Abstract
Background
Dysembryoplastic neuroepithelial tumors (DNETs) are benign glioneuronal tumors that occur in children. These tumors are characterized by seizures, lack of neurologic deficits, and a seemingly benign course after resection.
Methods
We conducted a retrospective review of data relating to 11 children diagnosed with DNET between January 1988 and December 2007 at St. Jude Children's Research Hospital. This report documents the clinical features, neurocognitive function, and treatment outcomes in our institutional series.
Results
Our patient cohort included 8 boys and 3 girls (median age at diagnosis, 10 years); all patients presented with seizures: 4 complex partial, 3 generalized tonic clonic, 2 absence, 1 partial simple, and 1 not classified. Of the 11 patients, 1 died of cardiac fibrosis, and tumors recurred or progressed in 4 (36%). Seizure control was achieved in all patients but 1. Of the 9 patients who completed neuropsychologic testing, only 3 (23%) functioned at or above the expected level of same-age peers.
Conclusion
The high recurrence and progression rates of DNETs and the high rate of abnormal neurocognitive test results in this study highlight the need for regular follow-up and appropriate academic counseling of children with these tumors.
Keywords: Dysembryoplastic neuroepithelial tumors (DNETs), pediatric, recurrence, neuropsychologic outcome
Introduction
Dysembryoplastic neuroepithelial tumors (DNETs), first described in 1988 by Daumas-Duport,1 are rare benign glioneuronal tumors occurring most commonly in children and young adults. Clinically, DNETs present as seizures before the age of 20 years in patients with a normal intelligence quotient (IQ).1,2 Pathologically, the characteristic features of these tumors are a nodular architecture and a specific glioneuronal element (SGNE), which typically contains oligodendrocyte-like cells (OLCs) in a columnar or alveolar pattern and “floating neurons” against a myxoid matrix.1 Neuroimaging findings confirm the cortical location and lack of edema or mass effect of DNETs.1,3-5 Local recurrence occurs rarely after gross total resection (GTR).1-3,6,7Here we report clinical, pathologic, radiologic, and outcome data for 11 children with a diagnosis of DNET treated at St. Jude Children's Research Hospital (SJCRH), with emphasis on data on recurrent disease and neurocognition.
Materials and Methods
We conducted a retrospective study of the patients diagnosed with DNET between January 1988 and December 2007 at SJCRH. The fourth edition (2007) of the World Health Organization's classification of tumors of the nervous system8 was used to determine the pathologic criteria for diagnosis; presence of an SGNE was required to make a firm diagnosis of DNET. DNETs were divided into “simple” and “complex” forms, as described in the WHO classification; essentially, complex DNETs contain glial nodules but simple DNETs do not. Of 1589 patients with newly diagnosed brain tumors, 11 patients (0.7%) were diagnosed with DNETs during the study period. Medical records were reviewed for clinical data as well as radiologic and pathologic features at diagnosis. Results of comprehensive neurologic exam by a neurologist, visual field testing, and neurocognitive evaluations were also obtained and reviewed.
Neurocognitive evaluations were completed for 9 of 11 patients. A retrospective review revealed that evaluations were conducted by using various measures according to the presenting clinical question, age, and year of evaluation. Intellect and academic achievement were assessed by the Wechsler Intelligence Scales for Children (WISC-R or WISCIII), Wechsler Adult Intelligence Scale (WAIS-III), Woodcock Johnson Test of Academic Achievement (WJ-R or WJ-Ach), Wide Range Achievement Test (WRAT-R or WRAT-III), Wechsler Individual Achievement Test (WIAT), Kaufmann Survey of Early Academic and Language Skills (K-SEALS), or McCarthy Scales of Children's Abilities. The Developmental Test of Visual-Motor Integration (VMI) was also used. Measures of memory function were derived from the Wide Range Assessment of Memory and Learning (WRAML), Wechsler Intelligence Scales for Children (WISCIII), or the McCarthy Scales of Children's Abilities. Scores from these tests were adjusted for age by using general population norms with a mean of 100 and a standard deviation of 15.
Parents were asked to complete the Behavioral Rating Inventory of Executive Function (BRIEF) and the Child Behavior Checklist (CBCL). The BRIEF is a questionnaire completed by parents and provides a measure of 8 aspects of executive function, processes by which a child can direct thought, action, and emotion. The CBCL provides 3 measures of competence (Activities, Social, and School) as well as measures of Internalizing and Externalizing behavior. Syndromes scored from the CBCL are Aggressive Behavior; Anxious/Depressed; Attention Problems; Rule-Breaking Behavior; Social Problems; Somatic Complaints; Thought Problems; and Withdrawn/Depressed. Six additional diagnostic-oriented areas are also assessed: Affective Problems; Anxiety Problems; Somatic Problems; Attention Deficit/Hyperactivity Problems; Oppositional Defiant Problems; and Conduct Problems.
