Abstract
Progenitor cell retention and release are largely governed by the binding of stromal-cell-derived factor 1 (SDF-1) to CXC chemokine receptor 4 (CXCR4) and by α4-integrin signaling. Both of these pathways are dependent on c-kit activity: the mobilization of progenitor cells in response to either CXCR4 antagonism or α4-integrin blockade is impaired by the loss of c-kit kinase activity; and c-kit–kinase inactivation blocks the retention of CXCR4-positive progenitor cells in the bone marrow. SDF-1/CXCR4 and α4-integrin signaling are also crucial for the retention of progenitor cells in the ischemic region, which may explain, at least in part, why clinical trials of progenitor cell therapy have failed to display the efficacy observed in preclinical investigations. The lack of effectiveness is often attributed to poor retention of the transplanted cells and, to date, most of the trial protocols have mobilized cells with injections of granulocyte colony-stimulating factor (G-CSF), which activates extracellular proteases that irreversibly cleave cell-surface adhesion molecules, including α4-integrin and CXCR4. Thus, the retention of G-CSF-mobilized cells in the ischemic region may be impaired, and the mobilization of agents that reversibly disrupt SDF-1/CXCR4 binding, such as AMD3100, may improve patient response. Efforts to supplement SDF-1 levels in the ischemic region may also improve progenitor cell recruitment and the effectiveness of stem cell therapy.
I. Introduction
Over the last decade, a compelling body of evidence has accumulated to suggest that progenitor cells of bone marrow origin, such as endothelial pro-genitor cells (EPCs) and mesenchymal stem cells (MSCs), play a significant role in postnatal physiological and pathophysiological vasculogenesis1–7 and could provide a promising new therapeutic approach for the treatment of ischemic disease.8–15 These cells form the structural components of the new vasculature, mediate favorable cell–cell contacts, and release growth factors that contribute to vessel growth and protect against cell death in the ischemic tissue.14,16,17 Furthermore, abnormally low levels of peripheral blood EPCs are closely associated with risk factors for cardiovascular disease, cardiovascular events, and mortality.18,19
Currently, most clinical trials of cell therapy for the treatment of ischemic heart disease have used progenitor cells of bone marrow origin,20–22 which are usually administered via intracoronary infusion or transplanted directly into the ischemic region. In general, the trials have found evidence of therapeutic benefit, but with only modest efficacy,21–26 and the absence of more definitive results is often attributed to poor retention and survival of the transplanted cells.21,22,27 Because increases in circulating progenitor cell levels are expected to enhance the number of cells recruited to the ischemic tissue,28–31 techniques that promote progenitor cell mobilization are being rigorously investigated.32–36 The effectiveness of this strategy has been demonstrated in numerous preclinical studies30,31,35–38 and has led to frequent investigations of progenitor-cell-mobilizing agents in early clinical trials.28,29,39–50 Granulocyte colony-stimulating factor (G-CSF) has been the most commonly used mobilizing agent, but the results from these trials have not met the expectations, despite substantial increases in peripheral blood progenitor cell counts.28,29,44,46,48,51,52 Thus, a better understanding of how progenitor cells interact with the microenvironment in the bone marrow and in the ischemic region could lead to the development of more effective cell-based therapies.
II. Progenitor Cell Mobilization
The mobilization of progenitor cells from bone marrow to the peripheral circulation is highly regulated under both normal physiological conditions and stress.53,54 In adult bone tissue, progenitor cells are retained predominantly in specialized microenvironments near the endosteum (i.e., the osteoblast niche), where they interact with spindle-shaped, N-cadherin-expressing osteoblasts,55,56 and in the sinusoids (i.e., the vascular niche), where they interact with SDF-1-expressing reticular cells.57–59 Many different cell types, matrix proteins, and soluble factors cooperatively regulate the self-renewal, differentiation, and maintenance of progenitor cells55–57,60–65; however, the bulk of experimental evidence suggests that progenitor cell retention and release are largely governed by two pathways, one of which is dependent on stromal-cell-derived factor 1 (SDF-1, also called CXC chemokine ligand 12 [CXCL12]) and the SDF-1 receptor CXC chemokine receptor 4 (CXCR4), and the other on α4β1-integrin (also called very late antigen-4 [VLA-4]).57,59,60,66–69 Initially, SDF-1/CXCR4 and α4β1-integrin signaling appear to proceed independently; for example, the α4β1-integrin antagonist Groβ can mobilize progenitor cells in mice transplanted with CXCR4-knockout bone marrow.70 However, results from our recent studies suggest that c-kit, a receptor tyrosine kinase that binds stem cell factor (SCF), is an integral downstream component of both pathways.71
A. SDF-1/CXCR4
CXCR4 is a G protein-coupled receptor composed of 352 amino acids with seven transmembrane helices72–74 and is broadly expressed by both mononuclear cells and progenitor cells in the bone marrow.72–78 The ligand for CXCR4, SDF-1, is a secreted or membrane-bound protein that is abundantly expressed by osteoblasts, endothelial cells, and a subset of reticular cells in the osteoblast and vascular niches.57,79–81 SDF-1/CXCR4 signaling induces the directional migration of cells and is involved in many biological processes, including cardiovascular organogenesis, hematopoiesis, immune response, and cancer metastasis. Interactions between SDF-1 and CXCR4 are crucial for maintaining populations of hematopoietic stem cells (HSCs) in adult animals,57,66,82–87 and mice that lack either SDF-1 or CXCR4 exhibit nearly identical phenotypes characterized by late gestational lethality and defects in bone marrow colonization, B-cell lymphopoiesis, blood vessel formation, and cardiac septum formation.83,85,88–90 Thus, the SDF-1/CXCR4 axis appears to have a fundamental role in both vasculogenesis and cardiogenesis.
