Abstract
Non-viral gene delivery has been extensively explored as the replacement for viral systems. Among various non-viral approaches, electroporation has gained increasing attention because of its easy operation and no restrictions on probe or cell type. Several effective systems are now available on the market with reasonably good gene delivery performance. To facilitate broader biological and medical applications, micro-/nanofluidics based technologies were introduced in cell electroporation during the past two decades and their advances are summarized in this perspective. Compared to the commercially available bulk electroporation systems, they offer several advantages, namely, (1) sufficiently high pulse strength generated by a very low potential difference, (2) conveniently concentrating, trapping, and regulating the position and concentration of cells and probes, (3) real-time monitoring the intracellular trafficking at single cell level, and (4) flexibility on cells to be transfected (from single cell to large scale cell population). Some of the micro-devices focus on cell lysis or fusion as well as the analysis of cellular properties or intracellular contents, while others are designed for gene transfection. The uptake of small molecules (e.g., dyes), DNA plasmids, interfering RNAs, and nanoparticles has been broadly examined on different types of mammalian cells, yeast, and bacteria. A great deal of progress has been made with a variety of new micro-/nanofluidic designs to address challenges such as electrochemical reactions including water electrolysis, gas bubble formation, waste of expensive reagents, poor cell viability, low transfection efficacy, higher throughput, and control of transfection dosage and uniformity. Future research needs required to advance micro-/nanofluidics based cell electroporation for broad life science and medical applications are discussed.
INTRODUCTION
Efficient delivery of exogenous cargos (such as nucleic acids, proteins, and small drugs) has long been pursued to increase our understanding of gene regulation mechanisms and to yield promising pharmaceutical and/or medical benefits in drug discovery, cancer treatment, and regenerative medicine.1, 2 The intracellular delivery barriers have been tackled by a variety of approaches including viral infection or non-viral perturbation. Viral vectors could efficiently mediate gene delivery via lipid membrane fusion. Classical chemical transfection methods including lipoplex and polyplex-based nanoparticles are often much less inefficient as the delivery relies on endocytosis and endosomal escape.3 In comparison, physical approaches are capable of delivering genes safely and efficiently because these methods can directly transfer naked genes into cells. Among them, biolistic transfection (i.e., hand-held gene gun) can be applied to a wide variety of cell/tissue types, but it causes significant physical damage to cells, and gold/tungsten particle carriers may have a negative impact on cell functions. Micro-injection is a precise tool which is widely used to generate transgenic animal models for biomedical research. The advantage of this technique is that the gene of interest is directly and precisely delivered into mammalian cells or specific tissues in a more controlled manner. Nevertheless, it requires specialized equipment, a highly skilled practitioner; and the quantity of injected cells is limited within a fixed time. The procedure is also harmful, particularly for small cells used in nuclear reprogramming.
Among non-viral approaches, electroporation (EP) has been rapidly adopted by researchers and clinicians for its simplicity, convenient operation, and almost no restriction on cell type and exogenous material properties.4, 5, 6 It has been used as a research tool in vitro to understand biological functions and transport of various molecular probes at the cellular level as well as clinical tools in vivo to deliver anticancer drugs and various genes, oligo DNA, and interference RNA.7, 8, 9, 10, 11 In conventional bulk electroporation, cells are treated with short, high-voltage pulses to create temporary pathways on the cell membrane to facilitate the uptake of molecule probes.6 The transient and reversible breakdown occurs when the transmembrane potential (ΔVm) surpasses the cell membrane capacitance (∼0.2–1 V), which depends on the applied electric field strength and the size of subjected cell.7 The proper pulse strength range for reversible electroporation of mammalian cells is ∼50–1000 V/cm, varying with the electric properties of cells, medium, and electroporation systems.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 An increase of the pulse amplitude would facilitate the uptake of more exogenous materials. But that is often tied with the low cell viability caused by slow membrane recovery, excessive swelling and/or loss of intracellular components, serious electrochemical damage, and Joule heating.18, 19, 20 For commercial bulk EP (BEP) systems, many empirical protocols have been established with different pulse application strategies, while their performance varies largely by cell types, medium, and the instrumental parameters.
Further improvements come from two approaches: searching for the most effective electroporation solutions, or the creation of the best electroporation system. The former relies on regulating the cell membrane permeabilization with special chemicals/biological additions in the electroporation solution. A representative commercial technology, Nucleofector™ from Lonza, has significantly improved the transfection efficiency of their maxGFP plasmids in many cell lines by introducing cell-type specific electroporation solutions.21, 22, 23, 24 For hard-to-transfect leukemia cells, up to 75% for K562 and 60% for HL 60 gene transfection efficiency has been reported, compared to an average of ∼15% for K562 and ∼30% for HL-60 in some other electroporation systems.11, 24 However, researchers are often frustrated with less effective results when switching to other probes and/or unlisted cell systems (e.g., primary cells) because of the secret compositions of their electroporation solution additions, unclear promotion mechanism, and the lack of flexibility on protocol optimization.
On the contrary, most attempts to create an ideal electroporation system came from process and system improvement to minimize the adverse effects in electroporation, namely, high-voltage pulses and associated problems (e.g., unwanted electrochemical reactions and pH variations, Joule heating, gas bubbles near the electrode), inaccurate treatment on subjected cells, and limitations on cell throughput in each treatment.25, 26, 27 The early efforts from this approach focused on finding optimal electroporation conditions (e.g., pulse amplitude, duration, and number) through trial-and-error processes to find a compromise of the acceptable transfection efficiency and cell viability. The recent emerging of micro-/nanotechnologies opened new routes to create better electrode designs, improved operation platforms, and more advanced regulations and controls on the electric pulses, cells, and pH and/or temperature variations.25, 26, 27 A number of new electroporation systems with micro-/nanoscale features, including microfluidic-electroporation (MEP) and nanofluidic-electroporation (NEP), have been introduced, which offer various advantages over available commercial systems towards improved performance (i.e., high transfection efficiency and high cell viability) from the following aspects: (1) in situ monitoring of intracellular content transport in the electroporation process and dynamics at the single cell level,28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 (2) very low potential differences (can be as low as 1 V/cm) while sufficient to upset the cell membrane to avoid unwanted electrochemical reactions, pH variations, Joule heating, and gas bubble formation,35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 (3) better accuracy and flexibility on cell handling and manipulation to achieve dosage control and specific treatment for different sizes of cell population.50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81 These new approaches have been applied to cell lysis, cell fusion, and cell electroporation with focuses varying from single cell analysis to large scale process towards clinic use. In this perspective, we will briefly review and comment on the new progress in this field.
