Abstract
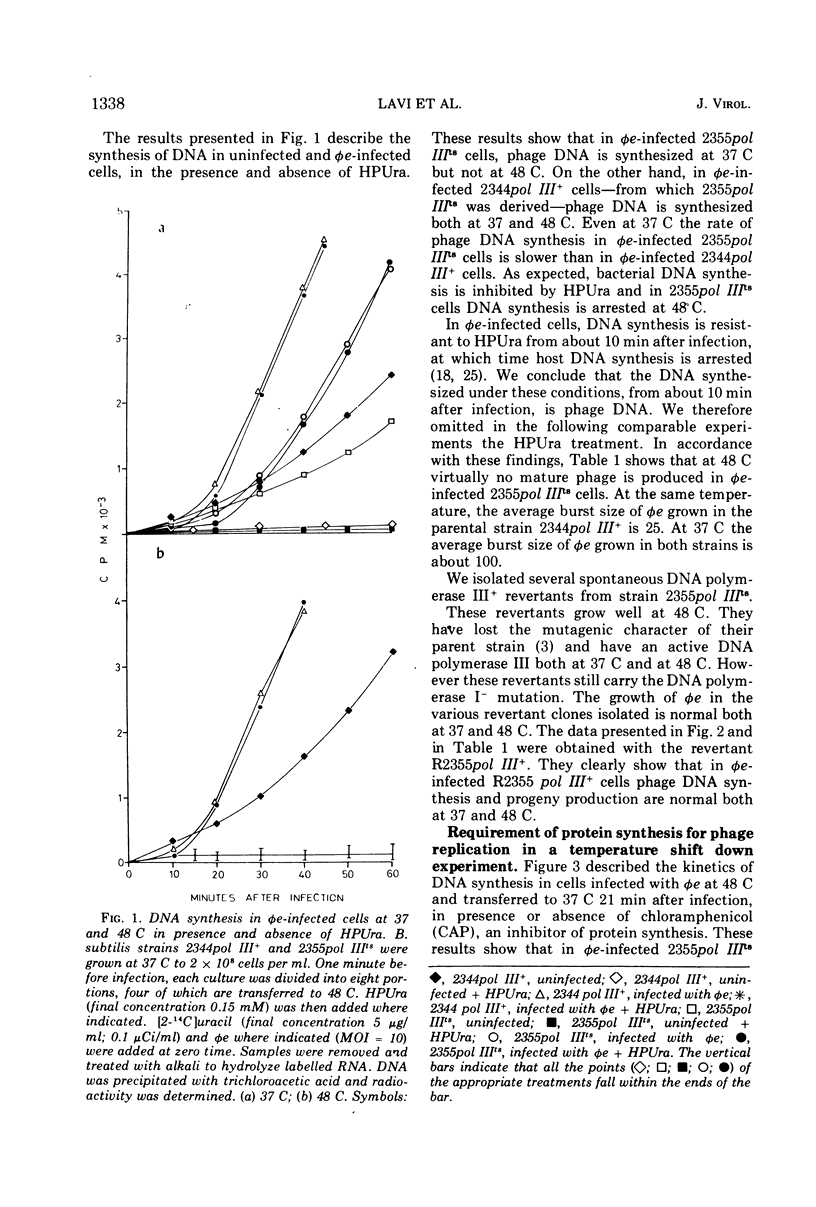
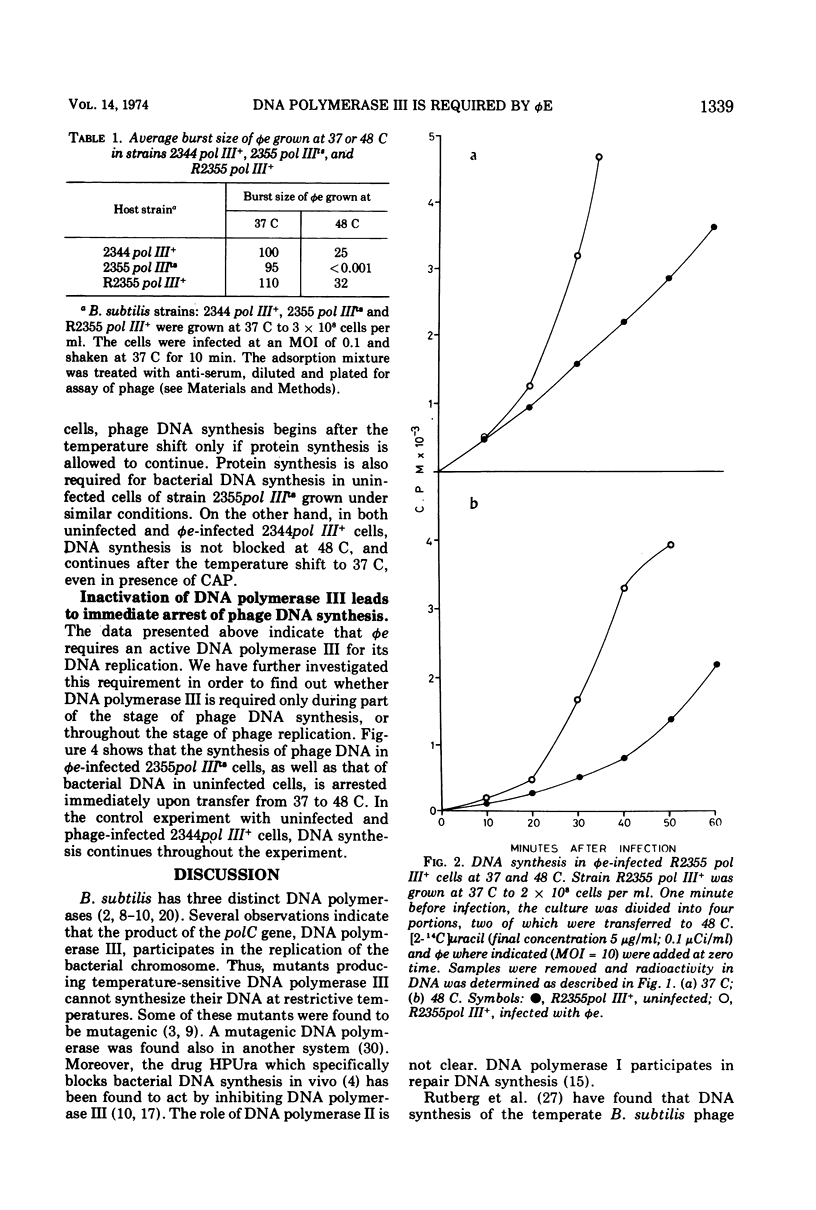
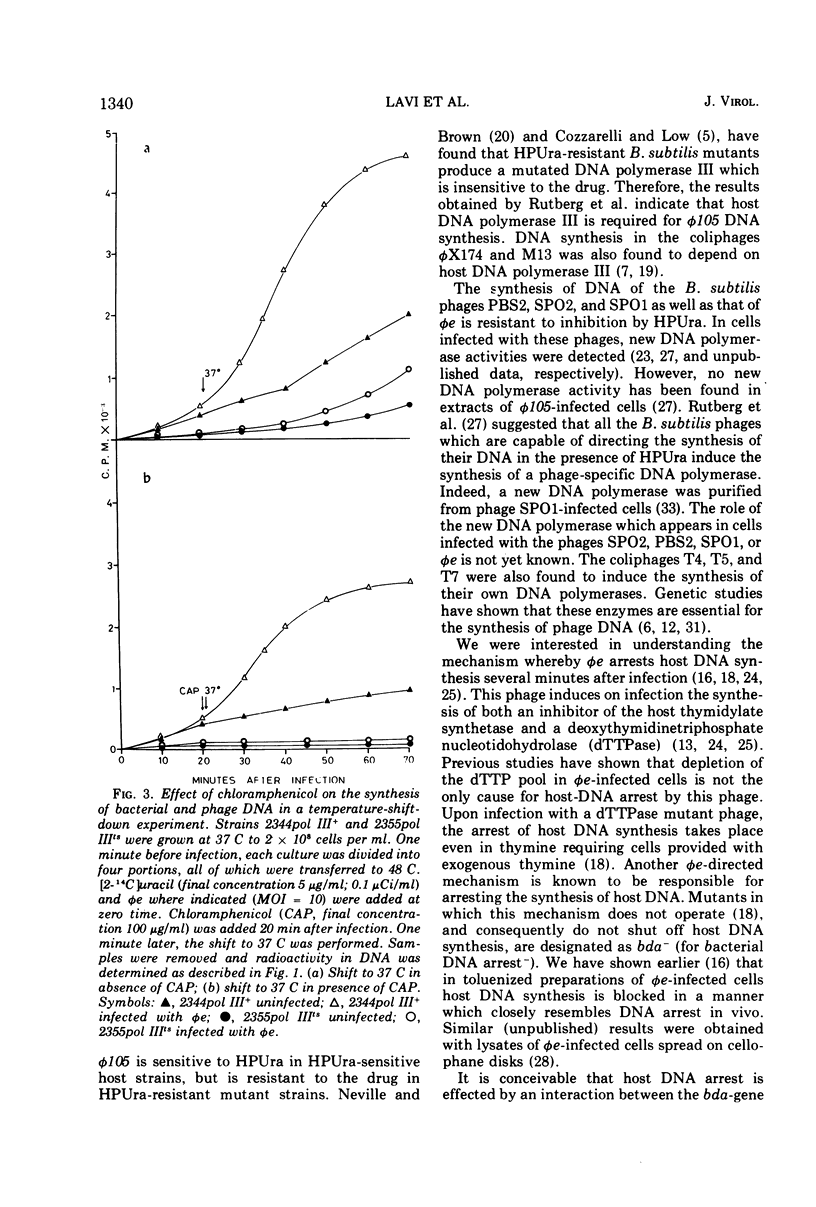
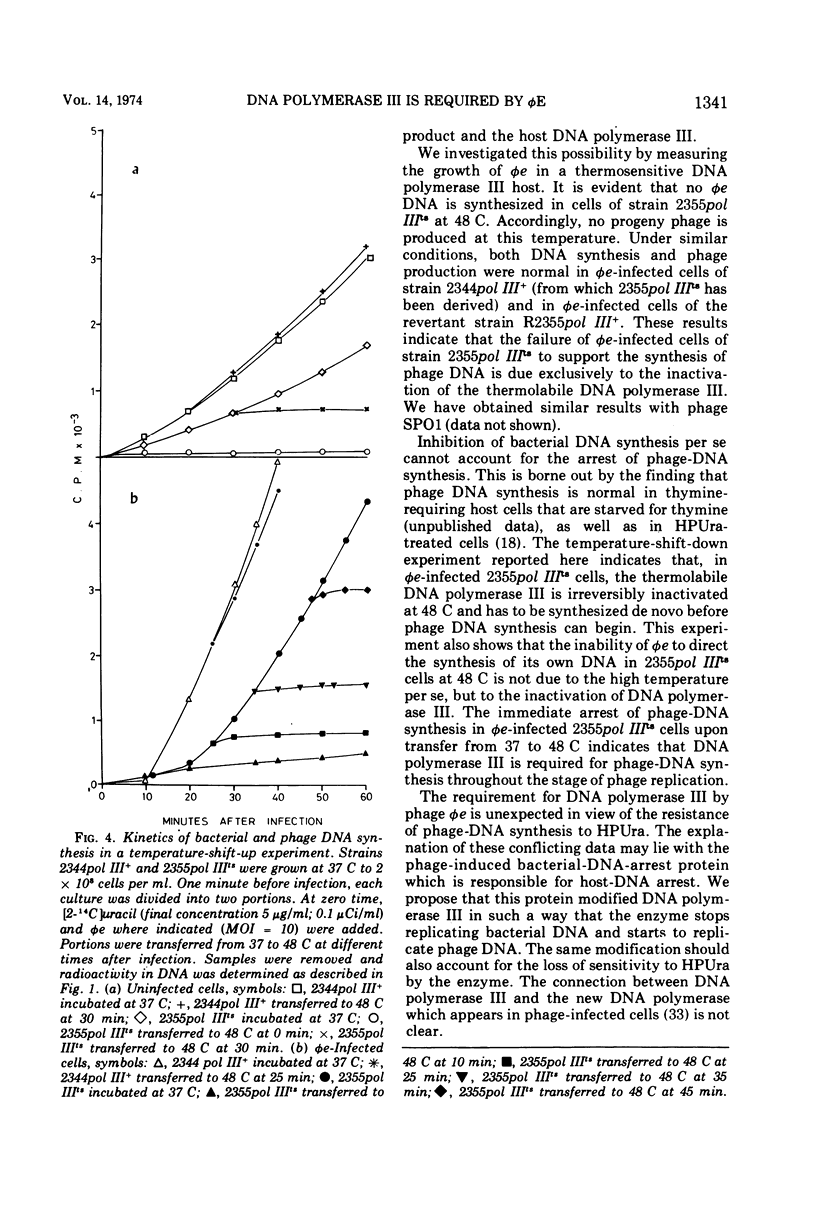
The virulent phage φe of Bacillus subtilis which contains hydroxymethyluracil in its DNA requires host DNA polymerase III for its DNA replication. DNA polymerase IIIts mutant cells infected with φe at restrictive temperatures do not support phage DNA synthesis. However, φe grows normally both at low and high temperatures in the mutant's parent strain and in spontaneous DNA polymerase III+ revertants isolated from the mutant strain. Temperature-shift-down experiments with φe-infected cells having thermosensitive DNA polymerase III (pol IIIts) indicate that at 48 C the thermolabile DNA polymerase III is irreversibly inactivated and has to be synthesized de novo after the shift to 37 C, before phage DNA synthesis can begin. Temperature-shift-up experiments with φe-infected mutant cells show that phage replication is arrested immediately after the temperature shift and indicate that φe requires DNA polymerase III throughout its replication stage.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APOSHIAN H. V. A DTMPASE FOUND AFTER INFECTION OF BACILLUS SUBTILIS WITH PHAGE SP5C. Biochem Biophys Res Commun. 1965 Jan 18;18:230–235. doi: 10.1016/0006-291x(65)90745-x. [DOI] [PubMed] [Google Scholar]
- Bazill G. W., Gross J. D. Effect of 6-(p-hydroxyphenyl)-azouracil on B. subtilis DNA polymerases. Nat New Biol. 1972 Nov 15;240(98):82–83. doi: 10.1038/newbio240082a0. [DOI] [PubMed] [Google Scholar]
