Abstract
Objective
To retrospectively evaluate the effect of 3D automated ultrasound (3D-AUS) as an adjunct to digital breast tomosynthesis (DBT) on radiologists’ performance and confidence in discriminating malignant and benign breast masses.
Methods
Two-view DBT (CC and MLO or Lateral) and single-view 3D-AUS were acquired on 51 patients with (subsequently) biopsy-proven masses (13 malignant, 38 benign). Six experienced radiologists rated, on a 13-point scale, the likelihood of malignancy of an identified mass, first by reading the DBT alone, followed immediately by reading the DBT with automatically co-registered 3D-AUS. The diagnostic performance of each method was measured using ROC analysis. and changes in sensitivity and specificity with the McNemar test. After each reading, radiologists took a survey to rate their confidence level in using DBT alone vs combined DBT/3D-AUS as potential screening modalities.
Results
The six radiologists had an average area under the ROC curve of 0.92 for both modalities (range 0.89–0.97 for DBT, 0.90–0.94 for DBT/3D-AUS). With BI-RADS rating of 4 as the threshold for biopsy recommendation, the average sensitivity of the radiologists increased from 96% to 100% (p>0.08) with 3D-AUS, while the specificity decreased from 33% to 25% (p>0.28). Survey responses indicated an increased confidence in potentially using DBT for screening when 3D-AUS was added (p<0.05 for each reader).
Conclusions
In this initial reader study, no significant difference in ROC performance was found with the addition of 3D-AUS to DBT. However, a trend to an improved discrimination of malignancy was observed when adding 3D-AUS. Radiologists’ confidence also improved with DBT/3DAUS compared to DBT alone.
Keywords: Automated Ultrasound, Tomosynthesis, Breast, Reader Study
Introduction
Ultrasound is essential for characterization of breast masses1. It is commonly employed as an adjunct to diagnostic clinical mammography, the standard imaging method for detecting breast cancer. The interpretation of hand-held ultrasound images by experienced radiologists leads to an accuracy close to 100% for differentiating simple cysts from other lesions2, and can improve distinction of malignant from benign breast masses3–5.
3D automated ultrasound (3D-AUS) scanners are currently being investigated6–15 as a means to reduce ultrasound breast examination time, measure additional tissue properties such as speed of sound and acoustic attenuation16, and provide 3D volumes that can be reviewed post-examination. When compared to hand-held ultrasound, 3D-AUS has been found to provide similar image quality and diagnostic information13, BI-RADS ratings7, 12, 13 and mass detection rates7. As an adjunct to mammography, 3D-AUS improved mass characterization17, and, in a recent screening study6, 18, 3D-AUS improved cancer detection and accuracy and reduced callback rates..
We have utilized a combined Digital Breast Tomosynthesis (DBT) and 3D-AUS imaging system, developed by GE Global Research with our collaboration and modifications. The breast is imaged sequentially with DBT and 3D-AUS under the same mammographic compression using a special dual-modality compression paddle. Imaging in the same geometry8–11 provides coregistration between the two modalities8. Coregistration potentially reduced the potential for misidentification of the location of abnormalities as it can occur when hand-held ultrasound is combined with mammography because the two modalities are performed in different geometries19, 20.
DBT is of interest for breast screening and diagnosis because of its 3D imaging capabilities and its potential to alleviate a limitation of mammography, the masking of noncalcified cancers by other dense tissue21. DBT has shown clinical potential compared to screen-film and digital mammography21, by subjectively providing better image quality22–25, increasing the number of detected abnormalities23, 26–28 and the accuracy in classification of masses22, 29, 30. In clinical practice, its sensitivity was found to be similar to the sensitivity of mammography31.
Whether ultrasound, and in particular 3D-AUS, could be as useful an adjunct to DBT for masses as it is for mammography currently in diagnostic evaluations has not been significantly investigated. We report here the results from an initial reader study. Our purposes were to preliminary evaluate the effect of adding 3D-AUS to DBT on radiologists’ abilities to discriminate malignant and benign breast masses, to assess radiologists’ abilities to detect lesions on 3D-AUS using co-registration, and to measure radiologists’ confidence in using combined DBT/3D-AUS as an indicator of its utility in screening and/or diagnostic breast imaging.
Materials and methods
Dataset
Institutional Review Board approval was granted for this investigation. Informed consent, including consent for future retrospective data analysis was obtained for each patient. The study was compliant with the Health Insurance Portability and Accountability Act.
Ninety-four patients, each with a mass assessed as suspicious or highly suggestive of malignancy (BI-RADS 4 and 5) based on clinical diagnostic mammography and ultrasound, were recruited between 2006 and 2009. All underwent DBT and 3D-AUS research scans before biopsy or FNA.
Inclusion criteria for the reader study were: visible mass on DBT, mass in the field of view (FOV) of the 3D-AUS, and pathology results available. Forty-three patients were excluded for the following reasons: technical problems during 3D-AUS acquisition (7), biopsy aborted (7), DBT negative (8) and mass excluded from FOV for 3D-AUS (21). The 7 cases excluded due to ‘technical problems’ were excluded due to communications issues between the main computer and the ultrasound scanner, resulting in asynchronous triggering signals and in random number of slices in the ultrasound datasets. The last case was excluded because of an issue during the DBT acquisition, resulting in poor image quality. Both issues took place in the early stage of the study, and were subsequently corrected for.
The final case set thus consisted of 51 patients (mean age 50, range 29–79 years old) for a total of 51 masses (mean diameter =1.8±1.1 cm). Thirteen masses were malignant (10 invasive ductal carcinoma, 2 invasive lobular carcinoma and 1 metaplastic carcinoma) and 38 were benign (13 cysts, 12 fibroadenomas, 2 papillomas, 1 lobular carcinoma in situ, and 10 other benign breast diagnoses).
In two of the 51 cases, the 3D-AUS was negative, as was the clinical hand-held ultrasound.
Imaging procedures and equipment
A CC- and either a lateral or an MLO-view DBT were obtained with the patient in a seated position using a DBT unit prototype8. Breast compression was applied, similar to, but often slightly reduced from, that of mammography. Twenty-one projection views were acquired in 7.5 sec with a total mean glandular dose approximately 1.4 times that for a conventional screen-film mammogram as estimated with an American College of Radiology (ACR) phantom. Image reconstruction was performed with a simultaneous algebraic reconstruction technique32 for a pixel size the same as the detector element pitch (0.1 mm × 0.1 mm) and slice spacing set to 1 mm.
Following the second view of DBT, a single automated whole breast ultrasound scan was acquired in the same view during the same compression10, using either a solid plastic polymethylpentene10, 11, 33, 34 or, in a few cases, a fiber mesh35 compression paddle, with the contact surface of the breast and the proximal breast periphery covered by ultrasonic coupling gel. A linear matrix array ultrasound transducer (GE-M12L) operated at 10 MHz was translated across the compression paddle with a computer-driven motorized transducer carriage. The image plane of the transducer was perpendicular to the chest wall while the motion of the carriage was parallel. Images were acquired with a GE LOGIQ 9 ultrasound system (GE Healthcare, Milwaukee, WI). Image spacing of 0.4 mm was achieved using an external image frame trigger that was developed for the LOGIQ 9 system.