RECENT PROGRESS IN MICRO-/NANOFLUIDICS BASED ELECTROPORATION
Monitoring in situ electroporation dynamics and intracellular transport and cell trapping
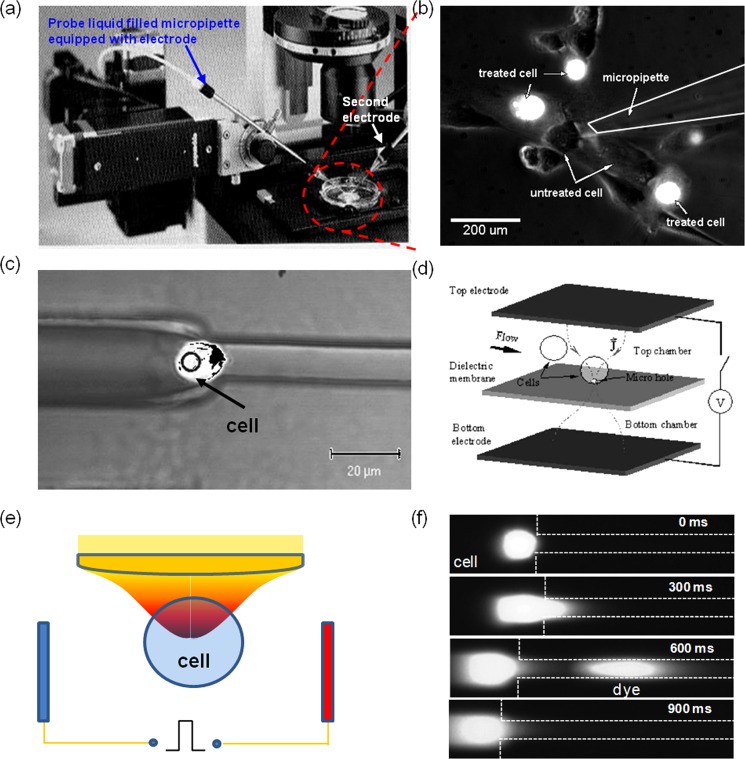
Many micro-/nanoscale electroporation systems have two-dimensional planar configurations, which offer convenient space to integrate optical detecting components for real-time monitoring the electroporation dynamics. Setups like a glass capillary electroporator combined with a precise micro-manipulator29, 30, 31, 32, 33 have long been used to break down a single targeted cell to analyze its intracellular components and/or to study the transportation of exogenous probes. This has been the key focus in early single cell electroporation research. Because of its time-consuming targeting process, more sophisticated microfluidic electroporation platforms were developed in 1990s to facilitate the handling of multiple cells in parallel. As illustrated in Fig. 1, those micro-devices usually consist of a large flow channel for cells to freely pass and one or an array of embedded constrictions (e.g., orifices or side microchannels with the key dimensions about half of the cell size or smaller to help trap cells at desired locations).42, 43, 44, 45, 46, 47, 48, 49 Electrodes were generally closely spaced and patterned so that a low potential difference is enough for electroporation and/or measurements of other electrical signal (e.g., electric conductivity or impedance) at the presence of electrolysis disturbance. To firmly hold cells, a gentle vacuum was sometimes used to pull a small portion of cells inside the microscale constrictions.46, 47, 48, 49, 55, 56, 57, 58 If cell lysis is necessary, more efficient lysis electrode designs such as inter-digitated electrodes with a saw-tooth structure could be used. Besides trapping cells with physical contact, optical tweezers technology (a technology that creates radiation pressure with an intensified laser beam to facilitate non-contact optical trapping to colloids) was also introduced to help grab and relocate cells remotely in electroporation micro-devices (Fig. 1e).46, 59 In these single cell electroporation experiments, the to-be-delivered reagents and the intracellular compartments were often labeled with fluorescence probes so that intracellular trafficking could be conveniently tracked.
Figure 1.
Micro/nanofluidic electroporation systems for single cell analysis: (a)–(b) traditional capillary electroporation setup (a) and probe-loading result (b); (c)–(d) typical microfluidic designs for cell trapping via microchannel constriction (c)46 and micro-orifice (d);42 (e) schematics of the use of optical tweezers technology for cell trapping in electroporation; and (f) the typical result on intracellular transport recording.46 Panel (c) reprinted with permission from N. R. Munce, J. Z. Li, P. R. Herman, and L. Lilge, Anal. Chem. 76, 4983 (2004). Copyright 2004 American Chemical Society. Panel (d) reprinted with permission from Y. Huang and B. Rubinsky, Sens. Actuators, A 89, 242 (2001). Copyright 2001 Elsevier.
The polarization dynamics of the cell membrane during electroporation could also be monitored to help better understand the electroporation mechanism.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 55, 59 In some studies, low molecular weight fluorescence dyes, such as propidium iodide (PI) and YOYO, were used as indicator regents for cell membrane permeabilization (these fluorescent dyes are membrane-impermeable to normal cells) as shown in Fig. 1f. To mimic the real situation of individual cells in bulk electroporation, optical tweezers were used to trap one, or multiple K562 cells and position them at various locations and patterns between two electrodes.59 It helped reveal the cell membrane permeability change dynamics at the presence of other cells during and after an electric pulse in an electroporation cuvette. This design, for the first time, provides valuable guidelines on cell-cell interaction effects when establishing electroporation protocols from single cell electroporation analysis and theory.
Pulse focusing and local perturbation
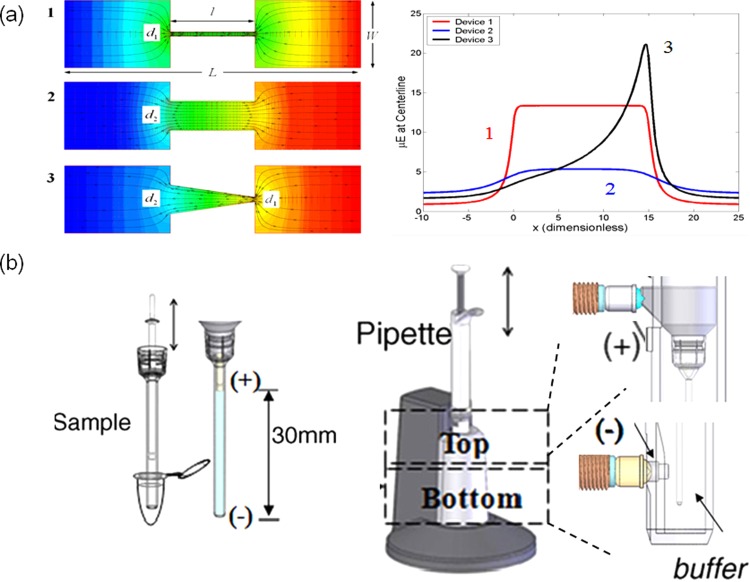
As mentioned earlier, a major problem associated with commercial electroporation systems is its harsh electric pulsation. The use of pulses with high amplitude and long duration is to increase the permeabilized area on the cell membrane to facilitate the uptake of more exogenous agents, and to ensure that the majority of cells randomly dispersed between electrodes will be properly porated. However, high-voltage pulses often lead to low cell viability resulting from slow membrane recovery, excessive swelling and/or loss of intracellular components, serious electrochemical damage, and Joule heating. Therefore, when selecting electroporation conditions, one faces the dilemma of choosing either high transfection efficiency or high cell viability, and in many cases, must compromise. Micro-/nanotechnologies have helped break this dilemma by providing the following advantages: (i) Focusing the applied electric pulses on a small area allows the use of a very low potential difference for cell poration. For instance, if two electrodes can be placed very close, e.g., ∼20 μm, a low voltage (1–2 V) can generate pulses with high enough amplitude (e.g., 500–1000 V/cm) to make cell membrane permeable.28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 49, 54 Such low voltage eliminates damages caused by electrolysis (i.e., pH variations near the electrodes and gas bubble burst) and associated Joule heating. (ii) The localized perturbation on the cell membrane minimizes cell damage, and allows the cell to recover without excessive loss of intracellular components.
The early micro-devices for low-voltage electroporation rely on various configurations of solid electrodes in close proximity.30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 Aluminum or polysilicon was used as electrode materials in early designs but later switched to precious metals (e.g., Ag/AgCl, Au, or Pt) for better signal sensitivity and less formation of harmful ions, such as Al3+ in electroporation medium.20, 60 Using bipolar pulses, or asymmetric electrode geometry could further reduce pH variation and bubble formation.60 An alternative way to resolve these issues is to place the solid electrodes away from cells while locally focusing the pulse strength with micro-channel constrictions since the electric field strength could be raised by reducing the cross-section area of a micro-channel according to the continuity equation of the electric field (i.e., ▽·Ε = 0). Micro-channels or micro-pores used for cell trapping could also be employed to generate high amplitude pulses with a low voltage potential difference. With critical dimensions of micro-channels around 4–20 μm, the electric fields strength can be easily increased to 0.5–1.0 kV/cm at voltages less than 10 V, enough to lyse or electroporate cells (Fig. 2a).
