Abstract
Polycytidylic acid [poly(rC)] covalently linked to cyanogen bromide-activated agarose is an effective affinity matrix for the RNA-dependent DNA polymerase from avian myeloblastosis virus. Poly(rC)-agarose is capable of binding large quantities of avian myeloblastosis DNA polymerase, which is then eluted by using a linear KCl gradient of increasing concentration. The DNA polymerase isolated from crude, detergent-disrupted virions by a single pass through columns of poly(rC)-agarose appears nearly homogeneous (approximately 90% pure) as determined by sodium dodecyl sulfate-polyacrylamide disc gel electrophoresis. Complete recovery of input enzymatic activity was obtained. Results suggest that polyribonucleotide columns may provide a high-yield, rapid method for the purification of oncornaviral DNA polymerase.
Full text
PDF
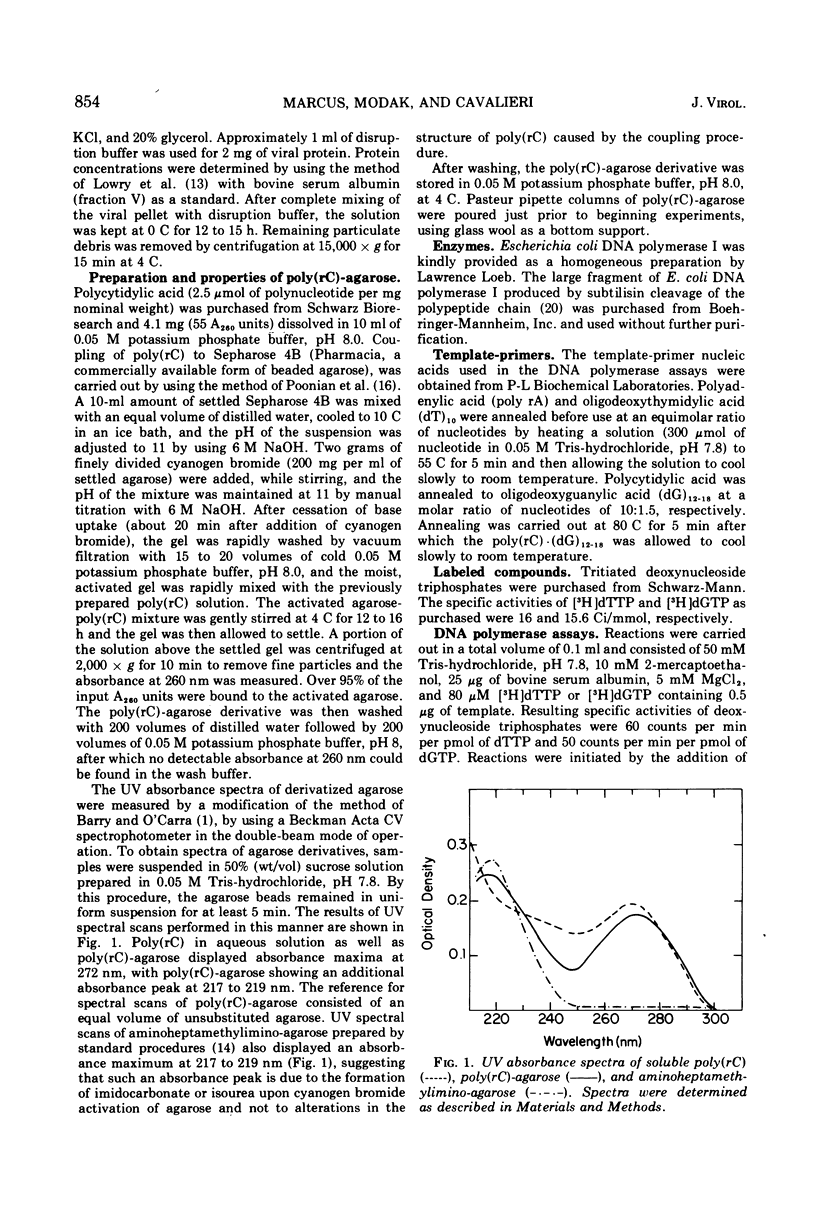


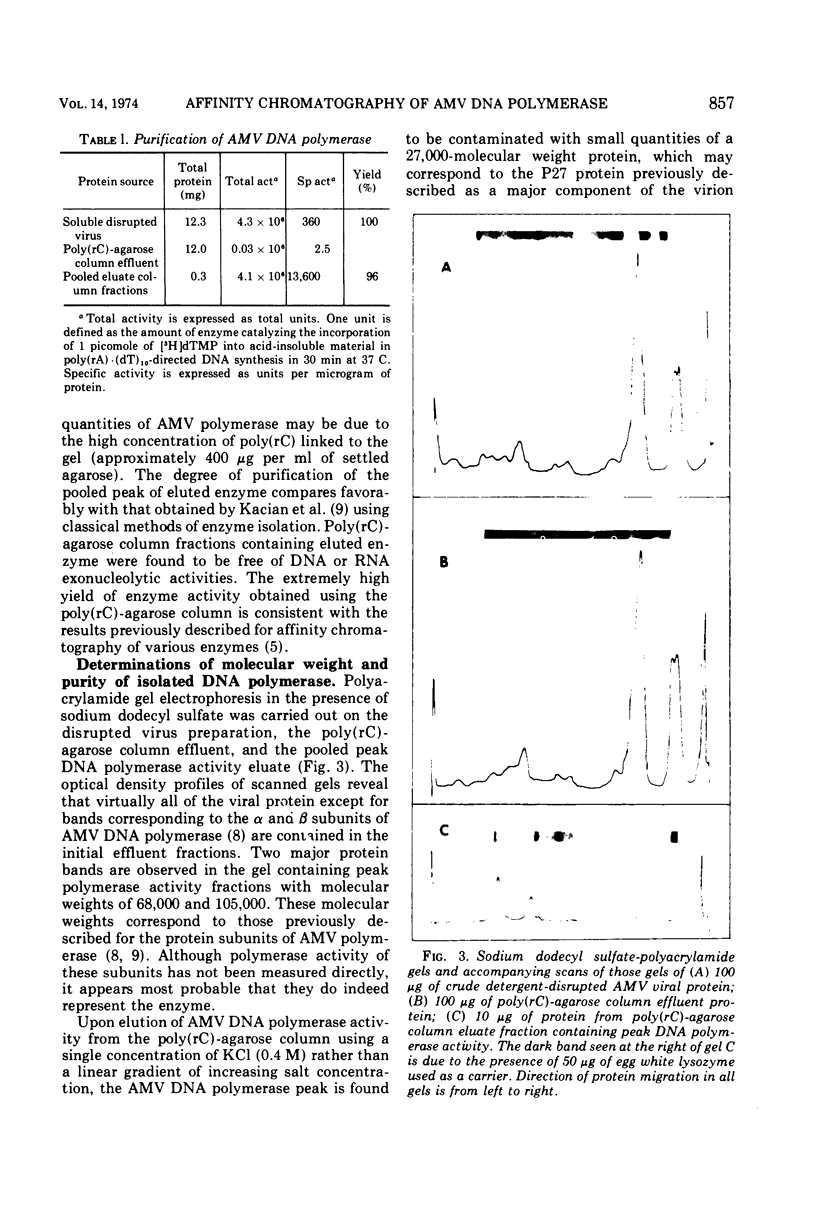
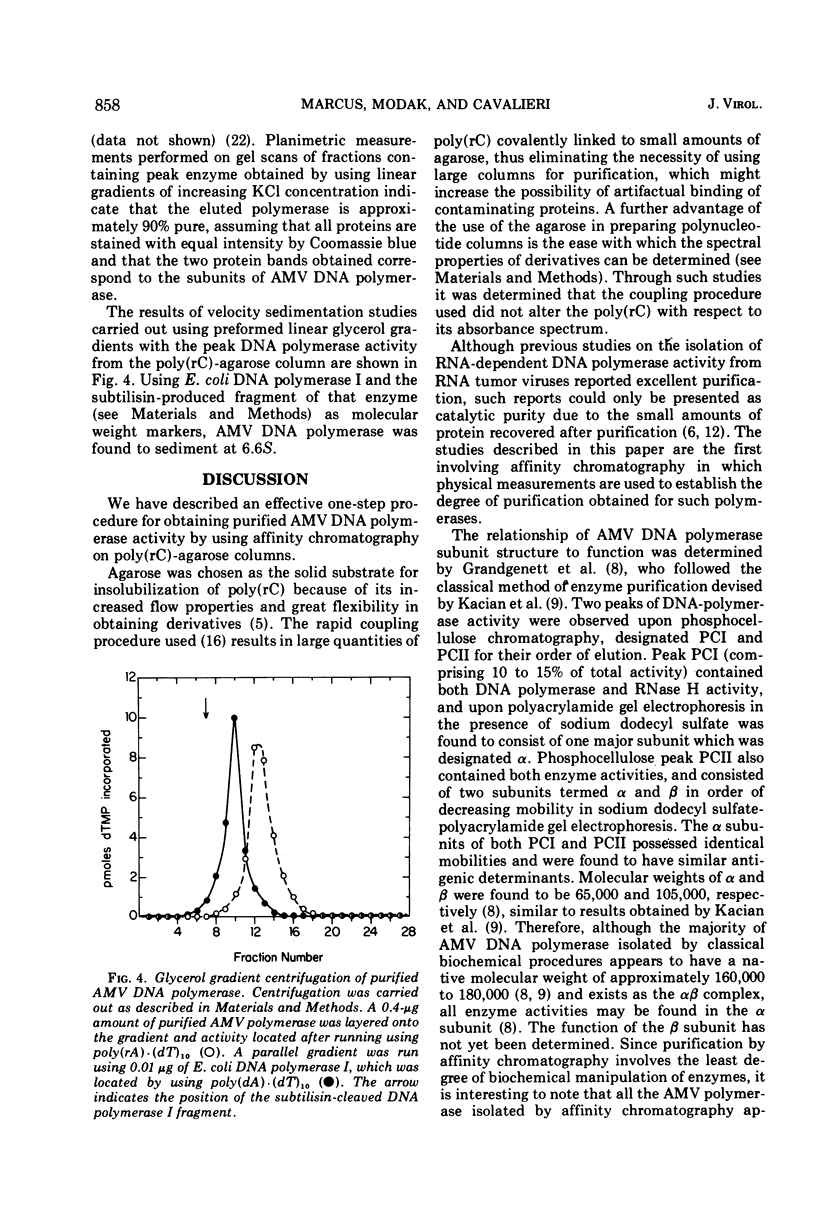

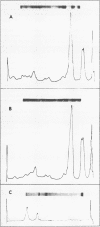
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry S., O'Carra P. Affinity chromatography of nicotinamide-adenine dinucleotide-linked dehydrogenases on immobilized derivatives of the dinucleotide. Biochem J. 1973 Dec;135(4):595–607. doi: 10.1042/bj1350595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya J., Xuma M., Reitz M., Sarin P. S., Gallo R. C. Utilization of mammalian 70S RNA by a purified reverse transcriptase from human myelocytic leukemic cells. Biochem Biophys Res Commun. 1973 Sep 5;54(1):324–334. doi: 10.1016/0006-291x(73)90926-1. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography of macromolecules. Adv Enzymol Relat Areas Mol Biol. 1972;36:29–89. doi: 10.1002/9780470122815.ch2. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Gerwin B. I., Milstien J. B. An oligonucleotide affinity column for RNA-dependent DNA polymerase from RNA tumor viruses. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2599–2603. doi: 10.1073/pnas.69.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman N. C., Spiegelman S. Distinguishing reverse transcriptase of an RNA tumor virus from other known DNA polymerases. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2203–2206. doi: 10.1073/pnas.68.9.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Gerard G. F., Green M. A single subunit from avian myeloblastosis virus with both RNA-directed DNA polymerase and ribonuclease H activity. Proc Natl Acad Sci U S A. 1973 Jan;70(1):230–234. doi: 10.1073/pnas.70.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacian D. L., Watson K. F., Burny A., Spiegelman S. Purification of the DNA polymerase of avian myeloblastosis virus. Biochim Biophys Acta. 1971 Sep 24;246(3):365–383. doi: 10.1016/0005-2787(71)90773-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis B. J., Abrell J. W., Smith R. G., Gallo R. C. Human DNA polymerase 3 (R-DNA polymerase): distinction from DNA polymerase I and reverse transcriptase. Science. 1974 Mar 1;183(4127):867–869. doi: 10.1126/science.183.4127.867. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Scolnick E. M., Parks W. P., Todaro G. T. Affinity chromatography of RNA-dependent DNA polymerase from RNA tumor viruses on a solid phase immunoadsorbent. Proc Natl Acad Sci U S A. 1972 Feb;69(2):393–397. doi: 10.1073/pnas.69.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S. L., Balbinder E. Use of affinity matrices in determining steric requirements for substrate binding: binding of anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase from Salmonella typhimurium to Sepharose-Anthranilate derivatives. Anal Biochem. 