Visualization software
In-house developed software was used to display DBT and 3D-AUS images. The tomosynthesis slices were displayed on an IBM Model T221 9-megapixel 22.2-inch-diagonal LCD monitor, while the 3D-AUS slices were displayed on an adjacent 2-megapixel 24-inch-diagonal monitor. Both the DBT and the 3D-AUS images were displayed with the original pixel resolution without sub-sampling. Readers could adjust contrast, brightness and zoom, and scroll through the slices for viewing. The software performed automatic registration of the lesion location from the DBT to the 3D-AUS volumes. The accuracy of the registration was estimated to be 0.8±0.3 mm for a rigid calibration phantom8. For patient data, a spatial discrepancy of up to 5 mm in the registration was noticed, due to usual patient movements, a tendency of the patients to pull back over the several minutes of compression, and variations in the speed of sound in the breast and refraction of the ultrasound beam.
Mass localization
The true positions of the biopsied masses on the DBT and 3D-AUS volumes were established by a Mammography Quality Standards Act (MQSA)-qualified radiologist (CP), who had access to clinical images (mammography and hand-held ultrasound) and clinical information. An independent verification was performed by a second qualified radiologist (MR).
Reader study design
Six academic breast radiologists (AJ, KK, AN, MN, SP, RP), with 3–20 (median 13) years of experience in mammographic and breast US interpretation, participated as observers. All were MQSA qualified and 5 were fellowship-trained in breast imaging.
These readers read the cases in randomized order during an average of 3 reading sessions. The radiologists were not informed about the cancer prevalence in the dataset and the results of their assessments were not discussed with them before the entire study was completed.
In the two-step sequential reading design used, the radiologists first examined the images of the mass in the CC and MLO (or lateral) DBT views. The position of the mass in these volumes was marked by a region of interest (ROI). The radiologists first categorized the masses from 1 to 5 using the ACR BI-RADS classification system., Category 0 (additional imaging needed) was not allowed. The likelihoods of malignancy (LM) were also rated, using a 13-point scale, with one (1) being ‘normal’, two (2) ‘benign’, three (3) probably benign with 0–2% risk of malignancy, four (4) suspicious with 3–10% risk, five (5) suspicious with 11–20% risk…. and thirteen (13) highly suggestive of malignancy with a risk higher than 94%. Readers were reminded at the beginning of each session that a rating greater than 2% LM (BI-RADS 4 or 5) implied a biopsy recommendation. The radiologists then selected descriptors of the shape, margin and mass density, according to the ACR BI-RADS. The presence/absence of calcifications was recorded. All assessments were recorded through the use of an interactive graphical user interface.
Immediately after reading the DBT images for a case, the radiologists examined the 3D-AUS volume corresponding to the last DBT view for the case, while the DBT volumes were still displayed. This mode is referred to as DBT/3D-AUS, below. The ROI of the mass in the DBT was co-registered and displayed on the AUS volume. The radiologists were informed of the potential imprecision of the coregistration. They first localized the position of the mass on the 3D-AUS images by marking an ROI. They then selected descriptors from a list of terms from the US BI-RADS of the ACR to characterize mass shape, orientation, margin, echo pattern, posterior acoustic features, lesion boundary and surrounding tissues. They finally provided a BI-RADS classification and a LM rating using combined information retrieved from DBT and 3D-AUS.
After each reading of DBT and combined DBT/3D-AUS volumes, radiologists rated confidence in using DBT and combined DBT/3D-AUS for simultaneous screening and diagnostic examinations. The survey question that was asked after each DBT reading was:
“If screening were performed by tomosynthesis in this fashion, would tomosynthesis alone allow you to avoid categorizing this case as BIRADS 0 and instead enable you to categorize it as BIRADS 2,3,4,5, without any additional imaging? (Assume no previous comparison mammograms)?”
The survey question that was asked after each combined DBT/3D-AUS reading was:
“If screening were performed by combined tomosynthesis and ultrasound as we presented, are these images sufficient to allow you to avoid categorizing this case as BIRADS 0 and instead enable you to categorize it as BIRADS 2,3,4,5 without any additional imaging? (Assume no previous comparison mammograms)?”
Answers were recorded using a 10-point scale, 1 being ‘No’, 5 being ‘Maybe’, and 10 being ‘Yes’.
Because the main objective of the study was to preliminary evaluate the effect of adding 3D-AUS to DBT on radiologists’ abilities to discriminate malignant and benign breast masses, it was necessary to have the reading of the mass of interest on both modalities for all the radiologists. In order to make sure that the correct mass were detected on the 3D-AUS, two reference radiologists reviewed the mass localizations on the 3D-AUS for each reader. The cases with incorrectly identified masses of interest on 3D-AUS were identified. The correct areas of the masses of interest were then marked on the 3D-AUS images. At least two months after the readers last reading session, the five readers who had incorrectly identified the locations of one or more masses of interest on the 3D-AUS were recalled to again read those cases. For the second readings, the masses of interest were already marked on the 3D-AUS. For cases in which there were such second readings, the ratings for the second readings replaced the ratings for the first readings in the analyses of the readers’ performances.
Data and statistical analysis
The observer performances in terms of LM ratings were analyzed using the Receiver-Operating Characteristics (ROC) methodology36,37. The statistical significance of the difference in the area under the ROC curve (Az) between the two modalities was tested using the Dorfman-Berbaum-Metz multireader multicase methodology38, 39 (http://wwwradiology.uchicago.edu/krl/KRL_ROC/software_index6.htm). This methodology takes into account both the reader and the case sample variations by means of an analysis of variance approach. The results can thus be generalized to the population of readers and to the population of samples.
To analyze changes in sensitivity and specificity, cases were classified as ‘no biopsy’ recommended (BI-RADS ratings 1, 2 or 3) or as ‘biopsy’ recommended (BI-RADS ratings 4 or 5). Individual change in sensitivity for a given radiologist with 3D-AUS was investigated using the McNemar test (WinStat, version 2005.1; R. Fitch Software, Lehigh Valley, Pa) with consideration of the number of beneficial and detrimental changes in biopsy recommendation for malignant masses. For a malignant mass, a change from no-biopsy with DBT to biopsy with combined DBT/3D-AUS was defined as beneficial, and a change from biopsy to no-biopsy was defined as detrimental. The McNemar test was similarly applied to the benign masses to assess changes in specificity. Finally, the change in the average ratings for the two survey questions was assessed using the Student’s two-tailed paired t-test.