Figure 2.
Micro/nanofeatures for pulse focusing and localized electroporation: (a) the simulation results on the electric field focusing in microchannels of various geometries; (b) a commercial capillary electroporation system, NeonTM.60 Panel (b)reprinted with permission from J. A. Kim, K. Cho, M. S. Shin, W. G. Lee, N. Jung, C. Chung, and J. K. Chang, Biosens. Bioelectron. 23, 1353 (2008). Copyright 2008 Elsevier.
Although pulse focusing and local perturbation were initially designed for single cell electroporation in micro-devices, similar concepts could also benefit bulk electroporation systems for better transfection performance. For example, by focusing the pulse strength in a small capillary via asymmetric electrode geometry, Neon™ from Life Technology recently demonstrated competitive performance to Nucleofector™ technology while using only common electroporation solutions (Fig. 2b).60, 61 Such asymmetric electrode setup also keeps gas bubbles and unwanted electrochemical reactions away from cells in their device.62
Cell and probe concentration
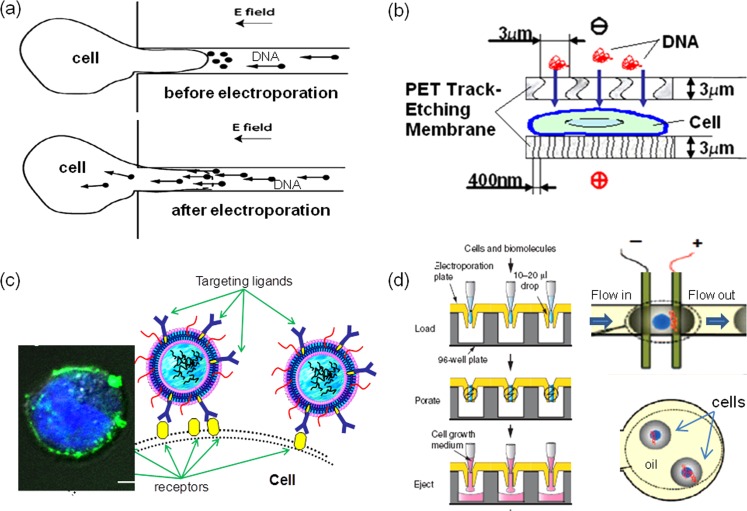
For the delivery of large molecule probes (e.g., DNA transfection), having cells and probes in close proximity is critical for successful electroporation. In typical bulk electroporation, 1–10 μg DNA plasmids are added to ensure effective transfection of 106 cells. Each cell is surrounded by ∼0.5–1.0 × 106 DNA molecules on average. However, the transfection performance suggests that only a small percentage of added DNA molecules are actually taken by the treated cells. To reduce the consumption of molecule probes and to improve the delivery efficiency, there is a need to concentrate DNA molecules onto the cell membrane surface, particularly when it becomes permeable. In this way, more molecules could enter the cell cytoplasm before the cell membrane recovers and closes the transient pores.
The probe pre- and post-concentration could be achieved by applying a low directional voltage right before and after the high voltage electroporation (Fig. 3a). When applying after pulsation, electrophoresis could help speed up the transport of negatively charged DNA molecules cross the cell membrane before its full recovery.63 If using prior to the cell membrane polarization, DNA probes are brought close to cells. Lin et al. successfully applied a low DC voltage (<1 V) in a micro-chamber to attract DNA plasmids towards the anode surface where cells were previously attached.53 The micro-channel constrictions used for pulse focusing and local perturbation could also be used here to pre- or post-concentrate probes and to facilitate their delivery if an additional DC bias (∼300 mV) is added, as demonstrated by several groups.56, 63 We found that a membrane sandwich electroporation configuration could also achieve the similar probe pre-concentration effect through the confinement effect (Fig. 3b).57 In these approaches, cells are either completely attached to the electrode surface in advance or gently held around the micro-channel constrictions by vacuum suction or confined in a micron-sized gap by the sandwich design prior to DNA attraction/pre-concentration. To pre-concentrate DNA probes around cells in a suspension state as in bulk electroporation, we adopted a specific binding approach where targeting ligands placed on the probe assemble to interact with receptors on the cell membrane (Fig. 3c). As an example, we successfully pre-concentrated an antisense oligo DNA (ODN G3139) onto K562 leukemia cells through Tf-TfR specific binding by encapsulating ODNs in transferrin (Tf) ligands targeted lipoplex nanoparticles because K562 cells over-express transferrin receptors, TfR, on their cell membrane surface.64 As clearly shown in a confocal microscopy image (Fig. 3c inset), many fluorescence-labeled Tf-lipoplex nanoparticles (Tf-LNs) were bound onto the cell membrane surface. When such Tf-LNs were used for in vitro delivery of encapsulated ODN G3139 in our flow-through electroporation system, substantial improvement was achieved for ODN delivery. Interestingly, we achieved >75% cell viability via MTS assay and did not observe high cytotoxicity commonly associated with the lipoplex/electroporation combination approaches. As ligands-receptor targeting becomes more widely used in biological applications other than immunology, this new approach provides an effective alternative for probe pre-concentration. Other concentration strategies include encapsulating cells and molecule probes in micro-droplets by pulsating the cell/probe containing droplets through a continuous oil flow stream either in a hydrodynamic focusing flow or in a concentric flow format (Fig. 3d).65, 66, 67
Figure 3.
Micro/nanofeatures for cell and probe concentration: (a) and (b) DNA probes were concentrated around cell membrane through electrophoresis in a single microchannel (a)63 and a membrane sandwich setup (b);57 in (b) cells were also fixed on membrane pores with gentle vacuum suction; (c) schematic and a confocal microscopic image on bringing probes around cell membrane through ligand-receptor specific binding approach;64 (d) confining cells and probes in microdroplets of buffer or oil prior to electroporation.65, 66 Panel (a) reprinted with permission from C. Ionescu-Zanetti, A.Blatz, and M. Khine, Biomed. Microdevices. 10, 113 (2008). Copyright 2008 Kluwer Academic. Panel (b) reprinted with permission from Z. Fei, S. Wang, Y. Xie, C.-G. Koh, B. Henslee, and L. James Lee, Anal. Chem. 79, 5719 (2007). Copyright 2007 American Chemical Society. Panel (c) reprinted with permission from S. Wang, X.Zhang, B. Yu, R. Lee, and L. J. Lee, Biosens. Bioelectron. 26, 778 (2010). Copyright 2010 Elsevier. Panel (d) reprinted with permission from E. G. Guignet and T. Meyer, Nat. Methods 5, 393 (2008). Copyright 2008 Macmillan and Y. H. Zhan, J. Wang, N. Bao, and C. Lu, Anal. Chem. 81, 2027 (2009). Copyright 2009 American Chemical Society.