Assessment reports generated by the evaluating clinician were reviewed. The reports provided either actual standard scores, descriptive labels for the level of performance, or both. Descriptive labels for standard scores are as follows: Average (90-109), High average (110-119), Superior (120-129) Very superior (130+), Low average (80-89), Borderline (70-79), and Deficient (≤69). Clinically significant levels of each measure derived from the parent report were also described.
In addition, radiologic characteristics, surgical extent of resection, relapse patterns, and outcome data were reviewed. The extent of resection was classified into 1of 5 categories on the basis of the surgeon's report and postoperative images as follows: (1) gross total resection (GTR), no visible tumor left; (2) near total resection (NTR), removal of more than 90% but less than 100% of tumor; (3) subtotal resection (STR): removal of 50% to 89% of the tumor; (4) partial resection (PR), removal of 10% to 49% of the tumor; or (5) biopsy (BX), removal of less than 10% of the tumor. The follow-up time was calculated as the time between the date of diagnosis (i.e., date of first craniotomy) and the date of last contact.
Results
Clinical characteristics
Our patient cohort comprised 8 boys and 3 girls, with a median age at diagnosis of 10 years (range, 5.1–16.2 years). All patients presented with seizures at diagnosis: 4 had complex partial (CP), 3 had generalized tonic clonic (GTC), 2 had absence, and 1 had simple partial (SP); in 1 patient, the type of seizure could not be classified. In patient 9, the CP seizure evolved to a GTC seizure. The median age of seizure onset was 9 years (range, 4–14 years), and the median time between seizure onset and surgery was 12 months (range, 0.5–60 months). Patients had a family history for seizures (n = 4), multiple sclerosis (n = 1), and bipolar disorder (n = 1).
Radiologic and histopathologic features
Radiologic data were consistent with those from literature 3-5 with regard to cortical location, hypointensity on T1 and hyperintensity on T2, enhancement, cystic component, edema, calcification, and bone remodeling (Figures 1 and 2). Also, the histopathologic features were consistent with those published previously (Figure 3).1,2,6-8
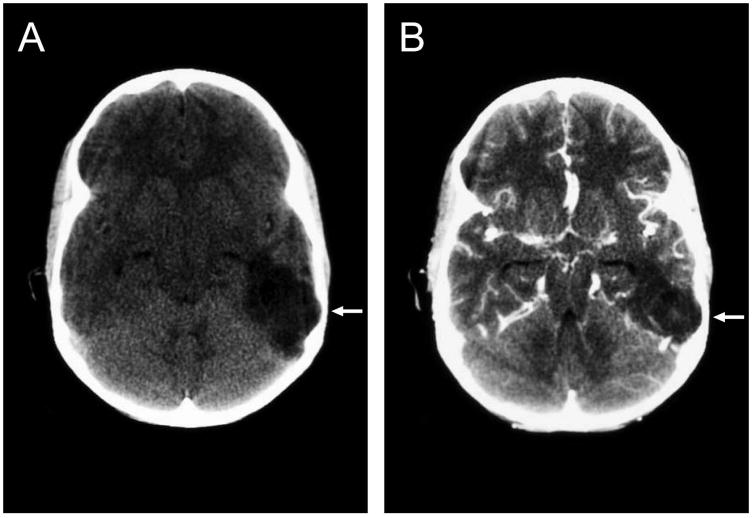
Figure 1.
Computed tomography scan of the brain of patient 6 showing a hypodense lesion (a) with no enhancement after injection of contrast dye (b). Bone remodeling is evident on both images (arrow).
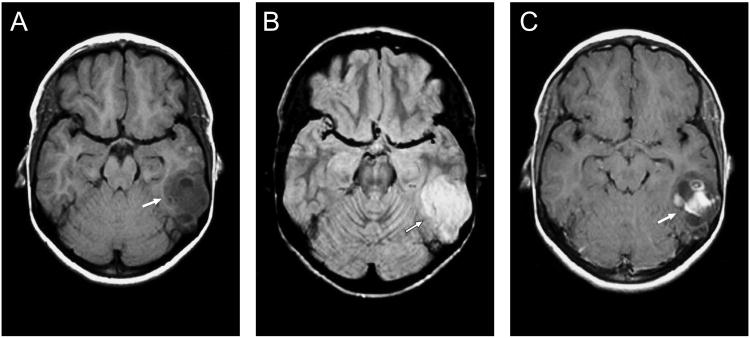
Figure 2.