Results
ROC analysis
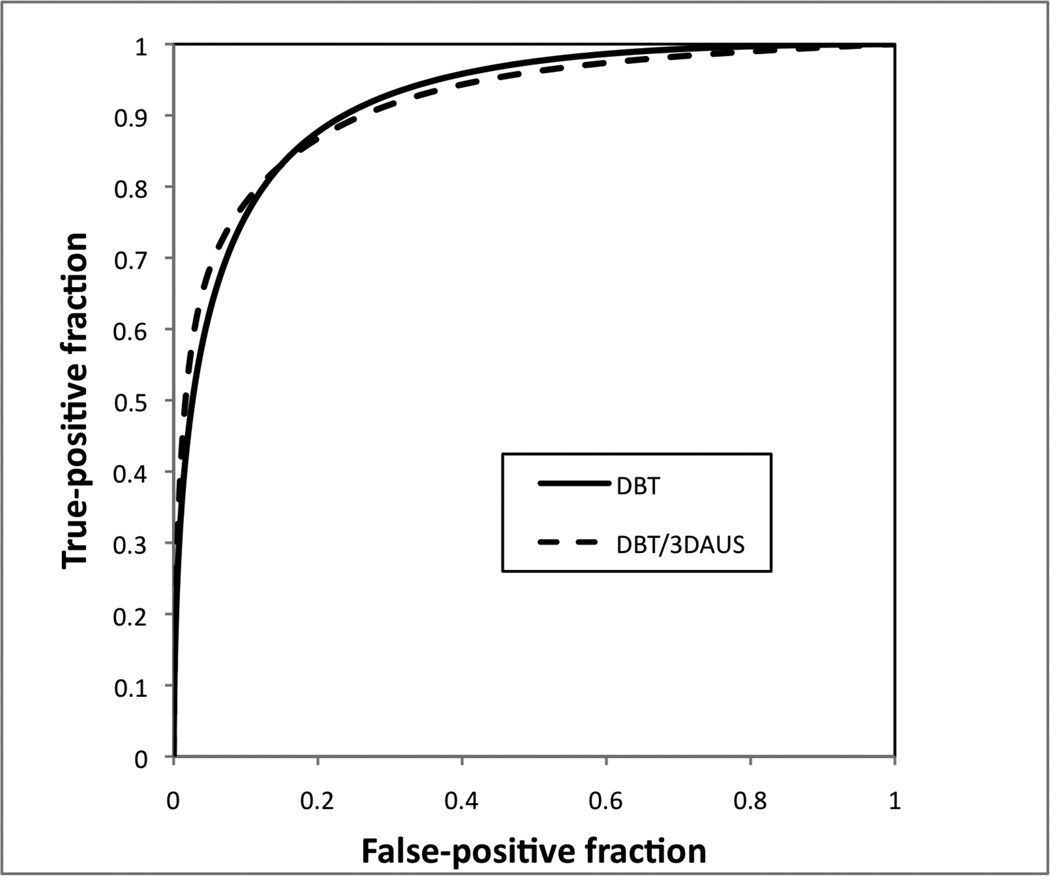
The average Az for LM across the 6 readers was 0.92 both for DBT alone (range 0.89–0.97) and combined DBT/3D-AUS (range 0.90–0.94), see Table 1 and Figure 1. The Az values increased for three radiologists, and decreased for two others, but these changes did not achieve statistical significance.
Table 1.
Az Values for 6 Radiologists in Characterization of Masses on DBT and DBT/3D-AUS
| Az | |||
|---|---|---|---|
| Radiologist No. | DBT | DBT/3D-AUS | P Value* |
| 1 | 0.92 | 0.93 | 0.67 |
| 2 | 0.91 | 0.91 | 0.88 |
| 3 | 0.93 | 0.94 | 0.77 |
| 4 | 0.89 | 0.90 | 0.78 |
| 5 | 0.92 | 0.91 | 0.76 |
| 6 | 0.97 | 0.94 | 0.29 |
| Average | 0.92 | 0.92 | 0.89 |
Note:
The significance of the change in Az value for the group of radiologists and for each radiologist was estimated using the Dorfman-Berbaum-Metz method.
Figure 1.
Graph shows the ROC curves for DBT alone and combined DBT/3D-AUS. The curves were constructed by taking the median of the True Positive Fraction among the readers for each value of the False Positive Fraction.
Sensitivity, specificity and changes in biopsy recommendations
With BI-RADS rating of 4 as the threshold for biopsy recommendation, the average sensitivity of radiologists increased from 96% for DBT alone to 100% for combined DBT/3-D AUS, while the specificity decreased from 33% to 25% (Table 2). With addition of 3D-AUS, three readers upgraded the same malignant mass to BI-RADS ≥4, and all malignant masses were correctly diagnosed by all six radiologists. The changes in sensitivity, specificity and biopsy recommendations did not achieve statistical significance (McNemar test, P values >0.99 for sensitivity and from 0.13 to 0.55 for specificity), except for radiologist #3, for whom the decrease in specificity was significant (P<0.05, McNemar test). On average per radiologist, the number of correct biopsy recommendations per reader (Table 3) was increased by 0.5/51 and the number of incorrect biopsy recommendations increased by 3.2/51 cases.
Table 2.
Sensitivity and Specificity for each radiologist at a decision threshold of BI-RADS 4
| Sensitivity | Specificity | |||||||
|---|---|---|---|---|---|---|---|---|
| Radiologist No. |
DBT | DB/3D-AUS | DBT | DB/3D-AUS | ||||
| 1 | 0.92 | (12) | 1.00 | (13) | 0.55 | (21) | 0.47 | (18) |
| 2 | 0.92 | (12) | 1.00 | (13) | 0.55 | (21) | 0.18 | (7)* |
| 3 | 1.00 | (13) | 1.00 | (13) | 0.45 | (17) | 0.26 | (10) |
| 4 | 0.92 | (12) | 1.00 | (13) | 0.29 | (11) | 0.21 | (8) |
| 5 | 1.00 | (13) | 1.00 | (13) | 0.00 | (0) | 0.11 | (4) |
| 6 | 1.00 | (13) | 1.00 | (13) | 0.16 | (6) | 0.26 | (10) |
| Average | 0.96 | (12.5) | 1.00 | (13) | 0.33 | (12.7) | 0.25 | (9.5) |
Note: The significance of the change in sensitivity and specificity for individual readers was estimated using the McNemar's test. Data in parentheses are the number of correctly classified lesions, i.e. true positive in the Sensitivity column and true negative in the Specificity column. The total numbers of malignant and benign lesions are 13 and 38, respectively.
P<0.01 (McNemar test)
Table 3.
Number of Biopsy Recommendations at a decision threshold of BI-RADS 4.
| DBT | DBT/3D-AUS | |||||
|---|---|---|---|---|---|---|
| Radiologist No. |
TP | FP | Total | TP | FP | Total |
| 1 | 12 | 17 | 29 | 13 | 20 | 33 |
| 2 | 12 | 17 | 29 | 13 | 31 | 44 |
| 3 | 13 | 22 | 35 | 13 | 27 | 40 |
| 4 | 12 | 27 | 39 | 13 | 31 | 44 |
| 5 | 13 | 38 | 51 | 13 | 34 | 47 |
| 6 | 13 | 32 | 45 | 13 | 28 | 41 |
| Average | 12.5 | 25.3 | 37.8 | 13 | 28.5 | 41.5 |
Note: Data are the numbers of biopsy recommendations for the malignant (TP :True Positive) and benign (FP : False Positive) cases. The total numbers of malignant and benign masses are 13 and 38, respectively.
Our study included a case of lobular carcinoma in situ, categorized as benign. This mass was recommended for biopsy by four readers with DBT and by three readers with DBT/3D-AUS.
Cyst cases
The changes in biopsy recommendations were analyzed specifically for the subgroup of the cyst cases. On average across the 6 readers, the number of biopsy recommendations for the 13 cyst cases decreased from 6.7 for DBT to 5.3 for combined DBT/3D-AUS (Tables 4 and 5). This was associated with a decrease in the average BI-RADS ratings per case, from 3.49 to 3.10 (p<0.1, Student ‘s two-tailed paired t-test).