- Bazill G. W., Gross J. D. Mutagenic DNA polymerase in B. subtilis. Nat New Biol. 1973 Jun 20;243(129):241–243. doi: 10.1038/newbio243241a0. [DOI] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Low R. L. Mutational alteration of Bacillus subtilis DNA polymerase 3 to hydroxyphenylazopyrimidine resistance: polymerase 3 is necessary for DNA replication. Biochem Biophys Res Commun. 1973 Mar 5;51(1):151–157. doi: 10.1016/0006-291x(73)90521-4. [DOI] [PubMed] [Google Scholar]
- De Waard A., Paul A. V., Lehman I. R. The structural gene for deoxyribonucleic acid polymerase in bacteriophages T4 and T5. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1241–1248. doi: 10.1073/pnas.54.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas L. B., Miller C. A. Replication of bacteriophage phiX174 DNA in a temperature-sensitive dnaE mutant of Escherichia coli C. J Virol. 1973 Jun;11(6):848–855. doi: 10.1128/jvi.11.6.848-855.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Ganesan A. T., Yehle C. O., Yu C. C. DNA replication in a polymerase I deficient mutant and the identification of DNA polymerases II and 3 in Bacillus subtilis. Biochem Biophys Res Commun. 1973 Jan 4;50(1):155–163. doi: 10.1016/0006-291x(73)91077-2. [DOI] [PubMed] [Google Scholar]
- Gass K. B., Cozzarelli N. R. Further genetic and enzymological characterization of the three Bacillus subtilis deoxyribonucleic acid polymerases. J Biol Chem. 1973 Nov 25;248(22):7688–7700. [PubMed] [Google Scholar]