Magnetic resonance image of the brain of patient 6 showing a hypointense lesion on T1 (a) and a hyperintense lesion on T2 (b). Post-contrast imaging shows partial enhancement (c) (arrow).
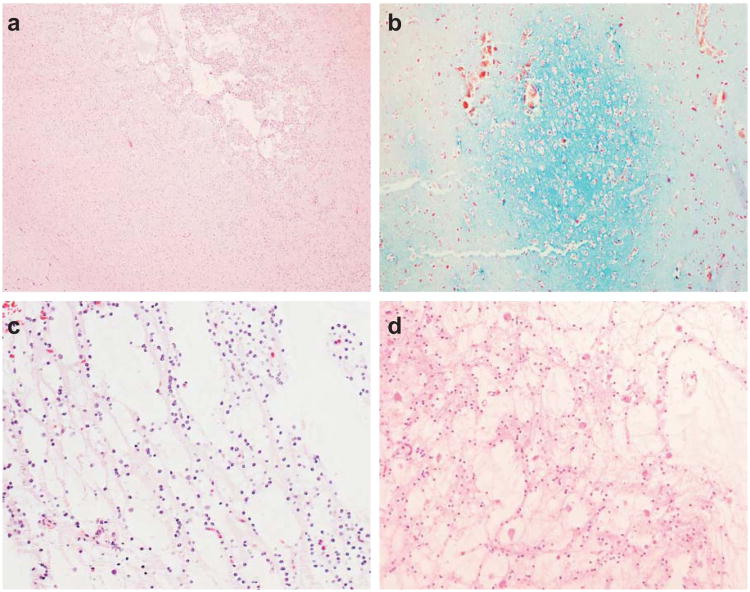
Figure 3.
Microcystic nodules (a) are characteristic of dysembryoplastic neuroepithelial tumors and demonstrate myxoid degeneration (b). The specific glioneuronal element is characterized by columns of OLCs between microcysts (c) and neurons that ‘float’ within the microcysts (d). (a, c, d: hematoxylin & eosin staining; b: alcian blue and periodic acid Schiff staining)
Patient outcome
At the time of last contact, all patients were alive: 8 had no evidence of radiologic disease (NED) and 3 had stable radiologic disease (SD). The median follow-up time was 9.7 years (range, 1–15.7 years). One patient whose status had been NED at the time of last contact died of cardiac fibrosis 6 years after the last contact.
A total of 18 surgical resections were performed: 6 patients underwent 1 resection each, 3 patients underwent 2 resections each, and 2 patients underwent 3 surgical resections each (Table 1). The reasons for the repeated craniotomies were recurrent disease that was detected radiologically post-GTR (n = 3), progression of a residual tumor (n = 2), worsening seizures (n = 1), and residual disease (n = 1).
Table 1. Clinical, Surgical and Seizure Data.
| Patient | Age at DX (years) | Tumor Location | VF defect | Seizure type | AED | Duration: seizure to surgery (years) | Seizure control post surgery | Extent of 1st resection | Extent of 2nd resection | Extent of 3rd resection | Disease status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.2 | Temp | QA | SP-Sens | Carbamazepine Levetiracetam | 3.8 | Resolved | GTR | – | – | NED |
| 2 | 5.1 | Front | – | CP | Carbamazepine | 1 | Resolved | GTR | – | – | NED |
| 3 | 9.2 | Temp | – | Absencee | Carbamazepine | 0.3 | Resolved | GTR | GTR | – | NED |
| 4 | 15 | Par | Normal | NA | Phenytoin | 5g | Resolved | NTR | – | – | SD |
| 5 | 16.2 | Temp | – | GTC | Phenytoin | 2 | Resolved | GTR | – | – | NED |
| 6 | 9 | Temp | QAc | Absencee | Carbamazepine | 1 | Resolved | GTR | GTR | GTR | NED |
| 7 | 13.7 | Temp-Par-Occ | QA | CP | Carbamazepine | 0.4 | Resolved | NTR | GTR | – | NED |
| 8 | 15.1 | Temp-Par | – | GTC | Levetiracetam | 0.0h | Controlled | NTR | – | – | SD |
| 9 | 5.5 | Temp-Par | HHd | CP → GTC | Carbamazepine | 0.8 | Resolved | GTR | – | – | NEDi |
| 10a | 6 | Temp | – | CP | Zonisamidef Oxcarbazepine | 1.5 | Resolved | STR | STR | – | SD |
| 11b | 11.6 | Front | Normal | GTC | Valproic acid Phenobarbital | 1.8 | Resolved | BX | GTR | GTR | NED |
Abbreviations: AED, antiepilepsy drugs; BX, biopsy; CP, complex partial; Front, frontal; GTC, generalized tonic clonic; GTR, gross total resection; HH, homonymous hemianopia; NA, not available; NED, no evidence of disease; NTR, near-total resection; Occ, occipital; Par, parietal; QA, quadrantanopia; SD, stable disease; Sens, sensory; SP, simple parietal; STR, subtotal resection; Temp, temporal; VF, visual field.