Dosage control with nanochannel electroporation
Many transfection techniques can deliver biomolecules into cells, but the dose cannot be controlled precisely. Delivering well-defined amounts of materials into cells is important for various biological studies and therapeutic applications, such as nuclear reprogramming and induced pluripotent stem (iPS) cells.68, 69 Nuclear reprogramming-based technologies have not been used to date to treat patients due to several obstacles. In particular, current methodologies used to induce nuclear reprogramming result in the admixture of “partially” reprogrammed cells with the “fully” reprogrammed cells, resulting in prolonged and costly testing of candidate cell lines in order to validate their reprogrammed status. These barriers are caused in part by the inability to control the precise quantity of DNA or RNA used to induce reprogramming in each cell by use of the standard infection or transfection-based technologies. Not controlling the gene dosage that each cell receives results in stochastic gene expression. That is, some cells get too little and others too much of the reprogramming genes. In addition, a high variability in the quality and capabilities of iPS cell colonies exists.70
Electroporation has been used as a research tool for investigating the biological functions of various therapeutic materials in stem cells and in cancer cells. In addition to in vitro studies, electroporation is also used as a clinical tool for delivering anticancer drugs (e.g., bleomycin and cisplatin) and DNA, RNA, or DNA vaccines for gene therapy and DNA vaccination. However, the transport of large reagents such as nucleic acids into cells still relies on endocytosis and endosomal escape. The electric field-induced nanopores on the cell surface mainly facilitate the binding of the reagent molecules onto the cell surface (i.e., electrically mediated endocytosis). The very high electric voltage used also results in low cell viability in most cases. Except for micro-injection, the aforementioned methods are all stochastic in nature, i.e., transfecting a large cell population randomly, consequently, the transfection level is non-uniform from cell to cell. Recently, microfluidics-based electroporation has been proposed as a new technology by a number of researchers including our group. MEP offers several advantages over BEP including low potential differences for cell poration (as low as 1 V/cm), more uniform electroporation and better transfection efficiency, less reagents needed, and single cell poration.25 However, the delivery mechanism is still similar to that in BEP (i.e., diffusion-based internalization and endocytosis) and it cannot achieve precise dose control.
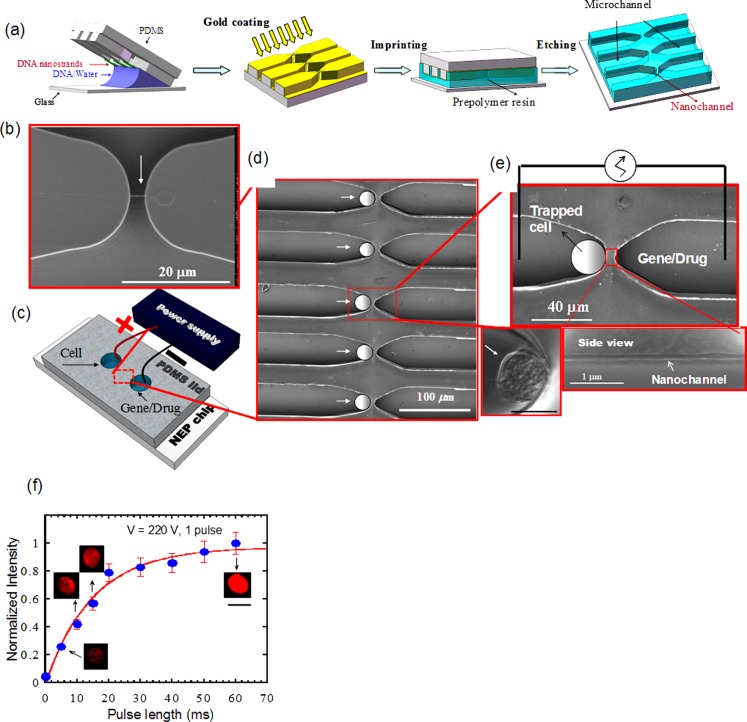
We recently developed a nanochannel electroporation (NEP) method consisting of two microchannels connected by a nanochannel with diameters ranging from 5 to 100 nm (Figs. 4a, 4b, 4c, 4d, 4e).71 This NEP device can be made by a simple and low-cost DNA combing and imprinting (DCI) method72 or a cleanroom-based process. The cell to be transfected is positioned in one microchannel against the nanochannel and the other microchannel is filled with the agent to be delivered. A voltage pulse(s) lasting milliseconds (ms) is delivered between the two microchannels causing transfection. Dose control is achieved by adjusting the duration and number of pulses, voltage manipulation, and/or the agent concentration. Although NEP and MEP devices share the same concept of focusing the electric field to a small area on the individual cell surface, the cell surface area affected by NEP is less than 1% that in MEP, leading to much less damage to the cell. In NEP, the “shot” is generated by the large electrophoretic force imposed on the to-be-delivered biomolecules both within the channel and through the cell membrane. For this purpose, a high electric voltage is necessary to generate a large electric field and a correspondingly high electrophoretic force. Dose control is achieved because transport of transfection agents is dominated by electrophoresis occurring during the electrical pulse. In comparison, voltages used in MEP result in low electric fields within the microchannel and cannot produce meaningful electrophoretic forces. Consequently, for MEP, delivery of biomolecules into the cell still has to rely largely on molecular diffusion and endocytosis after poration. If a higher voltage (say 100 V) was used in MEP to increase molecule acceleration, the cells would not survive. Experimentally, we observed the transition of the “shot” (or injection) dominated delivery to the diffusion dominated delivery when the channel diameter was increased from 90 nm to 1 and 5 μm.71 To show dosage control by NEP, cells were transfected with ODN, G3139 conjugated with Cy3 to allow fluorescent detection. NEP, using a single 220 V/2 mm pulse of varying durations was carried out and the fluorescence signal from the ODN uptake was measured and is summarized in Fig. 4f. The amount of ODN transfected is a monotonic function of the pulse duration and near-linear from 5 to 20 ms and the cell-to-cell variation in the amount of ODN delivered was ±10% and ±12%, respectively, a significant improvement compared to conventional transfection methods.
Figure 4.
Apparatus and operation of NEP.71 (a) Fabrication schematic of NEP chip by DCI, (b) a SEM image of a DNA nanostrand “combed” across two polymer “microridges.” (c) schematic of a NEP nanochannel-microchannel array covered by a PDMS lid with electrodes placed in reservoirs. (d) and (e) SEM images of several elements in a NEP array and close-ups of a single element showing a side view cut of a nanochannel. (f) Jurket cells transfected with Cy3-ODN using a single 220 V/2 mm pulse. Dose control was achieved by varying the pulse length and quantified by the measured fluorescence signal.
High throughput for large scale cell transfection
Depending on applications, the number of cells to be transfected varies. Single or few cell transfection is suitable for cell biology analysis and transgenesis. Transfection of small cell population (∼100 cells as in our NEP chip) maybe acceptable for iPS cells because they have a high proliferative index and are immortal due to telomerase expression, However, nuclear reprogramming of other more delicate and/or less proliferative cell lineages (e.g., induced neurons) will require larger-scale transfection in the order of 100 000 to millions cells. In the cases of therapeutic protein production by cell based bioreactors, the preferred cell numbers are in billions or higher. Clearly, high throughput or large scale cell electroporation is needed to address various applications. Current commercial bulk electroporation systems are capable of transfecting millions of cells in one shot. Most MEP/NEP devices, however, have much less cell capacity because of their planar and/or array-type designs. Nevertheless, there are efforts to achieve high throughput in MEP and NEP using array-type and flow-through setups.65, 66, 67, 71, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85 In these devices, either electric pulses or a DC field is imposed on the cell solution occupied the entire flow channel, or as solution droplets containing multiple cells. These technologies are briefly summarized here.
