Abstract
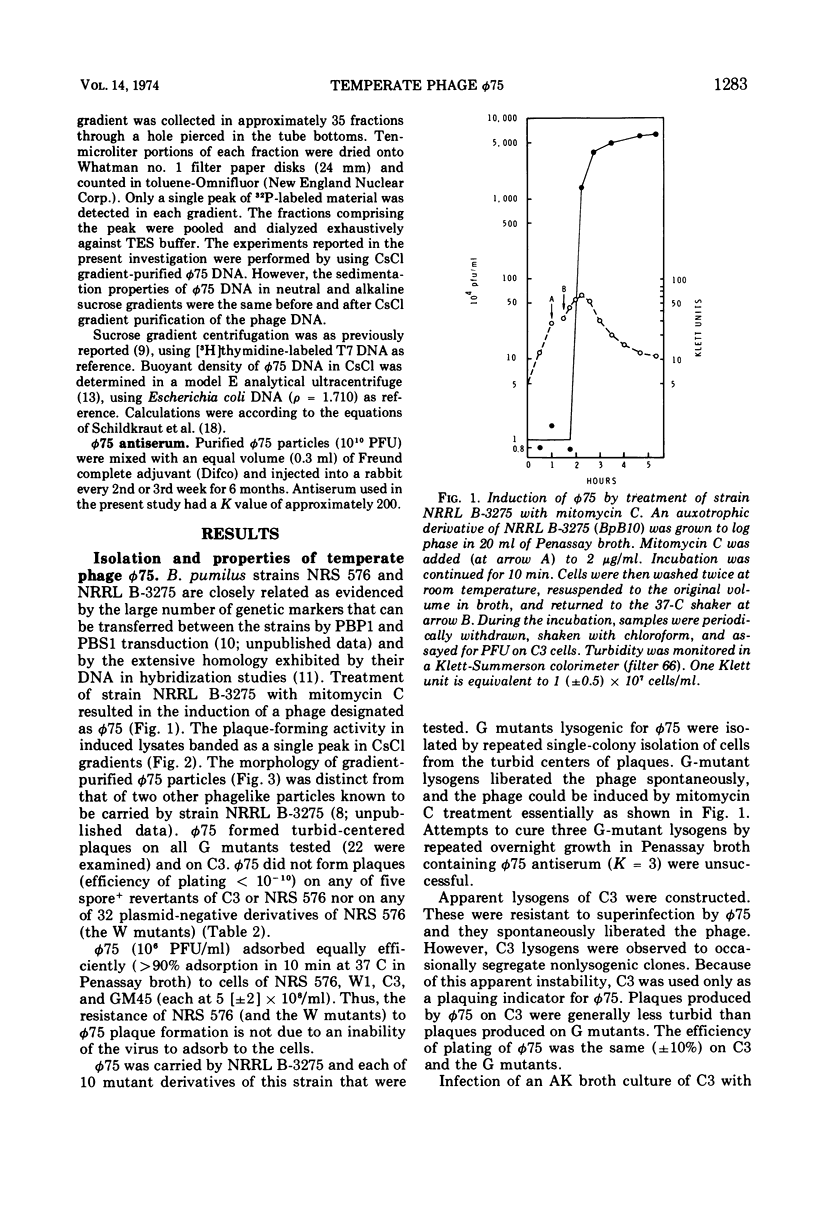
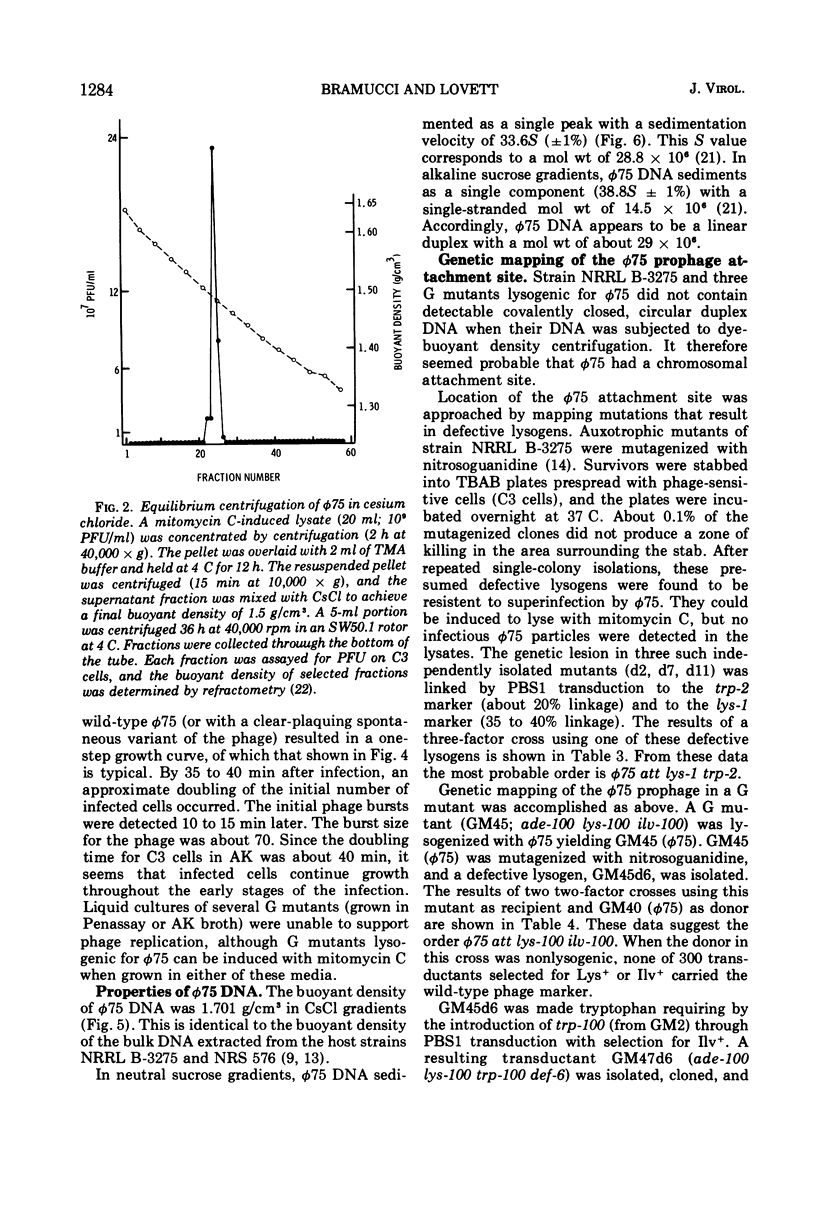
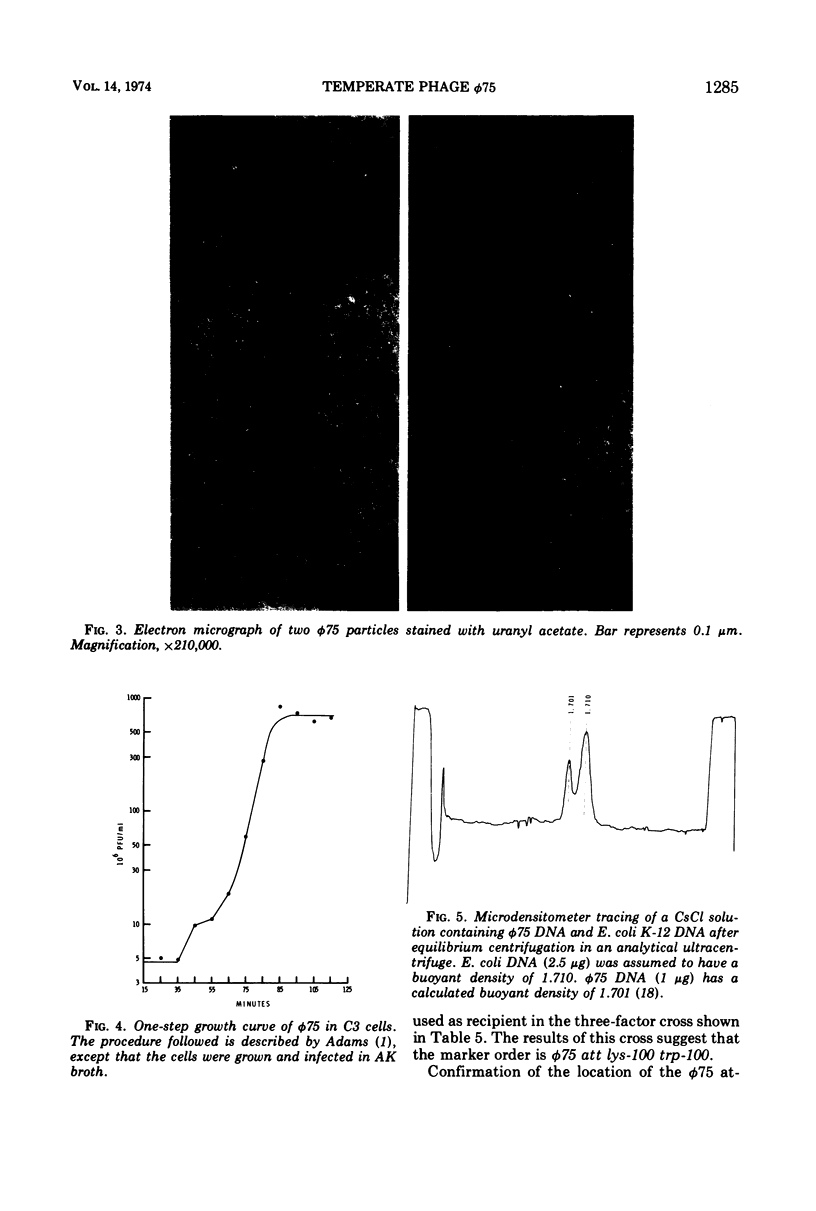
Bacillus pumilus strain NRRL B-3275 is lysogenic for an inducible, nondefective temperate bacteriophage φ75. φ75 infects and lysogenizes several asporogenic mutants of B. pumilus strain NRS 576 but does not productively infect the spore+ parent. φ75 DNA is a linear duplex with a mol wt of about 29 × 106 and a buoyant density of 1.701 g/cm3. The location of the φ75 prophage attachment site on the chromosome of both host strains is adjacent to a lysine marker. The apparent order is φ75 att lys trp.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Mathews J. L. Chromosomal location of pleiotropic negative sporulation mutations in Bacillus subtilis. Genetics. 1973 Feb;73(2):215–228. doi: 10.1093/genetics/73.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Marmur J. Characterization of inducible bacteriophages in Bacillus licheniformis. J Virol. 1970 Feb;5(2):237–246. doi: 10.1128/jvi.5.2.237-246.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Meinke W., Hathaway G., Spizizen J. Studies on Bacillus subtilis bacteriophage phi 15. Virology. 1973 Nov;56(1):110–122. doi: 10.1016/0042-6822(73)90291-2. [DOI] [PubMed] [Google Scholar]
