Abstract
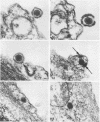
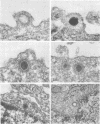
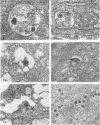
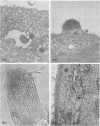
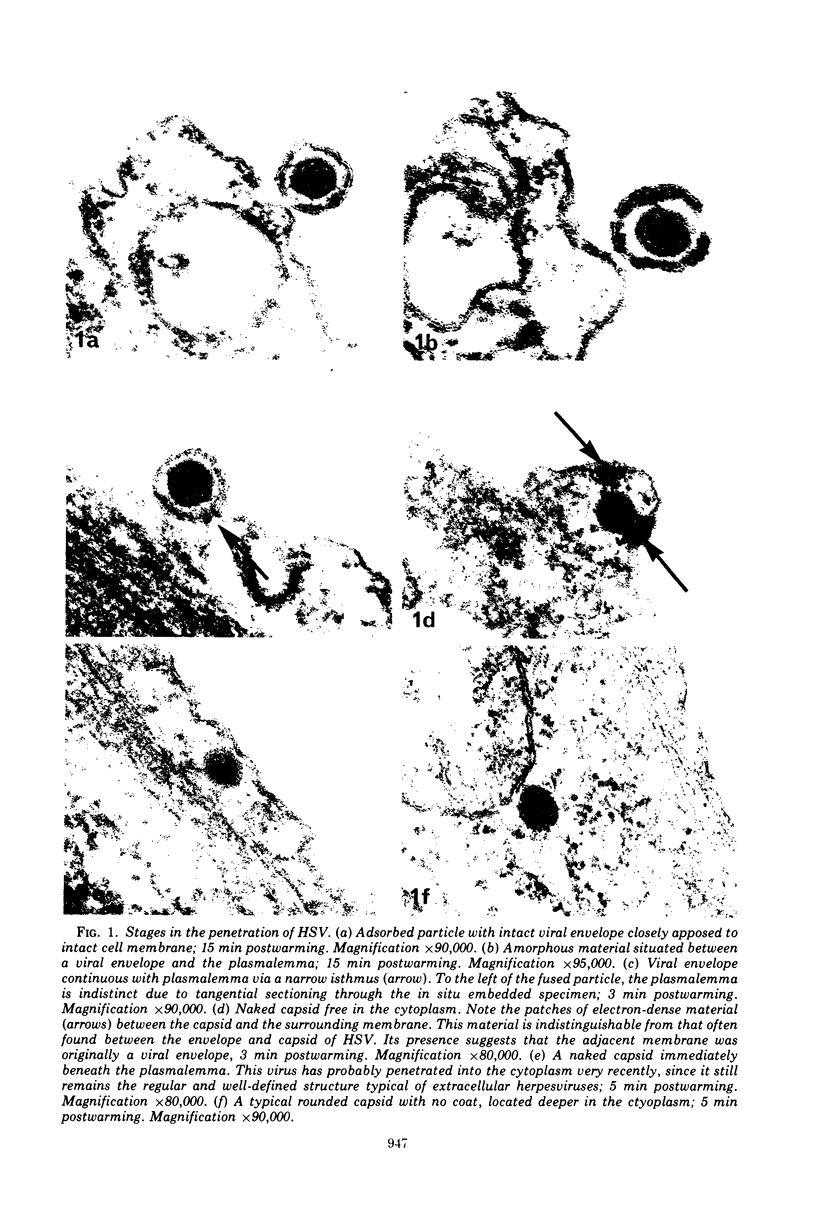
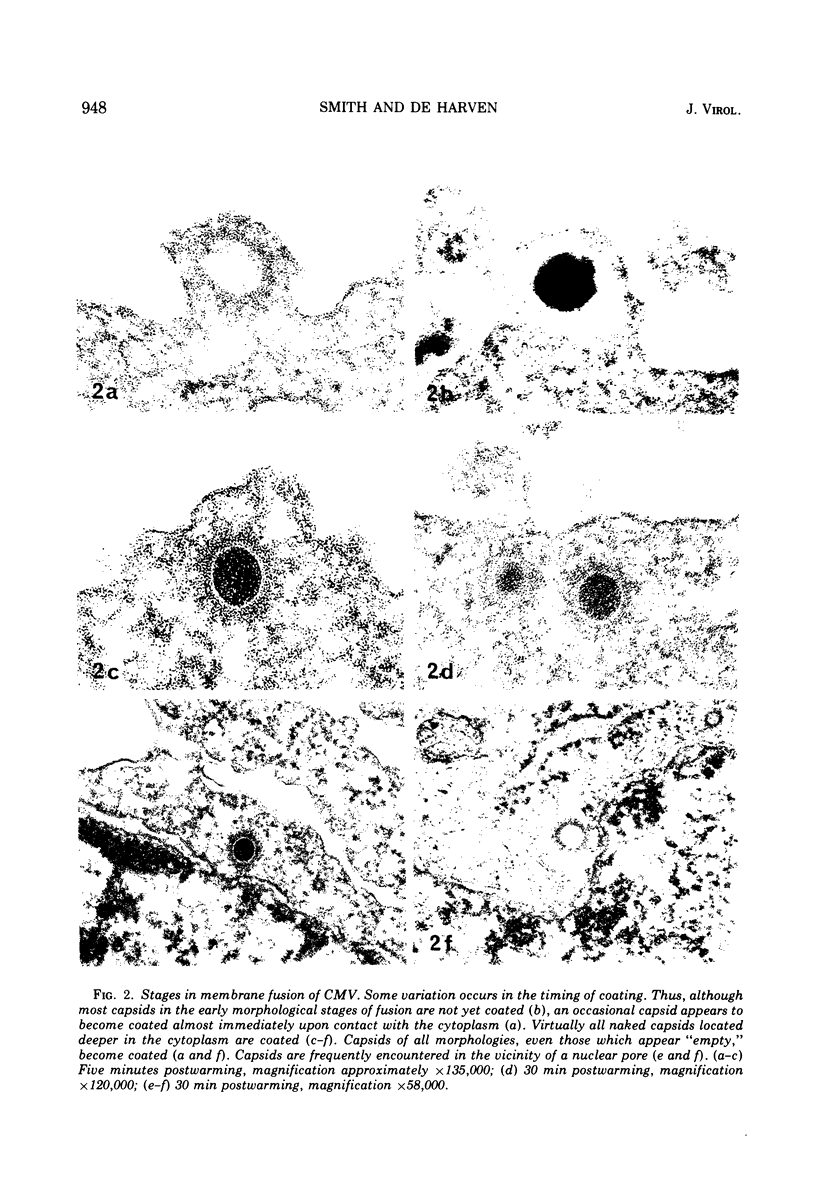
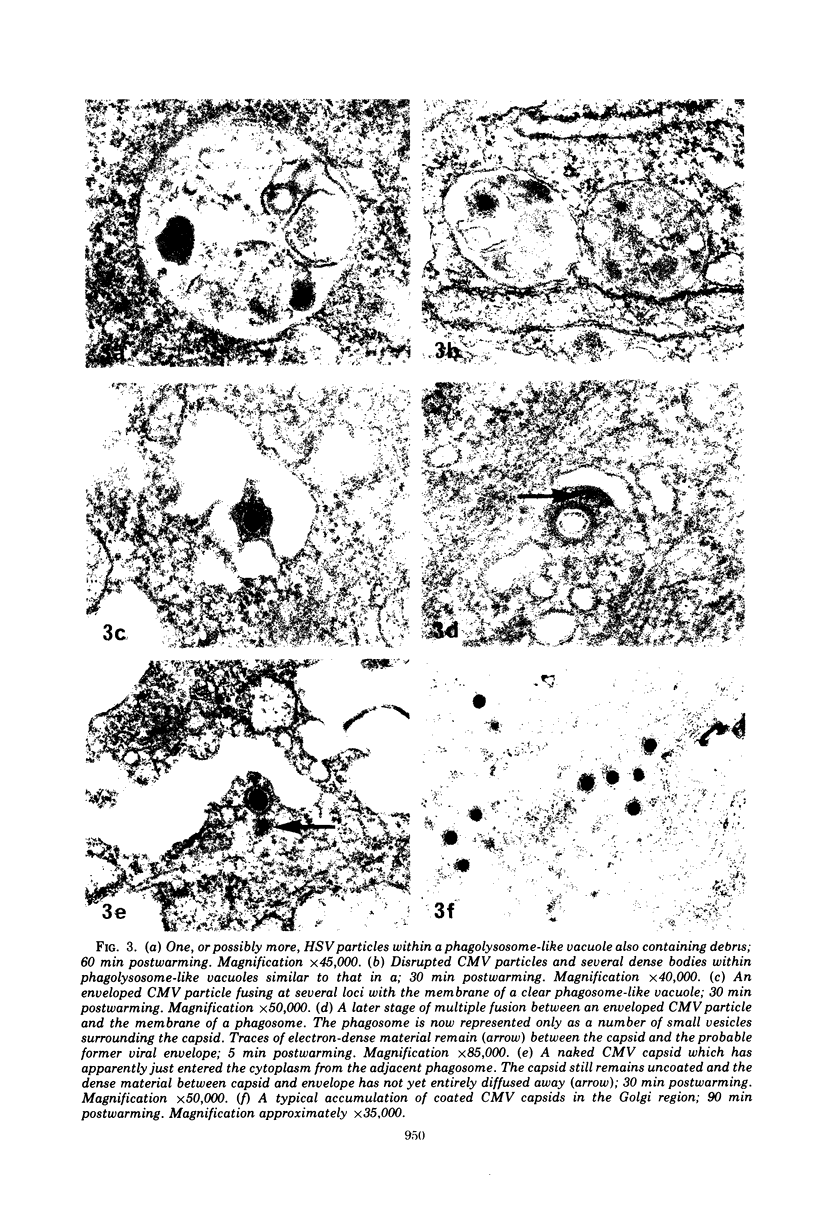
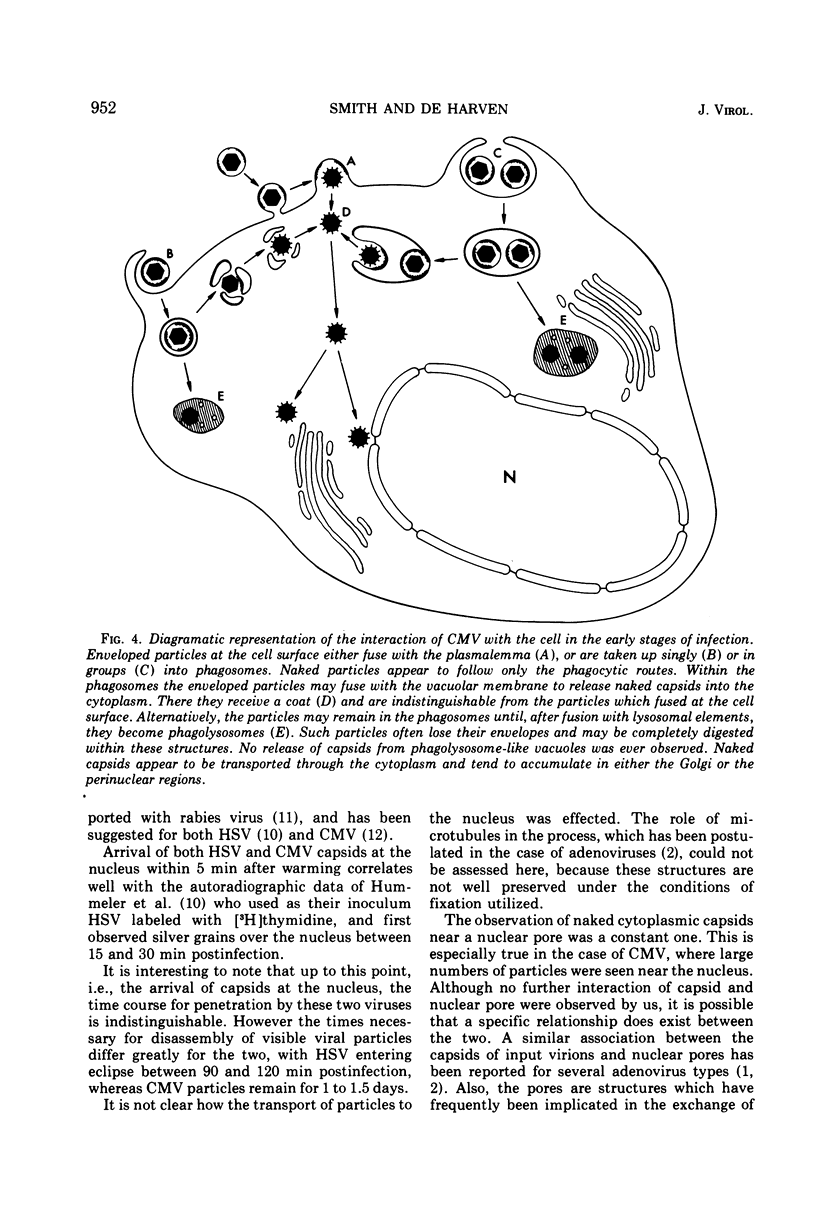
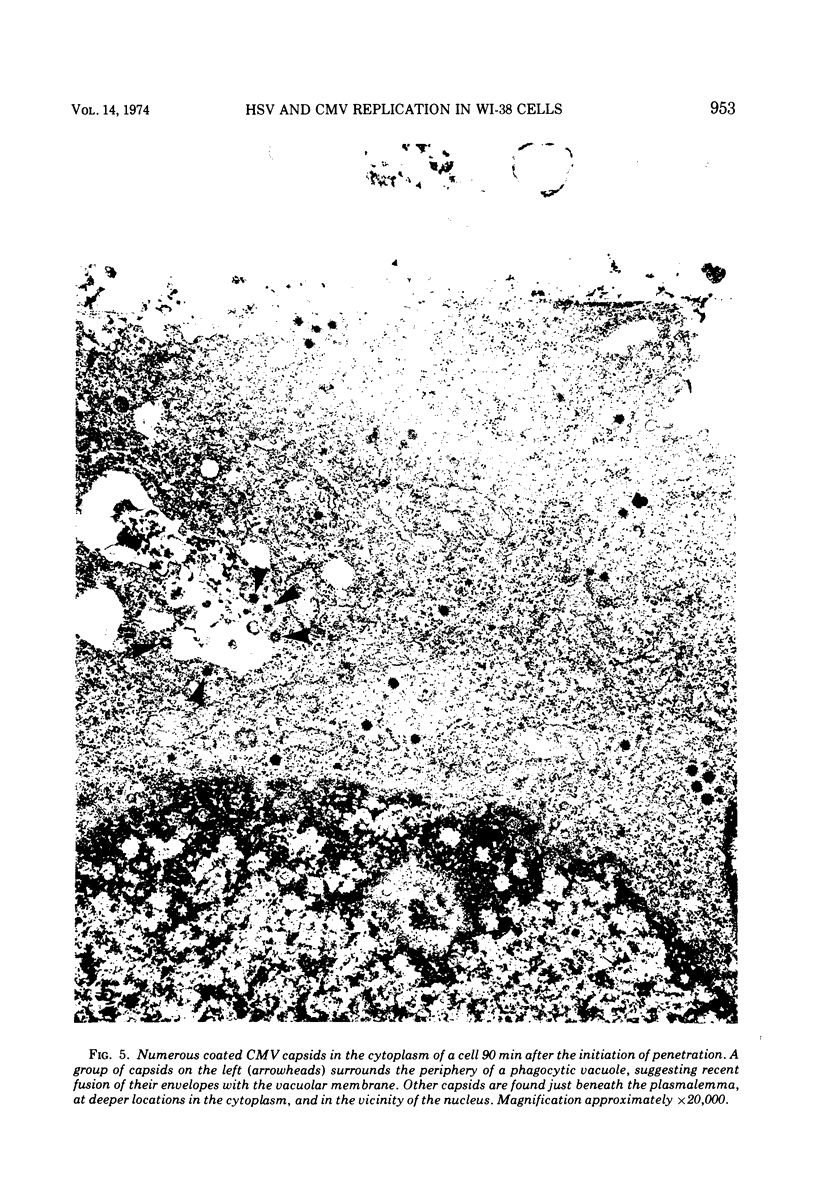
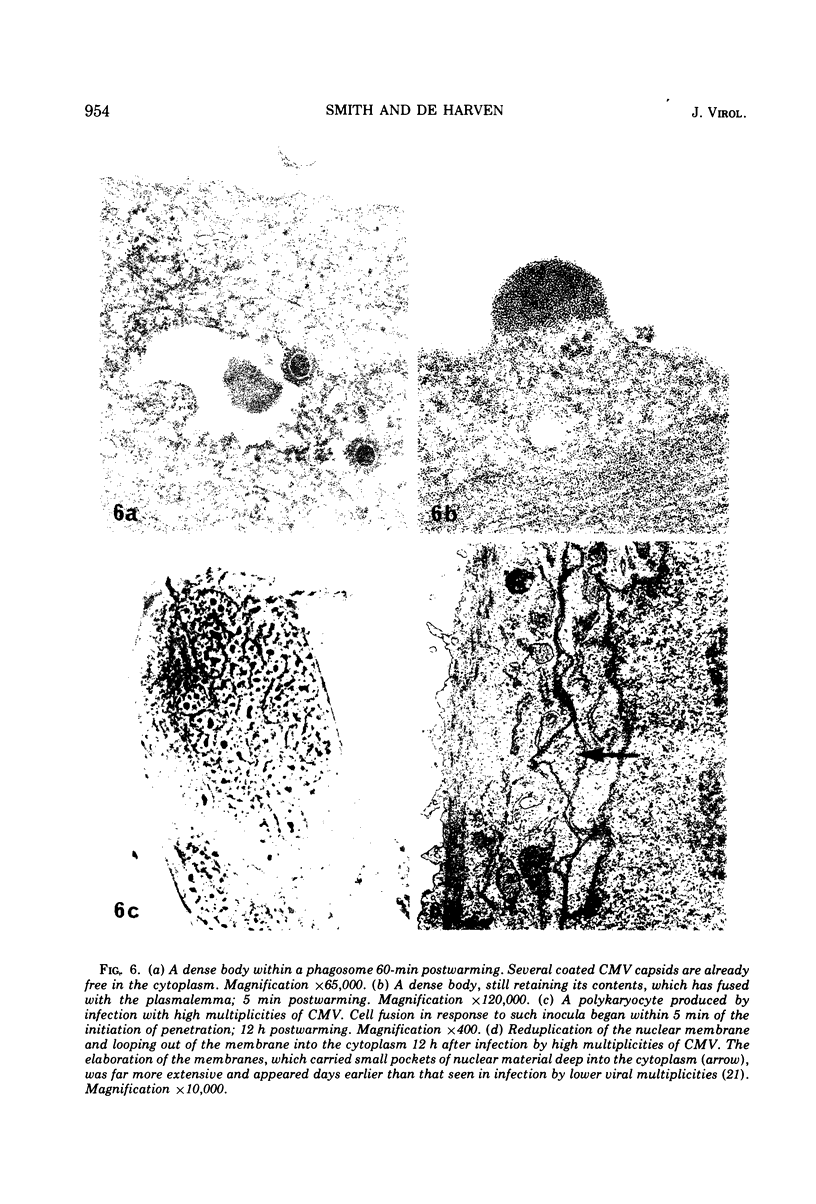
An electron microscope study was carried out on the early minutes of herpes simplex virus (HSV) and cytomegalovirus (CMV) penetration into WI-38 cells. Both HSV and CMV entered cells either by fusion of the viral envelope with a limiting cell membrane, or via phagocytosis. Both fusion and phagocytosis occurred within 3 min after the initiation of penetration. After fusion, the naked capsids of CMV free in the cytoplasm became coated with a fine, fibrillar material. CMV capsids thus coated retained a well-defined and easily identifiable morphology until the eclipse of visible viral particles between 1 