.1111/j.1742-4658.2006.05571.x. [DOI] [PubMed] [Google Scholar]
- Kim PM, Duan X, Huang AS, Liu CY, Ming GL, Song HJ, Snyder SH. Aspartate racemase, generating neuronal D-aspartate, regulates adult neurogenesis. Proc Natl Acad Sci U S A. 2010;107(7):3175–3179. doi: 10.1073/pnas.0914706107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich GA, Roussie J, Manglapus M, Randles J. Characterization of Na+-coupled Glutamate/Aspartate transport by a rat brain astrocyte line expressing GLAST and EAAC1. J Membr Biol. 2001;182(1):17–30. doi: 10.1007/s00232-001-0025-1. [DOI] [PubMed] [Google Scholar]
- Kochhar S, Christen P. Mechanism of racemization of amino acids by aspartate aminotransferase. Eur J Biochem. 1992;203(3):563–569. doi: 10.1111/j.1432-1033.1992.tb16584.x. [DOI] [PubMed] [Google Scholar]
- Koyama H, Adachi M, Sekine M, Katane M, Furuchi T, Homma H. Cytoplasmic localization and efflux of endogenous D-aspartate in pheochromocytoma 12 cells. Arch Biochem Biophys. 2006;446(2):131–139. doi: 10.1016/j.abb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Koyama H, Sekine M, Furuchi T, Katane M, Nimura N, Shimamoto K, Nakajima T, Homma H. A novel L-glutamate transporter inhibitor reveals endogenous D-aspartate homeostasis in rat pheochromocytoma MPT1 cells. Life Sci. 2005;76(25):2933–2944. doi: 10.1016/j.lfs.2004.10.057. [DOI] [PubMed] [Google Scholar]
- Kuwahara O, Mitsumoto Y, Chiba K, Mohri T. Characterization of D-aspartic acid uptake by rat hippocampal slices and effect of ischemic conditions. J Neurochem. 1992;59(2):616–621. doi: 10.1111/j.1471-4159.1992.tb09414.x. [DOI] [PubMed] [Google Scholar]
- Lamont HC, Staudenbauer WL, Strominger JL. Partial purification and characterization of an aspartate racemase from Streptococcus faecalis. J Biol Chem. 1972;247:5103–5106. [PubMed] [Google Scholar]
- Lapainis T, Rubakhin SS, Sweedler JV. Capillary electrophoresis with electrospray ionization mass spectrometric detection for single-cell metabolomics. Anal Chem. 2009;81(14):5858–5864. doi: 10.1021/ac900936g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapainis T, Sweedler JV. Contributions of capillary electrophoresis to neuroscience. J Chromatogr A. 2008;1184(1–2):144–158. doi: 10.1016/j.chroma.2007.10.098. [DOI] [PubMed] [Google Scholar]
- Lau CL, Beart PM, O’Shea RD. Transportable and Non-transportable Inhibitors of L-glutamate Uptake Produce Astrocytic Stellation and Increase EAAT2 Cell Surface Expression. Neurochem Res. 2010;35(5):735–742. doi: 10.1007/s11064-010-0130-6. [DOI] [PubMed] [Google Scholar]
- Lee JA, Homma H, Sakai K, Fukushima T, Santa T, Tashiro K, Iwatsubo T, Yoshikawa M, Imai K. Immunohistochemical localization of D-aspartate in the rat pineal gland. Biochem Biophys Res Commun. 1997;231(2):505–508. doi: 10.1006/bbrc.1996.5902. [DOI] [PubMed] [Google Scholar]
- Lee JA, Homma H, Tashiro K, Iwatsubo T, Imai K. D-aspartate localization in the rat pituitary gland and retina. Brain Res. 1999;838(1–2):193–199. doi: 10.1016/s0006-8993(99)01718-7. [DOI] [PubMed] [Google Scholar]
- Liu YM, Schneider M, Sticha CM, Toyooka T, Sweedler JV. Separation of amino acid and peptide stereoisomers by nonionic micelle-mediated capillary electrophoresis after chiral derivatization. J Chromatogr. 1998;800(2):345–354. doi: 10.1016/s0021-9673(97)01137-0. [DOI] [PubMed] [Google Scholar]
