Abstract
Epigenetic silencing of the tumor suppressor gene p15Ink4b (CDKN2B) is a frequent event in blood disorders like acute myeloid leukemia and myelodysplastic syndromes. The molecular function of p15Ink4b in hematopoietic differentiation still remains to be elucidated. Our previous study demonstrated that loss of p15Ink4b in mice results in skewing of the differentiation pattern of the common myeloid progenitor towards the myeloid lineage. Here, we investigated a function of p15Ink4b tumor suppressor gene in driving erythroid lineage commitment in hematopoietic progenitors. It was found that p15Ink4b is expressed more highly in committed megakaryocyte–erythroid progenitors than granulocyte–macrophage progenitors. More importantly, mice lacking p15Ink4b have lower numbers of primitive red cell progenitors and a severely impaired response to 5-fluorouracil- and phenylhydrazine-induced hematopoietic stress. Introduction of p15Ink4b into multipotential progenitors produced changes at the molecular level, including activation of mitogen-activated protein kinase\extracellular signal-regulated kinase (MEK/ERK) signaling, increase GATA-1, erythropoietin receptor (EpoR) and decrease Pu1, GATA-2 expression. These changes rendered cells more permissive to erythroid commitment and less permissive to myeloid commitment, as demonstrated by an increase in early burst-forming unit-erythroid formation with concomitant decrease in myeloid colonies. Our results indicate that p15Ink4b functions in hematopoiesis, by maintaining proper lineage commitment of progenitors and assisting in rapid red blood cells replenishment following stress.
Keywords: p15Ink4b, hematopoiesis, stem cell, cell fate, differentiation, erythropoiesis
Introduction
The myelodysplastic syndromes (MDS) are conditions characterized by ineffective production of white blood cells, red blood cells (RBCs) and platelets, and often progress to acute myeloid leukemia (AML). In the United States alone, approximately 20 000 people are diagnosed yearly with MDS, making it the most common form of hematological malignancy.1 Anemia is the most common symptom of MDS and is directly linked to poor quality of life. Lack of understanding of the molecular basis for these diseases hampers development of effective therapeutics.
A striking 60–80% of human leukemia and MDS cases have silenced expression of the CDKN2B (p15INK4b) gene (human chromosome 9p21 and mouse chromosome 4) that encodes the tumor suppressor p15INK4B.2, 3, 4 Lack of p15INK4B expression in MDS patients is positively associated with leukemic transformation and poor prognosis.5 In some studies, p15INK4B was also found to be a good prognostic marker for monitoring the response to treatment with DNA methylation inhibitors.6, 7, 8 Despite the compelling evidence supporting the important role of p15INK4B in the development and progression of MDS and leukemia, its function in normal blood cell formation is just beginning to emerge.
Previous examination of bone marrow from both embryonic and conditional myeloid-specific p15Ink4b knockout (Ink4bKO) models revealed skewing of blood cell formation towards granulocyte–macrophage progenitors (GMPs) at the expense of megakaryocyte–erythroid progenitors (MEPs).9, 10 Interestingly, loss of p15Ink4b does not alter cell proliferation, self-renewal or apoptosis in blood progenitors, implicating a specific role in cellular differentiation. Based upon these studies and its implied role in human blood diseases, we hypothesized that p15Ink4b has a role in commitment of blood progenitors to the erythroid lineage.
Methods
Animals
The Ink4bKO mice used in this study were previously described and were maintained on an 129/Sv background.11 Mice (8–12 weeks old) were used for the experiments, unless noted otherwise. All animals were housed at the NCI-SAIC-Fredrick facility. 5-Fluorouracil (5-FU) was injected intraperitoneally at a dose of 150 mg/kg and phenylhydrazine (PHZ) at 50 or 100 mg/kg. Retinoblastoma-floxed (Rbfl) mice were described previously and maintained on an FVB/129 background.12 Rbfl/fl animals were crossed with Ink4bKO animals to generate mice with an Ink4bKORbfl/fl genotype. Experiments were carried out according to the protocols approved by The Institutional Animal Care Committee at The National Cancer Institute, NIH.
Blood collection and analysis
Blood samples were collected for complete blood counts analysis using mandibular bleed. Samples were analyzed using a CDC Hemavet blood counter at the Pathology/Histology Laboratory, LASP (Frederick, MD, USA). For differential analysis, blood smears were stained with Diff-Quick (Siemens HealthCare Diagnostics, Tarrytown, NY, USA) and scored using an Olympus BH2 light microscope.
Tissue culture
The mouse hematopoietic progenitor cell line, EML, was maintained in Iscove's modified Dulbecco's medium supplemented with 20% heat-inactivated horse serum, 15% BHK/MKL conditioned medium (source of stem cell factor (SCF)) and penicillin–streptomycin (P/S) (Gibco-Invitrogen, Grand Island, NY, USA).13 A mouse myelomonocytic leukemia cell line, M1, was cultured in RPMI medium supplemented with 10% heat-inactivated horse serum, P/S and used as a positive control for p15Ink4b mRNA and protein detection.14 The HEK 293T-derived Lenti-X293T cell line (Clontech, Mountain View, CA, USA) was maintained in Dulbecco's modified Eagle's medium with high glucose medium supplemented with 10% heat-inactivated, Tet system-approved, fetal bovine serum (FBS), 4 mℳℒ-glutamine, 3.7 g/l sodium bicarbonate and 1 mℳ sodium pyruvate. Cells of passages 8–24 were used for high-titer virus production. The 293GP cells were maintained in Dulbecco's modified Eagle's medium with high glucose medium, supplemented with 10% FBS and P/S. A clone of NIH3T3 cells of passage number 7 were obtained from William Vass, and cultured in Dulbecco's modified Eagle's medium with 10% FBS and P/S. S17 stromal cells were obtained from Dr Giovanna Tosato and maintained in α-minimum essential medium supplemented with 5–10% FBS. Mouse erythroleukemia cells were obtained from Dr Sandra Ruscetti and were maintained as described previously.15, 16, 17
Hematopoietic colony assays
To quantify lineage-restricted and multipotential progenitors, MethoCult methylcellulose-based mediums were used (StemCell Technologies, Vancouver, BC, Canada). M3534 medium supports the growth of mouse colony-forming unit-granulocyte/macrophage (CFU-GM) colonies only, as it does not contain recombinant human Epo. M3436 medium is a serum-free medium supplemented with cytokines and recombinant human Epo and has been formulated to support optimal growth of early and late burst-forming unit-erythroid (BFU-E) only. Assays were carried out as recommended by the manufacturer. The following number of cells was plated per 35 mm pretested culture dish (StemCell Technologies): for M3436, 3000 EML cells or 37 500 whole bone marrow cells, or 10 000 lineage–negative (Lin−) cells per dish, or in vitro-differentiated progenitors; and for M3534, 3750 whole bone marrow cells or 1000 Lin− cells per dish, or in vitro-differentiated progenitors. MethoCult cultures were incubated at 37 °C, in 5% CO2 and 95% humidity for 10–14 days before scoring. Colonies were enumerated under a Leitz Fluovert inverted microscope and photographed at the NIH Visual Arts and Printing Services.
p15Ink4b-inducible expression system
The Lenti-X ProteoTunerGreen System (pLVX-PTuner-Green) (Clontech) allows for rapid, inducible and reversible control of protein levels in cells.18 This is achieved by expression of a destabilizing domain that is constitutively degraded in the mammalian cells. p15Ink4b cDNA was cloned into this vector downstream of the destabilizing domain using EcoRI and NotI sites. Positive clones were confirmed by restriction enzyme digestion and sequencing. Virus was produced as described below and ZSGreen1 protein was used as a marker for the selection of infected cells. Levels of p15Ink4b were regulated by the addition of a compound called Shield (SH). This synthetic membrane-permeable ligand bound the destabilizing domain of the fusion protein (DD-p15Ink4b), resulting in rapid and dose-dependent stabilization of p15Ink4b protein levels. Removal of SH by washing the cells resulted in a quick reduction of p15Ink4b protein to background levels. Cloning of the green fluorescent protein (GFP) upstream of the internal ribosome entry site element using EcoRI and NotI sites solved the problem of low fluorescence intensity of the empty pLVX-PTuner-Green vector in target cells and was used as a control vector.