The patient was seizure-free for 4 years, then seizures recurred and progressed during the next 8 years until a second resection was performed because of radiologic progression.
The patient underwent biopsy, and after 10 days, definite surgery. Five months later, the patient developed seizures, but the MRI was negative for a tumor. Approximately 4 years later, surgery was performed on the epileptic focus; no tumor was found.
The patient was blind in one eye because of congenital glaucoma and had QA in the other eye.
The visual field test used was a confrontational test administered by a neurologist.
The diagnosis of absence seizure was based on retrospective history from family and patients that was taken after the surgery; no EEG was performed to confirm.
The patient failed 11 different AED until the second surgery, where he needed only 2 AED after second surgery for 2 years and was able to stop AED without recurrence of seizures.
The patient had CT shortly after surgery and it showed a a mass, but the family deferred surgery for 5 years.
The duration from seizure to surgery in this patient was only 2 weeks.
The patient died from a cause not related to his disease after last contact.
Patient 10 developed a seizure that recurred 4 years after the first craniotomy and continued to worsen over 8 years, although there was no radiologic progression. Twelve years after his first operation, progressive disease was detected by magnetic resonance imaging (MRI); therefore, a second operation was performed, and the pathology report revealed the presence of a tumor. The patient became seizure-free 2 years after his second craniotomy.
Patient 11 showed no signs of radiologic progression, but craniotomy was performed for recurrent seizure; no tumor was found in the epileptic focus. The median time to recurrence or progression was 30 months (range, 23–144 months). Seizures of all patients except patient 8 were resolved by the time of last contact. The seizures of patient 8 (1 of the 3 patients with SD) are being controlled by single-agent anticonvulsant therapy.
Neurologic findings, visual field testing, and neurocognitive testing
Ten patients were comprehensively examined by a neurologist at least once during their follow-up. Except for visual field deficits, only 1 patient (patient 4) had abnormal neurologic findings, which were partly related to the surgery. Visual field testing was administered in 6 patients, and some defect was documented in 4 patients (67%): 3 had quadrantanopia and 1 had hemianopia. One patient (patient 6) was blind in the right eye because of congenital glaucoma and also had quadrantanopia in the left eye.
At the time of the retrospective chart review, 20 neurocognitive evaluations had been completed in 9 of 11 patients (1 evaluation, n = 4; 2 evaluations, n = 1; 3 evaluations, n = 2; 4 evaluations, n = 2). Patients were 5 to 19 years old at the time of evaluation and were 1 month to 6 years post-resection. Of the 9 patients, 8 received tests of overall intellect, 7 received tests of academic function, 6 received tests of visual motor integration and memory, and 2 received tests of processing speed. At the most recent evaluation, overall cognitive function and intellect tests showed that only 3 patients were functioning at or above average range for healthy same-age peers (Table 2). The remaining 5 patients (62.5%) who had been tested had low-average or deficient overall intellectual function. Assessment of academic achievement showed that only 3 patients were average or above average for reading and spelling whereas only 4 patients were average or above average for math. Of the 8 school-age patients, 5 (62.5%) either enrolled or had received a recommendation to enroll in support services such as special education classes, resource assistance, homebound education services, vocational rehabilitation, occupational therapy, physical therapy, and speech therapy. Clinical observation, parent reports, and direct assessment also indicated that patients had difficulty with attention and concentration, inhibition, auditory learning, and socialization. Two patients who were of eligible age were not able to complete their high-school education.
Table 2.