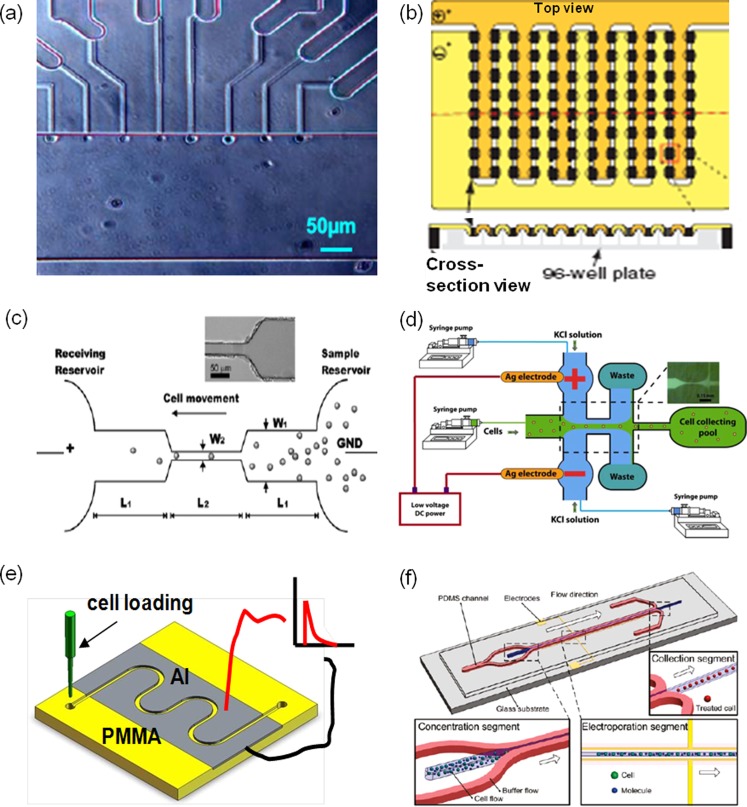
With a scanning process or array-type of setup, many single cell electroporation systems could be expanded easily for higher throughput treatment (Figs. 5a, 5b). By mounting a computer-controlled scanning module on a 3D micromanipulator, Olofsson et al. successfully transfected CHO cells and PC-12 cells with pmaxGFP plasmids and 9-base-pair oligonucleotides at a scanning speed of 0.1–1.0 mm/s.74 With different designs of microchannel arrays, several groups demonstrated electroporation with cytoplasmic chemicals (e.g., Calcein AM) or fluorescence dyes on leukemia cells and HeLa cells with a broad throughput range (10–105 cells/h).46, 55, 56, 63, 75 By sandwiching cells (∼104) between two pieces of track-etched membrane consisting of thousands of micropores, we successfully transfected 3T3 cells and mouse embryonic stem (mES) cells with better transfection efficiency (∼2–3 folds increase) when imposing low-voltage pulses (<1–35 V).57, 58 An alternative array-type system used a 96-well format to electroporate liquid droplets (10–20 μl) encapsulated with molecule probes and cells.65 With a multiple channel robotics, they effectively delivered dextran, siRNA, and cDNA into primary neurons, differentiated neutrophils, and some hard-to-transfect cells (e.g., HL-60) at a speed of ∼106 cells/min.
Figure 5.
High-throughput cell electroporation systems: (a) and (b) array-type expansion of single cell electroporation with microchannel arrays (a)63 and microdroplet generator arrays65 (b); (c)–(f) flow-through setups use DC electrical signals with wire-type electrodes in fluid reservoirs (c) and (d)76, 82 and pulse signals with planar electrodes along the entire flow channel (e) and (f)84, 85 in a shear flow (c) and (e) and a hydrodynamic focusing flow (d) and (f). The red and black lines illustrate electric wires which connect the Al electrodes to a pulse generator. Panel (a) reprinted with permission from C. Ionescu-Zanetti, A. Blatz, and M. Khine, Biomedical Microdevices 10, 113 (2008). Copyright 2008 Kluwer Academic. Panel (b) reprinted with permission from E. G. Guignet and T. Meyer, Nat. Methods 5, 393 (2008). Copyright 2008 Macmillan. Panel (c) reprinted with permission from H. Y. Wang, A. K. Bhunia, and C. Lu, Biosens. Bioelectron. 22, 582 (2006). Copyright 2006 Elsevier. Panel (d) reprinted with permission from T. Zhu, C. X. Luo, J.Y. Huang, C. Y. Xiong, Q. Ouyang, and J. Fang, Biomed. Microdevices 12, 35 (2010). Copyright 2010 Springer LLC. Panel (e) reprinted with permission from S. Wang, X. Zhang, W. Wang, and L. J. Lee, Anal. Chem. 81, 4414 (2009). Copyright 2009 American Chemical Society. Panel (f) reprinted with permission from Z. W. Wei, D.Y. Zhao, X. M. Li, M. X. Wu, W. Wang, H. Huang, X. X. Wang, Q. Du, Z. C. Liang, and Z. H. Li, Anal. Chem. 83, 5881 (2011). Copyright 2011 American Chemical Society.
Besides array-type setups, flow-through electroporation micro-devices were also developed for high throughput gene transfection. Lu's group developed a continuous flow electroporation system, in which cells were guided sequentially through a micro-channel while a constant DC electric bias was added.75, 76, 77, 78, 79 With geometric variation at some locations of the flow channel (from 213 to 33 μm), the electric field strength is highly focused (i.e., 6.5 times). When quickly passing these defined locations (1.2 to 2.7 μl/min), cells experience a number of field strength abruption, analogous to electric pulses in electroporation (Fig. 5c). Because of the application of a DC signal, cells in their system had to be suspended in a special electroporation buffer (8 mM Na2HPO4, 2 mM KH2PO4, and 250 mM sucrose) to suppress cell swelling. The devices were initially used for cell lysis or cell fusion,76, 77 but later successfully applied for mammalian transfection of pEGFP-C1 plasmids with the transfection efficiency of 25% on CHO cells.78, 79, 80 However, issues like water electrolysis, gas bubbles, and Joule heating impede its wide applications. To minimize these issues, electroporation with low-frequency alternative current (AC) signals (sine or square waves with a frequency of 10 Hz-10 kHz) was used in similar microfluidic devices by Lu's group.81 A shorter pulse duration (i.e., 1–4 ms) was chosen to yield better transfection efficiency of pEGFP-C1 DNA plasmids (∼25%–65%) with the cell viability of 80% or higher, while the improvements varied largely with the pulse duration and frequency. Alternatively, a hydrodynamic focusing microfluidics system was introduced to tackle the same issues.82 By sandwiching the cell suspension (the main flow stream) with two side flow streams consisting of KCl (3M in concentration), gas bubbles and heating were largely reduced in the side flow streams (KCl flow) so that the damage to cells in the central flow stream was largely avoided (Fig. 5d).