Table 4.
Changes in Biopsy Recommendations (Change in BI-RADS categories from DBT to DBT/3D-AUS)
| Malignant Masses | Benign masses | Cysts | ||||
|---|---|---|---|---|---|---|
| Radiologist No. | Beneficial | Detrimental | Beneficial | Detrimental | Beneficial | Detrimental |
| 1 | 1 | 0 | 4 | 7 | 2 | 1 |
| 2 | 1 | 0 | 3 | 17 | 3 | 5 |
| 3 | 0 | 0 | 7 | 12 | 6 | 4 |
| 4 | 1 | 0 | 4 | 8 | 2 | 3 |
| 5 | 0 | 0 | 4 | 0 | 4 | 0 |
| 6 | 0 | 0 | 5 | 1 | 4 | 0 |
| Total | 3 | 0 | 27 | 45 | 21 | 13 |
Note: Change in BI-RADS categories denotes a change from category 1, 2 or 3 (“no biopsy”) to category 4, or 5 (“biopsy recommended”). A beneficial change for a malignant mass is a decision based on DBT/3D-AUS to recommend biopsy for the mass that was not recommended for biopsy based on DBT alone. A beneficial change for a benign mass (including the cysts) is a decision based on DBT/3D-AUS not to recommend biopsy for the mass that was recommended for biopsy based on DBT alone.
Table 5.
Number of Biopsy Recommendations for the cysts cases
| Number of Biopsy Recommendations |
||
|---|---|---|
| Radiologist No. | DBT | DBT/3D-AUS |
| 1 | 3 | 2 |
| 2 | 4 | 6 |
| 3 | 6 | 4 |
| 4 | 7 | 8 |
| 5 | 12 | 8 |
| 6 | 8 | 4 |
| Average | 6.7 | 5.3 |
Note: The change in biopsy recommendations was not significant for any of the 6 readers (p > 0.13, McNemar test). The number of cyst cases is 13.
Mass detection
Overall, masses were correctly located in 3D-AUS volumes in 96% of the readings of the study. Using the automated co-registration, one radiologist correctly located the masses on the ultrasound in all the 51 cases, one radiologist in 50 cases, one radiologist in 49 cases, two radiologists in 48 cases and a fifth radiologist in 47 of the cases. There were a total of four cases for which missed registration of the mass on 3D-AUS occurred. Of these 4 cases, there were a total of 13 false positive detections composed of 6 marks at wrong locations and 7 incorrect negative calls. From the ratings of the second reading, i.e. of the correct mass marked on 3D-AUS by the reference radiologists prior to the reading, it was observed that the readers recommended on average 2.5 of these 4 cases for biopsy with DBT, 3.5 with combined DBT/3D-AUS.
There were two 3D-AUS negative cases and each was called negative by all readers.
Survey question
The confidence of each reader to perform simultaneously screening and diagnostic examinations was significantly improved with the addition of 3D-AUS (p<0.05, Student’s two-tailed paired t-test, Table 6). Four radiologists perceived combined DBT/3D-AUS as potentially useful in some cases for screening and diagnosis during the same examination; two readers expressed a more neutral opinion: The average confidence ratings for the six readers were 4.5, 4.8, 4.6, 4.5, 3.1, 2.1 for the use of DBT alone, and respectively 6.3, 7.8, 8.0, 9.4, 7.9, 4.5 for the use of combined DBT/3D-AUS. The radiologists expressed an increased confidence when 3D-AUS was added, compared to DBT alone, in 80% of the cases, resulting in an 86% increase in the overall mean.
Table 6.
Average ratings on a 1–10 scale for the survey question concerning confidence of using DBT and combined DBT/3D-AUS for screening and diagnosis after reading DBT and DBT/3D-AUS volumes.
| Radiologist No. | DBT | DBT/3D-AUS |
|---|---|---|
| 1 | 4.5 | 6.3 |
| 2 | 4.8 | 7.8 |
| 3 | 4.6 | 8.0 |
| 4 | 4.5 | 9.4 |
| 5 | 3.1 | 7.9 |
| 6 | 2.1 | 4.5 |
Note: For every reader, the mean ratings with the two modalities were statistically different (p<0.05, Student’s two-tailed paired T-test).
Discussion
Half of all of the readers had a sensitivity of 100% with DBT alone. With the addition of 3D-AUS, the sensitivity for all readers was 100%. The average change in the number of true positives was small, +0.5 TP/reader with the addition of 3D-AUS, because half of the readers reached a sensitivity of 100% with DBT alone. The specificity decreased with the addition of 3D-AUS, and for one of the readers it decreased significantly. These results are reasonably consistent with other studies, given the design and small size of this initial study of a new technique. Specifically, in the other studies, when used in addition to mammography, 3D-AUS (and hand-held ultrasound40) increased the number of call-backs when used for screening6 or increased the number of false positives for mass characterization17.
Three readers slightly improved their Az with the addition of 3D-AUS. One had a substantial drop. Overall, no significant changes in the average Az across the 6 readers were observed. This result is not unexpected considering the high value of Az obtained with DBT alone in this study, leaving small room for improvement. In a previous study, it was found that when used as an adjunct to mammography for mass characterization17, 3D-AUS significantly improved the Az value from 0.87 (mammography alone) to 0.93 (mammography/3D-AUS) whereas we observed a stable Az value of 0.92 for DBT and combined DBT/3D-AUS.
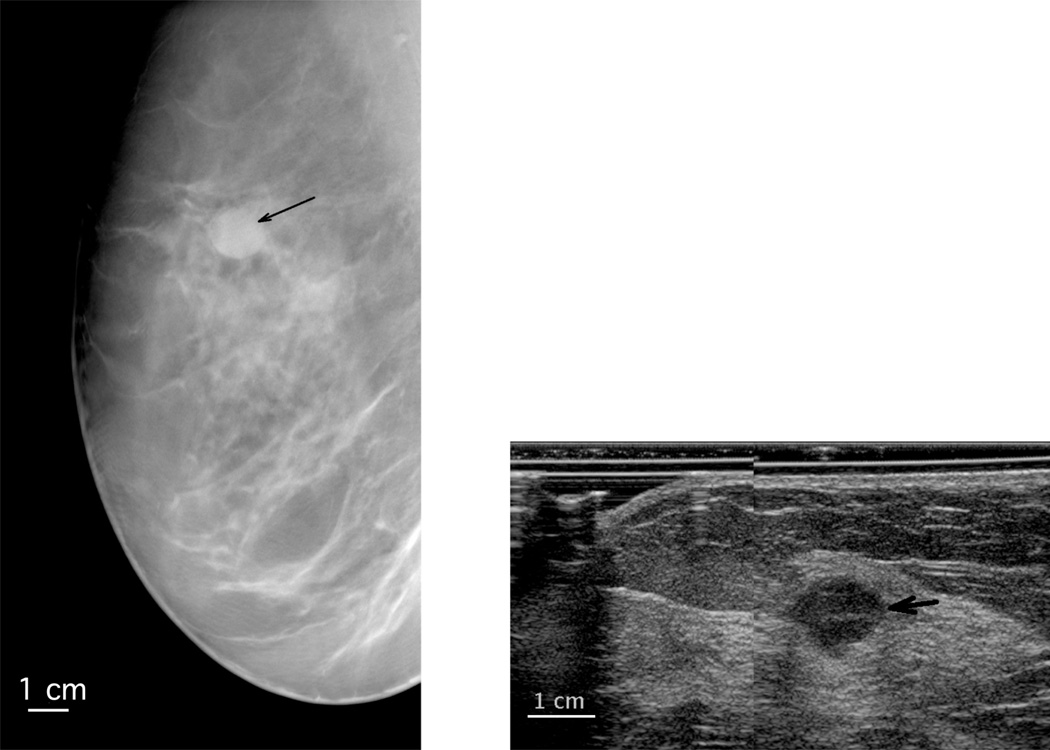
Despite our experience of improved mass visibility on DBT over mammography21, 26, 28, 30, the lack of outstanding results for DBT in much of the literature41 and the malignant case that was misdiagnosed in our present study by three radiologists with DBT alone and was corrected with the addition of 3D-AUS, suggest a continuing need for the use of ultrasound for mass characterization. The DBT and 3D-AUS images of the case misdiagnosed by three radiologists with DBT alone are shown in Figure 2. The tomosynthesis image demonstrates an oval mass with circumscribed margins, features that would lead a radiologist to believe that it would likely be a cyst or a fibroadenoma. The 3D-AUS image demonstrates that the mass is hypoechoic, and has slightly indistinct margins. These features indicate that it is not a simple cyst. If the mass were new or unknown in stability, it would need FNA or biopsy to determine whether it was a complicated cyst, a fibroadenoma, or a malignancy.