- Long Z, Lee JA, Okamoto T, Nimura N, Imai K, Homma H. D-aspartate in a prolactin-secreting clonal strain of rat pituitary tumor cells (GH(3)) Biochem Biophys Res Commun. 2000;276(3):1143–1147. doi: 10.1006/bbrc.2000.3573. [DOI] [PubMed] [Google Scholar]
- Long ZQ, Homma H, Lee JA, Fukushima T, Santa T, Iwatsubo T, Yamada RH, Imai K. Biosynthesis of D-aspartate in mammalian cells. FEBS Lett. 1998;434(3):231–235. doi: 10.1016/s0014-5793(98)00986-7. [DOI] [PubMed] [Google Scholar]
- Long ZQ, Sekine M, Adachi M, Furuchi T, Imai K, Nimura N, Homma H. Cell density inversely regulates D- and L-aspartate levels in rat pheochromocytoma MPT1 cells. Arch Biochem Biophys. 2002;404(1):92–97. doi: 10.1016/s0003-9861(02)00241-2. [DOI] [PubMed] [Google Scholar]
- Malthesorenssen D, Skrede KK, Fonnum F. Calcium-dependent release of d-[3H]aspartate evoked by selective electrical stimulation of excitatory afferent fibres to hippocampal pyramidal cells in vitro. Neuroscience. 1979;4(9):1255–1263. doi: 10.1016/0306-4522(79)90155-6. [DOI] [PubMed] [Google Scholar]
- Masuda W, Nouso C, Kitamura C, Terashita M, Noguchi T. Free D-aspartic acid in rat salivary glands. Arch Biochem Biophys. 2003;420(1):46–54. doi: 10.1016/j.abb.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Miao H, Rubakhin SS, Scanlan CR, Wang LP, Sweedler JV. d-Aspartate as a putative cell-cell signaling molecule in the Aplysia californica central nervous system. J Neurochem. 2006a;97(2):595–606. doi: 10.1111/j.1471-4159.2006.03891.x. [DOI] [PubMed] [Google Scholar]
- Miao H, Rubakhin SS, Sweedler JV. Subcellular analysis of D-Aspartate. Anal Chem. 2005;77(22):7190–7194. doi: 10.1021/ac0511694. [DOI] [PubMed] [Google Scholar]
- Miao H, Rubakhin SS, Sweedler JV. Confirmation of peak assignments in capillary electrophoresis using immunoprecipitation. Application to D-aspartate measurements in neurons. J Chromatogr A. 2006b;1106(1–2):56–60. doi: 10.1016/j.chroma.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Morikawa A, Hamase K, Inoue T, Konno R, Zaitsu K. Alterations in D-amino acid levels in the brains of mice and rats after the administration of D-amino acids. Amino Acids. 2007;32(1):13–20. doi: 10.1007/s00726-005-0357-8. [DOI] [PubMed] [Google Scholar]
- Muzzolini A, Bregola G, Bianchi C, Beani L, Simonato M. Characterization of glutamate and [H-3]D-aspartate outflow from various in vitro preparations of the rat hippocampus. Neurochem Int. 1997;31(1):113–124. doi: 10.1016/S0197-0186(96)00129-5. [DOI] [PubMed] [Google Scholar]
- Nagasaki H. Gender-related differences of mouse liver D-aspartate oxidase in the activity and response to administration of D-aspartate and peroxisome proliferators. Int J Biochem. 1994;26(3):415–423. doi: 10.1016/0020-711X(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Homma H, Lee JA, Imai K. D-aspartate stimulation of testosterone synthesis in rat Leydig cells. FEBS Lett. 1999a;444(2–3):160–164. doi: 10.1016/s0014-5793(99)00045-9. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Homma H, Matsumoto M, Imai K. Stimulation of steroidogenic acute regulatory protein (StAR) gene expression by D-aspartate in rat Leydig cells. FEBS Lett. 1999b;454(3):317–320. doi: 10.1016/s0014-5793(99)00840-6. [DOI] [PubMed] [Google Scholar]
- Nakatsuka S, Hayashi M, Muroyama A, Otsuka M, Kozaki S, Yamada H, Moriyama Y. D-aspartate is stored in secretory granules and released through a Ca2+-dependent pathway in a subset of rat pheochromocytoma PC12 cells. J Biol Chem. 2001;276(28):26589–26596. doi: 10.1074/jbc.M011754200. [DOI] [PubMed] [Google Scholar]
