Abstract
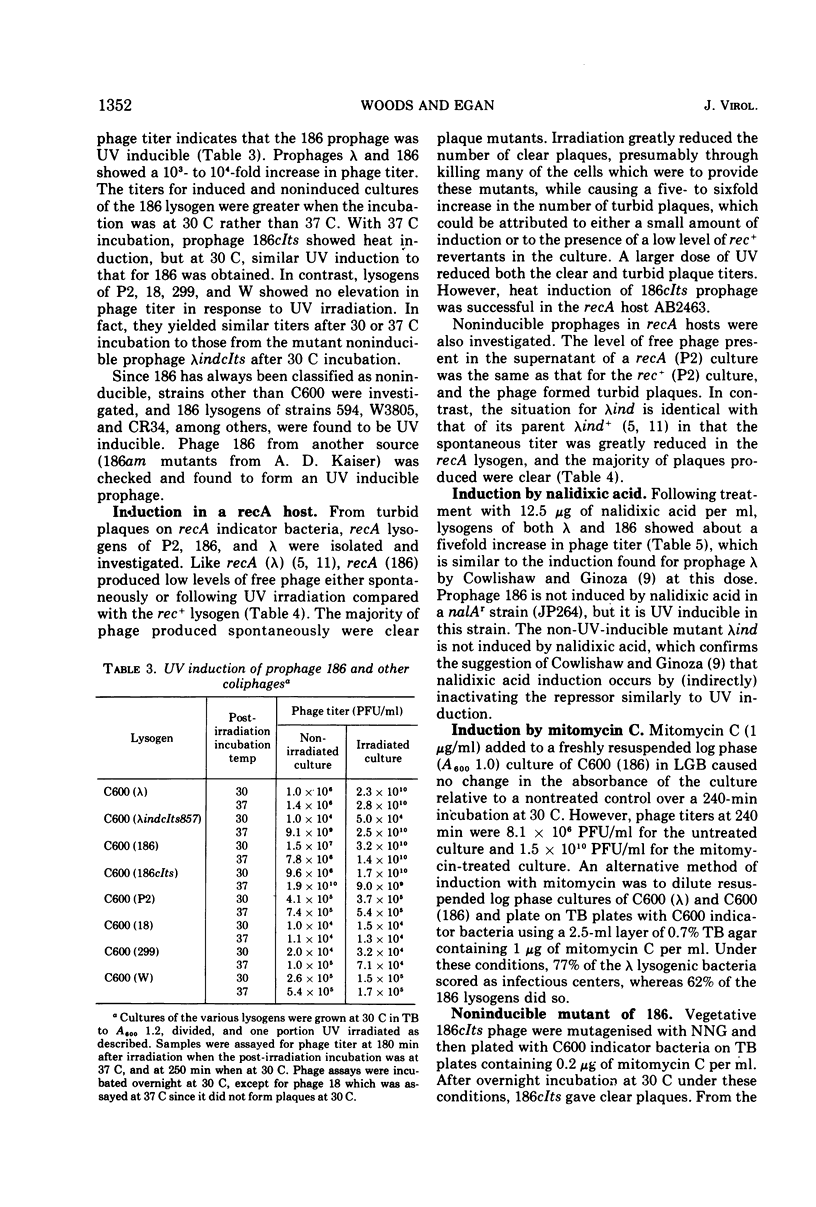
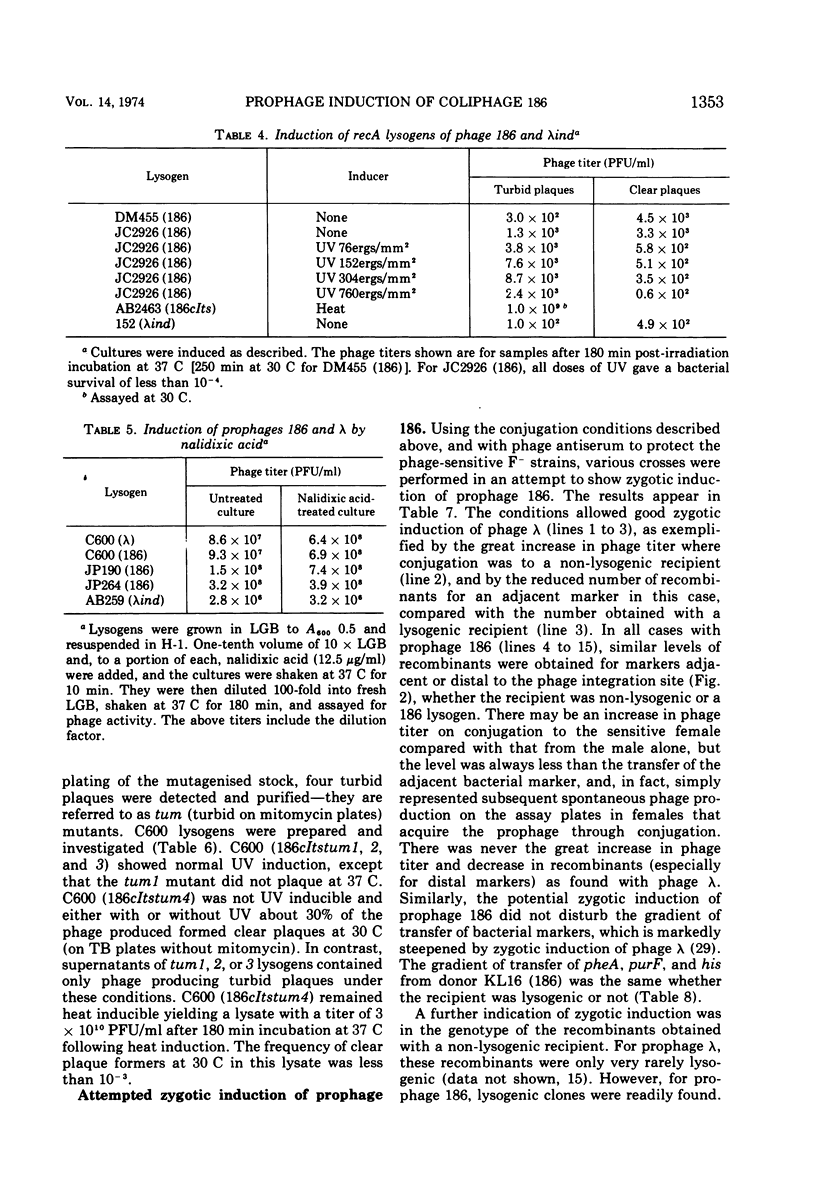
Coliphage 186 has been regarded as a member of the noninducible group of coliphages. Evidence that prophage 186 is induced by ultraviolet irradiation or by treatment with nalidixic acid or mitomycin C is now presented. The phage yields were similar to those from lysogens of the inducible phage lambda, and the induction required a recA+ host. A noninducible mutant of 186 was isolated from its heat-inducible derivative, 186cIts, that was no longer inducible by ultraviolet irradiation but remained heat inducible. That zygotic induction of 186 after transfer from a lysogenic male to a non-lysogenic recipient did not occur is indicated by the following findings: (i) there was only a slight increase in phage titer; (ii) similar levels of recombinants were obtained for markers adjacent or distal to the phage integration site, whether the recipient was lysogenic or not, and there was no effect on the gradient of marker transfer; (iii) lysogenic recombinants were readily found and the co-transfer of 186 with adjacent markers was the same to lysogenic or non-lysogenic recipients. Thus, 186 formed an inducible prophage that did not display zygotic induction. Nevertheless, it shared many properties with the noninducible phage P2 as outlined in the discussion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L., Barrand P., Fritsch A., Goldthwait D. A., Jacob F. Cohesive sites on the deoxyribonucleic acids from several temperate coliphages. J Mol Biol. 1966 Jun;17(2):343–357. doi: 10.1016/s0022-2836(66)80146-8. [DOI] [PubMed] [Google Scholar]
- Bertani L. E., Bertani G. Genetics of P2 and related phages. Adv Genet. 1971;16:199–237. doi: 10.1016/s0065-2660(08)60359-4. [DOI] [PubMed] [Google Scholar]
- Brooks K., Clark A. J. Behavior of lambda bacteriophage in a recombination deficienct strain of Escherichia coli. J Virol. 1967 Apr;1(2):283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calendar R., Lindahl G., Marsh M., Sunshine M. Temperature-inducible mutants of P2 phage. Virology. 1972 Jan;47(1):68–75. doi: 10.1016/0042-6822(72)90240-1. [DOI] [PubMed] [Google Scholar]