Hydrodynamic flows not only provide continuous treatment of cells for high throughput but also contribute to better cell membrane permeabilization and improved electroporation performance if mild flow stress is applied. A curved traveling path might benefit the mixing of gene and cells, heat transfer, and the uniform breakdown of cell membrane. Lu's group found that when a spiral shape micro-channel format was used, the transfection efficiency in their DC electroporation system was greatly enhanced.83 They believe that the secondary vortex flows along the curved flow paths are attributed to such improvements. We have also used a serpentine channel in a semi-continuous flow electroporation (SFE) device (Fig. 5e). Besides shear-enhanced permeabilization, the serpentine channel design offers better mixing of gene and cell suspensions and more importantly, better suppression of the Joule heating and gas bubble issues. Cells mixed with plasmid DNA were flowed through the micro-channel comprised of aluminum walls, which also serve as electrodes for electric pulse application. With a limited electrode surface area and a more efficient heat transfer configuration, heat generated during electroporation could be quickly conducted away, particularly from locations where cells stayed. This also restricted the growth and the size of gas bubbles which were rapidly flushed out into large open fluid reservoirs to further reduce the bubble burst damage to cells. Better transgene expression (10%–15% higher) of pWizGFP plasmid and a 50% or higher cell viability on K562 cells and mouse embryonic stem cells were achieved.84 To further minimize the electrolysis issue, a similar flow-through electroporation setup integrated with hydrodynamic focusing flow pattern was recently tested by Wei et al. (Fig. 5f).85 Great improvements were observed for the delivery of plasmid DNA and synthetic siRNA to several types of cells including HEK-239, C2C12, PC12, Neuro-2A, and HeLa cells. To avoid the toxicity from the corrosion of Al electrode, another similar design with thick gold electrodes (30–50 μm in height) all along the entire flow channel was fabricated through MEMS technologies followed by electroforming process.86
CONCLUSIONS AND PERSPECTIVES
Micro-/nanofluidics technologies were introduced in the past two decades for cell electroporation to broaden its acceptance in biological research and medical applications. Compared to the commercial bulk electroporation systems, they offer several advantages: (1) generating sufficient high electric field strength with low-voltage pulses to suppress unwanted electrochemical reactions; (2) conveniently concentrating, trapping, and manipulating the position and concentration of cells and probes to reduce reagent consumption; (3) real-time monitoring intracellular content transport to help establish optimal electroporation protocols; and (4) being flexible on cell numbers needed in each treatment (from single cell to high throughput). Some of those approaches focused on cell lysis or fusion and analysis of intracellular contents and their transport, while many others handled gene transfection to a large number of cells. Both batch-type and flow-through designs were made available to tackle several existing challenges in current electroporation systems. The uptake of small molecules (e.g., dyes), DNA plasmids, and RNA has been successfully tested on a variety of cell types, including mammalian cells, yeast, and bacteria.
Despite all new advances the micro-/nanofluidics based electroporation technologies can offer, their delivery performance and reliability have not yet been comprehensively measured or integrated with biological and clinics protocols and standards to exhibit their full potential to biological and medical users. This is attributed to several drawbacks associated with the current micro-/nanofluidics based electroporation systems: (1) relatively complicated operation procedure to biological and medical users; (2) existing limitations in each design regarding the cost and performance; and (3) insufficient database on clinically important molecule probes and cells to verify their relevance.
Excellent performance and reliability are essential for the commercialization of the aforementioned new technologies, while easy-to-use is the key to their success. With its rapid development in the past 20 years, scientists and engineers are getting used to micro-/nanofluidics technologies. However, they need to turn the new technologies into easy-to-use new products for end users. For more complicated designs, high level automation could lead to more user-friendly systems. Most current array-type and flow-through systems can match well with the robotic operation. When integrated, the complicated techniques could be hidden behind the easy-to-use operation interface to gain customer's acceptance as iPhone and iPAD in the IC industry.
Even though we have introduced many major advances in micro-/nanofluidics based electroporation, they have not yet been well integrated into one single system to promote the full potential of cell electroporation. For example, in situ visualization of single cell analysis is a well established technique; however, it has not been well integrated with gene transfection in electroporation. Through moving and affixing single or multiple cells at various locations or in a desirable pattern, we started closely mimicking the actual electroporation conditions and investigated how the relative positions of cells to the electrodes and each other affect electroporation efficiency with a single cell electroporation approach.59 In this way, the single cell analysis could not only reveal the actual electroporaiton dynamics and mechanism but also provide guidelines in protocol optimization for bulk electroporation. More work is needed to get these two approaches joining hands to overcome the complicated local electric conditions and large variations for individual cells in multi-cell setups.
Although high throughput electroporation has been addressed by both array-type and flow-through designs, it is difficult to achieve dosage control and uniformity, large cell numbers, high cell viability, and easy-to-use, all in the same device. The flow-through type designs are good for large cell numbers and may be easier-to-use, but their transfection mechanism is still similar to that in bulk electroporation and, consequently, is difficult to achieve good dosage control. The array-type designs, particularly in NEP, have potential to achieve much better dosage uniformity at the single cell level. However, their cell throughput may be limited even using the 3D type designs with a massively parallel array of nanochannels in the vertical direction. Some sort of semi-flow through and semi-array type combined systems may be able to take advantages of both designs.
When the nanochannel diameter in NEP is reduced to several nanometers, e.g., 3.4 nm, a recent study showed the successful transfection of a large 20 kbp DNA at very low electric voltages (e.g., 2 V).87 This essentially combines the advantages of both NEP (dose control and minimum cell damage) and MEP (minimum electrochemical reactions, bubble formation, and Joule heating), and is particularly useful to transfect a large number of cells. However, the amount of gene it delivered, particularly for larger DNA/RNA, may be too low to achieve practical use. More efforts should be spent in this area to realize an ideal electroporation system.
So far, most micro-/nanofluidics based cell electroporation approaches focus on the delivery of dyes, small chemicals, and DNA plasmids, which are convenient for quick concept demonstration. But, our focus should move to the delivery of valuable probes in the emerging biological and clinical fields which currently lack efficient delivery tools to analyze or treat hard-to-transfect cells. The rapid development of drug discovery, RNA interference, and regenerative medicine demands more efficient and accuracy delivery of various biomolecule probes. The micro-/nanofluidics based electroporation approaches have great potential to contribute to emerging applications, such as DNA vaccine delivery, stem cell transfection, induced pluripotent stem cells reprogramming, and neuronal analysis. In these applications, high delivery efficiency is essential while dose control, accurate delivery, and the consequent cell viability are also of great importance. The delivered probes in these applications are expected to have a long-term effect on cells and precisely regulate some specific cell functions. More attention should be given to a variety of cancer treatments (e.g., tumor progression, oncogenic regulation, and cancer invasion control) and neuron-related transfection analysis in the future. In addition, some reprogramming models and/or approaches (e.g., RNA-based) will require repeated transfections or sequential transfection of different biomolecules to the same cells over a certain period of time (>24 h) with good dose control and cell viability, which cannot be easily achieved with the current transfection systems. This provides another challenge and opportunity for micro-/nanofluidics based electroporation approaches.
ACKNOWLEDGMENTS
We acknowledge financial support from National Institute of Health under Grant No. R15CA156146 and National Science Foundation under Grant No. EEC-0914790.