- Negri A, Ceciliani F, Tedeschi G, Simonic T, Ronchi S. The primary structure of the flavoprotein D-aspartate oxidase from beef kidney. J Biol Chem. 1992;267 (17):11865–11871. [PubMed] [Google Scholar]
- Neidle A, Dunlop DS. Developmental changes in free D-aspartic acid in the chicken embryo and in the neonatal rat. Life Sci. 1990;46(21):1517–1522. doi: 10.1016/0024-3205(90)90424-p. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Fukuda M, Sasaoka K. Occurrence of D-amino acid aminotransferase in pea seedlings. Biochem Biophys Res Commun. 1973;52:998–1002. doi: 10.1016/0006-291x(73)91036-x. [DOI] [PubMed] [Google Scholar]
- Okuma E, Abe H. Simultaneous determination of D- and L-amino acids in the nervous tissues of crustaceans using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate and reversed-phase ion-pair high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1994;660(2):243–250. doi: 10.1016/0378-4347(94)00304-1. [DOI] [PubMed] [Google Scholar]
- Page JS, Rubakhin SS, Sweedler JV. Single-neuron analysis using CE combined with MALDI MS and radionuclide detection. Anal Chem. 2002;74 (3):497–503. doi: 10.1021/ac0156621. [DOI] [PubMed] [Google Scholar]
- Palmer AM, Reiter CT. Comparison of the superfused efflux of preaccumulated D-[3H]aspartate and endogenous L-aspartate and L-glutamate from rat cerebrocortical minislices. Neurochem Int. 1994;25(5):441–450. doi: 10.1016/0197-0186(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Pampillo M, Scimonelli T, Bottino MC, Duvilanski BH, Rettori V, Seilicovich A, Lasaga M. The effect of D-aspartate on luteinizing hormone-releasing hormone, alpha-melanocyte-stimulating hormone, GABA and dopamine release. Neuroreport. 2002;13(17):2341–2344. doi: 10.1097/01.wnr.0000044986.13025.9d. [DOI] [PubMed] [Google Scholar]
- Ramachandran B, Houben K, Rozenberg YY, Haigh JR, Varpetian A, Howard BD. Differential expression of transporters for norepinephrine and glutamate in wild type, variant, and WNT1-expressing PC12 cells. J Biol Chem. 1993;268 (32):23891–23897. [PubMed] [Google Scholar]
- Raucci F, Di Fiore MM. The maturation of oocyte follicular epithelium of Podarcis s. sicula is promoted by D-aspartic acid. J Histochem Cytochem. 2010;58(2):157–171. doi: 10.1369/jhc.2009.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucci F, Di Fiore MM. D-Asp: A new player in reproductive endocrinology of the amphibian Rana esculenta. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(29):3268–3276. doi: 10.1016/j.jchromb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Raucci F, Santillo A, D’Aniello A, Baccari GC. D-aspartate modulates transcriptional activity in Harderian gland of frog, Rana esculenta: Morphological and molecular evidence. J Cell Physiol. 2005;204(2):445–454. doi: 10.1002/jcp.20316. [DOI] [PubMed] [Google Scholar]
- Rubakhin SS, Romanova EV, Nemes P, Sweedler JV. Profiling metabolites and peptides in single cells. Nat Methods. 2011;8(4 Suppl):S20–29. doi: 10.1038/nmeth.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Homma H, Lee JA, Fukushima T, Santa T, Tashiro K, Iwatsubo T, Imai K. Emergence of D-aspartic acid in the differentiating neurons of the rat central nervous system. Brain Res. 1998;808(1):65–71. doi: 10.1016/s0006-8993(98)00599-x. [DOI] [PubMed] [Google Scholar]