- Calendar R., Lindqvist B., Sironi G., Clark A. J. Characterization of REP- mutants and their interaction with P2 phage. Virology. 1970 Jan;40(1):72–83. doi: 10.1016/0042-6822(70)90380-6. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Inman R. B. Origin and direction of replication of bacteriophage 186 DNA. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1768–1771. doi: 10.1073/pnas.70.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowlishaw J., Ginoza W. Induction of lambda prophage by nalidixic acid. Virology. 1970 Jun;41(2):244–255. doi: 10.1016/0042-6822(70)90076-0. [DOI] [PubMed] [Google Scholar]
- Fuerst C. R., Siminovitch L. Characterization of an unusual defective lysogenic strain of Escherichia coli K-12(lambda). Virology. 1965 Nov;27(3):449–451. doi: 10.1016/0042-6822(65)90131-5. [DOI] [PubMed] [Google Scholar]
- Garen A., Garen S., Wilhelm R. C. Suppressor genes for nonsense mutations. I. The Su-1, Su-2 and Su-3 genes of Escherichia coli. J Mol Biol. 1965 Nov;14(1):167–178. doi: 10.1016/s0022-2836(65)80238-8. [DOI] [PubMed] [Google Scholar]
- HAYES W. The kinetics of the mating process in Escherichia coli. J Gen Microbiol. 1957 Feb;16(1):97–119. doi: 10.1099/00221287-16-1-97. [DOI] [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Sur les processus de conjugaison et de recombinaison chez Escherichia coli. I. L'induction par conjugaison ou induction zygotique. Ann Inst Pasteur (Paris) 1956 Oct;91(4):486–510. [PubMed] [Google Scholar]
- JACOB F., WOLLMAN E. L. Sur les processus de conjugaison et de recombinaison génétique chez Escherichia coli. IV. Prophages inductibles et mesure des segments génétiques transferés au cours de la conjugaison. Ann Inst Pasteur (Paris) 1958 Nov;95(5):497–519. [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- KELLY B. Localization of P2 prophage in two strains of Escherichia coli. Virology. 1963 Jan;19:32–39. doi: 10.1016/0042-6822(63)90021-7. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Sironi G., Bialy H., Calendar R. Bacteriophage lambda; abortive infection of bacteria lysogenic for phage P2. Proc Natl Acad Sci U S A. 1970 Jul;66(3):587–594. doi: 10.1073/pnas.66.