References
- Gene and Cell Therapy: Therapeutic Mechanism and Strategies, 2nd ed., edited by Templeton N. S. (Marcel Dekker, Inc., New York, 2004). [Google Scholar]
- Toneguzzo F. and Keating A., Proc. Natl. Acad. Sci. U.S.A. 83, 3496 (1986). 10.1073/pnas.83.10.3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack D. W., Hoffman A. S., Pun S., and Stayton P. S., Nat. Rev. Drug Discovery 4, 581 (2005). 10.1038/nrd1775 [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., and Hofschneider P. H., Eur. Mol. Biol. Organ. J. 1, 841 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. C., Chassy B. M., and Saunder J. A., Guide to Electroporation and Electrofusion (Academic, San Diego, 1992). [Google Scholar]
- Gehl J., Acta Physiol. Scand. 177, 437 (2003). 10.1046/j.1365-201X.2003.01093.x [DOI] [PubMed] [Google Scholar]
- Teissie J. and Rols M. P., Biophys. J. 65, 409 (1993). 10.1016/S0006-3495(93)81052-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E. and Kakorin S., Radiol. Oncol. 32, 7 (1998). [Google Scholar]
- Hamm A., Krott N., Breibach I., Blindt R., and Bosserhoff A. K., Tissue Eng. 8, 235 (2002). 10.1089/107632702753725003 [DOI] [PubMed] [Google Scholar]
- Schakowski F., Buttgereit P., Mazur M., Marten A., Schottker B., Gorschluter M., and Schmidt-Wolf I., Genet. Vaccines Ther. 2, 1 (2004). 10.1186/1479-0556-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q. and Chang D. C., Biochim. Biophys. Acta 1088, 104 (1991). 10.1016/0167-4781(91)90158-I [DOI] [PubMed] [Google Scholar]
- Ho S. Y. and Mittal G. S., Crit. Rev. Biotechnol. 16, 349 (1996). 10.3109/07388559609147426 [DOI] [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y., and Kinosita K., Biochim. Biophys. Acta 812, 779 (1985). 10.1016/0005-2736(85)90272-X [DOI] [PubMed] [Google Scholar]
- Jordan E. T., Collins M., Terefe J., Ugozzoli L., and Rubio T., J. Biomol. Tech. 19, 328 (2008). [PMC free article] [PubMed] [Google Scholar]
- Prasanna G. L. and Panda T., Bioprocess Eng. 16, 261 (1997). 10.1007/s004490050319 [DOI] [Google Scholar]
- Kotnik T., Bobanovic F., and Miklavcic D., Bioelectrochem. Bioenerg. 43, 285 (1997). 10.1016/S0302-4598(97)00023-8 [DOI] [Google Scholar]
- Dev S. B., Rabussay D. P., Widera G., and Hofmann G. A., IEEE Trans. Plasma Sci. 28, 206 (2000). 10.1109/27.842905 [DOI] [Google Scholar]
- Lee R. C., River L. P., Pan F. S., Ji L., and Wollmann R. L., Proc. Natl. Acad. Sci. U.S.A. 89, 4524 (1992). 10.1073/pnas.89.10.4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rols M. P., Delteil C., Serin G., and Teissie J., Nucleic Acids Res. 22, 540 (1994). 10.1093/nar/22.3.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis-Husselbee J. W., Cullen P. J., Irvine R. F., and Dawson A. P., Biochem. J. 277, 883 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz P., Harnack U., and Morgenstern R., Biotechnol. Lett. 26, 1589 (2004). 10.1023/B:BILE.0000045658.33723.d6 [DOI] [PubMed] [Google Scholar]
- Lakshmipathy U., Pelacho B., Sudo K., Linehan J. L., Coucouvanis E., Kaufman D. S., and Verfaillie C. M., Stem Cells 22, 531 (2004). 10.1634/stemcells.22-4-531 [DOI] [PubMed] [Google Scholar]
- Siemen H., Nix M., Endl E., Koch P., Itskovitz-Eldor J., and Brüstle O., Stem Cells Dev. 14, 378 (2005). 10.1089/scd.2005.14.378 [DOI] [PubMed] [Google Scholar]
- See http://www.lonza.com for more information on the working principles, transfection protocols and performance of NucleofectorTM technology.
- Fox M. B., Esveld D. C., Valero A., Luttge R., Mastwijk H. C., Bartels P. V., van den Berg A., and Boom R. M., Anal. Bioanal. Chem. 385, 474 (2006). 10.1007/s00216-006-0327-3 [DOI] [PubMed] [Google Scholar]
- Lee W. G., Demirci U., and Khademhosseini A., Integr. Biol. 1, 242 (2009). 10.1039/b819201d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Hwang I., Britain D., Chung T. D., Sun Y., and Kim D. H., Lab Chip 11, 3941 (2011). 10.1039/c1lc20766k [DOI] [PubMed] [Google Scholar]
- Gabriel B. and Teissié J., Biophys. J. 73, 2630 (1997). 10.1016/S0006-3495(97)78292-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Cell 22, 479 (1998). 10.1016/0092-8674(80)90358-X [DOI] [PubMed] [Google Scholar]
- Lundqvist J. A., Sahlin F., Aberg M. A., Stromberg A., Eriksson P. S., and Orwar O., Proc. Natl. Acad. Sci. U.S.A. 95, 10356 (1998). 10.1073/pnas.95.18.10356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung E. S., J. Chromatogr. A 830, 243 (1999). 10.1016/S0021-9673(98)00920-0 [DOI] [PubMed] [Google Scholar]
- Stephen D. J. and Pepperkok R., Proc. Natl. Acad. Sci. U.S.A. 98, 4295 (2001). 10.1073/pnas.081065198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolkrantz K., Farre C., Brederlau A., Karlsson R. I., Brennan C., Eriksson P. S., Weber S. G., Sandberg M., and Orwar O., Anal. Chem. 73, 4469 (2001). 10.1021/ac010403x [DOI] [PubMed] [Google Scholar]
- Golzio M., Teissié J., and Rols M. P., Proc. Natl. Acad. Sci. U.S.A. 99, 1292 (2002). 10.1073/pnas.022646499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obataya I., Nakamura C., Han S., Nakamura N., and Miyake J., Nano Lett. 5, 27 (2005). 10.1021/nl0485399 [DOI] [PubMed] [Google Scholar]
- Han S., Nakamura C., Obataya I., Nakamura N., and Miyake J., Biochem. Biophys. Res. Commun. 332, 633 (2005). 10.1016/j.bbrc.2005.04.059 [DOI] [PubMed] [Google Scholar]
- Han S., Nakamura C., Obataya I., Nakamura N., and Miyake J., Biosens. Bioelectron. 20, 2120 (2005). 10.1016/j.bios.2004.08.023 [DOI] [PubMed] [Google Scholar]
- Chen X., Kis A., Zettl A., and Bertozzi C. R., Proc. Natl. Acad. Sci. U.S.A. 104, 8218 (2007). 10.1073/pnas.0700567104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaratha D., Unal K., and Wickramasinghe H. K., Appl. Phys. Lett. 93, 153111 (2008). 10.1063/1.2981568 [DOI] [Google Scholar]
- Huang Y. and Rubinsky B., Biomed. Microdevices 2, 145 (1999). 10.1023/A:1009901821588 [DOI] [Google Scholar]
- Lee S.-W. and Tai Y.-C., Sens. Actuators, A 73, 74 (1999). 10.1016/S0924-4247(98)00257-X [DOI] [Google Scholar]
- Huang Y. and Rubinsky B., Sens. Actuators, A 89, 242 (2001). 10.1016/S0924-4247(00)00557-4 [DOI] [Google Scholar]
- Huang Y., Sekhon N. S., Borninski J., Chen N., and Rubinsky B., Sens. Actuators, A 105, 31 (2003). 10.1016/S0924-4247(03)00084-0 [DOI] [Google Scholar]
- Lu H., Schmidt M. A., and Jensen K. F., Lab Chip 5, 23 (2005). 10.1039/b406205a [DOI] [PubMed] [Google Scholar]
