Abstract
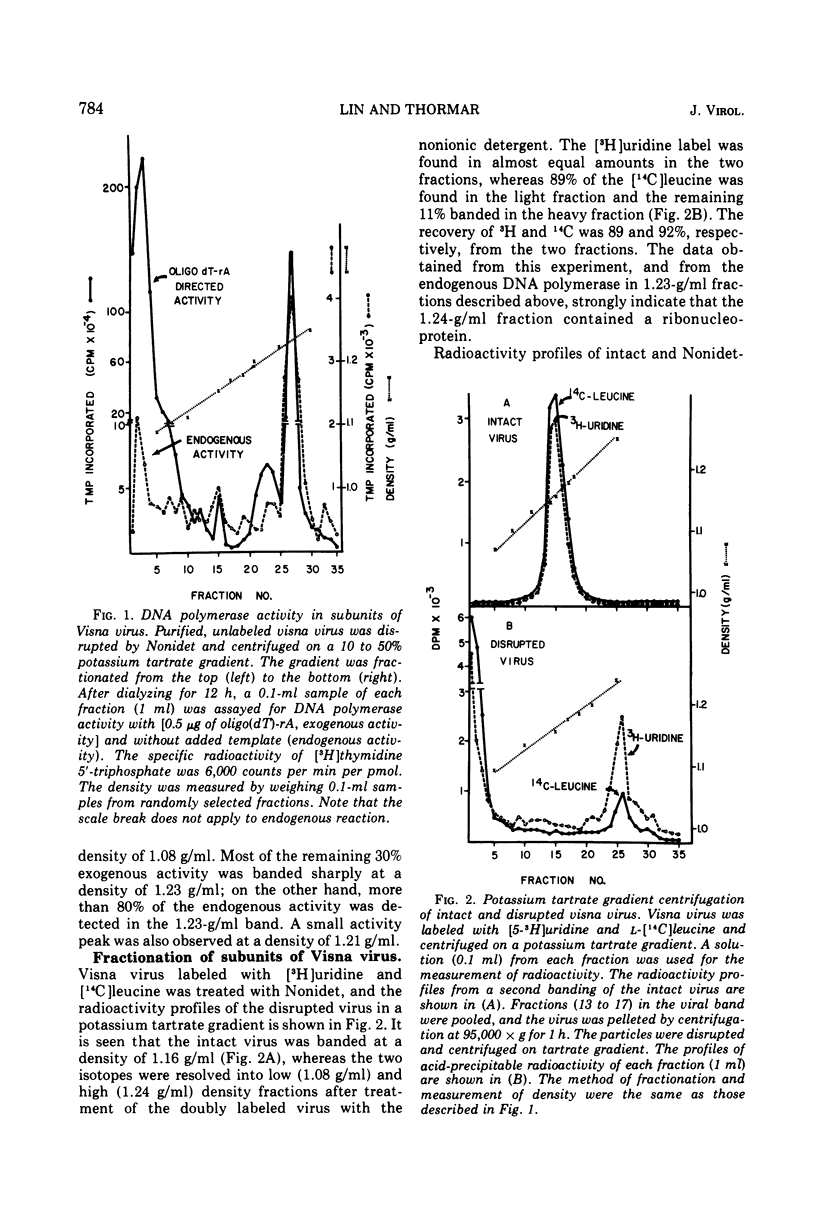
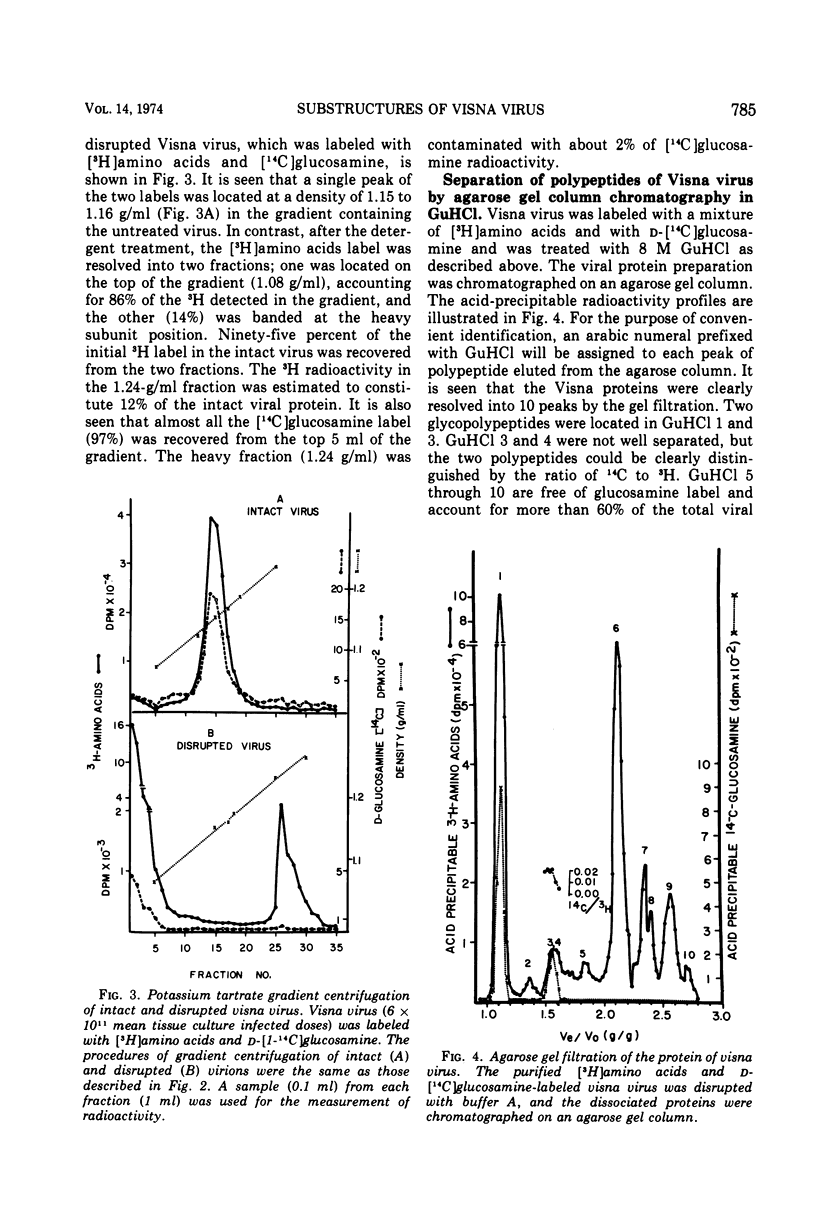
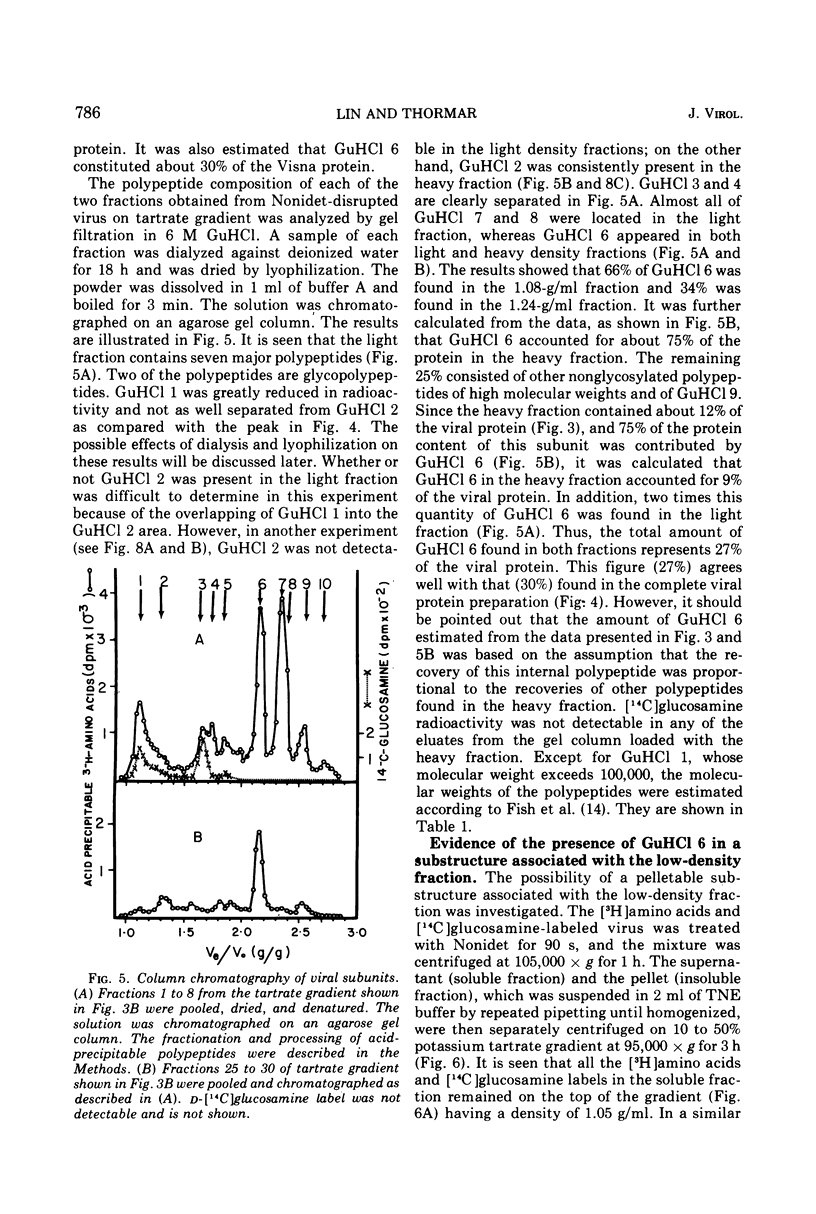
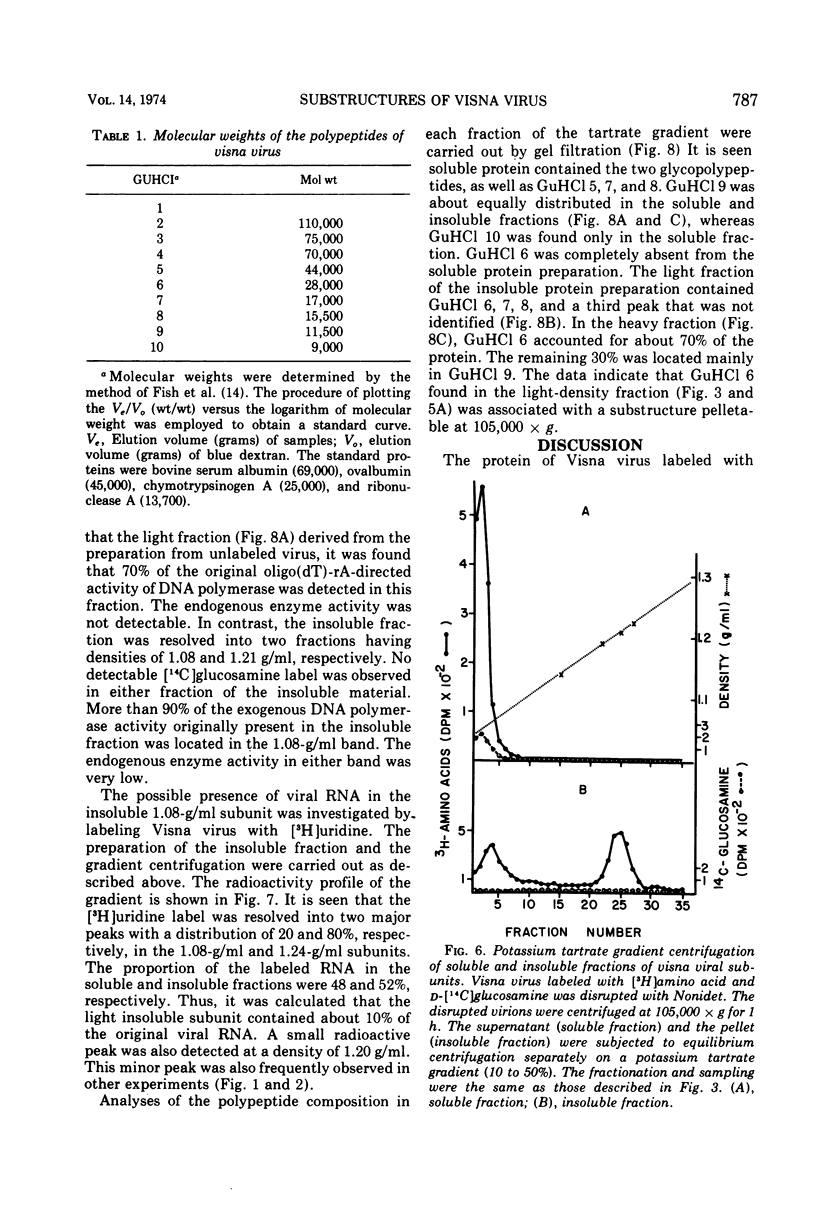
The protein of Visna virus, disrupted by 8 M guanidine hydrochloride and heating, was resolved into 10 polypeptides by agarose gel column chromatography in 6 M guanidine hydrochloride. Two of the peaks contained glycopolypeptides. Nonidet-disrupted virions were resolved into two fractions by potassium tartrate gradient centrifugation, with densities of 1.08 and 1.24 g/ml, respectively. About 70% of the viral DNA polymerase directed by added template was released into the light fraction, in which very little endogenous enzyme activity was detected. Also released into the light fraction were all of the glycopolypeptides, 50% of the viral RNA, and a part of each of the other viral protein components. The data indicate that extensive degradation of subviral structures occurred, even under mild conditions for virion disruption. The 1.24-g/ml fraction was composed of 50% of the viral RNA, most of the endogenous DNA polymerase activity (80%), and a major internal polypeptide (GuHCl6) with an estimated mol wt of 28,000. Two other polypeptides were also consistently detected in the heavy fraction, but they constituted less than 25% of the ribonucleoprotein complex, compared with 75% for GuHCl6.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P., Brown N. R., Bader A. V. Characteristics of cores of avian leuko-sarcoma viruses. Virology. 1970 Aug;41(4):718–728. doi: 10.1016/0042-6822(70)90436-8. [DOI] [PubMed] [Google Scholar]