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Mandel M., Berg A. Cohesive sites and helper phage function of P2, lambda, and 186 DNA's. Proc Natl Acad Sci U S A. 1968 May;60(1):265–268. doi: 10.1073/pnas.60.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Berg A. Melting temperatures of the cohesive ends of some non-inducible coliphage DNA's. J Mol Biol. 1968 Nov 28;38(1):137–139. doi: 10.1016/0022-2836(68)90135-6. [DOI] [PubMed] [Google Scholar]
- Murray K., Murray N. E. Terminal nucleotide sequences of DNA from temperate coliphages. Nat New Biol. 1973 May 30;243(126):134–139. doi: 10.1038/newbio243134a0. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Wu R. Nucleotide sequence analysis of DNA. IV. Complete nucleotide sequence of the left-hand cohesive end of coliphage 186 DNA. J Mol Biol. 1972 Apr 14;65(3):447–467. doi: 10.1016/0022-2836(72)90201-x. [DOI] [PubMed] [Google Scholar]
- Skalka S. A., Hanson P. Comparisons of the distribution of nucleotides and common sequences in deoxyribonucleic acid from selected bacteriophages. J Virol. 1972 Apr;9(4):583–593. doi: 10.1128/jvi.9.4.583-593.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLMAN E. L., JACOB F. Lysogénie et recombinaison génétique chez Escherichia coli K12. C R Hebd Seances Acad Sci. 1954 Aug 2;239(5):455–456. [PubMed] [Google Scholar]
- Wang J. C. Cyclization of coliphage 186 DNA. J Mol Biol. 1967 Sep 28;28(3):403–411. doi: 10.1016/s0022-2836(67)80089-5. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Schwartz H. Noncomplementarity in base sequences between the cohesive ends of coliphages 186 and lambda and the formation of interlocked rings between the two DNA's. Biopolymers. 1967;5(10):953–966. doi: 10.1002/bip.1967.360051008. [DOI] [PubMed] [Google Scholar]
- Wiman M., Bertani G., Kelly B., Sasaki I. Genetic map of Escherichia coli strain C. Mol Gen Genet. 1970;107(1):1–31. doi: 10.1007/BF00433220. [DOI] [PubMed] [Google Scholar]
- Woods W. H., Egan J. B. Integration stie of noninducible coliphage 186. J Bacteriol. 1972 Aug;111(2):303–307. doi: 10.1128/jb.111.2.303-307.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