Virus production and concentration
Murine stem cell virus-internal ribosome entry site-GFP-based constructs (MIG) were packaged in 293GP cells by co-transfection of 12 μg each of the plasmid of interest and the vesicular stomatitis virus-G envelop protein containing plasmid using Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA) in serum-free medium. Transfection medium was replaced with fresh medium 4 h later. Virus containing supernatant was collected 48–72 h following the transfection, filtered through a 0.45-μm CA filter (Corning, Manassas, VA, USA) and concentrated at 22 000 r.p.m. for 2 h using a Beckman Coulter Optima L-90 ultracentrifuge equipped with an SW 28 rotor. The viral stock was titered by infecting NIH3T3 cells in the presence of 4 μg/ml polybrene and the resulting GFP-positive cells were enumerated using flow cytometry. Lenti-X-pTuner constructs were packaged using the Lenti-X HTX Packaging System (Clontech) according to the manufacturer's protocol. Briefly, 5.0 × 106 Lenti-X293T cells were plated per 10 cm plate the day before transfection in 10 ml of growth medium. The following day, cells, at 80–90% confluence, were co-transfected with the Lenti-X-pTuner vector and HTX Packaging Mix and incubated for 4 h. Subsequently, the medium was replaced with fresh complete growth medium and incubated for an additional 48 h before harvesting. The viral stock was filtered through a 0.45 μm CA filter (Corning) and concentrated 100 × using a Lenti-X Concentrator (Clontech) according to the manufacturer's protocol. Viral stocks were stored at −80 °C. For infecting primary cells and the EML cell line, viral stocks with a titer 108 infectious unit/ml or higher were used.
Introduction of p15Ink4b into hematopoietic progenitors and in vitro differentiation
Bone marrow cells extracted from the femur and tibia of 8- to 12-week-old animals were filtered through a 70-μm nylon filter (BD Biosciences, San Jose, CA, USA) and enriched for hematopoietic progenitors using an EasySep Mouse Hematopoietic Cell Enrichment Kit. Cells were infected overnight in plates coated with 5 μg/cm2 RetroNectin (Takara-Bio, Mountain View, CA, USA) and immobilized virus. Virus-bound plates were prepared using the centrifugation method. Briefly, 6- or 12-well untreated plates (BD Biosciences) were coated with RetroNectin overnight and blocked with phosphate-buffered saline containing 2% bovine serum albumin. Then, a highly concentrated viral stock of 0.5–1.5 ml was added per well and centrifuged for 2 h at 1500 g at 32 °C to facilitate attachment of virus particles onto RetroNectin. Following a wash, cells were introduced to the wells in Iscove's modified Dulbecco's medium containing 10% FBS, 20 ng/ml SCF (R&D Systems, Minneapolis, MN, USA), 20 ng/ml Flt-3L, 10 ng/ml interleukin-11 and 50 mℳ β-mercaptoethanol. Cells were briefly centrifuged at 200 g for 0.5–1 h at 32 °C to enhance infection efficiency. Following an overnight infection, cells were differentiated on irradiated S17 stromal cells for 60 h in RPMI medium containing 10% FBS, 20 ng/ml SCF and 100 nℳ SH before plating them in methylcellulose medium. Alternatively, cells were expanded in Iscove's modified Dulbecco's medium containing 10% FBS, 20 ng/ml SCF, 20 ng/ml Flt-3L, 10 ng/ml interleukin-11, 20 ng/ml interleukin-3 and 20 ng/ml thrombopoietin for 3–5 days to assess infection efficiency.19 The transduction efficiency was assessed by fluorescence-activated cell sorting analysis of GFP-positive cells and typically 30–60% positive cells were found. In the experiments utilizing bone marrow from Ink4bKORbfl/fl mice, Lin− cells were infected with two viral constructs (MIG- and Lenti-X-pTuner-based constructs) simultaneously using a mixture of highly concentrated viral supernatant (>108 infectious unit/ml each).
Introduction of p15Ink4b into the EML cell line and in vitro differentiation
EML cells were infected with the lentiviral vector pLVX-pTuner-p15Ink4b-Green, as described above for primary hematopoietic progenitors. Cells positive for ZSGreen were sorted and expanded as a cell line designated EMLp15Tuner. Expression of p15Ink4b was induced by the addition of SH (5–500 nℳ) into the culture medium. Following the induction of p15Ink4b, cells were counted and either plated directly into MethoCult or differentiated in liquid culture into myeloid and erythroid lineages as described previously.13
Hematopoietic progenitor sorting
Bone marrow cells extracted from femur, tibia and hip of 8- to 12-week-old animals were enriched for hematopoietic progenitors using the EasySep Mouse Hematopoietic Cell Enrichment Kit (StemCell Technologies). Cells were stained with the following dye-conjugated anti-mouse antibodies: APC-eFluor 780-c-kit (2B8 clone; eBiosciences, San Diego, CA, USA), APC-Sca1 (D7 clone; eBiosciences), PE-Cy7-interleukin-7Rα (A7R34 clone; eBiosciences), PerCP-eFluor 710Flt-3 (A2F10 clone; eBiosciences), FITC-CD34 or eFluor 450-CD34 (RAM34clone; eBiosciences) and PE-CD16/CD32 (2.4G2 clone; BD Biosciences Pharmingen, San Jose, CA, USA). Cells were sorted according to the previously described methods for isolation of common myeloid progenitor (CMP), MEP, GMP, LT-HSC, ST-HSC, MPP using BD FACSAria and BD FACSVantage SE with DiVa option (BD Biosciences).20
Additional antibodies used in this study include: FITC-Ter119 (Ly-76 clone; eBiosciences), PE-Ter119 (Ly-76 clone; BD Biosciences Pharmingen), PE-CD71 (C2 clone; BD Biosciences Pharmingen), FITC-Mac1 (M1/70 clone; BD Biosciences Pharmingen), PE-Gr1 (RB6-8C5 clone; BD Biosciences Pharmingen) and PE-Sca1 (D7 clone; eBiosciences). Data were acquired using BD FACSCantoII or BD LSRII instruments. Data were analyzed using FlowJo (Tree Star Inc., Ashland, OR, USA).
Cell cycle analysis
Cell cycle analysis was performed according to a published protocol.21 The data were analyzed using the ModFit LT software (Verity Software House, Topsham, ME, USA).
Real-time reverse transcription-polymerase chain reaction
Total RNA was isolated from sorted progenitors using a miRCURY RNA Isolation Kit (Exiqon, Woburn, MA, USA) and RNA concentration and quality was assessed using an Agilent 2100 bioanalyzer and RNA Pico Chips (Agilent, Palo Alto, CA, USA). RNA used for this study had an RNA integrity number of 9.0–10. For the EML cells, total RNA was extracted using Trizol reagent (Invitrogen). Predesigned gene-specific primers, 18SrRNA internal control primers and MGB-FAM-labeled probes were purchased from Applied Biosystems (Carlsbad, CA, USA). cDNA was prepared using a High Capacity cDNA Reverse Transcription Kit and amplified using TaqMan Gene Expression Master Mix (Applied Biosystems). The ΔΔCt method was used to calculate relative fold changes in mRNA levels. Samples with a low concentration of RNA were preamplified using TaqMan PreAmp MasterMix according to the manufacturer's protocol (Applied Biosystems).
Western blot analysis
Western blot analysis was performed as described previously with the following modifications.22 The gel was transferred onto a 0.25-μm polyvinylidene difluoride membrane for the detection of small proteins such as p15Ink4b. A high-sensitivity substrate was utilized for the detection of the signal (Millipore, Billerica, MA, USA). The following antibodies were used: goat polyclonal anti-p15 (M20), rat monoclonal anti-GATA-1 (N6), mouse monoclonal anti-GATA-2 (CG2-96), rabbit polyclonal anti-Pu.1 (T21), rabbit polyclonal anti-EpoR (M20), goat anti-rat IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-human Rb protein (BD), rabbit monoclonal anti-actin (EP184E) (Millipore), rabbit anti-goat IgG-HRP, goat anti-mouse IgG-HRP and goat anti-rabbit IgG-HRP (KPL, Gaithersburg, MD, USA).
Statistical analysis
Statistical analysis was carried out using Microsoft Excel and GraphPad Prism software. The unpaired two-tailed Student's t-test, Mann–Whitney and log-rank tests were used to calculate P-values.