- Gass K. B., Low R. L., Cozzarelli N. R. Inhibition of a DNA polymerase from Bacillus subtilis by hydroxyphenylazopyrimidines. Proc Natl Acad Sci U S A. 1973 Jan;70(1):103–107. doi: 10.1073/pnas.70.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo P., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6867–6873. [PubMed] [Google Scholar]
- KALLEN R. G., SIMON M., MARMUR J. The new occurrence of a new pyrimidine base replacing thymine in a bacteriophage DNA:5-hydroxymethyl uracil. J Mol Biol. 1962 Aug;5:248–250. doi: 10.1016/s0022-2836(62)80087-4. [DOI] [PubMed] [Google Scholar]
- Laipis P. J., Ganesan A. T. A deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis. J Biol Chem. 1972 Sep 25;247(18):5867–5871. [PubMed] [Google Scholar]
- Lavi U., Marcus M. Arrest of host DNA synthesis in Bacillus subtilis infected with phage phi e. Virology. 1972 Sep;49(3):668–674. doi: 10.1016/0042-6822(72)90523-5. [DOI] [PubMed] [Google Scholar]
- Mackenzie J. M., Neville M. M., Wright G. E., Brown N. C. Hydroxyphenylazopyrimidines: characterization of the active forms and their inhibitory action on a DNA polymerase from Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):512–516. doi: 10.1073/pnas.70.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Newlon M. C. Control of DNA synthesis in Bacillus subtilis by phage phi e. Virology. 1971 Apr;44(1):83–93. doi: 10.1016/0042-6822(71)90155-3. [DOI] [PubMed] [Google Scholar]
- Mitra S., Stallions D. R. Role of dna genes of Escherichia coli in M13 phage replication. Virology. 1973 Apr;52(2):417–424. doi: 10.1016/0042-6822(73)90336-x. [DOI] [PubMed] [Google Scholar]