and 1.5 days postinfection. In contrast, naked HSV capsids free in the cytoplasm were never coated. Rather, within minutes after penetration, they assumed a rounded, less regular outline, and were no longer detectable by 90 to 120 min postinfection. The free naked capsids of both viruses appeared to migrate across the cytoplasm toward the nucleus and to become located near nuclear pores. Both HSV and CMV capsids reached the nucleus as early as 5 min after the initiation of penetration. No further interaction with the nucleus could be documented. Particles were also consistently identified in the Golgi region. Phagocytosed particles generally remained within phagosomes, where they appeared to be degraded. However, stages were identified in what is believed to be the escape of enveloped viruses from phagosomes into the cytoplasm via fusion of their envelope with the phagosomal membrane.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. II. Comparative observations on the penetration of types 1, 5, 7, and 12. Virology. 1970 Mar;40(3):478–485. doi: 10.1016/0042-6822(70)90190-x. [DOI] [PubMed] [Google Scholar]
- DALES S., GOMATOS P. J., HSU K. C. THE UPTAKE AND DEVELOPMENT OF REOVIRUS IN STRAIN L CELLS FOLLOWED WITH LABELED VIRAL RIBONUCLEIC ACID AND FERRITIN-ANTIBODY CONJUGATES. Virology. 1965 Feb;25:193–211. doi: 10.1016/0042-6822(65)90199-6. [DOI] [PubMed] [Google Scholar]
- Dales S., Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973 Dec;56(2):465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- Dales S., Silverberg H. Viropexis of herpes simplex virus by HeLa cells. Virology. 1969 Mar;37(3):475–480. doi: 10.1016/0042-6822(69)90232-3. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., HUMMELER K., BERKALOFF A. THE ENTRY AND DISTRIBUTION OF HERPES VIRUS AND COLLOIDAL GOLD IN HELA CELLS AFTER CONTACT IN SUSPENSION. J Exp Med. 1964 Feb 1;119:291–302. doi: 10.1084/jem.119.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARNHAM A. E., NEWTON A. A. The effect of some environmental factors on herpes virus grown in HeLa cells. Virology. 1959 Apr;7(4):449–461. doi: 10.1016/0042-6822(59)90073-x. [DOI] [PubMed] [Google Scholar]