Results
Defect in early erythroid progenitor production in Ink4bKO animals
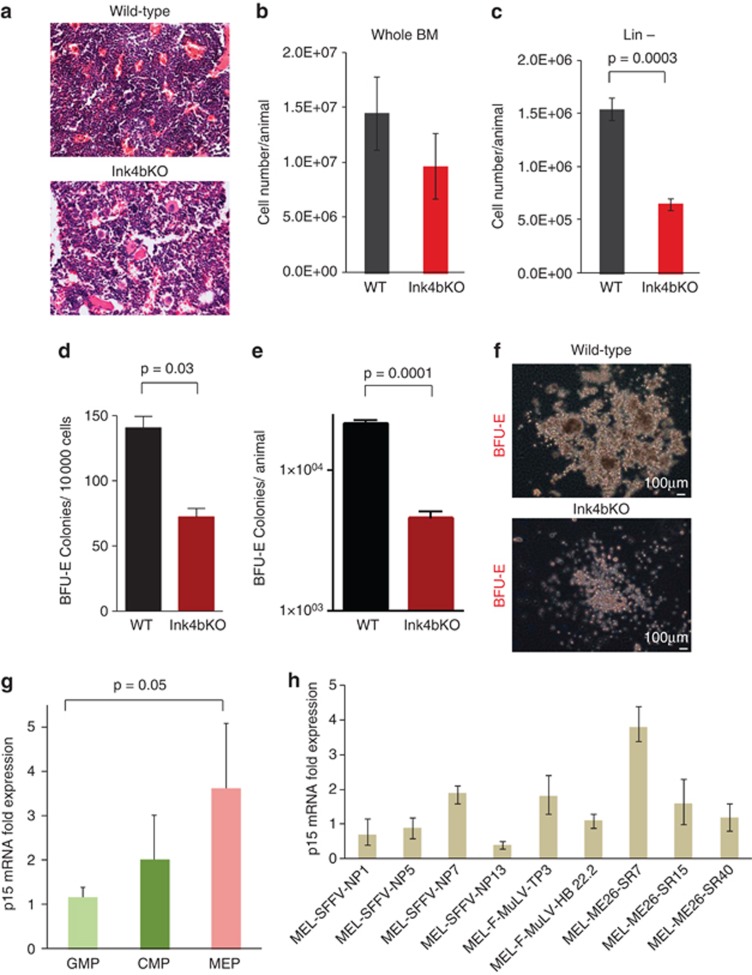
Bone marrow histopathology and analysis of peripheral blood of Ink4bKO animals showed no significant differences in the more mature stages of the erythroid and myeloid cells compared to that of wild-type animals (Figure 1a and Supplementary Figure S1). The whole bone marrow cellularity of Ink4bKO mice was slightly lower as compared with wild-type mice, and the number of Lin− cells was significantly decreased, confirming that the hematopoietic defect was limited to immature progenitor cells (Figure 1b and c). Our previous observation, showing a bias of CMPs of Ink4bKO mice towards the myeloid lineage, prompted us to investigate the function of p15Ink4b in erythroid fate decision of uncommitted progenitors.9, 23 The exact precursor cell for the erythroid progenitor still remains controversial. For this reason, in our studies, we focused on examination of the whole Lin− population instead of a subpopulation like CMP. To determine how the loss of p15Ink4b expression might affect the formation of committed erythroid progenitor cells, we employed a methylcellulose-based colony forming assay (MethoCult) that allows the detection of early RBC precursor cells termed BFU-E.24 Bone marrow of mice lacking p15Ink4b gave rise to significantly lower numbers of early BFU-E colonies compared with mice carrying a normal p15Ink4b locus (wild type) (Figure 1d and e). BFU-E colonies generated from bone marrow of Ink4bKO mice also showed changes in morphology and were notably smaller than those found in cultures initiated from wild-type marrow (Figure 1f).
Figure 1.
Function of p15Ink4b in erythroid lineage commitment. (a) Decalcified, paraffin-embedded, hematoxylin and eosin (H&E) sections of bone marrow. (b) Total number of bone marrow cells isolated from the femur and tibia of wild-type (WT) and Ink4bKO mice (n=3). (c) Total number of Lin− cells isolated from the femurs and tibias of WT and KO mice (n=3). (d) Number of BFU-E colonies obtained from plating 10 000 of Lin− bone marrow cells in M3436 methylcellulose medium (n=6). (e) Total number of BFU-E colonies contained in the femurs and tibias of WT and Ink4bKO mice (n=6). (f) Representative pictures of BFU-E colonies. (g) Expression of p15Ink4b mRNA in sorted hematopoietic progenitor populations of WT mice. 18SrRNA was used as an internal control and depicted as fold change in mRNA compared with GMPs (n=9). (h) Expression of p15Ink4b mRNA in a panel of mouse erythroleukemia (MEL) cell lines. Cell lines were established from mice inoculated with spleen focus-forming virus (SFFV), Friend murine leukemia virus (F-MuLV) or a murine myb-ets-containing virus (ME26). 18SrRNA was used as an internal control and depicted as fold change of mRNA compared with the NP1 cell line (n=3).
Expression of p15Ink4b during lineage commitment of hematopoietic progenitors
Next, we examined p15Ink4b expression in mouse primary bone marrow progenitors at various stages of myeloid and erythroid lineage commitment. To accomplish this, we used fluorescence-activated cell sorting to purify multilineage progenitor cells that are capable of forming both myeloid and erythroid cell types (CMPs), as well as those more committed to the erythroid (MEP) and myeloid lineages (GMP). Quantitative real-time polymerase chain reaction analysis of cDNA derived from these cells determined that MEPs expressed twofold higher levels of p15Ink4b mRNA compared with CMPs, and fourfold higher levels than GMPs (Figure 1g). Of further interest, the expression of p16Ink4a, a gene whose locus is physically linked to and is often concomitantly expressed with p15Ink4b, was not detected in any of the progenitor populations of wild-type mice (data not shown). However, low levels of p16Ink4a were detected in the progenitor populations of Ink4bKO mice (Supplementary Figure S2). Although these two genes function cooperatively in many tissues to inhibit the cell cycle through the binding of cyclin-dependent kinases (Cdks), our findings suggest a novel role for p15Ink4b in MEPs. The association of p15Ink4b expression with erythroid commitment was further supported by the identification of mRNA encoding p15Ink4b in several erythroleukemia cell lines that are blocked at early stages of RBC development (Figure 1h).
Response of Ink4bKO animals to 5-FU treatment
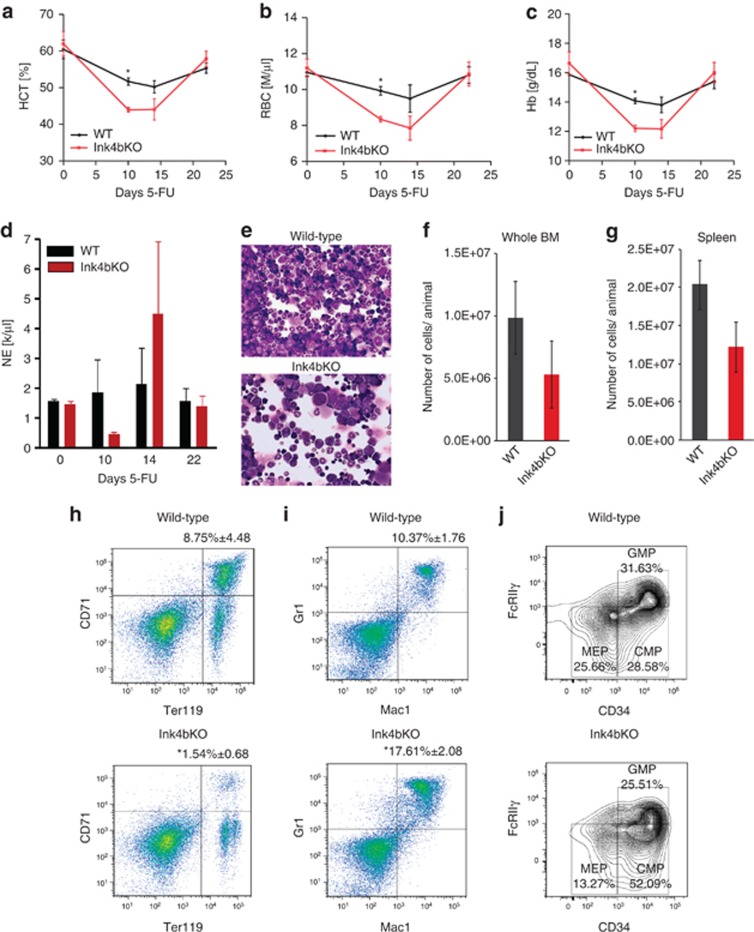
We believe that, evolutionarily, mice have developed strong compensation mechanisms that can mask defects in RBC and leukocyte development. However, alterations in development might be more easily observed under severe stress conditions. Therefore, we set out to investigate the ability of Ink4bKO animals to initiate erythropoiesis under conditions of severe anemic stress. For these experiments, knockout and wild-type mice were treated with two different stimuli, both inducing anemia, but acting through different cellular mechanisms: 5-FU and PHZ. Ink4bKO animals challenged with a moderate dose of 5-FU developed a more severe anemia than the wild-type mice, as evidenced by reduced levels of RBCs, hemoglobin and hematocrit in the peripheral blood (Figure 2a–c). The neutrophil counts were lower in Ink4bKO animals at day 10 postinjection (Figure 2d) and the bone marrow contained fewer mature cells (Figure 2e and f). Cellularity of spleens of Ink4bKO animals was also decreased (Figure 2g), all consistent with a slower recovery rate from the hematopoietic stress. Examination of the spleen and bone marrow of Ink4bKO mice 10 days after 5-FU treatment revealed a decreased frequency of early erythroid cells that were double positive for CD71 and Ter119 markers (Figure 2h). At the same time, there was an increased frequency of myeloid cells that were Mac1 and Gr1 positive (Figure 2i). Analysis of blood progenitor populations also showed reduced numbers of MEPs (Figure 2j) in the bone marrow of knockout animals. These data suggest that p15Ink4b facilitates RBC formation under conditions of severe anemic stress.