- Baltimore D. RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature. 1970 Jun 27;226(5252):1209–1211. doi: 10.1038/2261209a0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Bauer H. Polypeptides of avian RNA tumor viruses. 1. Isolation and physical and chemical analysis. Virology. 1970 Dec;42(4):1097–1112. doi: 10.1016/0042-6822(70)90357-0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Gelderblom H., Bauer H., Mölling K., Hüper G. Polypeptides of avian RNA tumor viruses. V. Analysis of the virus core. Virology. 1972 Mar;47(3):567–578. doi: 10.1016/0042-6822(72)90546-6. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Luftig R., Shaper J. H. Localization of RNA tumor virus polypeptides. I. Isolation of further virus substructures. Virology. 1973 Dec;56(2):549–564. doi: 10.1016/0042-6822(73)90057-3. [DOI] [PubMed] [Google Scholar]
- Brahic M., Tamalet J., Chippaux-Hyppolite C. Virus Visna: isolement d'une molécute d'acide ribonucléique de haut poids moléculaire. C R Acad Sci Hebd Seances Acad Sci D. 1971 Apr 19;272(16):2115–2118. [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Comparison of Rous sarcoma virus-specific deoxyribonucleic acid polymerases in virions of Rous sarcoma virus and in Rous sarcoma virus-infected chicken cells. J Virol. 1971 May;7(5):625–634. doi: 10.1128/jvi.7.5.625-634.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. On the structure of RNA tumor viruses. Curr Top Microbiol Immunol. 1970;51:78–104. [PubMed] [Google Scholar]