The roles of SDF-1 and CXCR4 in bone marrow progenitor cell retention and release are well established.66 Selective antagonism of CXCR4 with the pharmacological agent AMD3100 rapidly and potently mobilizes bone marrow progenitor cells in both animals and humans,86,91–93 and systemically injected bone marrow progenitor cells accumulate predominantly in subdomains of bone marrow microvessels that are rich in SDF-1 expression.68,94 Notably, both SDF-1 and CXCR4 expression are upregulated by relatively low oxygen tension (hypoxia) in discrete regions of the bone marrow and by the activation of hypoxia inducible factor 1 (HIF-1).87,95–100
B. α4-integrin
Integrins are heterodimeric transmembrane receptors composed of non-covalently joined α and β subunits and have the remarkable ability to transmit both incoming and outgoing signals across the cell membrane.101,102 Integrins usually induce signaling pathways by acting synergistically with growth-factor receptors to regulate cell shape, adhesion, migration, proliferation, and differentiation; but both can also function independently.103,104 The α4-integrins, α4β1 and α4β7, bind to vascular cell adhesion molecule 1 (VCAM-1), which is expressed on the surface of endothelial and stromal cells105–109 and to fibro-nectin in the extracellular matrix. These binding interactions are crucial for the adhesion of progenitor cells to the microenvironment and, consequently, to progenitor cell retention and recruitment.61,64,65 The expression of α4-integrin is downregulated during progenitor cell mobilization,110,111 and the cleavage of VCAM-1 and α4-integrin is a critical step during cytokine-induced bone marrow progenitor cell mobilization.112 In adult mice, deletion of α4-integrin persistently alters the distribution of progenitor cells,108,113,114 and antibody-mediated α4-integrin blockade mobilizes progenitor cells in both animals and humans.62,115,116 The level of α4-integrin expression on mobilized peripheral blood progenitor cells is inversely correlated with bone marrow homing and predicts the rate of engraftment in patients who have received autologous progenitor cell transplantation.117 Furthermore, we have shown that the transient blockade of α4-integrin activity leads to higher peripheral blood EPC levels, greater EPC-mediated neovascularization, and less adverse cardiac remodeling after myocardial infarction, and that α4-integrin antibodies can release bone marrow EPCs from immobilized VCAM-1 or bone marrow stromal cells.31 Thus, α4-integrin antibodies appear to mobilize EPCs from the bone marrow by disrupting VCAM-1:α4-integrin binding.
C. c-kit
c-kit (also called CD117) is a type III receptor tyrosine kinase expressed predominantly in bone marrow stem/progenitor cells118 and has recently been identified as a marker for EPC and cardiac progenitor cell identity.119–121 The ligand for c-kit, namely, SCF, is expressed in bone marrow endothelial cells and stromal cells as either a membrane-bound protein or a soluble one.122,123 Dimers of SCF bind to c-kit, which triggers c-kit homodimerization and the phosphorylation of specific c-kit tyrosine residues.124 The pattern of c-kit phosphorylation determines which signaling event is activated and can induce both positive and negative pathways.124–126 SCF/c-kit signaling is essential for embryonic hematopoiesis,127,128 and mutations that lead to the loss of c-kit (i.e., the W mutation), c-kit kinase activity (e.g., the W42 mutation), or SCF (the Sl mutation)129 cause severe macrocytic anemia and death in utero or during the perinatal period. Notably, defects in c-kit activity are also associated with impaired vascular development and angiogenesis,130–132 and cancer therapies that target c-kit are cardiotoxic.133,134 c-kit also supports progenitor cell maintenance135–137 and is a crucial component of cardiac regeneration.138 After myocardial infarction, c-kit-positive bone marrow cells are recruited to the ischemic myocardium and facilitate cardiac repair by differentiating into cardiac cell lineages and by expressing angiogenic cytokines.35,131
The mobilization of progenitor cells in response to α4-integrin blockade is markedly blunted in c-kitW/W-V mutant mice, which are defective in c-kit kinase activity but have normal levels of c-kit expression and SCF binding at the cell surface.131,139–141 Thus, the kinase activity of c-kit appears to be crucial for progenitor cell mobilization, but the mechanism by which c-kit participates in the retention and release of progenitor cells is unclear. In the bone marrow, membrane-bound SCF can be cleaved by SDF-1 to form soluble SCF, which subsequently activates c-kit and leads to progenitor cell mobilization,59 but the functional blockade of c-kit (with the c-kit–neutralizing antibody ACK2) has also been shown to mobilize bone marrow HSCs to the peripheral circulation and to enhance the engraftment of systemically injected donor bone marrow cells.142 Thus, both the activation and blockade of c-kit activity have been associated with progenitor cell mobilization.59,142,143 Furthermore, c-kit is the only known receptor for SCF, but SCF is not always required for c-kit activity,144–146 and neither SCF nor an SCF-neutralizing antibody are potent mobilizers,147,148 so SCF-binding alone cannot adequately explain the role of c-kit in progenitor cell mobilization.142,147,148
D. SDF-1/CXCR4–c-kit Signaling
The kinetics of bone marrow progenitor cell mobilization induced by a c-kit-neutralizing antibody (i.e., ACK2) and by antagonism of CXCR4 with the pharmacological CXCR4 antagonist AMD3100 are similar, so we investigated whether c-kit is involved in CXCR4-mediated bone marrow progenitor cell trafficking. Peripheral blood progenitor cell levels significantly increased, and bone marrow progenitor cell levels significantly declined after AMD3100 was injected into wild-type mice, but not after it was injected into c-kit kinase-defective (c-kitW/W-V) mice.71 To determine which specific subpopulations of bone marrow cells were affected by the c-kit kinase deficiency, we developed a short-term, in vivo bone marrow clearance/repopulation assay. AMD3100 was administered to wild-type and c-kitW/W-V mice, and then labeled bone marrow mononuclear cells were injected into the peripheral circulation and allowed to repopulate the bone marrow. Significantly fewer systemically administered CXCR4-expressing progenitor cells were observed in the bone marrow of c-kitW/W-V mice than in the bone marrow of wild-type mice.
AMD3100 also failed to mobilize bone marrow progenitor cells that expressed a constitutively active c-kit kinase (c-kitD816V) mutation. Thus, both the loss and the constitutive activation of c-kit kinase activity impaired AMD3100-induced bone marrow progenitor cell mobilization, which may seem contradictory. However, bone marrow progenitor cell levels were lower in c-kit kinase-defective mice than in wild-type mice before mobilization, and, after mobilization, systemically administered CXCR4-positive progenitor cells could not repopulate the bone marrow of c-kit kinase-defective mice. These observations suggest that c-kit kinase inactivation blocks the retention of CXCR4-positive progenitor cells and, consequently, that the cells susceptible to AMD3100-induced mobilization are (in effect) already mobilized.