- Neville M. M., Brown N. C. Inhibition of a discrete bacterial DNA polymerase by 6-(p-hydroxyphenylazo)-uracil and 6-(p-hydroxyphenylazo-)-isocytosine. Nat New Biol. 1972 Nov 15;240(98):80–82. doi: 10.1038/newbio240080a0. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A., Sueoka N. Sequential replication of the Bacillus subtilis chromosome. IV. Genetic mapping by density transfer experiment. J Mol Biol. 1967 Jul 28;27(2):349–368. doi: 10.1016/0022-2836(67)90025-3. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Price A. R., Cook S. J. New deoxyribonucleic acid polymerase induced by Bacillus subtilis bacteriophage PBS2. J Virol. 1972 Apr;9(4):602–610. doi: 10.1128/jvi.9.4.602-610.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H. Synthesis of DNA in phage-infected Bacillus subtilis. Virology. 1969 Aug;38(4):527–537. doi: 10.1016/0042-6822(69)90173-1. [DOI] [PubMed] [Google Scholar]
- Roscoe D. H. Thymidine triphosphate nucleotidohydrolase: a phage-induced enzyme in Bacillus subtilis. Virology. 1969 Aug;38(4):520–526. doi: 10.1016/0042-6822(69)90172-x. [DOI] [PubMed] [Google Scholar]
- Roscoe D. H., Tucker R. G. The biosynthesis of 5-hydroxymethyldeoxyuridylic acid in bacteriophage-infected Bacillus subtilis. Virology. 1966 May;29(1):157–166. doi: 10.1016/0042-6822(66)90205-4. [DOI] [PubMed] [Google Scholar]