- Duesberg P. H., Robinson H. L., Robinson W. S., Huebner R. J., Turner H. C. Proteins of Rous sarcoma virus. Virology. 1968 Sep;36(1):73–86. doi: 10.1016/0042-6822(68)90118-9. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Nucleic acid and proteins isolated from the Rauscher mouse leukemia virus (MLV). Proc Natl Acad Sci U S A. 1966 Jan;55(1):219–227. doi: 10.1073/pnas.55.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi P., Brahic M., Tamalet J., Delbecchi L. L'ADN polymérase ARN dépendante du virus visna. Etude des produits de la réaction. C R Acad Sci Hebd Seances Acad Sci D. 1972 Oct 2;275(14):1567–1570. [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Fleissner E. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. I. Avian leukemia-sarcoma viruses. J Virol. 1971 Nov;8(5):778–785. doi: 10.1128/jvi.8.5.778-785.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Takemoto K., Robert M., Gallo R. C. Polyadenylic acid in Visna virus RNA. Science. 1973 Mar 30;179(4080):1328–1330. doi: 10.1126/science.179.4080.1328. [DOI] [PubMed] [Google Scholar]
- Green M., Cartas M. The genome of RNA tumor viruses contains polyadenylic acid sequences. Proc Natl Acad Sci U S A. 1972 Apr;69(4):791–794. doi: 10.1073/pnas.69.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Baringer J. R. The structural polypeptides of RNA slow viruses. Virology. 1974 Jan;57(1):238–250. doi: 10.1016/0042-6822(74)90124-x. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Robinson H. L., Robinson W. S. Isolation and characterization of proteins from Rous sarcoma virus. Virology. 1971 Jan;43(1):251–266. doi: 10.1016/0042-6822(71)90243-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Characterization of ribonucleic acid from visna virus. J Virol. 1971 May;7(5):582–587. doi: 10.1128/jvi.7.5.582-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. On visna virus: purification and nucleic acid content. Virology. 1970 Dec;42(4):1140–1143. doi: 10.1016/0042-6822(70)90363-6. [DOI] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Ribonucleic acid-dependent deoxyribonucleic acid polymerase in visna virus. J Virol. 1970 Nov;6(5):702–704. doi: 10.1128/jvi.6.5.702-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle W. E., Harter D. H., Choppin P. W. The proteins of visna virus. Virology. 1972 Feb;47(2):542–545. doi: 10.1016/0042-6822(72)90299-1. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Fleissner E., Sarkar N. H., Aoki T. Chromatographic separation and antigenic analysis of proteins of the oncornaviruses. II. Mammalian leukemia-sarcoma viruses. J Virol. 1972 Feb;9(2):359–366. doi: 10.1128/jvi.9.2.359-366.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P., Rifkin D. B., Compans R. W. Isolation and characterization of ribonucleoprotein substructures from Rous sarcoma virus. Virology. 1972 Oct;50(1):65–75. doi: 10.1016/0042-6822(72)90346-7. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Baluda M. A. The nucleic acid from avian myeloblastosis virus compared with the RNA from the Bryan strain of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1686–1692. doi: 10.1073/pnas.54.