In isolated bone marrow mononuclear cells, SDF-1/CXCR4 signaling upregulates, and the antagonism or genetic deletion of CXCR4 downregulates, c-kit phosphorylation. These results, as well as the lack of AMD3100-induced progenitor cell mobilization in mice with bone marrow cells that expressed a constitutively active c-kit mutant, suggest that CXCR4-mediated mobilization requires c-kit deactivation. Thus, AMD3100 and G-CSF appear to induce progenitor cell mobilization through fundamentally different mechanisms, because G-CSF-induced mobilization requires an increase in c-kit activation.59 Furthermore, G-CSF-induced mobilization occurs 3–5 days after administration and is accompanied by an increase in the number of progenitor cells present in the perivascular niche,149 whereas AMD3100-induced mobilization occurs within a few hours and, consequently, is unlikely to be preceded by the perivascular accumulation of progenitor cells. The two agents also appear to mobilize different subpopulations of progenitor cells, and more cells are mobilized when G-CSF and AMD3100 are combined than when G-CSF is administered alone.150–155 Collectively, these observations would suggest that G-CSF and other slow-acting agents increase c-kit phosphorylation by upregulating SCF, which (by itself) has only a modest effect on mobilization but potently promotes progenitor cell proliferation; if so, G-CSF-induced mobilization may be delayed until an adequate surplus of progenitor cells is available for release to the peripheral blood.149 Conversely, fast-acting agents, such as AMD3100, may mobilize progenitor cells directly by reducing c-kit phosphorylation in the perivascular niche.156 This hypothesis is also supported by recent evidence that progenitor cells can be rapidly mobilized by the administration of a c-kit neutralizing antibody.142
E. α4-integrin–c-kit Signaling
Because c-kit also appears to participate in progenitor cell mobilization through an α4-integrin-mediated mechanism,140 and interactions between α4-integrin and VCAM-1 support the adhesion and retention of mononuclear cells in the bone marrow,31 we performed a series of in vitro experiments to determine whether the phosphorylation state of c-kit is altered by α4-integrin-mediated adhesion. Wild-type bone marrow mononuclear cells were applied to VCAM-1-coated or uncoated plates, allowed to adhere for 15 min, incubated with or without AMD3100 for another 15 min, and then c-kit phosphorylation at tyrosine 719 was evaluated. Phosphorylated c-kit levels were notably higher in adherent wild-type cells (i.e., cells from VCAM-1– coated plates) than in nonadherent wild-type cells (i.e., cells from uncoated plates), and treatment with an α4-integrin-blocking antibody reduced phosphorylated c-kit levels, whereas treatment with the CXCR4 ligand SDF-1 markedly increased c-kit phosphorylation. Furthermore, both SDF-1 and SCF induced c-kit phosphorylation, but c-kit levels were highest when the cells were incubated with both factors, and AMD3100 treatment suppressed SDF-1-induced, but not SCF-induced, c-kit phosphorylation. Collectively, these observations suggest that α4-integrin-mediated mononuclear cell adhesion is associated with an increase in phosphorylated c-kit levels, and that in adherent mononuclear cells, SDF-1 upregulates, and AMD3100 downregulates, c-kit phosphorylation. Thus, SDF-1- and SCF-induced c-kit activation may occur independently and regulate different cellular activities (Fig. 1).
FIG. 1.
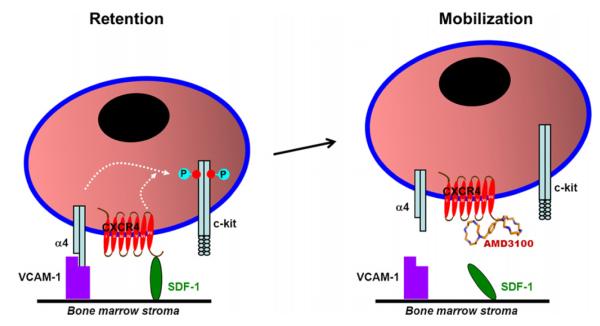
c-kit is a common component of two signaling pathways that regulate progenitor cell trafficking. Progenitor cell retention and release are largely governed by two pathways, one of which is dependent on the binding of SDF-1 to CXCR4 and the other on α4-integrin/VCAM-1 binding. Both interactions lead to the phosphorylation of c-kit, which is crucial for the retention of progenitor cells in the bone marrow. AMD3100 disrupts the SDF-1/CXCR4 interaction, which reduces c-kit phosphorylation and mobilizes progenitor cells from the bone marrow. SCF also increases phosphorylated c-kit levels by binding directly to c-kit, but disruption of the SCF/c-kit interaction does not appear to induce progenitor cell mobilization. Furthermore, AMD3100 suppresses SDF-1-induced, but not SCF-induced, c-kit phosphorylation, and phosphorylated c-kit levels are higher when cells are incubated with both SDF-1 and SCF than with either individual factor. Thus, SDF-1 and SCF appear to regulate c-kit phosphorylation independently and likely coordinate different cellular activities.