- Gao J., Yin X. F., and Fang Z. L., Lab Chip 4, 47 (2004). 10.1039/b310552k [DOI] [PubMed] [Google Scholar]
- Munce N. R., Li J. Z., Herman P. R., and Lilge L., Anal. Chem. 76, 4983 (2004). 10.1021/ac0496906 [DOI] [PubMed] [Google Scholar]
- Valero A., Merino F., Wolbers F., Luttge R., Vermes I., Andersson S. M. H., and van den Berg A., Lab Chip 5, 49 (2005). 10.1039/b415813j [DOI] [PubMed] [Google Scholar]
- Kurosawa O., Oana H., Matsuoka S., Noma A., Kotera H., and Washizu M. S., Meas. Sci. Technol. 17, 3127 (2006). 10.1088/0957-0233/17/12/S02 [DOI] [Google Scholar]
- Valero A., Post J. N., van Nieuwkasteele J. W., Ter Braak P. M., Kruijer W., and van den Berg A., Lab Chip 8, 62 (2008). 10.1039/b713420g [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Jen C. M., Huang M. Y., Wu C. Y., and Lin X. Z., Sens. Actuators B 79, 137 (2001). 10.1016/S0925-4005(01)00859-0 [DOI] [Google Scholar]
- Huang Y. and Rubinsky B., Sens. Actuators, A 104, 205 (2003). 10.1016/S0924-4247(03)00050-5 [DOI] [Google Scholar]
- McClain M. A., Culbertson C. T., Jacobson S. C., Allbritton N. L., Sims C. E., and Ramsey J. M., Anal. Chem. 75, 5646 (2003). 10.1021/ac0346510 [DOI] [PubMed] [Google Scholar]
- Lin Y., Li M., and Wu C., Lab Chip 4, 104 (2004). 10.1039/b312804k [DOI] [PubMed] [Google Scholar]
- Shin Y. S., Cho K., Kim J., Lim S., Park C., Lee K., Park Y., Chun C., Han D., and Chang J., Anal. Chem. 76, 7045 (2004). 10.1021/ac0496291 [DOI] [PubMed] [Google Scholar]
- Khine M., Lau A., Ionescu-Zanetti C., Seo J., and Lee L. P., Lab Chip 5, 38 (2005). 10.1039/b408352k [DOI] [PubMed] [Google Scholar]
- Khine M., Zanetti C. I., Blatz A., Wang L. P., and Lee L. P., Lab Chip 7, 457 (2007). 10.1039/b614356c [DOI] [PubMed] [Google Scholar]
- Fei Z., Wang S., Xie Y., Koh C.-G., Henslee B., and James Lee L., Anal. Chem. 79, 5719 (2007). 10.1021/ac070482y [DOI] [PubMed] [Google Scholar]
- Fei Z., Hu X., Choi H.-W., Wang S., Farson D., and Lee L. J., Anal. Chem. 82, 353 (2010). 10.1021/ac902041h [DOI] [PubMed] [Google Scholar]
- Brian B., Morss A., Hu X., Lafyatis G. P., and Lee L. J., Anal. Chem. 83, 3998 (2011). 10.1021/ac1019649 [DOI] [PubMed] [Google Scholar]
- Kim J. A., Cho K., Shin M. S., Lee W. G., Jung N., Chung C., and Chang J. K., Biosens. Bioelectron. 23, 1353 (2008). 10.1016/j.bios.2007.12.009 [DOI] [PubMed] [Google Scholar]
- See http://www.invitrogen.com for more information on the working principles, transfection protocols and performance of Neon® transfection system.
- Lee W. G., Bang H., Yun H., Kim J. A., Cho K., Chung C., Chang J. K., and Han D. C., Curr. Appl. Phys. 8, 696 (2008). 10.1016/j.cap.2007.04.046 [DOI] [Google Scholar]
- Ionescu-Zanetti C., Blatz A., and Khine M., Biomed. Microdevices 10, 113 (2008). 10.1007/s10544-007-9115-x [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang X., Yu B., Lee R., and Lee L. J., Biosens. Bioelectron. 26, 778 (2010). 10.1016/j.bios.2010.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guignet E. G. and Meyer T., Nat. Methods 5, 393 (2008). 10.1038/nmeth.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y. H., Wang J., Bao N., and Lu C., Anal. Chem. 81, 2027 (2009). 10.1021/ac9001172 [DOI] [PubMed] [Google Scholar]
- Xiao K., Zhang M., Chen S., Wang L., Chang D., and Wen W., Electrophoresis 31, 3175 (2010). 10.1002/elps.201000155 [DOI] [PubMed] [Google Scholar]
- Takahashi K. and Yamanaka S., Cell 126, 663 (2006). 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Okita K., Nakagawa M., and Yamanaka S., Nat. Protoc. 2, 3081 (2007). 10.1038/nprot.2007.418 [DOI] [PubMed] [Google Scholar]
- Hu B.-Y., Weick J. P., Yu J., Ma L.-Y., Zhang X.-Q., Thomson J. A., and Zhang S.-C., Proc. Natl. Acad. Sci. U.S.A. 107, 4335 (2010). 10.1073/pnas.0910012107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukany P. E., Morss A., Liao W., Henslee B., Jung H. C., Zhang X., Yu B., Wang X., Wu Y., Li L., Gao K., Hu X., Zhao X., Hemminger O., Lu W., Lafyatis G. P., and Lee L. J., Nat. Nanotechnol. 6, 747 (2011). 10.1038/nnano.2011.164 [DOI] [PubMed] [Google Scholar]
- Guan J., Boukany P. E., Hemminger O., Chiou N.-R., Zha W., Cavanaugh M., and Lee L. J., Adv. Mater. 22, 3993 (2010). 10.1002/adma.201000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain T. and Muthuswamy J., Lab Chip 7, 1004 (2007). 10.1039/b707479d [DOI] [PubMed] [Google Scholar]
- Olofsson J., Levin M., Strömberg A., Weber S. G., Ryttsén F., and Orwar O., Anal. Chem. 79, 4410 (2007). 10.1021/ac062140i [DOI] [PubMed] [Google Scholar]
- Kim J. A., Cho K., Shin Y. S., Jung N., Chung C., and Chang J. K., Biosens. Bioelectron. 22, 3273 (2007). 10.1016/j.bios.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Bhunia A. K., and Lu C., Biosens. Bioelectron. 22, 582 (2006). 10.1016/j.bios.2006.01.032 [DOI] [PubMed] [Google Scholar]
- Wang J. and Lu C., Appl. Phys. Lett. 89, 234102 (2006). 10.1063/1.2402122 [DOI] [Google Scholar]
- Wang H. Y. and Lu C., Anal. Chem. 78, 5158 (2006). 10.1021/ac060733n [DOI] [PubMed] [Google Scholar]
- Wang H. Y. and Lu C., Biotechnol. Bioeng. 95, 1116 (2006). 10.1002/bit.21066 [DOI] [PubMed] [Google Scholar]
- Wang H. Y. and Lu C., Biotechnol. Bioeng. 100, 579 (2008). 10.1002/bit.21784 [DOI] [PubMed] [Google Scholar]
- Zhan Y., Cao Z., Bao N., Li J., Wang J., Geng T., Lin H., and Lu C., J. Controlled Release 160, 570 (2012). 10.1016/j.jconrel.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Zhu T., Luo C. X., Huang J. Y., Xiong C. Y., Ouyang Q., and Fang J., Biomed. Microdevices 12, 35 (2010). 10.1007/s10544-009-9355-z [DOI] [PubMed] [Google Scholar]
- Wang J., Zhan Y. H., Ugaz V. M., and Lu C., Lab Chip 10, 2057 (2010). 10.1039/c004472e [DOI] [PubMed] [Google Scholar]
- Wang S., Zhang X., Wang W., and Lee L. J., Anal. Chem. 81, 4414 (2009). 10.1021/ac9002672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z. W., Zhao D. Y., Li X. M., Wu M. X., Wang W., Huang H., Wang X. X., Du Q., Liang Z. C., and Li Z. H., Anal. Chem. 83, 5881 (2011). 10.1021/ac200625b [DOI] [PubMed] [Google Scholar]
- Dalmay C., Villemejane J., Joubert V., Français O., Mir L. M., and Pioufle B. L., Sens. Actuators B 160, 1573 (2011). 10.1016/j.snb.2011.09.009 [DOI] [Google Scholar]
- Nelson E. M., Kurtz V., Shim J., Timp W., and Timp G., Analyst 137, 3020 (2012). 10.1039/c2an35571j [DOI] [PMC free article] [PubMed] [Google Scholar]