Neurocognitive evaluations of children with dysembryoplastic neuroepithelial tumors (DNETs)*.
| Patient | Age at Surgery (years,months) | Evaluation | Time from Surgery (months) | Overall Intellect (standard score1) | Reading (standard score1) | Spelling (standard score1) | Mathematics (standard score1) | Visual Motor Integration | Memory (standard score1) | Processing Speed (standard score1) | Support Services | Parent-reported Measures & Assessment Reports2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.2 | 1 | 10 | Average (NA) | Average (97) | Average (107) | High Average (119) | Average (97) | No | |||
| 2 | 5.1 | 1 | 5 | Average (94) | No | Difficulty with sustained attention | ||||||
| 2 | 8 | Occupational | Diagnosed with ADHD | |||||||||
| 3 | 16 | Border line (73) | Average (NA) | Borderline (NA) | Low Average (NA) | Occupational Speech PT |
Difficulties with impulsivity, mental flexibility, and auditory learning | |||||
| 4 | 15 | 1 | 60 | Deficient (55) | Borderline (77) | Special education | Unable to graduate from high school because of learning disabilities | |||||
| 5 | 16.2 | 1 | 20 | Low average (82) | Average (110) | Average (103) | High average (118) | Deficient (68) | No | Adjustment difficulties | ||
| 6 | 9 | 1 | 1 | Borderline (72) | Borderline (78) | Deficient (69) | Deficient (58) | Low Average (88) | Low Average (NA) | Borderline (72) | Education support recommended | |
| 2 | 13 | Borderline (75) | Deficient (58) | Deficient (62) | Low average (81) | Average (96) | Education support recommended | Difficulties with social competency | ||||
| 3 | 24 | Borderline (79) | Deficient (46) | Deficient (69) | Borderline (75) | Average (105) | Low average (86) | Special education | Problems with sustained attention Reading-related learning disability |
|||
| 4 | 63 | Average (90) | Borderline (76) | Borderline (73) | Borderline (70) | Average (97) | Borderline (NA) | Low average (NA) | Resource assistance 3×/wk | |||
| 7 | 13.7 | 1 | 1 | Average (102) | Average (106) | Low average (87) | Average (90) | Significant problems with sustained attention, socialization, and aggression | ||||
| 2 | 75 | Low average (87) | Recommended to Vocational Rehabilitation |
Did not graduate from high school. Behavioral misconduct and incarceration; reactive depression; alcohol and drug abuse |
||||||||
| 9 | 5.5 | 1 | 1 | Deficient (66) | Deficient (NA) | Deficient (NA) | Pre-school age | Auditory processing and memory for auditory input significantly deficient; receptive language deficiency Difficulties with sustained attention |
||||
| 10 | 6 | 1 | 14 | High Average (113) | High Average (114) | Average (105) | High Average (110) | Average (92) | Superior (NA) | No | Slow psychomotor speed | |
| 2 | 30 | Superior (123) | High Average (119) | High Average (118) | High Average (112) | Average (98) | No | Slow psychomotor speed | ||||
| 3 | 50 | High Average (119) | Average (108) | Average (104) | Average (102) | High Average (117) | No | Recovery of visual motor skills | ||||
| 4 | 69 | Superior (122) | Very superior (133) | High Average (119) | High average (116) | High average (113) | Very superior (NA) | No | ||||
| 11 | 11.6 | 1 | 6 | Low average (81) | Deficient (64) | Deficient (64) | Borderline (71) | Borderline (75) | Education support recommended | Difficulty with sustained attention, concentration, and recall of verbally presented material | ||
| 2 | 21 | Average (90) | Borderline (70) | Deficient (61) | Deficient (67) | Average (NA) | Special education | Social withdrawal and anxiety | ||||
| 3 | 42 | Low Average (81) | Deficient (69) | Deficient (65) | Deficient (61) | Average (NA) | Homebound–special education | Verbal skills deficit Visual-spatial skills deficit Weak self-esteem |
Retrospective chart review of children diagnosed with DNET between January 1988 and December 2007 at St. Jude Children's Research Hospital
Abbreviations: ADHD, attention deficit hyperactivity disorder not otherwise specified; NA, not available; PT, physical therapy
Population Mean=100, Standard Deviation=15
Information derived from Behavioral Rating Inventory of Executive Function (BRIEF), the Child Behavior Checklist (CBCL), and assessment reports.