III. Progenitor Cell Recruitment and Retention
Mobilized progenitor cells are recruited from the peripheral circulation to the ischemic region, where they become incorporated into the growing vasculature.14,157 Several of the intermediate steps during progenitor cell recruitment, including chemotaxis, transendothelial migration, and adhesion to single layers of mature endothelial cells and integrin, are regulated by SDF-1/CXCR4 binding,30,99,158–160 but the mechanisms and downstream components of SDF-1/CXCR4 signaling at the injury site are poorly understood. SDF-1 expression is significantly elevated in the plasma of patients with acute myocardial infarction,161 and the expression of both CXCR4 and SDF-1 is elevated in ischemic myocardium,158,162,163 whereas the blockade of SDF-1/CXCR4 signaling diminishes progenitor cell recruitment,99,138,162,164 and impairments in CXCR4 signaling contribute to the reduced angiogenic potency of EPCs from patients with coronary artery disease and related conditions such as aging and diabetes.164–167 SDF-1 and CXCR4 may also participate in vascular remodeling by recruiting smooth muscle progenitor cells168 and protect cardiomyocytes against ischemia/reperfusion damage by activating the antiapoptotic kinases Akt and extracellular-regulated kinase.169
Hypoxia induces SDF-1 expression at the injury site,163 where platelets are an important source of SDF-1 expression,170,171 and we have recently shown that hypoxic preconditioning enhances the recruitment of cardiosphere-derived Lin-negative, c-kit-positive progenitor (CLK) cells (i.e., cardiac progenitor cells) by inducing CXCR4 expression.138 CXCR4 expression is much lower in CLK cells than in bone marrow mononuclear cells under normoxic conditions, but increases significantly in response to hypoxia. The increase is accompanied by elevations in SDF-1 expression and preceded by the upregulation of HIF-1α, whereas the siRNA-mediated inactivation of HIF-1α abolishes CXCR4 upregulation. Hypoxic treatment also increased the migration of isolated CLK cells toward SDF-1 in a CXCR4-dependent manner, and hypoxic preconditioning was associated with a 2.5-fold increase in the recruitment of systemically injected CLK cells to the ischemic myocardium of mice after surgically induced myocardial infarction. The recruited cells expressed cardiac troponin I, von Willebrand factor, and smooth muscle actin, indicating that CLK cells can differentiate into cardiomyocytes, endothelial cells, and vascular smooth muscle cells, respectively.
IV. Therapeutic Implications
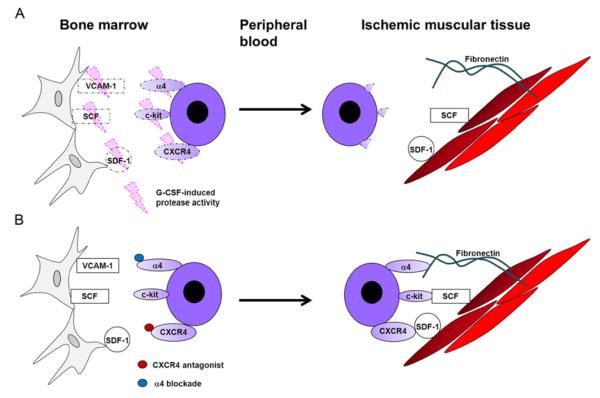
Both the release of progenitor cells from the bone marrow to the peripheral blood and the recruitment and retention of progenitor cells in ischemic tissue are regulated by interactions between SDF-1 and CXCR4.37,66,68,75,85,91,99,158,162,163,172–174 The interaction must be disrupted before progenitor cells can be mobilized from the bone marrow to the peripheral circulation and restored to enable retention of the mobilized cells in the ischemic tissue. Thus, the effectiveness of progenitor cell therapy is crucially dependent on how mobilization is induced and on the level of SDF-1 expression in the ischemic region at the time of cell administration. To date, G-CSF is the most frequently used mobilizing agent in clinical trials of progenitor cell therapy,28,29,44,46,48,51,52 but the efficacy results from many of these trials have been disappointing, perhaps because G-CSF mobilizes progenitor cells by activating extracellular proteases that irreversibly cleave cell-surface adhesion molecules, including α4-integrin, VCAM-1, and CXCR4.92,175 Thus, the retention of G-CSF-mobilized cells in the ischemic region may be impaired, and mobilizing agents that reversibly disrupt SDF-1/CXCR4 binding, such as AMD3100, may improve the effectiveness of cell therapy37 (Fig. 2).
FIG. 2.
Mechanisms of therapeutic progenitor cell mobilization. (A) Growth factors (e.g., G-CSF) mobilize progenitor cells by activating extracellular proteases that irreversibly cleave cell-surface adhesion molecules, including α4-integrin, c-kit, and CXCR4, which are crucial for progenitor cell retention in the ischemic region. (B) Receptor antagonists, such as the CXCR4 antagonist AMD3100 or the α4-integrin-blocking antibody Natalizumab, mobilize progenitor cells by reversibly blocking interactions that bind progenitor cells to the bone marrow substrate without cleaving the adhesion molecules. Thus, the use of reversible antagonists, rather than growth factors, for therapeutic progenitor cell mobilization may increase the number of mobilized cells retained in the ischemic region.
Cardiac SDF-1 expression is upregulated within minutes to an hour after myocardial infarction but declines 4–7 days later.158 Thus, if progenitor cells are administered days after, or even years after (i.e., in patients with established coronary disease), the infarct event, retention of the administered cells is likely to be poor in the ischemic region. Thus, several approaches to increase SDF-1 levels in the ischemic region are currently being investigated. Locally delivered SDF-1 protein increased vascular growth in the injured limbs of mice after surgically induced hind-limb ischemia,176 and SDF-1 also improved cardiac function after ischemic myocardial injury by increasing progenitor cell recruitment and angiogenesis and by reducing scar formation.177 However, local SDF-1 delivery is limited by the rapid diffusion of the administered protein and by the activity of proteases in the inflammatory environment of the injury. Furthermore, the induction of SDF-1/CXCR4 signaling also stimulates the production of matrix metalloproteases (MMPs),178–181 including MMP-2, which (in concert with several exopeptidases) cleaves SDF-1 to produce a neurotoxin that has been implicated in some forms of dementia.163,182 To overcome this limitation, Segers et al. designed an SDF-1 variant that retains the chemotactic properties of the native molecule but is resistant to MMP-2 and exopeptidase cleavage. Nanofiber-mediated delivery of this construct, S-SDF-1 (also called S4V), promoted progenitor cell recruitment and improved cardiac function in a murine model of myocardial infarction.183 Furthermore, sustained SDF-1 release has been achieved by covalently linking it to a polyethylene glycol fibrin patch.174 When the patch was applied to the surface of infarcted mouse hearts, SDF-1 continued to be released for 28 days, and the treatment was associated with greater numbers of incorporated c-kit-positive cells (i.e., progenitor cells) and with improvements in left ventricular function.