Figure 2.
Sections extracted from the LMO views of a metaplastic carcinoma in a right breast (left DBT, right 3D-AUS, the black arrow points toward the mass), diagnosed as benign on DBT by three readers. The AUS image is from a plane normal to the image plane of the DBT at the level of the white arrows on the DBT. The ultrasound is magnified relative to the DBT.
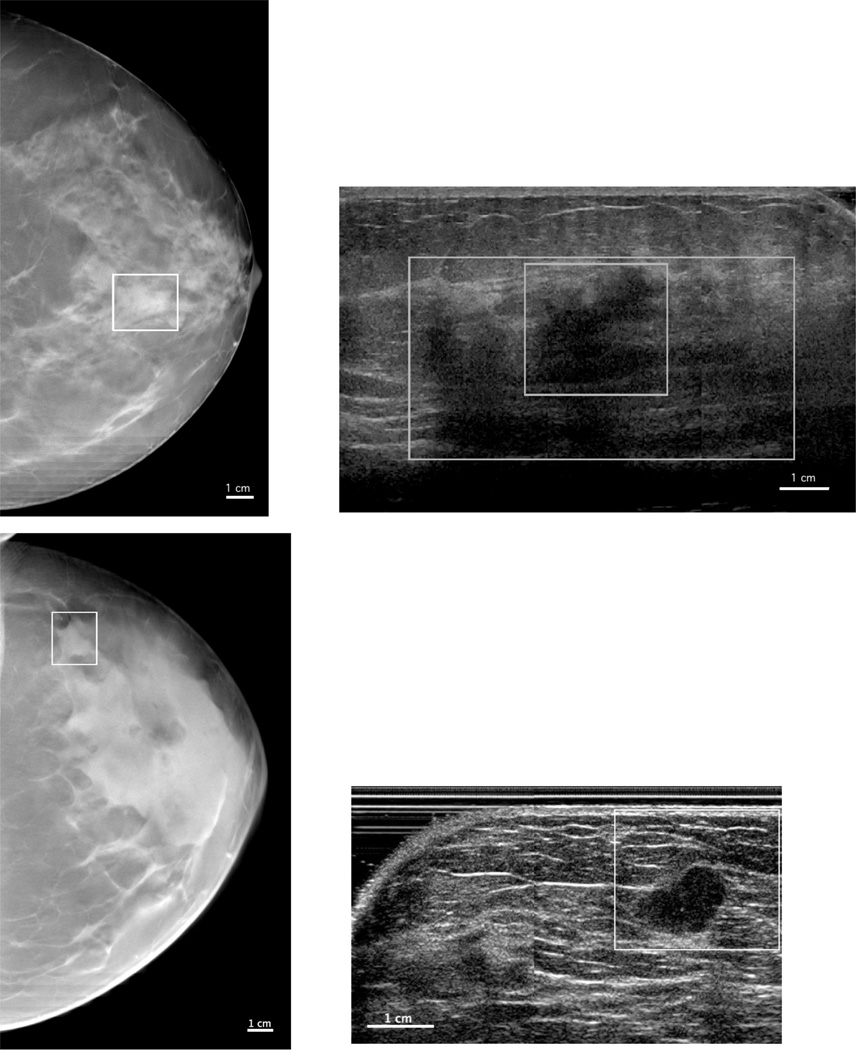
3D-AUS also could be important for delineating complete extension of masses and for imaging masses in dense breasts, as illustrated in Figure 3. For the top case in this figure, 3D-AUS shows the complete delineation of the mass (large box), which is larger than initially seen on DBT and confirmed by pathology after mass excision. For the bottom case in this figure, DBT, and also the mammogram were so dense that the mass was only distinguishable because of the surrounding fat on three sides, while the mass is clearly evident in the 3D-AUS images.
Figure 3.
DBT slices (left) and 3D-AUS sections (right) for two invasive ductal carcinomas. The solid line boxes indicate the boundaries of the region of interest containing the mass on the DBT sections and the co-registered ROI on the 3D-AUS sections. The large box on the top-right 3D-AUS image shows the complete delineation of the mass as confirmed by pathology after mass excision.