Figure 2.
Response of mice lacking p15Ink4b to hematopoietic stress induced by 5-FU. (a–d) Hematocrit (HCT), red blood cells (RBC), hemoglobin (Hb) and neutrophil (NE) counts in the peripheral blood of wild-type (WT) and Ink4bKO animals injected with 100 mg/kg 5-FU. *P=0.02 at 10 days and *P=0.03 at 14 days (n=6). (e) Representative picture of Diff-Quick-stained cytospins of whole bone marrow of WT and Ink4bKO animals 10 days after injection with 5-FU. (f) Total number of bone marrow cells isolated from the femurs and tibias of WT and Ink4bKO mice 10 days after injection with 5-FU (n=3). (g) Total number of cells isolated from spleens of WT and Ink4bKO mice 10 days after injection with 5-FU (n=3). (h) Frequency of early erythroid cells that are double positive for Ter119 and CD71 markers in the spleen of Ink4bKO animals 10 days after injection with 5-FU. *P=0.05 (n=3). (i) Frequency of myeloid cells that are double positive for Mac-1 and Gr-1 markers in the bone marrow of Ink4bKO animals 10 days after injection with 5-FU. *P=0.02 (n=3). (j) Frequency of MEPs, CMPs and GMPs present in the bone marrow of WT and Ink4bKO animals on day 14 after 5-FU injection. Bone marrows from three animals were pooled together and analyzed.
Response of Ink4bKO animals to PHZ treatment
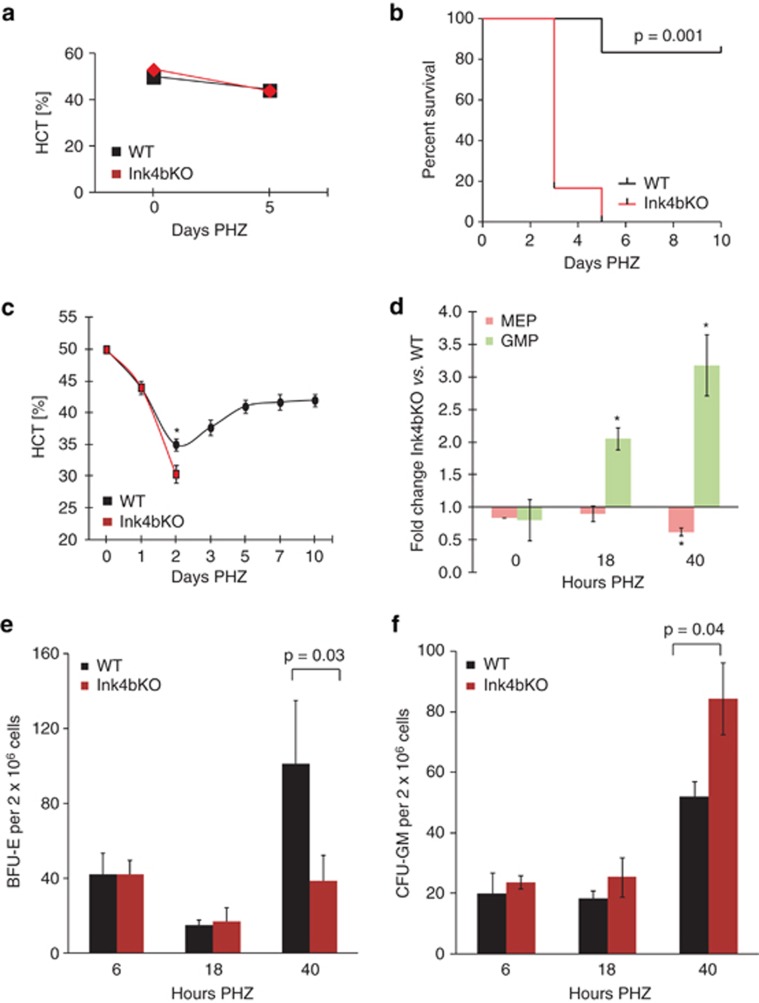
Treatment of animals with a low dose of PHZ (50 mg/kg) did not discriminate between Ink4bKO and wild-type animals (Figure 3a). However, exposure to higher dose of PHZ (100 mg/kg) was lethal to animals lacking p15Ink4b (Figure 3b). This was in direct contrast to wild-type mice, of which 80% survived PHZ treatment. The time of death for Ink4bKO animals was 3–5 days post treatment, a time point that correlated with the lowest levels of circulating RBCs in wild-type mice and was followed by a profound period of recovery in surviving animals (Figure 3c). As the most immediate response to the anemia caused by PHZ comes from the spleen,25 we compared the frequency of blood progenitor cells in the spleens of PHZ-treated wild-type and Ink4bKO mice by both flow cytometry and methylcellulose-based culture assays (Figure 3d–f). We observed that the animals lacking p15Ink4b showed no increase in MEP (Figure 3d) and BFU-E (Figure 3e) when treated with PHZ, whereas wild-type mice showed a continual increase in these cells over a 40-h period post treatment. Remarkably, the spleens of PHZ-treated knockout mice contained a greater number of both GMPs (Figure 3d) and CFU-GM (Figure 3f) compared with wild-type animals, indicating that the Ink4bKO animals in response to PHZ are not able to increase the number of MEPs and instead overproduce GMPs. In all, loss of p15Ink4b in mice impairs the balance of erythroid and myeloid progenitor cell formation, preventing sufficient erythropoiesis to allow recovery from anemia.
Figure 3.
Response of mice lacking p15Ink4b to hematopoietic stress induced by PHZ. (a) Hematocrit (HCT) levels following injection of 50 mg/kg PHZ on two consecutive days (n=10). (b) Survival curve of mice following single injection of 100 mg/kg PHZ (wild type (WT), n=6; Ink4bKO, n=7). (c) Hematocrit levels following injection of 100 mg/kg PHZ. *P=0.03 (n=3). (d) Relative decrease in the number of MEPs and increase in the number of GMPs in the spleen of Ink4bKO animals as compared with WT animals following PHZ injection. Comparison of Ink4bKO vs WT mice. *P=0.02 at 18 h and *P=0.03 at 40 h (n=4). (e and f) Decreased numbers of erythroid BFU-E colonies and increased numbers of myeloid CFU-GM colonies in the spleen of Ink4bKO animals as compared with WT animals following PHZ injection (n=3).