Local SDF-1 expression has also been increased through the administration of genetically engineered MSCs.184,185 Intravenous injections of either unmodified MSCs or MSCs that overexpressed SDF-1 to rats after acute myocardial infarction were associated with improvements in cardiac function, and the beneficial effects appeared to evolve primarily through the preservation of preexisting cardiomyocytes rather than the generation of new cardiomyocytes within the infarct zone.184 Vascular density, cardiomyocyte survival, and cardiac myosin-positive area were greater in animals treated with the modified cells than in those treated with unmodified cells, and SDF-1 overexpression also increased the number of small cardiac myosin-expressing cells that had not differentiated into mature cardiac myocytes, but were capable of depolarizing and, consequently, may have contributed to improvements in contractile function.185
V. Summary
Progenitor cell retention and release are largely governed by SDF-1/ CXCR4 and α4-integrin signaling. The initial steps of these two pathways appear to proceed independently, but both regulate c-kit phosphorylation,71 and the mobilization of progenitor cells in response to either CXCR4 antagonism or α4-integrin blockade is impaired by the loss of c-kit kinase activity.131,139–141 Furthermore, bone marrow progenitor cell levels are lower in c-kit kinase-defective mice than in wild-type mice before mobilization, and systemically administered CXCR4-positive progenitor cells cannot repopulate the bone marrow in the absence of c-kit kinase activity.71 Collectively, these observations suggest that c-kit-kinase inactivation blocks the retention of CXCR4-positive progenitor cells in the bone marrow, and that c-kit could function as a final common mediator of fundamental importance to the regulation of bone marrow progenitor cell trafficking.
SDF-1/CXCR4 and α4-integrin signaling are also crucial for the retention of progenitor cells in the ischemic region, which may explain, at least in part, why clinical trials of progenitor cell therapy have failed to display the efficacy observed in preclinical investigations. The lack of effectiveness is often attributed to poor retention of the transplanted cells,21,22,27 and, to date, most of the trial protocols have mobilized cells via G-CSF administration, which activates extracellular proteases that irreversibly cleave cell-surface adhesion molecules, including α4-integrin and CXCR4.92,175 Thus, the retention of G-CSF-mobilized cells in the ischemic region may be impaired, and mobilizing agents that reversibly disrupt SDF-1/CXCR4 binding, such as AMD3100, may improve patient response.37 Efforts to supplement SDF-1 levels in the ischemic region may also improve progenitor cell recruitment and the effectiveness of stem cell therapy.
Acknowledgment
We thank W. Kevin Meisner, PhD, for editorial assistance.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- 3.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 5.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–40. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 7.Schuleri KH, Amado LC, Boyle AJ, Centola M, Saliaris AP, Gutman MR, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–11. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 8.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–36. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–72. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 10.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 12.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 13.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 14.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–83. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–6. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 19.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 20.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 21.Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation. 2010;121:325–35. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]
- 22.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–15. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 23.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–7. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 24.Rosenzweig A. Cardiac cell therapy—mixed results from mixed cells. N Engl J Med. 2006;355:1274–7. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 25.Reffelmann T, Konemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. J Am Coll Cardiol. 2009;53:305–8. doi: 10.1016/j.jacc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 28.Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003–10. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 29.Ripa RS, Jorgensen E, Wang Y, Thune JJ, Nilsson JC, Sondergaard L, et al. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–92. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 30.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin G, Ii M, Silver M, Wecker A, Bord E, Ma H, et al. Functional disruption of alpha4 integrin mobilizes bone marrow-derived endothelial progenitors and augments ischemic neovascularization. J Exp Med. 2006;203:153–63. doi: 10.1084/jem.20050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams GB, Scadden DT. A niche opportunity for stem cell therapeutics. Gene Ther. 2008;15:96–9. doi: 10.1038/sj.gt.3303063. [DOI] [PubMed] [Google Scholar]
- 33.Aicher A, Kollet O, Heeschen C, Liebner S, Urbich C, Ihling C, et al. The Wnt antagonist Dickkopf-1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ Res. 2008;103:796–803. doi: 10.1161/CIRCRESAHA.107.172718. [DOI] [PubMed] [Google Scholar]
- 34.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–23. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, et al. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–14. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- 37.Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:11008–13. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roncalli J, Renault MA, Tongers J, Misener S, Thorne T, Kamide C, et al. Sonic hedgehog-induced functional recovery after myocardial infarction is enhanced by AMD3100-mediated progenitor-cell mobilization. J Am Coll Cardiol. 2011;57:2444–52. doi: 10.1016/j.jacc.2010.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voors AA, Belonje AM, Zijlstra F, Hillege HL, Anker SD, Slart RH, et al. A single dose of erythropoietin in ST-elevation myocardial infarction. Eur Heart J. 2010;31:2593–600. doi: 10.1093/eurheartj/ehq304. [DOI] [PubMed] [Google Scholar]
- 40.Belonje AM, Voors AA, van Gilst WH, Anker SD, Slart RH, Tio RA, et al. Effects of erythropoietin after an acute myocardial infarction: rationale and study design of a prospective, randomized, clinical trial (HEBE III) Am Heart J. 2008;155:817–22. doi: 10.1016/j.ahj.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 41.Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–9. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi N, Nakamura T, Sawada T, Matsubara K, Furukawa K, Hadase M, et al. Erythropoietin prevention trial of coronary restenosis and cardiac remodeling after ST-elevated acute myocardial infarction (EPOC-AMI): a pilot, randomized, placebo-controlled study. Circ J. 2010;74:2365–71. doi: 10.1253/circj.cj-10-0267. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura R, Takahashi A, Yamada T, Miyai N, Irie H, Kinoshita N, et al. Erythropoietin in patients with acute coronary syndrome and its cardioprotective action after percutaneous coronary intervention. Circ J. 2009;73:1920–6. doi: 10.1253/circj.cj-09-0219. [DOI] [PubMed] [Google Scholar]