- Ito J., Spizizen J. Abortive infection of sporulating Bacillus subtilis 168 by phi 2 bacteriophage. J Virol. 1971 Apr;7(4):515–523. doi: 10.1128/jvi.7.4.515-523.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Bramucci D., Bramucci M. G., Burdick B. D. Some properties of the PBP1 transduction system in Bacillus pumilus. J Virol. 1974 Jan;13(1):81–84. doi: 10.1128/jvi.13.1.81-84.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Bramucci M. G. Biochemical studies of two Bacillus pumilus plasmids. J Bacteriol. 1974 Oct;120(1):488–494. doi: 10.1128/jb.120.1.488-494.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S. PBPI: a flagella specific bacteriophage mediating transduction in Bacillus pumilus. Virology. 1972 Mar;47(3):743–752. doi: 10.1016/0042-6822(72)90564-8. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Pizer L. I., Isom H. C. Enzymatic defects in three genetic classes of serine-requiring mutants of Bacillus pumilus. J Bacteriol. 1973 Nov;116(2):1075–1078. doi: 10.1128/jb.116.2.1075-1078.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S. Plasmid in Bacillus pumilus and the enhanced sporulation of plasmid-negative variants. J Bacteriol. 1973 Jul;115(1):291–298. doi: 10.1128/jb.115.1.291-298.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S. Spontaneous auxotrophic and pigmented mutants occurring at high frequency in Bacillus pumilus NRRL B-3275. J Bacteriol. 1972 Nov;112(2):977–985. doi: 10.1128/jb.112.2.977-985.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Genetic analysis in Bacillus pumilus by PBSI-mediated transduction. J Bacteriol. 1970 Feb;101(2):603–608. doi: 10.1128/jb.101.2.603-608.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Identification of Bacillus subtilis NRRL B-3275 as a strain of Bacillus pumilus. J Bacteriol. 1969 Nov;100(2):658–661. doi: 10.1128/jb.100.2.658-661.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Linkage groups in Bacillus pumilus determined by bacteriophage PBS1-mediated transduction. J Bacteriol. 1971 May;106(2):697–699. doi: 10.1128/jb.106.2.697-699.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SEAMAN E., TARMY E., MARMUR J. INDUCIBLE PHAGES OF BACILLUS SUBTILIS. Biochemistry. 1964 May;3:607–613. doi: 10.1021/bi00893a001. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]