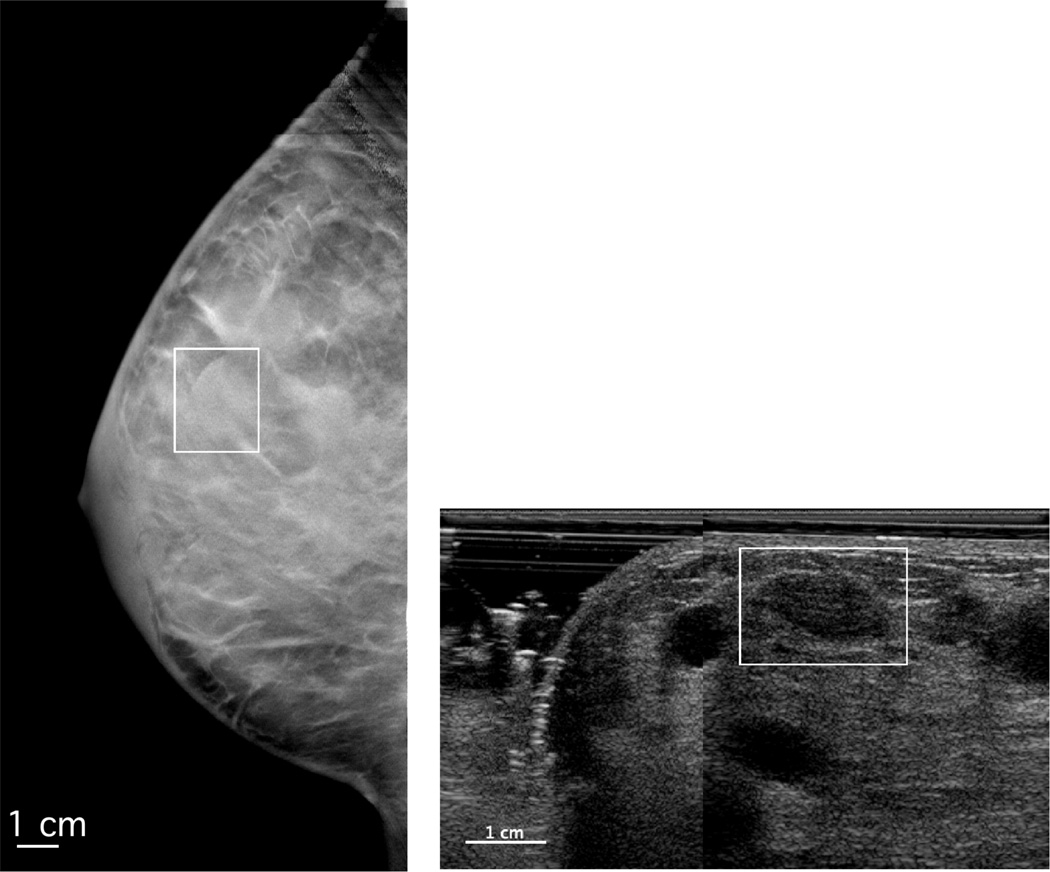
Some false positive results may be explained by suboptimal quality of some of the 3D-AUS images when scanned from only one side of the breast as in this study. Breast ultrasound imaging using mammographic compression may cause reverberation artifacts in cysts as illustrated in Figure 4. Reverberation artifacts are observed inside the mass on the 3-AUS image, probably due to reverberation involving the solid compression paddle and hyperechoic structures located above the cyst The orientations of these structures, in mammographic compression geometry, are more perpendicular to the direction of propagation of the ultrasound beam than they would be for a hand-held ultrasound scan, resulting in stronger echoes. Limitations in image quality may also result from an increased distance between the ultrasound probe and the target mass in mammographic compression geometry compared to the hand-held ultrasound, and from scattering by tissue structures42, particularly structures not as flattened as they would be in hand-held ultrasound optimized images, or from the presence of air bubbles in the coupling media7, 9, 12, 13, especially in the pari-areolar and lateral breast9. Also in 3D-AUS as performed here there is limited coverage of the breast, responsible for the exclusion of 25% of the research cases.
Figure 4.
DBT slice (left) and 3D-AUS (right) of a cyst case. The boxes indicate the boundary of the region of interest containing the mass on the DBT slice and the co-registered ROI on the 3D-AUS section. Reverberation artifacts can be observed inside the on the 3D-AUS image.
For almost ¾ of these excluded cases, the mass was located too far posterior, an area of the breast difficult to image with ultrasound when the transducer beam is oriented in a cranio-caudal direction while the breast in mammographic compression. Improvements should come from the use of multiple 3D-AUS views. We plan systematic acquisitions of two views of 3D-AUS in our future protocol, providing two volumes co-registered with two DBT volumes, improving the coverage and field of view for the ultrasound imaging, and potentially also allowing discrimination of acoustic shadowing artifacts. For the cases excluded because the mass was located at the edge of the breast, the issue usually arose from an ineffective coupling of the transducer/gel to the skin. We recently developed greatly improved techniques for gel positioning and maintenance in the large gaps between the transducer/compression paddle surface and the edge of the breast.
Substantial improvements in 3D-AUS image quality could also be obtained with the use of the compound imaging mode, speckle reduction, better acoustic coupling via new coupling gel control and application methods9, a new mesh paddle35, scanning from both sides of the breast43 with transducers shaped to allow imaging close to the chest wall, other enhanced scanning and acquisition techniques44, 45 and multiple 3D-AUS views.
In our study, automatic registration was perceived as very useful by the radiologists, and resulted in the correct identification of masses in 96% of the readings. Missed registrations were concentrated in four cases, for which 3D-AUS volumes were found very difficult to interpret due to a variety of limitations: the depth and very small size of the masses, or an offset in the registration probably caused by an inadvertent posterior pull-back of the breast by the patient between the DBT and 3D-AUS scans.
The majority of the radiologists perceived combined DBT/3D-AUS as potentially useful for screening and diagnosis of some cases during the same examination. The question of the usefulness of screening with ultrasound is still debated, but several major studies reported improved incremental cancer detection with hand-held ultrasound1, 40, 46, 47 and AUS6, 18. Similar BI-RADS classification of cysts between hand-held ultrasound and 3D-AUS has also been reported7. If ultrasound’s full potential to differentiate simple cysts from solid masses can be confirmed in a system combining it with DBT, as reported here, that system could be useful for both screening and diagnostic workup of many cases in a single examination. This is especially important because simple cysts constitute 25–27% of all palpable or mammographically detected lesions in the general diagnostic population46, 48. This role of 3D-AUS should be particularly efficacious and could therefore improve the potential23 of DBT in screening and diagnostic breast imaging practices.
One commercial breast tomosynthesis system recently received FDA approval, and the role of tomosynthesis in breast imaging is likely to increase rapidly. 3D-AUS is compatible with the design of mammography and tomosynthesis systems and could be implemented using clinical ultrasound scanners, at the cost of the installation of ultrasound compatible compression paddle(s) with one or two motorized transducer carriages and controls. Reading time of combined system data could be reduced by the use of CAD49, 50,51 and automatic registration52, to reach an acceptable standard for clinical practice. The use of CAD could also improve mass characterization17, 53, particularly given coregistered ultrasound and DBT.
The prevalence of cancer in our study population was higher than that in a diagnostic population in clinical practice, but it has been reported that a prevalence between 2 and 28% would not significantly affect the outcomes of an ROC study54.
Our study has a number of limitations.
First, the inclusion criteria could introduce unintended bias towards tomosynthesis. This study being a characterization study, only cases for which the mass was seen on DBT and was in the field of view of the ultrasound were used. Second, because biopsy results were used as truth, all of the masses that were analyzed had been recommended for biopsy after mammographic and hand-held ultrasound imaging. Thus masses present in a diagnostic population diagnosed as benign after mammographic and hand-held ultrasound imaging were not included. Third, the study involved only 51 patients and 6 readers, thereby limiting our ability to assess small differences between the two modalities. Fourth, all observers from our study were experienced radiologists in breast imaging, with some experience with DBT reading. Our results may thus not generalize to radiologists with different experience.
In summary, we reported the first of one of the early studies of ultrasound with tomosynthesis, as they may trigger interest for automated ultrasound in this geometry, and show some directions for further improvements. In this pilot reader study, the characterization of masses by the radiologists was not statistically improved by the addition of 3D-AUS. However, we tentatively believe these preliminary results are encouraging in that: 1) the addition of 3D-AUS led to the diagnosis of one additional malignant case by half of the readers, and 2) a trend towards a decrease in the number of biopsy recommendations was observed for the cysts cases. The realized 3D-AUS image quality and breast coverage may be a limiting factor of the employed prototype, especially for masses identification, but substantial improvements are possible. The majority of the readers indicated an increased confidence in potentially performing simultaneous screening and diagnostic examinations with a combined DBT/3D-AUS system. Assessing the performance of a revised combined system in an enhanced screening population will be the objective of future studies.