- 44.Engelmann MG, Theiss HD, Hennig-Theiss C, Huber A, Wintersperger BJ, Werle-Ruedinger AE, et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48:1712–21. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 45.Valgimigli M, Rigolin GM, Cittanti C, Malagutti P, Curello S, Percoco G, et al. Use of granulocyte-colony stimulating factor during acute myocardial infarction to enhance bone marrow stem cell mobilization in humans: clinical and angiographic safety profile. Eur Heart J. 2005;26:1838–45. doi: 10.1093/eurheartj/ehi289. [DOI] [PubMed] [Google Scholar]
- 46.Ince H, Petzsch M, Kleine HD, Schmidt H, Rehders T, Korber T, et al. Preservation from left ventricular remodeling by front-integrated revascularization and stem cell liberation in evolving acute myocardial infarction by use of granulocyte-colony-stimulating factor (FIRSTLINE-AMI) Circulation. 2005;112:3097–106. doi: 10.1161/CIRCULATIONAHA.105.541433. [DOI] [PubMed] [Google Scholar]
- 47.Kang HJ, Kim HS, Koo BK, Kim YJ, Lee D, Sohn DW, et al. Intracoronary infusion of the mobilized peripheral blood stem cell by G-CSF is better than mobilization alone by G-CSF for improvement of cardiac function and remodeling: 2-year follow-up results of the Myocardial Regeneration and Angiogenesis in Myocardial Infarction with G-CSF and Intra-Coronary Stem Cell Infusion (MAGIC Cell) 1 trial. Am Heart J. 2007;153:237, e1–8. doi: 10.1016/j.ahj.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Ellis SG, Penn MS, Bolwell B, Garcia M, Chacko M, Wang T, et al. Granulocyte colony stimulating factor in patients with large acute myocardial infarction: results of a pilot dose-escalation randomized trial. Am Heart J. 2006;152:1051, e9–14. doi: 10.1016/j.ahj.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Takano H, Hasegawa H, Kuwabara Y, Nakayama T, Matsuno K, Miyazaki Y, et al. Feasibility and safety of granulocyte colony-stimulating factor treatment in patients with acute myocardial infarction. Int J Cardiol. 2007;122:41–7. doi: 10.1016/j.ijcard.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 50.Engelmann MG, Theiss HD, Theiss C, Huber A, Wintersperger BJ, Werle-Ruedinger AE, et al. G-CSF in patients suffering from late revascularized ST elevation myocardial infarction: analysis on the timing of G-CSF administration. Exp Hematol. 2008;36:703–9. doi: 10.1016/j.exphem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Hill JM, Syed MA, Arai AE, Powell TM, Paul JD, Zalos G, et al. Outcomes and risks of granulocyte colony-stimulating factor in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1643–8. doi: 10.1016/j.jacc.2005.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivas G, Anversa P, Frishman WH. Cytokines and myocardial regeneration: a novel treatment option for acute myocardial infarction. Cardiol Rev. 2009;17:1–9. doi: 10.1097/CRD.0b013e31817bd7ab. [DOI] [PubMed] [Google Scholar]
- 53.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–6. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 55.Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 57.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 59.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–30. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oostendorp RA, Dormer P. VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells. Leuk Lymphoma. 1997;24:423–35. doi: 10.3109/10428199709055581. [DOI] [PubMed] [Google Scholar]
- 62.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 63.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM, Harlan JM. Synergistic mobilization of hemopoietic progenitor cells using concurrent beta1 and beta2 integrin blockade or beta2-deficient mice. Blood. 2001;97:1282–8. doi: 10.1182/blood.v97.5.1282. [DOI] [PubMed] [Google Scholar]
- 64.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001;98:2403–11. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 65.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–96. [PubMed] [Google Scholar]
- 66.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 67.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–54. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–6. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–56. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 70.Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–9. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng M, Zhou J, Wu M, Boriboun C, Thorne T, Liu T, et al. CXCR4-mediated bone marrow progenitor cell maintenance and mobilization are modulated by c-kit activity. Circ Res. 2010;107:1083–93. doi: 10.1161/CIRCRESAHA.110.220970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–71. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loetscher M, Geiser T, O’Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–7. [PubMed] [Google Scholar]
- 74.Nomura H, Nielsen BW, Matsushima K. Molecular cloning of cDNAs encoding a LD78 receptor and putative leukocyte chemotactic peptide receptors. Int Immunol. 1993;5:1239–49. doi: 10.1093/intimm/5.10.1239. [DOI] [PubMed] [Google Scholar]
- 75.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–83. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 77.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–76. [PubMed] [Google Scholar]
- 78.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–43. [PubMed] [Google Scholar]
- 79.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–37. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- 81.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 82.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–71. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 83.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 84.Kawabata K, Ujikawa M, Egawa T, Kawamoto H, Tachibana K, Iizasa H, et al. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci USA. 1999;96:5663–7. doi: 10.1073/pnas.96.10.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 86.Shepherd RM, Capoccia BJ, Devine SM, Dipersio J, Trinkaus KM, Ingram D, et al. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 2006;108:3662–7. doi: 10.1182/blood-2006-06-030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–11. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 88.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 89.Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ-specific process of artery formation. Blood. 2005;105:3155–61. doi: 10.1182/blood-2004-07-2563. [DOI] [PubMed] [Google Scholar]
- 90.Ara T, Itoi M, Kawabata K, Egawa T, Tokoyoda K, Sugiyama T, et al. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170:4649–55. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 91.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–8. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–30. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 94.Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–73. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59:15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 96.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zagzag D, Krishnamachary B, Yee H, Okuyama H, Chiriboga L, Ali MA, et al. Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 2005;65:6178–88. doi: 10.1158/0008-5472.CAN-04-4406. [DOI] [PubMed] [Google Scholar]