- Sato M, Inoue F, Kanno N, Sato Y. The occurrence of N-methyl-D-aspartic acid in muscle extracts of the blood shell, Scapharca broughtonii. Biochem J. 1987;241 (1):309–311. doi: 10.1042/bj2410309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DD, Galindo R, Queen SA, Paxton LL, Allan AM. Characterization of electrically evoked H-3 -D-aspartate release from hippocampal slices. Neurochem Int. 2001;38(3):255–267. doi: 10.1016/s0197-0186(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Scanlan C, Shi T, Hatcher NG, Rubakhin SS, Sweedler JV. Synthesis, accumulation, and release of D-aspartate in the Aplysia californica CNS. J Neurochem. 2010;115(5):1234–1244. doi: 10.1111/j.1471-4159.2010.07020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Cooper OB, Snyder SH. D-aspartate localizations imply neuronal and neuroendocrine roles. Proc Natl Acad Sci U S A. 1997;94(5):2013–2018. doi: 10.1073/pnas.94.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoyama C, Miura R. Structural and functional characterization of the human brain D-aspartate oxidase. J Biochem. 1997;121 (4):798–803. doi: 10.1093/oxfordjournals.jbchem.a021655. [DOI] [PubMed] [Google Scholar]
- Shibata K, Sugaya N, Ono W, Abe K, Takahashi S, Kera Y. Determination of D-aspartate N-methyltransferase activity in the starfish by direct analysis of N-methyl-D-aspartate with high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sciw. 2011;879(29):3229–3234. doi: 10.1016/j.jchromb.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Shibata K, Watanabe T, Yoshikawa H, Abe K, Takahashi S, Kera Y, Yamada RH. Purification and characterization of aspartate racemase from the bivalve mollusk Scapharca broughtonii. Comp Biochem Physiol B Biochem Mol Biol. 2003;134(2):307–314. doi: 10.1016/s1096-4959(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Simonic T, Duga S, Negri A, Tedeschi G, Malcovati M, Tenchini ML, Ronchi S. cDNA cloning and expression of the flavoprotein D-aspartate oxidase from bovine kidney cortex. Biochem J. 1997;322:729–735. doi: 10.1042/bj3220729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli P, Brown ER, Ferrandino G, Branno M, Montarolo PG, D’Aniello E, Rastogi RK, D’Aniello B, Baccari GC, Fisher G, D’Aniello A. D-aspartic acid in the nervous system of Aplysia limacina: Possible role in neurotransmission. J Cell Physiol. 2006;206(3):672–681. doi: 10.1002/jcp.20513. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Takahashi T, Kera Y, Matsunaga R, Shibuya H, Yamada RH. Cloning and expression in Escherichia coli of the D-aspartate oxidase gene from the yeast Cryptococcus humicola and characterization of the recombinant enzyme. J Biochem. 2004;135(4):533–540. doi: 10.1093/jb/mvh068. [DOI] [PubMed] [Google Scholar]
- Takigawa Y, Homma H, Lee JA, Fukushima T, Santa T, Iwatsubo T, Imai K. D-aspartate uptake into cultured rat pinealocytes and the concomitant effect on L-aspartate levels and melatonin secretion. Biochem Biophys Res Commun. 1998;248(3):641–647. doi: 10.1006/bbrc.1998.8971. [DOI] [PubMed] [Google Scholar]
- Todoroki N, Shibata K, Yamada T, Kera Y, Yamada RH. Determination of N-methyl-D-aspartate in tissues of bivalves by high-performance liquid chromatography. J Chromatogr B. 1999;728(1):41–47. doi: 10.1016/s0378-4347(99)00089-4. [DOI] [PubMed] [Google Scholar]