- Rutberg L., Armentrout R. W., Jonasson J. Unrelatedness of temperate Bacillus subtilis bacteriophages SP02 and phi105. J Virol. 1972 May;9(5):732–737. doi: 10.1128/jvi.9.5.732-737.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Otto B., Nüsslein V., Huf J., Herrmann R., Bonhoeffer F. Deoxyribonucleic acid replication in vitro. J Mol Biol. 1972 Jan 28;63(2):183–200. doi: 10.1016/0022-2836(72)90369-5. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Roscoe D. H. The course of phage phi-e infection in sporulating cells of Bacillus subtilis strain 3610. Virology. 1969 Oct;39(2):265–275. doi: 10.1016/0042-6822(69)90047-6. [DOI] [PubMed] [Google Scholar]
- Speyer J. F. Mutagenic DNA polymerase. Biochem Biophys Res Commun. 1965 Oct 8;21(1):6–8. doi: 10.1016/0006-291x(65)90417-1. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Barnes J. E. Deoxyribonucleic acid synthesis in Escherichia coli infected with some deoxyribonucleic acid polymerase-less mutants of bacteriophage T4. Virology. 1966 Jan;28(1):100–107. doi: 10.1016/0042-6822(66)90310-2. [DOI] [PubMed] [Google Scholar]
- Yehle C. O., Ganesan A. T. Deoxyribonucleic acid synthesis in bacteriophage SP01-infected Bacillus subtilis. II. Purification and catalytic properties of a deoxyribonucleic acid polymerase induced after infection. J Biol Chem. 1973 Nov 10;248(21):7456–7463. [PubMed] [Google Scholar]
- Yehle C. O., Ganesan A. T. Deoxyribonucleic acid synthesis in bacteriophage SPO1-infected Bacillus subtilis. I. Bacteriophage deoxyribonucleic acid synthesis and fate of host deoxyribonucleic acid in normal and polymerase-deficient strains. J Virol. 1972 Feb;9(2):263–272. doi: 10.1128/jvi.9.2.263-272.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