Restoration of Ink4bKO mice corrects the observed skewing in hematopoietic cell differentiation
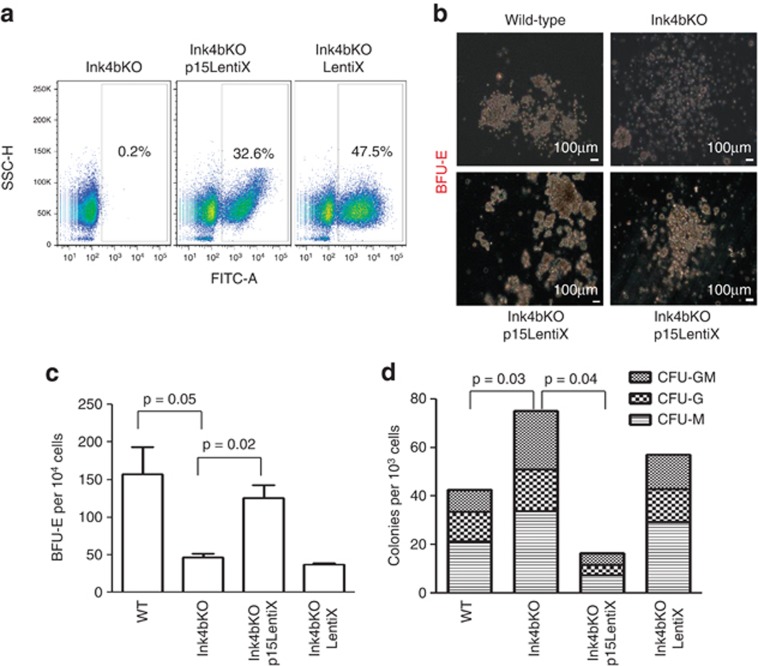
To determine whether these distinct changes in BFU-E-forming capacity were directly related to the loss of p15Ink4b expression, we used a lentivirus-based inducible protein expression system, ProteoTuner (pLVX-PTuner-Green), to restore p15Ink4b in bone marrow progenitors from knockout animals18 (Figure 4a). Using this system, we were able to efficiently induce expression of low levels of p15Ink4b by simply adding of an appropriate concentration of the inducer named SH. We chose this expression system because of the relatively low background as compared with other expression systems that we tested, namely a doxycycline-inducible system and murine stem cell virus-internal ribosome entry site-GFP expression vector. Expression of p15Ink4b in bone marrow derived from knockout mice restored the BFU-E colony morphology (Figure 4b) to resemble wild-type mice. In addition, it re-established the balance between the erythroid and myeloid progenitor compartments by increasing the number of BFU-E colonies (Figure 4c) and suppressing the formation of myeloid colonies (CFU-GM, CFU-G and CFU-M) (Figure 4d). Overall, this work demonstrates that p15Ink4b has a direct role in regulating the formation of early erythroid progenitor cells in normal bone marrow.
Figure 4.
Restoration of p15Ink4b expression in bone marrow cells of Ink4bKO mice restores erythoid/myeloid progenitor cell balance. (a) Infection efficiencies of Lin− bone marrow cells from Ink4bKO mice transduced with the pLVX-PTuner-Green vector containing murine p15Ink4b cDNA (p15LentiX) or empty vector (LentiX). (b) Representative pictures of BFU-E colonies obtained from plating p15Ink4b-transduced and in vitro-differentiated cells (S17 monolayer, SCF, SH) in M3436 methylcellulose medium. (c) Number of BFU-E colonies obtained by plating 10 000 of transduced and in vitro-differentiated cells in M3436 methylcellulose medium. (d) Number of myeloid colonies (CFU-GM, CFU-G and CFU-M) obtained by plating 1000 of the same cells as in (c) in M3534 medium. Following overnight infection, cells were differentiated for 60 h on S17 stromal cells in the presence of SH (n=9). Non-transduced wild-type (WT) and Ink4bKO cells were carried through the same experimental procedure as transduced cells for direct comparison within the experiment.
Induction of p15Ink4b increases the erythroid commitment of EML cells
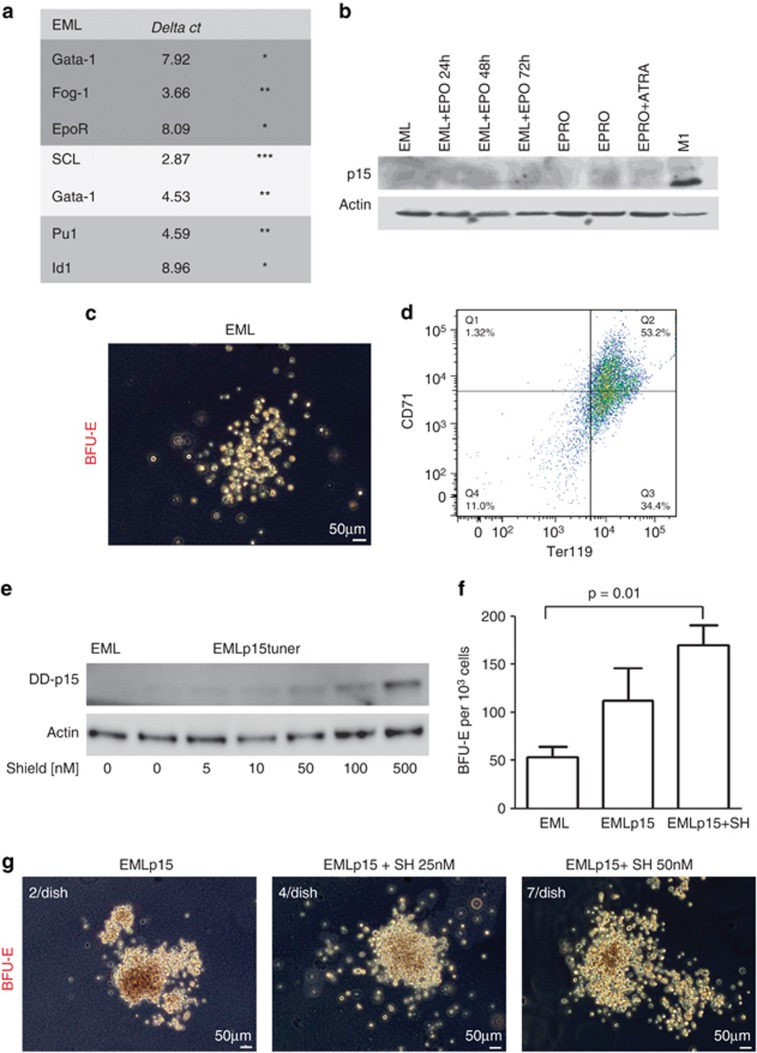
To gain molecular insight into the mechanisms through which p15Ink4b protein affects erythropoiesis, we explored the potential utility of the mouse multipotent blood progenitor cell line, EML. EML cells were developed by Tsai et al.13 from murine bone marrow cells infected with a dominant-negative form of a retinoic acid receptor and had been studied previously as a model for the differentiation of blood progenitors.26, 27 To provide further rationale for using EML cells in our study, we profiled mRNA expression of key regulators of hematopoietic differentiation. EML cells were found to express high levels of SCL, intermediate levels of GATA-2, Pu.1 and Fog-1, and low levels of GATA-1, EpoR and Id1, consistent with what has been reported previously for CMPs (Figure 5a).20 Using western blot analysis, we found no detectable expression of p15Ink4b protein in EML cells, even when cultured in the presence of the master erythroid cytokine, Epo. There was also no detectable expression along differentiation towards the myeloid lineage, suggesting loss of expression due to genetic or epigenetic changes (Figure 5b). Consistent with our data in purified mouse hematopoietic progenitors, EML cells were found to express low levels of p16Ink4a (10-fold lower than the levels detected in M1 cells that were used as a control). In accordance with an absence of p15Ink4b, we found that EML cells showed a limited capacity for erythroid progenitor formation as demonstrated by BFU-E formation in methylcellulose assays (Figure 5c and f). Despite the small size of colonies detected in this assay, they were confirmed to be BFU-E by the expression of Ter119 and CD71 markers (Figure 5d). We established a modified EML cell line based on the ProteoTuner p15Ink4b-inducible system, which we referred to as EMLp15Tuner. Using this system, we induced the expression of low levels of p15Ink4b protein that were physiologically relevant (Figure 5e). Induction of p15Ink4b in EML cells for just 24 h resulted in increased overall BFU-E number and an increased appearance of large colonies with a dense core, representing earlier (more primitive) erythroid progenitors. (Figure 5f and g). Interestingly, even extremely low levels of p15Ink4b that were constitutively produced in the absence of SH, due to leakage in the overexpression system, were able to induce an increase in numbers of colonies (Figure 5f) and alter morphology (Figure 5g), supporting the idea that low p15Ink4b protein levels are sufficient to produce a developmental change.
Figure 5.