Discussion
We retrospectively analyzed clinical data, radiologic and pathologic characteristics, and neurocognitive test results of 11 patients diagnosed with DNET at SJCRH from January 1988 to December 2007. The neurocognitive and neurologic findings of our study are significant: 67% of tested patients had a visual field defect, which is higher than the 5%-18% rate reported previously.1-3
In our study, only 3 patients had average or above-average cognitive function, and more than 5 of 8 (63%) of school-aged patients were receiving or were deemed eligible to receive special support services. These values are considerably higher than those reported previously. In 3 studies by Daumas-Duport, few tested patients had borderline IQ (i.e., 0 of 14,6 2 of 39,1 and 6 of 20 2), but further details were not provided. Cognitive testing and academic achievement of patients with DNETs have also not been well documented in other studies.9-11 In their series, Nolan et al. reported that 5 of 26 patients (19%) had a history of developmental delay or learning difficulty,12 but the details of tests used to evaluate these 5 patients were not provided.
Comprehensive neurocognitive evaluation is not routinely reported in studies on children with DNET and, when performed, includes only a small subset of the cohort study or data that is combined with that of adults. Minikin et al. performed neurocognitive evaluation of 6 of 24 (25%) patients: 5 with temporal tumors and 1 with a nontemporal tumor.13 Two patients with preoperative cognitive impairment remained impaired postoperatively. Hennessy et al. performed neurocognitive evaluation of children and adults with different temporal lobe lesions (n = 282) who had persistent seizures (n = 56, 20%) after temporal resection.14 In their series, 10 of 77 patients with DNET (13%) had persistent seizures and additional damage in the form of behavioral and/or cognitive disorders, GTC seizures, and spread abnormalities on electroencephalogram was evident in 6 of the 10 (60%) patients. No data were provided either on the 67 patients with controlled seizures or on the age of patients or details of neurocognitive tests used. However, Raymond et al. reported a detailed neurocognitive evaluation of 10 of 16 adults and children with DNET.15 Memory was affected in 8 of 10 (80%) patients and the mean verbal and performance IQ scores were 94.6% and 105%, respectively. There were 3 patients with developmental delays, of whom only 1 showed some improvement in the postsurgical developmental follow-up.
Ours is the first study in children with DNET in which comprehensive neurocognitive testing was performed in the majority of patients, and this may be the reason why our results of neurocognitive testing are different from those of previous studies. However, ours is a retrospective study on a small group of patients, and testing methods varied with patients' age at the time as well as the year of testing, which dictated the test version available. Moreover, factors such as anti-seizure medications (AEDs), family environment, preexisting conditions, or timing of evaluations may have affected the results.
Four patients in the present cohort were taking carbamazepine and 1 patient was taking phenytoin at the time of evaluation. Although previous literature has shown a relationship between AEDs and cognition,16 many studies do not account for the confounding effects of factors such as type of seizures, age of patient, length of exposure to medication, or the number of seizures. 17,18 In addition, results of large-scale studies have varied with the aspect of cognition being measured. For example, among children with seizures, children prescribed AEDs scored lower on processing speed, language, and verbal memory and learning than those not receiving medication. Children with a history of multiple seizures, even those not receiving medication, had lower scores on measures of attention and executive function than did children who had only 1 seizure.19
In the present study, patient 10 had high-achieving parents, which could be a family status–related variable contributing to why he performed better than the average child, especially in reading (Table 2). Patients 6 and 7 had preexisting conditions such as congenital glaucoma and behavioral issues, which may have contributed to their poor performance. Also, patient 9 underwent a neuropsychologic evaluation 1 month after surgery and had a borderline IQ score, suggesting that factors other than the tumor may influence cognition. Because of these reasons, it is impossible to determine the impact of DNET alone – isolated from other factors – on cognition in this study or previous studies. Prospective large-scale studies are needed that include serial neurocognitive evaluations conducted at diagnosis, before surgery, after surgery, and during regularly scheduled follow-up.
Protocol-driven assessment of cognitive function can decrease variability in testing procedures and improve comprehension of disease- and treatment-related impact on performance. Such assessments would also serve as an important conduit to identify patients in need of support services. For example, patient 6 exhibited improvement in performance 5 years after surgery, which the clinician attributed special educational support at school as well as continued resource assistance provided. Such services improved intellect level from the borderline to the average range and academic performance from the deficient to the borderline range. These gains may not have occurred without timely assessment, accurate diagnosis of function, and critical recommendations to the family and school.