Acknowledgments
We thank Mark Helvie, Chris Lashbrook, Lisa DeCaussin, Jeanne Hill, Becca Booi, PhD (currently with Sg2, Inc, Chicago, IL), and Ganesh Narayanasamy, PhD (currently with University of Kentucky, Lexington, KY), all from the Department of Radiology at the University of Michigan; Anne Hall, PhD, of GE Healthcare (Milwaukee, WI); Jeff Eberhard, PhD, and Andrea Schmitz, MS, for system development; Kai Thomenius, PhD, Carl Chalek, MS, and numerous other staff and scientists at GE Global Research (Niskayuna, NY); and Lorenzo Pesce, PhD, from the Department of Radiology at the University of Chicago, for fruitful discussions about receiver operating characteristic curve statistics. This study used a digital breast tomosynthesis unit prototype and an initial automated ultrasound system developed by General Electric Global Research with the support of the University of Michigan under National Institutes of Health grant RO1 CA 091713 and the Uniformed Services University of the Health Sciences under Office of Naval Research grant MDA9050210012.
Abbreviations
- AUS
automated ultrasound
- Az
area under the curve
- BI-RADS
Breast Imaging Reporting and Data System
- DBT
digital breast tomosynthesis
- ROC
receiver operating characteristic
- 3D
3-dimensional
- US
ultrasound
References
- 1.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002 Oct;225(1):165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 2.Jackson VP. The role of US in breast imaging. Radiology. 1990 Nov;177(2):305–311. doi: 10.1148/radiology.177.2.2217759. [DOI] [PubMed] [Google Scholar]
- 3.Skaane P, Engedal K. Analysis of sonographic features in the differentiation of fibroadenoma and invasive ductal carcinoma. AJR Am J Roentgenol. 1998 Jan;170(1):109–114. doi: 10.2214/ajr.170.1.9423610. [DOI] [PubMed] [Google Scholar]
- 4.Stavros AT, Thickman D, Rapp CL, et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995 Jul;196(1):123–134. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- 5.Taylor KJ, Merritt C, Piccoli C, et al. Ultrasound as a complement to mammography and breast examination to characterize breast masses. Ultrasound Med Biol. 2002 Jan;28(1):19–26. doi: 10.1016/s0301-5629(01)00491-4. [DOI] [PubMed] [Google Scholar]
- 6.Kelly KM, Dean J, Comulada WS, et al. Breast cancer detection using automated whole breast ultrasound and mammography in radiographically dense breasts. Eur Radiol. 2010 Mar;20(3):734–742. doi: 10.1007/s00330-009-1588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenkel E, Heckmann M, Heinrich M, et al. Automated breast ultrasound: Lesion detection and BI-RADS (TM) classification - a pilot study. Rofo-Fortschr Rontg. 2008 Sep;180(9):804–808. doi: 10.1055/s-2008-1027563. [DOI] [PubMed] [Google Scholar]
- 8.Goodsitt MM, Chan HP, Hadjiiski LM, et al. Automated registration of volumes of interest for a combined x-ray tomosynthesis and ultrasound breast imaging system. Lect Notes Comput Sc. 2008;5116:463–468. [Google Scholar]
- 9.Li J, Goodsitt MM, Padilla F, et al. Effect of a gel retainment dam on automated ultrasound coverage in a dual-modality breast imaging system. J Ultrasound Med. 2010 Jul;29(7):1075–1081. doi: 10.7863/jum.2010.29.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha SP, Goodsitt MM, Roubidoux MA, et al. Automated ultrasound scanning on a dual-modality breast imaging system: coverage and motion issues and solutions. J Ultrasound Med. 2007 May;26(5):645–655. doi: 10.7863/jum.2007.26.5.645. [DOI] [PubMed] [Google Scholar]
- 11.Sinha SP, Roubidoux MA, Helvie MA, et al. Multi-modality 3D breast imaging with X-Ray tomosynthesis and automated ultrasound. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:1335–1338. doi: 10.1109/IEMBS.2007.4352544. [DOI] [PubMed] [Google Scholar]
- 12.Kotsianos-Hermle D, Hiltawsky KM, Wirth S, et al. Analysis of 107 breast lesions with automated 3D ultrasound and comparison with mammography and manual ultrasound. Eur J Radiol. 2009 Jul;71(1):109–115. doi: 10.1016/j.ejrad.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Kotsianos-Hermle D, Wirth S, Fischer T, et al. First clinical use of a standardized three-dimensional ultrasound for breast imaging. Eur J Radiol. 2009 Jul;71(1):102–108. doi: 10.1016/j.ejrad.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Richter K, Prihoda H, Heywang-Köbrunner SH, et al. Description and first clinical use of a new system for combined mammography and automated clinical amplitude/velocity reconstructive imaging breast sonography. Invest Radiol. 1997;32(1):19–28. doi: 10.1097/00004424-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Richter K, Willrodt RG, Opri F, et al. Differentiation of breast lesions by measurements under craniocaudal and lateromedial compression using a new sonographic method. Invest Radiol. 1996;31(7):401–414. doi: 10.1097/00004424-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Callahan KS, Borup DT, Jonhson SA, et al. Transmission breast ultrasound imaging: Representative case studies of speed of sound and attenuation of sound computed tomographic images. Am. J. Clin. Oncol. 2007;30:458–459. [Google Scholar]
- 17.Sahiner B, Chan HP, Hadjiiski LM, et al. Multi-modality CADx: ROC study of the effect on radiologists' accuracy in characterizing breast masses on mammograms and 3D ultrasound images. Acad Radiol. 2009 Jul;16(7):810–818. doi: 10.1016/j.acra.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly KM, Dean J, Lee SJ, et al. Breast cancer detection: radiologists' performance using mammography with and without automated whole-breast ultrasound. Eur Radiol. 2010 Jul 15; doi: 10.1007/s00330-010-1844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway WF, Hayes CW, Brewer WH. Occult Breast Masses - Use of a Mammographic Localizing Grid for Us Evaluation. Radiology. 1991 Oct;181(1):143–146. doi: 10.1148/radiology.181.1.1653441. [DOI] [PubMed] [Google Scholar]
- 20.Berg WA, Woel BS. Mammographic–Sonographic Correlation. Ultrasound Clinics. 2007;1:567–591. [Google Scholar]
- 21.Helvie MA. Digital mammography imaging: breast tomosynthesis and advanced applications. Radiol Clin North Am. 2010 Sep;48(5):917–929. doi: 10.1016/j.rcl.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gennaro G, Baldan E, Bezzon E, et al. Clinical performance of digital breast tomosynthesis versus full-field digital mammography: Preliminary results. Lect Notes Comput Sc. 2008;5116:477–482. 769. [Google Scholar]
- 23.Good WF, Abrams GS, Catullo VJ, et al. Digital breast tomosynthesis: a pilot observer study. AJR Am J Roentgenol. 2008 Apr;190(4):865–869. doi: 10.2214/AJR.07.2841. [DOI] [PubMed] [Google Scholar]
