Abstract
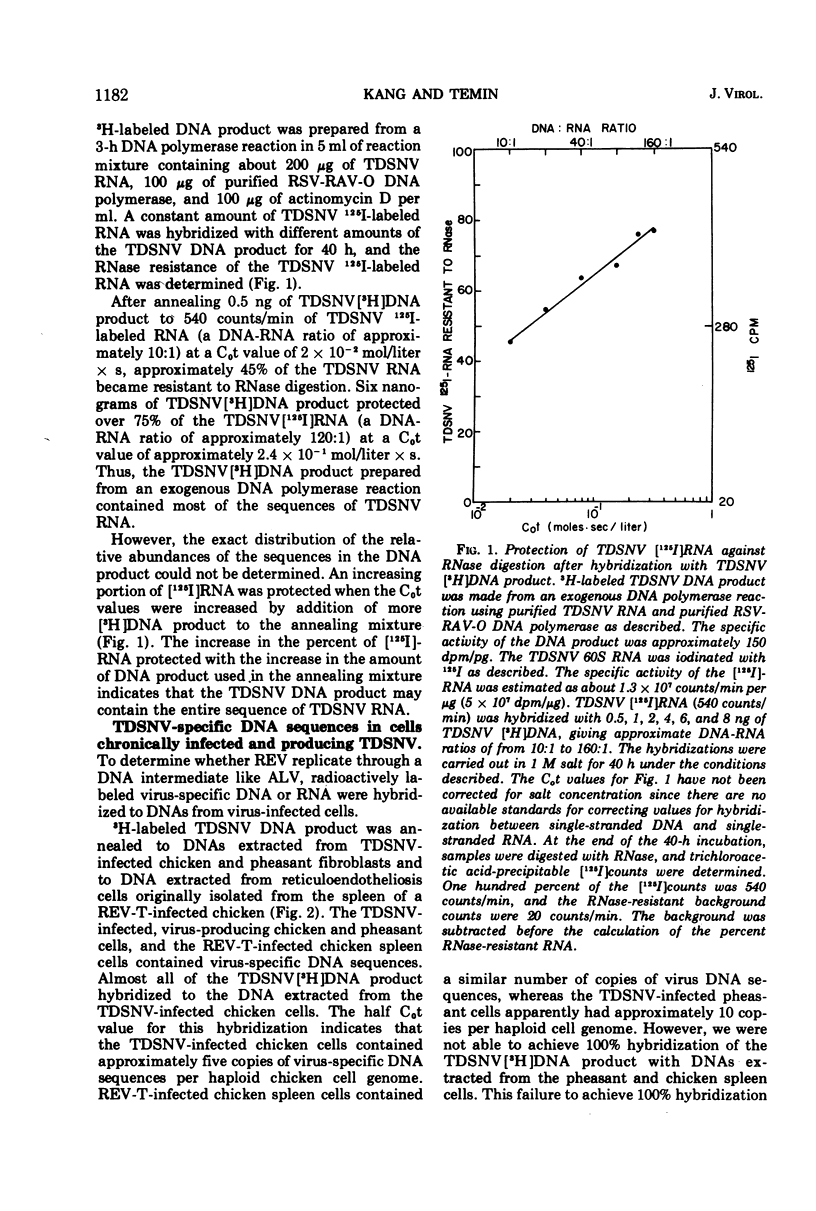
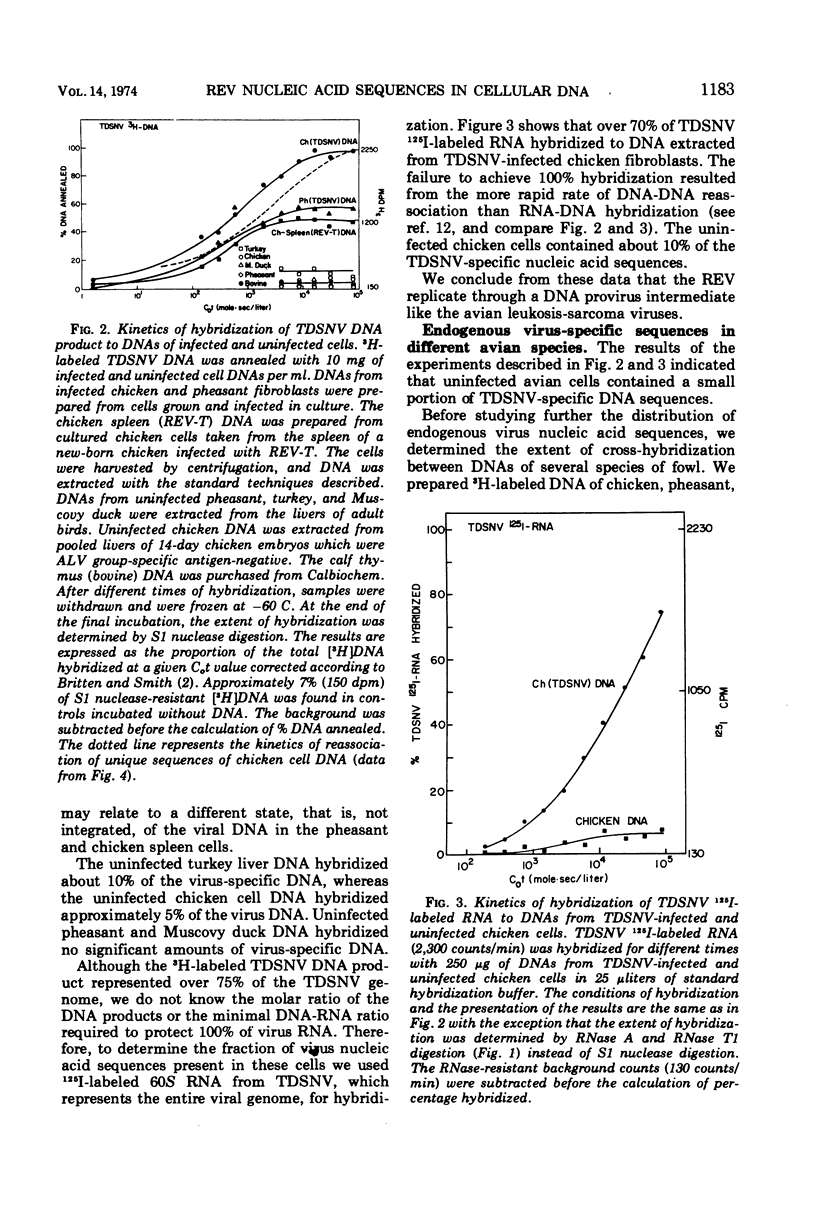
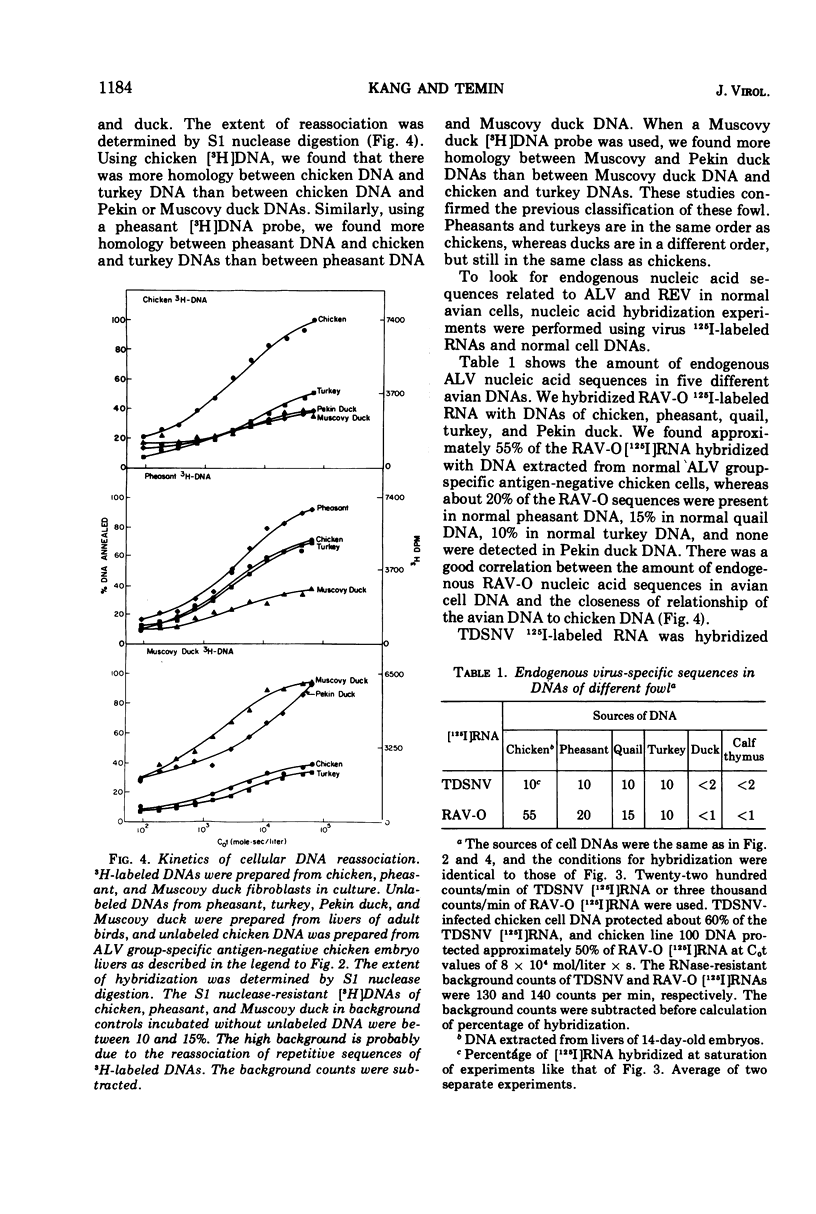
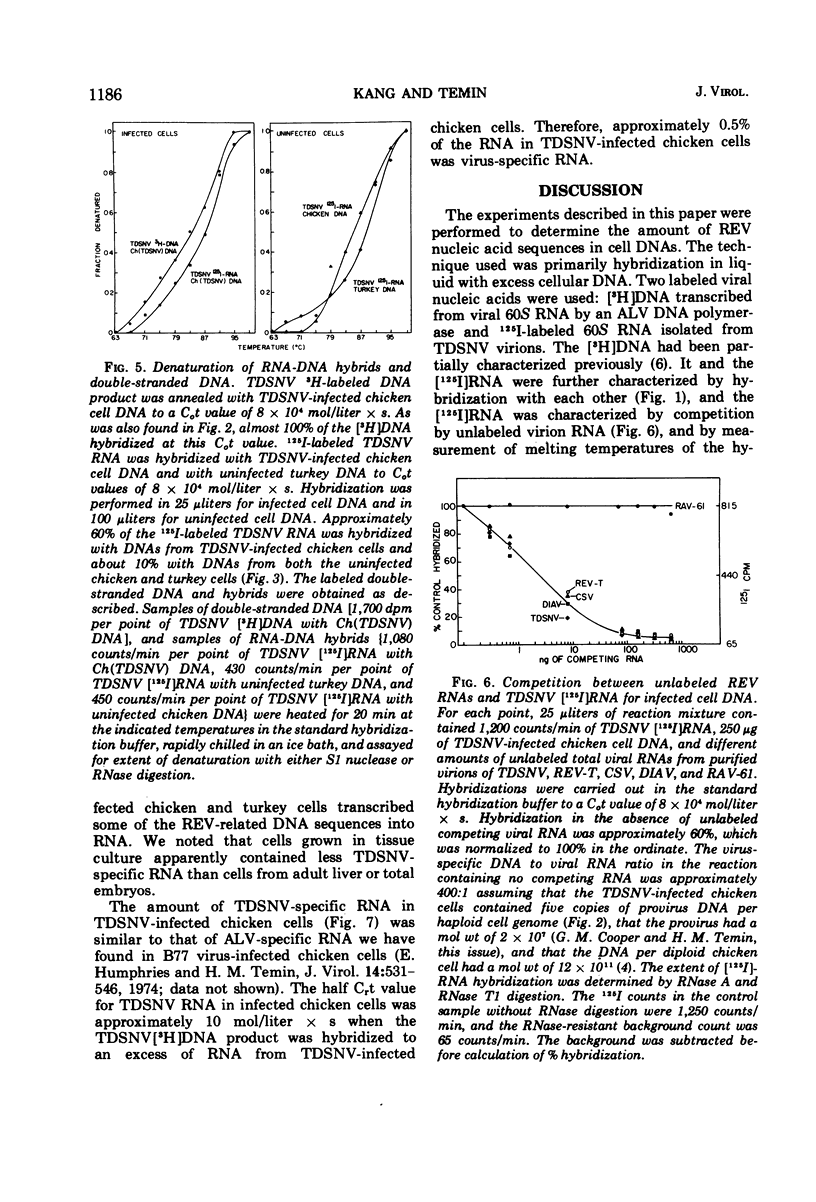
Reticuloendotheliosis virus 60S RNA labeled with 125I, or reticuloendotheliosis virus complementary DNA labeled with 3H, were hybridized to DNAs from infected chicken and pheasant cells. Most of the sequences of the viral RNA were found in the infected cell DNAs. The reticuloendotheliosis viruses, therefore, replicate through a DNA intermediate. The same labeled nucleic acids were hybridized to DNA of uninfected chicken, pheasant, quail, turkey, and duck. About 10% of the sequences of reticuloendotheliosis virus RNA were present in the DNA of uninfected chicken, pheasant, quail, and turkey. None were detected in DNA of duck. The specificity of the hybridization was shown by competition between unlabeled and 125I-labeled viral RNAs and by determination of melting