Support services recommended by clinicians can vary depending on the area(s) of weakness exhibited in detailed testing. Methods used to intervene with otherwise healthy populations having similar deficits can provide a basis for formulating these recommendations. Intervention programs can include pharmacotherapy, cognitive therapy, experimental interventions designed relative to specific deficits, or commercially available programs. An accommodation approach could also be taken that improves the survivor's instructional environment at home or school.20
The rate of recurrence and progression of DNET in our study was higher than that reported previously. Particularly, patient 6 is only the second reported patient to have had 2 recurrences post-GTR.13 Initial studies on patients with DNET did not identify risk factors for tumor recurrence because they did not find recurrent disease. In 5 studies reporting a total of 238 patients (some of whom were reported in more than 1 study), patients receiving adequate postoperative follow-up had no recurrent disease.1-3,6,7 However, later studies have reported tumor recurrence with incomplete resection in 1 of 52 patients (2%)16 and 3 of 26 patients (12%)10,12 or post-GTR in 1 of 14 patients (7%)5 and 1 of 24 patients (4%).13 Additional case studies have also been reported.21,22 Ray et al.22 reported 5 patients with recurrent DNET (3 adults and 2 children), with tumor recurrences in 2 patients post-GTR. Ray et al. provide a comprehensive review of 9 recurrent cases from the literature in their study.
We found no identified risk factors for DNET recurrence in our literature search. Most studies on DNET have focused on identifying risk factors for seizure control but not tumor control.9-14, 23 Our group of 4 patients who experienced recurrence and progression was too small to pinpoint histopathologic, radiologic, or clinical factors for tumor recurrence.
The time to progression or recurrence in our series ranged from 2 to 12 years (median 2.5 years). Ray et al reports a median time to recurrence of 6.8 years (range 1-11 years) on 5 cases.22; therefore, on the basis of these studies, Based on this and our data we recommend annual surveillance for patients with DNET. In patients who experience worsening seizures, imaging should be done sooner. The wide range in time to diagnosis generally reflects the lack of standard recommendations for the best time for surgical intervention. Our cohort had a lower variance in median time from seizure than that reported in other studies (onset to surgery, 3.5 years; range, 0.5- 24 years). In guidelines developed by O'Brien et al. for the diagnosis and management of DNET, 2 years is recommended as the standard time to medically control seizures.24 In addition, they accept observation only in patients with stable seizures.
Our work disputes the common observations with respect to risk of recurrence and neurocognitive testing in children with DNET . Although our study is limited by a small patient population and its retrospective nature, the results support the need for a registry that will facilitate prospective studies in patients with DNET.
Contributor Information
Ibrahim Qaddoumi, Department of Oncology, Division of Neuro-Oncology, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105.
David W. Ellison, Department of Pathology, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105.
E. Brannon Morris, Department of Oncology, Division of Neuro-Oncology, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105; Department of Oncology, Division of Neurology, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105.
Alberto Broniscer, Department of Oncology, Division of Neuro-Oncology, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105.
Frederick Boop, Department of Surgery, Division of Neurosurgery, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105.
Thomas Merchant, Department of Radiological Sciences, Division of Radiation Oncology, 262 Danny Thomas Place, Memphis, TN 38105.
Shawna L. Palmer, Department of Behavioral Medicine, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105.
Amar Gajjar, Department of Oncology, Division of Neuro-Oncology, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105.
References
- 1.Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, Laws ER, Jr, Vedrenne C. Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizures. Report of thirty-nine cases. Neurosurgery. 1988;23:545–556. doi: 10.1227/00006123-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Daumas-Duport C, Varlet P, Bacha S, Beuvon F, Cervera-Pierot P, Chodkiewicz JP. Dysembryoplastic neuroepithelial tumors: nonspecific histological forms -- a study of 40 cases. J Neurooncol. 1999;41:267–280. doi: 10.1023/a:1006193018140. [DOI] [PubMed] [Google Scholar]