Induction of p15Ink4b increases commitment of EML cells to the erythroid lineage. (a) Profiling of mRNA expression in EML cells of genes linked to erythroid (GATA-1, Fog-1, EpoR), HSC (SCL, GATA-2) or myeloid differentiation (Pu.1, Id1). Stars indicate the relative abundance of the transcript based on the ΔCt value. The Ct value inversely correlates with the gene expression. 18SrRNA was used as an internal control. (b) Lack of p15Ink4b expression in EML cells induced to differentiate towards the erythroid lineage (EML+Epo), promyelocytes (EPRO) or mature neutrophils (EPRO+ATRA). A lysate from the M1 cell line was used as a positive control. (c) Representative picture of a BFU-E colony obtained by plating 1000 EML cells in M3436 methylcellulose medium. (d) Fluorescence-activated cell sorting (FACS) analysis of the expression of Ter119 and CD71 antigens on BFU-E colonies isolated from cultured EML cells in M3436 medium for 18 days. (e) Induction of p15Ink4b in the EML cell line using the ProteoTuner system. EML cells were infected with the pLVX-PTuner-Green vector containing murine p15Ink4b cDNA cloned upstream of a destabilizing domain (DD, 12 kDa) that allowed induction of p15Ink4b expression at the protein level with minimal leakage via the addition of SH. ZSGreen-positive cells were sorted and expanded as a cell line (EMLp15Tuner, EMLp15). Expression of p15Ink4b was induced by the addition of SH to the culture medium at the indicated concentrations for 24 h. (f) Number of BFU-E colonies obtained by plating 1000 EML or EMLp15Tuner cells in M3436 methylcellulose medium pretreated with SH at a concentration of 100 nℳ for 24 h to induce the expression of p15Ink4b (n=3). (g) Representative pictures and number of large BFU-E colonies.
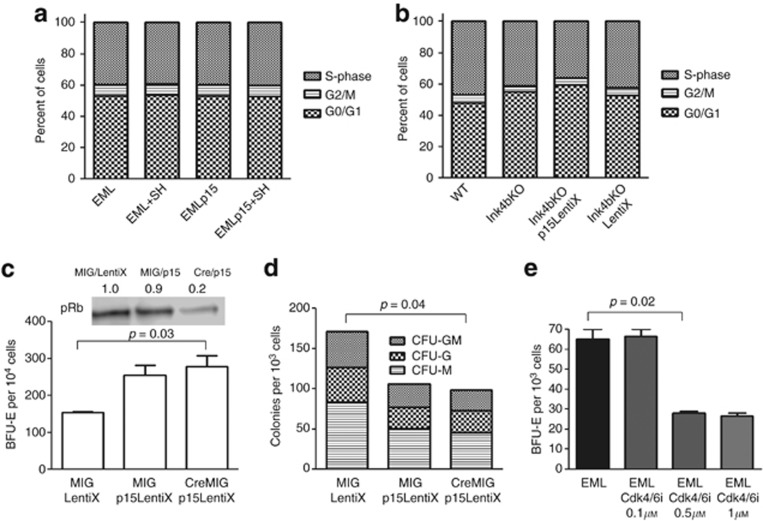
Function of p15Ink4b in erythroid differentiation is cell cycle independent
Interestingly, no significant cell cycle distribution changes were invoked by p15Ink4b expression either in bone marrow-derived blood progenitors or in the EMLp15Tuner cell line (Figure 6a and b). pRb has long been implicated in erythropoiesis.28, 29 To determine if pRb is required for p15Ink4b-mediated erythroid lineage commitment, we created Ink4bKO animals containing flRb alleles that could be efficiently removed by the expression of Cre recombinase in vitro (Figure 6c, upper panel). We then restored p15Ink4b expression in vitro in bone marrow progenitors of these mice using the ProteoTuner system and compared the frequency of erythroid and myeloid progenitors in the presence of Cre, to remove the Rb allele, or in the absence of Cre. As shown in Figure 6c and d, restoration of p15Ink4b expression in bone marrow progenitors returned the balance of myeloid and erythroid lineage commitment even in the absence of pRb. These results prompted us to test the effect of inhibition of Cdk4/6 on BFU-E colony formation in EML cells. Cells were pretreated with a specific pharmacological inhibitor of Cdk4/6 (Cdk4/6 IV; Calbiochem, San Diego, CA, USA) for 24 h before plating them in methylcellulose medium. Inhibition of Cdk4/6, unlike p15Ink4b expression, resulted in decreased numbers of BFU-E. Collectively, our data suggest that p15Ink4b when expressed at low levels does not affect Cdk4/6 and might have an additional cell-cycle-independent function (Figure 6e).
Figure 6.
Role of p15Ink4b in the regulation of hematopoietic progenitor fate might be independent of its canonical function in the cell cycle. (a) Cell cycle analysis of EML and EMLp15Tuner (EMLp15) cells treated for 48 h with SH (100 nℳ) to induce expression of p15Ink4b. A representative experiment is shown (n=2). (b) Cell cycle analysis of Lin− bone marrow-derived cells of wild-type (WT) or Ink4bKO mice following re-expression of p15Ink4b. Ink4bKO cells were infected overnight with either the p15Ink4b-expressing vector (p15LentiX) or empty vector (LentiX). Following overnight infection, cells were transferred onto a S17 stromal cell layer and differentiated for 60 h in the presence of SH (100 nℳ) to induce p15Ink4b expression. Subsequently, the cells were fixed in ethanol and stained with propidium iodide (PI). ModFit LT software was used to analyze the data. A representative experiment is shown (n=2). (c) Number of BFU-E colonies obtained by plating 10 000 Lin− Ink4bKORbfl/fl bone marrow cells transduced with either empty vectors, MIG or pLVX-PTuner-Green (LentiX), or p15Ink4b-expressing vector (p15LentiX), or Cre recombinase (CreMIG)-expressing vector in M3436 medium. Following overnight infection, cells were transferred onto an S17 stromal cell layer and differentiated for 60 h in the presence of SH (100 nℳ) to induce p15Ink4b expression (n=4). To assess the deletion efficiency of pRb, a fraction of cells following overnight infection was transferred into the expansion medium for 3–5 days before harvesting them for western blot analysis (upper panel). The blot was quantitated using ImageJ and normalized to actin. (d) Number of myeloid colonies (CFU-GM, CFU-G and CFU-M) obtained by plating 1000 of the same cells as in (c) in M3534 medium (n=4). (e) Number of BFU-E colonies obtained by plating 1000 EML cells, pretreated for 24 h with Cdk4/6 IV inhibitor (CDK4/6i) or left untreated in M3436 medium.
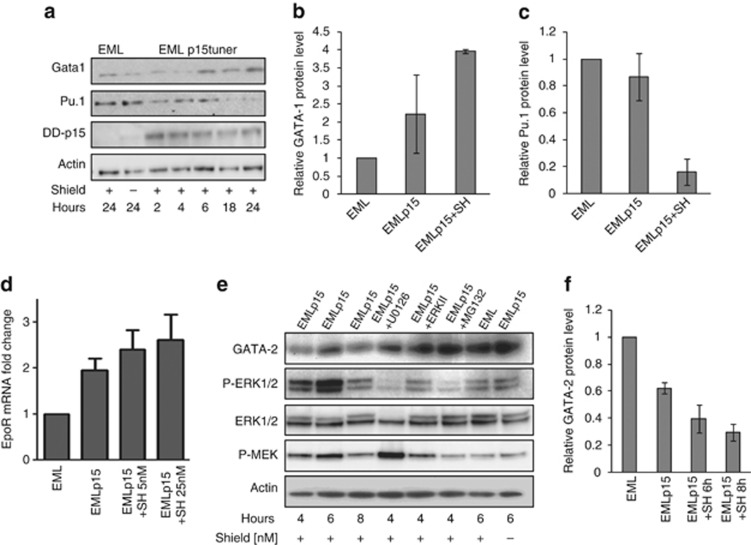
p15Ink4b regulates the expression of master regulators of erythroid differentiation
To begin to explore other potential mechanisms by which p15Ink4b could regulate myeloid and erythroid lineage commitment, the protein expression levels of transcription factors known to be associated with progenitor differentiation were examined. Induction of p15Ink4b in EMLp15Tuner cells was concomitant with increased expression of the erythroid-specific transcription factor GATA-1 and a decrease in the myeloid-specific transcription factor Pu.1 (Figure 7a–c) at the protein level. The observed dynamic changes following p15Ink4b expression were concomitant with transcriptional upregulation of the EpoR (Figure 7d), a target of GATA-1.30 We also observed that induction of p15Ink4b led to rapid decrease in the expression of another important transcription factor that regulates hematopoietic differentiation, GATA-2 (Figure 7e and f). It was of our interest to further understand what molecular pathways provoke the observed dynamic changes on the protein level shortly following p15Ink4b protein accumulation. As JAK-STAT, JNK and ERK-MAPK signaling cascades have been implicated in erythropoiesis,31, 32 we further utilized the EMLp15Tuner system to look at changes in these signal-transduction pathways following rapid accumulation of p15Ink4b. As shown in Figure 7e, the expression of p15Ink4b in EML cells specifically results in the phosphorylation of mitogen-activated protein kinase\extracellular signal-regulated kinase (MEK/ERK1/2), a signaling cascade shown previously to be essential for erythropoiesis.33 Treatment of p15Ink4b-expressing EML cells with inhibitors of ERK1/2 phosphorylation (U0126 and ERKII) or proteasome inhibitor (MG132) prevented the loss of GATA-2 (Figure 7e). GATA-2 is highly expressed in HSC and uncommitted hematopoietic progenitors. Our data suggest that p15Ink4b expression, through mechanisms that involve ERK1/2 phosphorylation, might regulate proteasome-mediated degradation of GATA-2, leading to the increase in GATA-1 and EpoR mRNA. Important to this study here, GATA-1 is known to induce EpoR, a critical step in erythroid differentiation, and at the same time to suppress activity of the myeloid-specific transcription factor Pu.1.34, 35
Figure 7.