temperatures. In contrast, 125I-labeled RNA of Rous-associated virus-O, an avian leukosis-sarcoma virus, hybridized 55% to DNA of uninfected chicken, 20% to DNA of uninfected pheasant, 15% to DNA of uninfected quail, 10% to DNA of uninfected turkey, and less than 1% to DNA of uninfected duck.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altaner C., Temin H. M. Carcinogenesis by RNA sarcoma viruses. XII. A quantitative study of infection of rat cells in vitro by avian sarcoma viruses. Virology. 1970 Jan;40(1):118–134. doi: 10.1016/0042-6822(70)90384-3. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Comparison of Rous sarcoma virus-specific deoxyribonucleic acid polymerases in virions of Rous sarcoma virus and in Rous sarcoma virus-infected chicken cells. J Virol. 1971 May;7(5):625–634. doi: 10.1128/jvi.7.5.625-634.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Early DNA-RNA complex from the endogenous RNA-directed DNA polymerase activity of uninfected chicken embryos. Nat New Biol. 1973 Apr 18;242(120):206–208. doi: 10.1038/newbio242206a0. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Kang C. Y., Temin H. M. Endogenous RNA-directed DNA polymerase activity in virions of RNA tumor viruses and in a fraction from normal chicken cells. Methods Enzymol. 1974;29:119–124. doi: 10.1016/0076-6879(74)29014-1. [DOI] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Lack of serological relationship among DNA polymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken cells. J Virol. 1973 Sep;12(3):440–448. doi: 10.1128/jvi.12.3.440-448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Specific serological relationships among partially purified DNA polymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and avian cells. J Virol. 1974 May;13(5):1020–1029. doi: 10.1128/jvi.13.5.1020-1029.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Rous sarcoma virus nucleotide sequences in cellular DNA: measurement by RNA-DNA hybridization. Science. 1972 Nov 17;178(4062):750–753. doi: 10.1126/science.178.4062.750. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., McMillin C., MacDonnell D. Nucleotide sequence relationships of avian RNA tumor viruses: measurement of the deletion in a transformation-defective mutant of Rous sarcoma virus. J Virol. 1974 Apr;13(4):837–846. doi: 10.1128/jvi.13.4.837-846.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberg N. H., Refetoff S. Hybridization of RNA labelled with 125 I to high specific activity. Nat New Biol. 1973 Apr 4;242(118):142–145. doi: 10.1038/newbio242142a0. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. The cellular and molecular biology of RNA tumor viruses, especially avian leukosis-sarcoma viruses, and their relatives. Adv Cancer Res. 1974;19(0):47–104. doi: 10.1016/s0065-230x(08)60052-4. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]