- Topo E, Soricelli A, D’Aniello A, Ronsini S, D’Aniello G. The role and molecular mechanism of D-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. Reprod Biol Endocrinol. 2009;7(120) doi: 10.1186/1477-7827-7-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topo E, Soricelli A, Di Maio A, D’Aniello E, Di Fiore MM, D’Aniello A. Evidence for the involvement of d-aspartic acid in learning and memory of rat. Amino Acids. 2010;38(5):1561–1569. doi: 10.1007/s00726-009-0369-x. [DOI] [PubMed] [Google Scholar]
- Vacca RA, Christen P, Malashkevich VN, Jansonius JN, Sandmeier E. Substitution of apolar residues in the active site of aspartate aminotransferase by histidine. Effects on reaction and substrate specificity. Eur J Biochem. 1995;227(1–2):481–487. doi: 10.1111/j.1432-1033.1995.tb20413.x. [DOI] [PubMed] [Google Scholar]
- Vacca RA, Giannattasio S, Graber R, Sandmeier E, Marra E, Christen P. Active-site Arg->Lys substitutions alter reaction and substrate specificity of aspartate aminotransferase. J Biol Chem. 1997;272(35):21932–21937. doi: 10.1074/jbc.272.35.21932. [DOI] [PubMed] [Google Scholar]
- Vanveldhoven PP, Brees C, Mannaerts GP. D-aspartate oxidase, a peroxisomal enzyme in liver of rat and man. Biochim Biophys Acta. 1991;1073(1):203–208. doi: 10.1016/0304-4165(91)90203-s. [DOI] [PubMed] [Google Scholar]
- Verdoorn TA, Dingledine R. Excitatory amino acid receptors expressed in Xenopus oocytes: agonist pharmacology. Mol Pharmacol. 1988;34:298–307. [PubMed] [Google Scholar]
- Wang H, Wolosker H, Morris JF, Pevsner J, Snyder SH, Selkoe DJ. Naturally occurring free D-aspartate is a nuclear component of cells in the mammalian hypothalamo-neurohypophyseal system. Neuroscience. 2002;109(1):1–4. doi: 10.1016/S0306-4522(01)00545-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Wolosker H, Pevsner J, Snyder SH, Selkoe DJ. Regulation of rat magnocellular neurosecretory system by D-aspartate: evidence for biological role(s) of a naturally occurring free D-amino acid in mammals. J Endocrinol. 2000;167(2):247–252. doi: 10.1677/joe.0.1670247. [DOI] [PubMed] [Google Scholar]
- Wang LP, Ota N, Romanova EV, Sweedler JV. A novel pyridoxal 5′-phosphate-dependent amino acid racemase in the Aplysia californica central nervous system. J Biol Chem. 2011;286(15):13765–13774. doi: 10.1074/jbc.M110.178228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JC, Jane DE. The glutamate story. Br J Pharmacol. 2006;147:S100–S108. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Huang AS, Beigneux A, Kim PM, Molliver ME, Blackshaw S, Young SG, Nelson RJ, Snyder SH. Behavioural alterations in male mice lacking the gene for D-aspartate oxidase. Behav Brain Res. 2006;171(2):295–302. doi: 10.1016/j.bbr.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Wolosker H, D’Aniello A, Snyder SH. D-aspartate disposition in neuronal and endocrine tissues: Ontogeny, biosynthesis and release. Neuroscience. 2000;100(1):183–189. doi: 10.1016/s0306-4522(00)00321-3. [DOI] [PubMed] [Google Scholar]
- Yamada R, Nagasaki H, Nagata Y, Wakabayashi Y, Iwashima A. Administration of D-aspartate increases D-aspartate oxidase activity in mouse liver. Biochim Biophys Acta. 1989;990(3):325–328. doi: 10.1016/S0304-4165(89)80053-4. [DOI] [PubMed] [Google Scholar]
- Yamada RH, Ujiie H, Kera Y, Nakase T, Kitagawa K, Imasaka T, Arimoto K, Takahashi M, Matsumura Y. Purification and properties of D-aspartate oxidase from Cryptococcus humicolus UJ1. Biochimica Et Biophysica Acta-Protein Structure and Molecular Enzymology. 1996;1294(2):153–158. doi: 10.1016/0167-4838(96)00012-x. [DOI] [PubMed] [Google Scholar]