- 3.Stanescu CR, Varlet P, Beuvon F, et al. Dysembryoplastic neuroepithelial tumors: CT, MR findings and imaging follow-up: a study of 53 cases. J Neuroradiol. 2001;28:230–240. [PubMed] [Google Scholar]
- 4.Koeller KK, Dillon WP. Dysembryoplastic neuroepithelial tumors: MR appearance. AJNR Am J Neuroradiol. 1992;13:1319–1325. [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez C, Girard N, Paz PA, Bouvier-Labit C, Lena G, Figarella-Branger D. The usefulness of MR imaging in the diagnosis of dysembryoplastic neuroepithelial tumor in children: a study of 14 cases. AJNR Am J Neuroradiol. 2003;24:829–834. [PMC free article] [PubMed] [Google Scholar]
- 6.Daumas-Duport C. Dysembryoplastic neuroepithelial tumours. Brain Pathol. 1993;3:283–295. doi: 10.1111/j.1750-3639.1993.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 7.Honavar M, Janota I, Polkey CE. Histological heterogeneity of dysembryoplastic neuroepithelial tumour: identification and differential diagnosis in a series of 74 cases. Histopathology. 1999;34:342–356. doi: 10.1046/j.1365-2559.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 8.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan CH, Bittar RG, Davis GA, Kalnins RM, Fabinyi GC. Long-term seizure outcome following surgery for dysembryoplastic neuroepithelial tumor. J Neurosurg. 2006;104:62–69. doi: 10.3171/jns.2006.104.1.62. [DOI] [PubMed] [Google Scholar]
- 10.Sakuta R, Otsubo H, Nolan MA, et al. Recurrent intractable seizures in children with cortical dysplasia adjacent to dysembryoplastic neuroepithelial tumor. J Child Neurol. 2005;20:377–384. doi: 10.1177/08830738050200041801. [DOI] [PubMed] [Google Scholar]
- 11.Sandberg DI, Ragheb J, Dunoyer C, Bhatia S, Olavarria G, Morrison G. Surgical outcomes and seizure control rates after resection of dysembryoplastic neuroepithelial tumors. Neurosurg Focus. 2005;18:E5. [PubMed] [Google Scholar]
- 12.Nolan MA, Sakuta R, Chuang N, et al. Dysembryoplastic neuroepithelial tumors in childhood: long-term outcome and prognostic features. Neurology. 2004;62:2270–2276. doi: 10.1212/01.wnl.0000130495.69512.6f. [DOI] [PubMed] [Google Scholar]
- 13.Minkin K, Klein O, Mancini J, Lena G. Surgical strategies and seizure control in pediatric patients with dysembryoplastic neuroepithelial tumors: a single-institution experience. J Neurosurg Pediatrics. 2008;1:206–210. doi: 10.3171/PED/2008/1/3/206. [DOI] [PubMed] [Google Scholar]
- 14.Hennessy MJ, Elwes RD, Binnie CD, Polkey CE. Failed surgery for epilepsy. A study of persistence and recurrence of seizures following temporal resection. Brain. 2000;123(Pt 12):2445–2466. doi: 10.1093/brain/123.12.2445. [DOI] [PubMed] [Google Scholar]
- 15.Raymond AA, Halpin SF, Alsanjari N, et al. Dysembryoplastic neuroepithelial tumor. Features in 16 patients. Brain. 1994;117:461–475. doi: 10.1093/brain/117.3.461. [DOI] [PubMed] [Google Scholar]
- 16.Park SP, Kwon SH. Cognitive effects of antiepileptic drugs. J Clin Neurol. 2008;4:99–106. doi: 10.3988/jcn.2008.4.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–72. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- 18.Mandelbaum DE, Burack GD, Bhise VV. Impact of antiepileptic drugs on cognition, behavior and motor skills in children with new-onset, idiopathic epilepsy. Epilepsy and Behavior. 2009;16:341–4. doi: 10.1016/j.yebeh.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Fasteneau PS, Johnson CS, Perkins SM, et al. Neuropsychological status at seizure onset in children. Neurology. 2009;73:526–34. doi: 10.1212/WNL.0b013e3181b23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer SL, Leigh L. Survivors of pediatric posterior fossa tumors: cognitive outcome, intervention, and risk-based care. Eur J Oncol Nurs. 2009;13:171–8. doi: 10.1016/j.ejon.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher CO, White JB, Scheithauer BW, Raffel C. Recurrence of dysembryoplastic neuroepithelial tumor following resection. Pediatr Neurosurg. 2008;44:333–336. doi: 10.1159/000138372. [DOI] [PubMed] [Google Scholar]
- 22.Ray WZ, Blackburn SL, Casavilca-Zambrano S, et al. Clinicopathologic features of recurrent dysembryoplastic neuroepithelial tumor and rare malignant transformation: a report of 5 cases and review of the literature. J Neurooncol. 2009;94:283–292. doi: 10.1007/s11060-009-9849-9. [DOI] [PubMed] [Google Scholar]
- 23.Hennessy MJ, Elwes RD, Honavar M, Rabe-Hesketh S, Binnie CD, Polkey CE. Predictors of outcome and pathological considerations in the surgical treatment of intractable epilepsy associated with temporal lobe lesions. J Neurol Neurosurg Psychiatry. 2001;70:450–458. doi: 10.1136/jnnp.70.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien DF, Farrell M, Delanty N, et al. The Children's Cancer and Leukaemia Group guidelines for the diagnosis and management of dysembryoplastic neuroepithelial tumours. Br J Neurosurg. 2007;21:539–549. doi: 10.1080/02688690701594817. [DOI] [PubMed] [Google Scholar]