1972 Aug;48(2):448–459. doi: 10.1016/0003-2697(72)90098-x. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Modak M. J., Cavalieri L. F. Evidence for template-specific sites in DNA polymerases. Biochem Biophys Res Commun. 1974 Jan 23;56(2):516–521. doi: 10.1016/0006-291x(74)90873-0. [DOI] [PubMed] [Google Scholar]
- Poonian M. S., Schlabach A. J., Weissbach A. Covalent attachment of nucleic acids to agarose for affinity chromatography. Biochemistry. 1971 Feb 2;10(3):424–427. doi: 10.1021/bi00779a011. [DOI] [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Sarin P. S., Reitz M. S., Gallo R. C. Reverse transcriptase activity of human acute leukaemic cells: purification of the enzyme, response to AMV 70S RNA, and characterization of the DNA product. Nat New Biol. 1972 Nov 15;240(98):67–72. doi: 10.1038/newbio240067a0. [DOI] [PubMed] [Google Scholar]
- Setlow P., Brutlag D., Kornberg A. Deoxyribonucleic acid polymerase: two distinct enzymes in one polypeptide. I. A proteolytic fragment containing the polymerase and 3' leads to 5' exonuclease functions. J Biol Chem. 1972 Jan 10;247(1):224–231. [PubMed] [Google Scholar]
- Smith R. G., Gallo R. C. DNA-dependent DNA polymerases I and II from normal human-blood lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2879–2884. doi: 10.1073/pnas.69.10.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg K., Hurley N. E., Davis N. L., Rueckert R. R., Fleissner E. Structural studies of avian myeloblastosis virus: comparison of polypeptides in virion and core component by dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1974 Feb;13(2):513–528. doi: 10.1128/jvi.13.2.513-528.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]