- HOLMES I. H., WATSON D. H. AN ELECTRON MICROSCOPE STUDY OF THE ATTACHMENT AND PENETRATION OF HERPES VIRUS IN BHK21 CELLS. Virology. 1963 Sep;21:112–123. doi: 10.1016/0042-6822(63)90309-x. [DOI] [PubMed] [Google Scholar]
- Hummeler K., Tomassini N., Zajac B. Early events in herpes simplex virus infection: a radioautographic study. J Virol. 1969 Jul;4(1):67–74. doi: 10.1128/jvi.4.1.67-74.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y., Furukawa T., Plotkin S., Koprowski H. Ultrastructural study on the sequence of human cytomegalovirus infection in human diploid cells. Arch Gesamte Virusforsch. 1973;40(3):311–324. doi: 10.1007/BF01242551. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Wiktor T. J., Koprowski H. Early events of rabies virus replicaton in tissue cultures. An electron microscopic study. Lab Invest. 1973 Feb;28(2):142–148. [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Howe C. Structure and development of viruses as observed in the electron microscope. IX. Entry of parainfluenza I (Sendai) virus. J Virol. 1968 Oct;2(10):1122–1132. doi: 10.1128/jvi.2.10.1122-1132.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M., Mednis B. Electron microscopy of herpes simplex virus. I. Entry. J Virol. 1968 May;2(5):507–516. doi: 10.1128/jvi.2.5.507-516.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rose H. M. Structure and development of viruses as observed in the electron microscope. 8. Entry of influenza virus. J Virol. 1968 Sep;2(9):925–936. doi: 10.1128/jvi.2.9.925-936.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Mednis B. Structure and development of viruses as observed in the electron microscope. V. Entry and uncoating of adenovirus. J Virol. 1969 Nov;4(5):777–796. doi: 10.1128/jvi.4.5.777-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., De Harven E. Concentration of herpesviruses. J Virol. 1973 Feb;11(2):325–328. doi: 10.1128/jvi.11.2.325-328.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., De Harven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J Virol. 1973 Oct;12(4):919–930. doi: 10.1128/jvi.12.4.919-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackpole C. W. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969 Jul;4(1):75–93. doi: 10.1128/jvi.4.1.75-93.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplin I., Schidlovsky G. Partial purification and electron microscopy of virus in the EB-3 cell line derived from a Burkitt lymphoma. Science. 1966 May 20;152(3725):1084–1085. doi: 10.1126/science.152.3725.1084. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B., SCOTT D. E. Serologic differentiation of viruses responsible for cytomegalic inclusion disease. Virology. 1960 Sep;12:130–132. doi: 10.1016/0042-6822(60)90156-2. [DOI] [PubMed] [Google Scholar]
- Zee Y. C., Talens L. Entry of infectious bovine rhinotracheitis virus into cells. J Gen Virol. 1971 Apr;11(1):59–63. doi: 10.1099/0022-1317-11-1-59. [DOI] [PubMed] [Google Scholar]