- 98.Vanbervliet B, Bendriss-Vermare N, Massacrier C, Homey B, de Bouteiller O, Briere F, et al. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med. 2003;198:823–30. doi: 10.1084/jem.20020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 100.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 101.Hemler ME, Lobb RR. The leukocyte beta 1 integrins. Curr Opin Hematol. 1995;2:61–7. doi: 10.1097/00062752-199502010-00009. [DOI] [PubMed] [Google Scholar]
- 102.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- 104.Liu S, Thomas SM, Woodside DG, Rose DM, Kiosses WB, Pfaff M, et al. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature. 1999;402:676–81. doi: 10.1038/45264. [DOI] [PubMed] [Google Scholar]
- 105.Liu S, Rose DM, Han J, Ginsberg MH. Alpha4 integrins in cardiovascular development and diseases. Trends Cardiovasc Med. 2000;10:253–7. doi: 10.1016/s1050-1738(00)00073-6. [DOI] [PubMed] [Google Scholar]
- 106.Katayama Y, Hidalgo A, Peired A, Frenette PS. Integrin alpha4beta7 and its counterreceptor MAdCAM-1 contribute to hematopoietic progenitor recruitment into bone marrow following transplantation. Blood. 2004;104:2020–6. doi: 10.1182/blood-2003-12-4157. [DOI] [PubMed] [Google Scholar]
- 107.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/ differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity. 1999;11:555–66. doi: 10.1016/s1074-7613(00)80131-4. [DOI] [PubMed] [Google Scholar]
- 108.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003;23:9349–60. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ulyanova T, Priestley GV, Nakamoto B, Jiang Y, Papayannopoulou T. VCAM-1 ablation in nonhematopoietic cells in MxCre+ VCAM-1f/f mice is variable and dictates their phenotype. Exp Hematol. 2007;35:565–71. doi: 10.1016/j.exphem.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prosper F, Stroncek D, McCarthy JB, Verfaillie CM. Mobilization and homing of peripheral blood progenitors is related to reversible downregulation of alpha4 beta1 integrin expression and function. J Clin Invest. 1998;101:2456–67. doi: 10.1172/JCI188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wagers AJ, Allsopp RC, Weissman IL. Changes in integrin expression are associated with altered homing properties of Lin(-/lo)Thy1.1(lo)Sca-1(+)c-kit(+) hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Exp Hematol. 2002;30:176–85. doi: 10.1016/s0301-472x(01)00777-9. [DOI] [PubMed] [Google Scholar]
- 112.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–97. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 113.Priestley GV, Ulyanova T, Papayannopoulou T. Sustained alterations in biodistribution of stem/progenitor cells in Tie2Cre+ alpha4(f/f) mice are hematopoietic cell autonomous. Blood. 2007;109:109–11. doi: 10.1182/blood-2006-06-026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 115.Zohren F, Toutzaris D, Klarner V, Hartung HP, Kieseier B, Haas R. The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood. 2008;111:3893–5. doi: 10.1182/blood-2007-10-120329. [DOI] [PubMed] [Google Scholar]
- 116.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci USA. 1993;90:9374–8. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hartz B, Volkmann T, Irle S, Loechelt C, Neubauer A, Brendel C. alpha4 integrin levels on mobilized peripheral blood stem cells predict rapidity of engraftment in patients receiving autologous stem cell transplantation. Blood. 2011;118:2362–5. doi: 10.1182/blood-2011-02-331918. [DOI] [PubMed] [Google Scholar]
- 118.Besmer P, Murphy JE, George PC, Qiu FH, Bergold PJ, Lederman L, et al. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986;320:415–21. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- 119.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 120.Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808–13. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 122.Flanagan JG, Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990;63:185–94. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- 123.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–64. [PubMed] [Google Scholar]
- 124.Yuzawa S, Opatowsky Y, Zhang Z, Mandiyan V, Lax I, Schlessinger J. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell. 2007;130:323–34. doi: 10.1016/j.cell.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 125.Arakawa T, Yphantis DA, Lary JW, Narhi LO, Lu HS, Prestrelski SJ, et al. Glycosylated and unglycosylated recombinant-derived human stem cell factors are dimeric and have extensive regular secondary structure. J Biol Chem. 1991;266:18942–8. [PubMed] [Google Scholar]
- 126.Blume-Jensen P, Jiang G, Hyman R, Lee KF, O’Gorman S, Hunter T. Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat Genet. 2000;24:157–62. doi: 10.1038/72814. [DOI] [PubMed] [Google Scholar]
- 127.McCulloch EA, Siminovitch L, Till JE, Russell ES, Bernstein SE. The cellular basis of the genetically determined hemopoietic defect in anemic mice of genotype Sl-Sld. Blood. 1965;26:399–410. [PubMed] [Google Scholar]
- 128.Barker JE. Sl/Sld hematopoietic progenitors are deficient in situ. Exp Hematol. 1994;22:174–7. [PubMed] [Google Scholar]
- 129.Tan JC, Nocka K, Ray P, Traktman P, Besmer P. The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science. 1990;247:209–12. doi: 10.1126/science.1688471. [DOI] [PubMed] [Google Scholar]
- 130.Heissig B, Werb Z, Rafii S, Hattori K. Role of c-kit/Kit ligand signaling in regulating vasculogenesis. Thromb Haemost. 2003;90:570–6. doi: 10.1160/TH03-03-0188. [DOI] [PubMed] [Google Scholar]
- 131.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–77. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:2304–9. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908–16. doi: 10.1038/nm1446. [DOI] [PubMed] [Google Scholar]
- 134.Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–32. [PubMed] [Google Scholar]
- 135.Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–24. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 136.Williams DE, Eisenman J, Baird A, Rauch C, Van Ness K, March CJ, et al. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990;63:167–74. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- 137.Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–33. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 138.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–16. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roberts AW, Foote S, Alexander WS, Scott C, Robb L, Metcalf D. Genetic influences determining progenitor cell mobilization and leukocytosis induced by granulocyte colony-stimulating factor. Blood. 1997;89:2736–44. [PubMed] [Google Scholar]
- 140.Papayannopoulou T, Priestley GV, Nakamoto B. Anti-VLA4/VCAM-1-induced mobilization requires cooperative signaling through the kit/mkit ligand pathway. Blood. 1998;91:2231–9. [PubMed] [Google Scholar]
- 141.Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, et al. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990;9:1805–13. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–9. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fazel SS, Chen L, Angoulvant D, Li SH, Weisel RD, Keating A, et al. Activation of c-kit is necessary for mobilization of reparative bone marrow progenitor cells in response to cardiac injury. FASEB J. 2008;22:930–40. doi: 10.1096/fj.07-8636com. [DOI] [PubMed] [Google Scholar]