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Tronick S. R., Scolnick E. M. Polyadenylate rich RNA in the 70 S RNA of murine leukemia-sarcoma virus. Virology. 1972 Jul;49(1):230–235. doi: 10.1016/s0042-6822(72)80025-4. [DOI] [PubMed] [Google Scholar]
- Schlom J., Harter D. H., Burny A., Spiegelman S. DNA polymerase activities in varions of visna virus, a causative agent of a "slow" neurological disease. Proc Natl Acad Sci U S A. 1971 Jan;68(1):182–186. doi: 10.1073/pnas.68.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone L. B., Scolnick E., Takemoto K. K., Aaronson S. A. Visna virus: a slow virus with an RNA dependent DNA polymerase. Nature. 1971 Jan 22;229(5282):257–258. doi: 10.1038/229257a0. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Stone L. B. Transformation of murine cells by two "slow viruses," visna virus and progressive pneumonia virus. J Virol. 1971 Jun;7(6):770–775. doi: 10.1128/jvi.7.6.770-775.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Mizutani S. RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature. 1970 Jun 27;226(5252):1211–1213. doi: 10.1038/2261211a0. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Scolnick E. M., Parks W. P. Reversible inactivation of the deoxyribonucleic acid polymerase of Rauscher leukemia virus. J Virol. 1972 Oct;10(4):885–888. doi: 10.1128/jvi.10.4.885-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