Molecular changes following the expression of p15Ink4b in EML cells. (a) Time-dependent changes in GATA-1, Pu.1 and p15Ink4b protein expression following the addition of SH (100 nℳ) to induce accumulation of p15Ink4b protein in EMLp15Tuner cells (EMLp15). EML cells treated with SH or untreated EMLp15Tuner cells, harvested at 24 h, were used as controls (n=3). (b) Increase in GATA-1 protein levels at 24 h following the addition of SH. (c) Decrease in Pu.1 protein levels at 24 h following the addition of SH. Blots were quantitated using ImageJ and normalized to actin (n=2). (d) EpoR mRNA expression in EMLp15Tuner cells following 24 h exposure to SH (5 and 25 nℳ). Expression was normalized to 18SrRNA and depicted as fold change of the mRNA levels in EML cells (n=3). (e) Time-dependent changes in GATA-2 expression and phosphorylation of MEK and ERK1/2 proteins following the addition of SH (100 nℳ).The observed decrease in GATA-2 is abolished by the addition of MEK1/2 inhibitor (U0126), ERK inhibitor (ERKII) or 26S proteasome inhibitor (MG132). EML cells treated with SH or untreated EMLp15Tuner cells, harvested at 6 h, were used as controls. (f) Relative decrease in GATA-2 protein levels at 6 and 8 h following the addition of SH. Blots were quantitated using ImageJ and normalized to actin (n=2).
Discussion
The AML tumor suppressor p15Ink4b is demonstrated here to have a novel biological function in erythropoiesis. Its function in regulating production of erythroid cells may provide an explanation for the anemia observed in MDS and AML patients, 80% of which show a methylation-mediated repression of p15INK4B expression. Based on our study, our view of the normal role of p15Ink4b in organisms is to assist the blood system in regulating the lineage fate of progenitor cells by promoting erythroid commitment while suppressing myeloid cell formation, a role that becomes exaggerated under anemic stress. Developmental processes, like blood formation, are orchestrated by transcriptional networks. Our functional demonstration of a role for p15Ink4b in erythropoiesis and blood progenitor homeostasis provides a missing link in the regulation of such networks. This knowledge will not only promote further investigation of p15Ink4b in cellular differentiation and regulation of signal-transduction pathways but will also advance our understanding of p15Ink4b in the etiology of the diseases like anemia and cancer.
Our experiments only begin to address possible mechanisms that can drive increases in erythroid progenitors at the expense of myeloid progenitors and factors that work downstream of the p15Ink4b protein. One observation made here was that expression of p15Ink4b results in phosphorylation of MEK and ERK1/2, a signaling cascade shown previously to be essential for erythropoiesis.33 Interestingly, ERK1/2 was also observed to be an important effector downstream of p15Ink4b in the development of dendritic cells.36 As shown here, the downstream effects of this signaling cause decreases in the expression of GATA-2 and Pu.1 and increases in GATA-1 as well as EPOR. p15Ink4b-induced signaling may impact a replacement of GATA-2 with GATA-1 at some promoters, a process known as the ‘GATA switch'.37, 38 Further research will be required to demonstrate if this is the case.
As GATA-2 is highly expressed in HSC and progenitors, the inability to downregulate its expression, in part, because of the loss of p15Ink4b might lead to increased cycling and exhaustion of HSC, providing an explanation for the severe pancytopenia observed in MDS patients. Indeed, altered expression of either p15INK4B or GATA-2 has been linked to poor prognosis in a high number of AML patients.39, 40 Moreover, it has been reported recently that heritable GATA-2 mutations are associated with familial MDS and AML.41
It has been demonstrated previously, using a large cohort of patients representing various types of hematological malignancies, that loss of p15INK4B but not p16INK4A is characteristic of adult and pediatric AML and pediatric B-ALL.2, 6 Inactivation of both genes was rather uncommon and occurred only in pediatric T-ALL and Burkitt's lymphoma.2, 6 The tumor suppressor function of p16INK4A has been predominantly linked to cancers of epithelial origin and it is associated to its ability to bind CDK4/6 and inhibit cell cycle.6, 42 Taking into consideration these data and the fact that these two genes show differential regulation on the transcriptional level, it is likely that they have distinct physiological functions.43 Indeed, we observed that p16Ink4a is not expressed in purified hematopoietic progenitor populations like CMP, MEP and GMP of wild-type mice. There was an increase in the expression of p16Ink4a in the absence of p15Ink4b that could represent a compensation mechanism. Taking into consideration the fact that Ink4bKO animals, despite an imbalance in hematopoietic progenitor pool, display no difference in peripheral blood counts, it is possible that p16Ink4a could partially compensate for some but not all of p15Ink4b function, especially under stress. Consistent with the previous studies demonstrating a lack of cell cycle perturbation in the hematopoietic progenitor populations of Ink4bKO mice, we show that erythroid lineage commitment evoked by p15Ink4b expression is independent of cell cycle and pRb levels.9, 23 This is also consistent with the current notion of pRb function in erythroid differentiation, especially in RBC maturation marked by cell cycle exit and enucleation.44 Interestingly, it has been demonstrated previously that p16Ink4a also has an additional pRb-independent function in preventing c-Jun phosphorylation by directly binding to JNK kinase.45 Our findings suggest the potential of a novel cell cycle-independent role for p15Ink4b in the bifurcation of myeloid and erythroid commitment.
Loss of p15Ink4b in mice impairs the balance between erythroid and myeloid progenitor cell formation, preventing sufficient erythropoiesis to allow recovery from anemia. On the other hand, the overproduction of myeloid progenitors that is evident under steady-state and exaggerated under stress provides a favorable condition for the development of myeloid neoplasia. Indeed, we have previously demonstrated that loss of p15Ink4b in mice results in monocytosis and predisposition to myeloid leukemia.10, 11
Acknowledgments
We thank G Tosato, W Vass, X Qian and S Ruscetti for the reagents, and technical support. B Taylor, S Banerjee from NCI FACS Core Facility, M Albaugh from SAIC and Z Valivullah for technical support. This work was supported by intramural research program of The National Cancer Institute, Center for Cancer Research.
Footnotes
Supplementary Information accompanies the paper on Blood Cancer Journal website (http://www.nature.com/bcj)
The authors declare no conflict of interest.