- 144.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89:1502–6. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsujimura T, Furitsu T, Morimoto M, Isozaki K, Nomura S, Matsuzawa Y, et al. Ligand-independent activation of c-kit receptor tyrosine kinase in a murine mastocytoma cell line P-815 generated by a point mutation. Blood. 1994;83:2619–26. [PubMed] [Google Scholar]
- 146.Mol CD, Lim KB, Sridhar V, Zou H, Chien EY, Sang BC, et al. Structure of a c-kit product complex reveals the basis for kinase transactivation. J Biol Chem. 2003;278:31461–4. doi: 10.1074/jbc.C300186200. [DOI] [PubMed] [Google Scholar]
- 147.Briddell RA, Hartley CA, Smith KA, McNiece IK. Recombinant rat stem cell factor synergizes with recombinant human granulocyte colony-stimulating factor in vivo in mice to mobilize peripheral blood progenitor cells that have enhanced repopulating potential. Blood. 1993;82:1720–3. [PubMed] [Google Scholar]
- 148.Andrews RG, Briddell RA, Knitter GH, Opie T, Bronsden M, Myerson D, et al. In vivo synergy between recombinant human stem cell factor and recombinant human granulocyte colony-stimulating factor in baboons enhanced circulation of progenitor cells. Blood. 1994;84:800–10. [PubMed] [Google Scholar]
- 149.Li Z, Li L. Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci. 2006;31:589–95. doi: 10.1016/j.tibs.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 150.Fruehauf S, Veldwijk MR, Seeger T, Schubert M, Laufs S, Topaly J, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11:992–1001. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- 151.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 152.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–74. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 153.Fowler CJ, Dunn A, Hayes-Lattin B, Hansen K, Hansen L, Lanier K, et al. Rescue from failed growth factor and/or chemotherapy HSC mobilization with G-CSF and plerixafor (AMD3100): an institutional experience. Bone Marrow Transplant. 2009;43:909–17. doi: 10.1038/bmt.2008.409. [DOI] [PubMed] [Google Scholar]
- 154.Blum A, Childs RW, Smith A, Patibandla S, Zalos G, Samsel L, et al. Targeted antagonism of CXCR4 mobilizes progenitor cells under investigation for cardiovascular disease. Cytotherapy. 2009;11:1016–9. doi: 10.3109/14653240903131640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, et al. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009;114:2530–41. doi: 10.1182/blood-2009-04-214403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res. 2008;78:413–21. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]
- 157.Vandervelde S, van Luyn MJ, Tio RA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol. 2005;39:363–76. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 158.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 159.Chavakis E, Carmona G, Urbich C, Gottig S, Henschler R, Penninger JM, et al. Phosphatidylinositol-3-kinase-gamma is integral to homing functions of progenitor cells. Circ Res. 2008;102:942–9. doi: 10.1161/CIRCRESAHA.107.164376. [DOI] [PubMed] [Google Scholar]
- 160.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–7. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 161.Chang LT, Yuen CM, Sun CK, Wu CJ, Sheu JJ, Chua S, et al. Role of stromal cell-derived factor-1alpha, level and value of circulating interleukin-10 and endothelial progenitor cells in patients with acute myocardial infarction undergoing primary coronary angioplasty. Circ J. 2009;73:1097–104. doi: 10.1253/circj.cj-08-0497. [DOI] [PubMed] [Google Scholar]
- 162.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 163.Penn MS. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circ Res. 2009;104:1133–5. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–51. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 165.Egan CG, Lavery R, Caporali F, Fondelli C, Laghi-Pasini F, Dotta F, et al. Generalised reduction of putative endothelial progenitors and CXCR4-positive peripheral blood cells in type 2 diabetes. Diabetologia. 2008;51:1296–305. doi: 10.1007/s00125-008-0939-6. [DOI] [PubMed] [Google Scholar]
- 166.Oh BJ, Kim DK, Kim BJ, Yoon KS, Park SG, Park KS, et al. Differences in donor CXCR4 expression levels are correlated with functional capacity and therapeutic outcome of angiogenic treatment with endothelial colony forming cells. Biochem Biophys Res Commun. 2010;398:627–33. doi: 10.1016/j.bbrc.2010.06.108. [DOI] [PubMed] [Google Scholar]
- 167.Tendera M, Wojakowski W, Ruzyllo W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) trial. Eur Heart J. 2009;30:1313–21. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 168.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, et al. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–91. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 169.Hu X, Dai S, Wu WJ, Tan W, Zhu X, Mu J, et al. Stromal cell derived factor-1 alpha confers protection against myocardial ischemia/reperfusion injury: role of the cardiac stromal cell derived factor-1 alpha CXCR4 axis. Circulation. 2007;116:654–63. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, et al. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–33. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, et al. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117:206–15. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 172.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–82. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 173.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009;29:1802–9. doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 174.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–71. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 175.Hill JM, Bartunek J. The end of granulocyte colony-stimulating factor in acute myocardial infarction? Reaping the benefits beyond cytokine mobilization. Circulation. 2006;113:1926–8. doi: 10.1161/CIRCULATIONAHA.106.623777. [DOI] [PubMed] [Google Scholar]
- 176.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 177.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–31. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fernandis AZ, Prasad A, Band H, Klosel R, Ganju RK. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157–67. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- 179.Janowska-Wieczorek A, Marquez LA, Dobrowsky A, Ratajczak MZ, Cabuhat ML. Differential MMP and TIMP production by human marrow and peripheral blood CD34(+) cells in response to chemokines. Exp Hematol. 2000;28:1274–85. doi: 10.1016/s0301-472x(00)00532-4. [DOI] [PubMed] [Google Scholar]
- 180.Samara GJ, Lawrence DM, Chiarelli CJ, Valentino MD, Lyubsky S, Zucker S, et al. CXCR4-mediated adhesion and MMP-9 secretion in head and neck squamous cell carcinoma. Cancer Lett. 2004;214:231–41. doi: 10.1016/j.canlet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 181.Spiegel A, Kollet O, Peled A, Abel L, Nagler A, Bielorai B, et al. Unique SDF-1-induced activation of human precursor-B ALL cells as a result of altered CXCR4 expression and signaling. Blood. 2004;103:2900–7. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- 182.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–8. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 183.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–92. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 184.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 185.Unzek S, Zhang M, Mal N, Mills WR, Laurita KR, Penn MS. SDF-1 recruits cardiac stem cell-like cells that depolarize in vivo. Cell Transplant. 2007;16:879–86. doi: 10.3727/096368907783338271. [DOI] [PubMed] [Google Scholar]