Supplementary Material
References
- Cogle CR, Craig BM, Rollison DE, List AF. Incidence of the myelodysplastic syndromes using a novel claims-based algorithm: high number of uncaptured cases by cancer registries. Blood 2011; 117: 7121–7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JG, Civin CI, Issa JP, Collector MI, Sharkis SJ, Baylin SB. Distinct patterns of inactivation of p15INK4B and p16INK4A characterize the major types of hematological malignancies. Cancer Res 1997; 57: 837–841. [PubMed] [Google Scholar]
- Drexler HG. Review of alterations of the cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18 and p19 in human leukemia-lymphoma cells. Leukemia 1998; 12: 845–859. [DOI] [PubMed] [Google Scholar]
- Markus J, Garin MT, Bies J, Galili N, Raza A, Thirman MJ et al. Methylation-independent silencing of the tumor suppressor INK4b (p15) by CBFbeta-SMMHC in acute myelogenous leukemia with inv(16). Cancer Res 2007; 67: 992–1000. [DOI] [PubMed] [Google Scholar]
- Tien HF, Tang JH, Tsay W, Liu MC, Lee FY, Wang CH et al. Methylation of the p15(INK4B) gene in myelodysplastic syndrome: it can be detected early at diagnosis or during disease progression and is highly associated with leukaemic transformation. Br J Haematol 2001; 112: 148–154. [DOI] [PubMed] [Google Scholar]
- Herman JG, Jen J, Merlo A, Baylin SB. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res 1996; 56: 722–727. [PubMed] [Google Scholar]
- Lehmann U, Dobbelstein C, Fenner M, Romermann D, Hasemeier B, Metzig K et al. Complete cytogenetic remission after decitabine treatment in a patient with secondary AML harbouring high p15INK4b gene methylation and high global DNA methylation. Ann Hematol 2009; 88: 275–277. [DOI] [PubMed] [Google Scholar]
- Paul TA, Bies J, Small D, Wolff L. Signatures of polycomb repression and reduced H3K4 trimethylation are associated with p15INK4b DNA methylation in AML. Blood 2010; 115: 3098–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosu-Myles M, Taylor BJ, Wolff L. Loss of the tumor suppressor p15Ink4b enhances myeloid progenitor formation from common myeloid progenitors. Exp Hematol 2007; 35: 394–406. [DOI] [PubMed] [Google Scholar]
- Bies J, Sramko M, Fares J, Rosu-Myles M, Zhang S, Koller R et al. Myeloid-specific inactivation of p15Ink4b results in monocytosis and predisposition to myeloid leukemia. Blood 2010; 116: 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L, Garin MT, Koller R, Bies J, Liao W, Malumbres M et al. Hypermethylation of the Ink4b locus in murine myeloid leukemia and increased susceptibility to leukemia in p15(Ink4b)-deficient mice. Oncogene 2003; 22: 9265–9274. [DOI] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev 2000; 14: 994–1004. [PMC free article] [PubMed] [Google Scholar]
- Tsai S, Bartelmez S, Sitnicka E, Collins S. Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant-negative retinoic acid receptor can recapitulate lymphoid, myeloid, and erythroid development. Genes Dev 1994; 8: 2831–2841. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y. Differentiation of a cell line of myeloid leukemia. J Cell Physiol 1969; 74: 223–234. [DOI] [PubMed] [Google Scholar]
- Sun-Hoffman L, Aurigemma RE, Sun B, Ruscetti SK. Transactivation of the GATA-1 promoter by a myb-ets-containing mouse retrovirus is mediated by CACCC elements. Oncogene 1996; 13: 1037–1042. [PubMed] [Google Scholar]
- Nishigaki K, Hanson C, Thompson D, Yugawa T, Ruscetti S. Activation of the Jun N-terminal kinase pathway by friend spleen focus-forming virus and its role in the growth and survival of friend virus-induced erythroleukemia cells. J Virol 2005; 79: 12752–12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L, Tambourin P, Ruscetti S. Induction of the autonomous stage of transformation in erythroid cells infected with SFFV: helper virus is not required. Virology 1986; 152: 272–276. [DOI] [PubMed] [Google Scholar]
- Banaszynski LA, Chen LC, Maynard-Smith LA, Ooi AG, Wandless TJ. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 2006; 126: 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzio A, Passegue E, Ayton PM, Karsunky H, Cleary ML, Weissman IL. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev 2003; 17: 3029–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000; 404: 193–197. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Li X, Bedner E. Use of flow and laser-scanning cytometry in analysis of cell death. Methods Cell Biol 2001; 66: 69–109. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Mishra PJ, Bertino JR, Banerjee D. Epigenetic reversal of acquired resistance to 5-fluorouracil treatment. Mol Cancer Ther 2009; 8: 1045–1054. [DOI] [PubMed] [Google Scholar]
- Rosu-Myles M, Wolff L. p15Ink4b: dual function in myelopoiesis and inactivation in myeloid disease. Blood Cells Mol Dis 2008; 40: 406–409. [DOI] [PubMed] [Google Scholar]
- Zhang J, Socolovsky M, Gross AW, Lodish HF. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood 2003; 102: 3938–3946. [DOI] [PubMed] [Google Scholar]
- Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood 2005; 105: 2741–2748. [DOI] [PubMed] [Google Scholar]
- Ye ZJ, Kluger Y, Lian Z, Weissman SM. Two types of precursor cells in a multipotential hematopoietic cell line. Proc Natl Acad Sci USA 2005; 102: 18461–18466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature 2008; 453: 544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Sankaran VG, Orkin SH. Rb and hematopoiesis: stem cells to anemia. Cell Div 2008; 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daria D, Filippi MD, Knudsen ES, Faccio R, Li Z, Kalfa T et al. The retinoblastoma tumor suppressor is a critical intrinsic regulator for hematopoietic stem and progenitor cells under stress. Blood 2008; 111: 1894–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, Oda N, Shen K, Noguchi CT. Regulation of transcription of the human erythropoietin receptor gene by proteins binding to GATA-1 and Sp1 motifs. Nucleic Acids Res 1995; 23: 3041–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard C, Chretien S, Pelus AS, Porteu F, Muller O, Mayeux P et al. Activation of the mitogen-activated protein kinases Erk1/2 by erythropoietin receptor via a G(i) protein beta gamma-subunit-initiated pathway. J Biol Chem 2003; 278: 11050–11056. [DOI] [PubMed] [Google Scholar]
- Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol 2009; 86: 237–250. [DOI] [PubMed] [Google Scholar]
- Geest CR, Buitenhuis M, Groot Koerkamp MJ, Holstege FC, Vellenga E, Coffer PJ. Tight control of MEK-ERK activation is essential in regulating proliferation, survival, and cytokine production of CD34+-derived neutrophil progenitors. Blood 2009; 114: 3402–3412. [DOI] [PubMed] [Google Scholar]
- Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene 2002; 21: 3368–3376. [DOI] [PubMed] [Google Scholar]
- Burda P, Laslo P, Stopka T. The role of PU.1 and GATA-1 transcription factors during normal and leukemogenic hematopoiesis. Leukemia 2010; 24: 1249–1257. [DOI] [PubMed] [Google Scholar]
- Fares J, Koller R, Humeniuk R, Wolff L, Bies J. The tumor suppressor p15Ink4b regulates the differentiation and maturation of conventional dendritic cells. Blood 2012; 119: 5005–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. GATA switches as developmental drivers. J Biol Chem 2010; 285: 31087–31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick EH, Martowicz ML, Pal S, Johnson KD. Developmental control via GATA factor interplay at chromatin domains. J Cell Physiol 2005; 205: 1–9. [DOI] [PubMed] [Google Scholar]
- Vicente C, Vazquez I, Conchillo A, Garcia-Sanchez MA, Marcotegui N, Fuster O et al. Overexpression of GATA2 predicts an adverse prognosis for patients with acute myeloid leukemia and it is associated with distinct molecular abnormalities. Leukemia 2011; 26: 550–554. [DOI] [PubMed] [Google Scholar]
- Shimamoto T, Ohyashiki JH, Ohyashiki K. Methylation of p15(INK4b) and E-cadherin genes is independently correlated with poor prognosis in acute myeloid leukemia. Leuk Res 2005; 29: 653–659. [DOI] [PubMed] [Google Scholar]
- Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet 2011; 43: 1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimpenfort P, Ijpenberg A, Song JY, van der Valk M, Nawijn M, Zevenhoven J et al. p15Ink4b is a critical tumour suppressor in the absence of p16Ink4a. Nature 2007; 448: 943–946. [DOI] [PubMed] [Google Scholar]
- Peters G. Cell cycle. Stifled by inhibitions. Nature 1994; 371: 204–205. [DOI] [PubMed] [Google Scholar]
- Dirlam A, Spike BT, Macleod KF. Deregulated E2f-2 underlies cell cycle and maturation defects in retinoblastoma null erythroblasts. Mol Cell Biol 2007; 27: 8713–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BY, Choi HS, Ko K, Cho YY, Zhu F, Kang BS et al. The tumor suppressor p16(INK4a) prevents cell transformation through inhibition of c-Jun phosphorylation and AP-1 activity. Nat Struct Mol Biol 2005; 12: 699–707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.