- 24.Poplack SP, Tosteson TD, Kogel CA, et al. Digital breast tomosynthesis: initial experience in 98 women with abnormal digital screening mammography. AJR Am J Roentgenol. 2007 Sep;189(3):616–623. doi: 10.2214/AJR.07.2231. [DOI] [PubMed] [Google Scholar]
- 25.Andersson I, Ikeda DM, Zackrisson S, et al. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur Radiol. 2008 Dec;18(12):2817–2825. doi: 10.1007/s00330-008-1076-9. [DOI] [PubMed] [Google Scholar]
- 26.Helvie MA, Roubidoux MA, Zhang Y, et al. Tomosynthesis mammography versus conventional mammography: lesion detection and reader preference - initial experience. Radiologic Society of North America 92nd Scientific Assembly and Annual Meeting; Chicago, IL. 2006. [Google Scholar]
- 27.Lo J, Durham N, Orman J, et al. Breast tomosynthesis: initial clinical experience with 100 human subjects. Radiologic Society of North America 92nd Scientific Assembly and Annual Meeting; Chicago, IL. 2006. [Google Scholar]
- 28.Helvie MA, Roubidoux MA, Hadjiiski LM, et al. Research Digital Tomosynthesis Mammography: Detection of T1 Invasive Breast Carcinomas not Diagnosed by Conventional Breast Imaging or Physical Exam. 94th Scientific Assembly and Annual Meeting of the Radiological Society of North America; Vol RSNA Program 2008; Chicago, IL. 2008. p. 468. [Google Scholar]
- 29.Helvie MA, Hadjiiski LM, Goodsitt MM, et al. Characterization of Benign and Malignant Breast Masses by Digital Breast Tomosynthesis Mammography. 94th Scientific Assembly and Annual Meeting of the Radiological Society of North America; Vol RSNA Program 2008; Chicago, IL. 2008. p. 325. [Google Scholar]
- 30.Helvie MA, Roubidoux MA, Hadjiiski LM, et al. Tomosynthesis Mammography vs Conventional Mammography: Comparison of Breast Masses Detection and Characterization. 93rd Scientific Assembly and Annual Meeting of the Radiological Society of North America; Vol RSNA Program 2007; Chicago, IL. 2007. p. 381. [Google Scholar]
- 31.Teertstra HJ, Loo CE, van den Bosch MAAJ, et al. Breast tomosynthesis in clinical practice: initial results. European Radiology. 2010 Jan;20(1):16–24. doi: 10.1007/s00330-009-1523-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Chan HP, Sahiner B, et al. A comparative study of limited-angle cone-beam reconstruction methods for breast tomosynthesis. Med Phys. 2006 Oct;33(10):3781–3795. doi: 10.1118/1.223754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booi RC, Krucker JF, Goodsitt MM, et al. Evaluating thin compression paddles for mammographically compatible ultrasound. Ultrasound Med Biol. 2007 Mar;33(3):472–482. doi: 10.1016/j.ultrasmedbio.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapur A, Carson PL, Eberhard J, et al. Combination of digital mammography with semi-automated 3D breast ultrasound. Technol Cancer Res Treat. 2004 Aug;3(4):325–334. doi: 10.1177/153303460400300402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blane CE, Goodsitt MM, Grimm JC, et al. New compression paddle for wire localization in mammography. Acad Radiol. 2010 Feb;17(2):142–145. doi: 10.1016/j.acra.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Wagner RF, Metz CE, Campbell G. Assessment of medical imaging systems and computer aids: a tutorial review. Acad Radiol. 2007 Jun;14(6):723–748. doi: 10.1016/j.acra.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Metz CE. Some practical issues of experimental design and data analysis in radiological ROC studies. Invest Radiol. 1989;24:234–245. doi: 10.1097/00004424-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Dorfman DD, Berbaum KS, Metz CE. Receiver Operating Characteristic Rating Analysis - Generalization to the Population of Readers and Patients with the Jackknife Method. Invest Radiol. 1992 Sep;27(9):723–731. [PubMed] [Google Scholar]
- 39.Pesce LL, Metz CE. Reliable and computationally efficient maximum-likelihood estimation of "proper" binormal ROC curves. Acad Radiol. 2007 Jul;14(7):814–829. doi: 10.1016/j.acra.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008 May 14;299(18):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diekmann F, Bick U. Breast Tomosynthesis. Semin Ultrasound Ct. 2011 Aug;32(4):281–287. doi: 10.1053/j.sult.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Hovanessian Larsen L, Wallman M, Johnson M, et al. Can Automated Whole Breast Ultrasound Replace Hand-held Ultrasound in a Busy Diagnostic Breast Center. RSNA 2008; Chicago, Illinois. 2008. [Google Scholar]
- 43.Carson PL, Fouzaan Z, van der Spek S, et al. Dual sided automated ultrasound system in the mammographic geometry. Paper presented at: IEEE International Ultrasonics Symposium; Orlando, FL. 2011. [Google Scholar]
- 44.Tozaki M, Isobe S, Yamaguchi M, et al. Optimal scanning technique to cover the whole breast using an automated breast volume scanner. Jpn J Radiol. 2010 May;28(4):325–328. doi: 10.1007/s11604-010-0424-2. [DOI] [PubMed] [Google Scholar]
- 45.Carson PL, Wang B, LeCarpentier GL, et al. Local Compression in Automated Breast Ultrasound in the Mammographic Geometry. Paper presented at: 2010 IEEE Int Ultrasonics Symp.; San Diego. 2010. [Google Scholar]
- 46.Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: Detection with screening US - Diagnostic yield and tumor characteristics. Radiology. 1998 Apr;207(1):191–199. doi: 10.1148/radiology.207.1.9530316. [DOI] [PubMed] [Google Scholar]
- 47.Berg WA. Rationale for a trial of screening breast ultrasound: American College of Radiology Imaging Network (ACRIN) 6666. AJR Am J Roentgenol. 2003 May;180(5):1225–1228. doi: 10.2214/ajr.180.5.1801225. [DOI] [PubMed] [Google Scholar]
- 48.Hilton SV, Leopold GR, Olson LK, et al. Real-time breast sonography: application in 300 consecutive patients. AJR Am J Roentgenol. 1986 Sep;147(3):479–486. doi: 10.2214/ajr.147.3.479. [DOI] [PubMed] [Google Scholar]
- 49.Chang RF, Chang-Chien KC, Takada E, et al. Rapid image stitching and computer-aided detection for multipass automated breast ultrasound. Medical Physics. 2010 May;37(5):2063–2073. doi: 10.1118/1.3377775. [DOI] [PubMed] [Google Scholar]
- 50.Ikedo Y, Fukuoka D, Hara T, et al. Computerized mass detection in whole breast ultrasound images: Reduction of false positives using bilateral subtraction technique; Paper presented at: Medical Imaging 2007: Computer-Aided Diagnosis, Proceedings of SPIE Volume: 65142007. [Google Scholar]
- 51.Chan HP, Wei J, Zhang YH, et al. Computer-aided detection of masses in digital tomosynthesis mammography: Comparison of three approaches. Medical Physics. 2008 Sep;35(9):4087–4095. doi: 10.1118/1.2968098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sinha SP, Roubidoux MA, Goodsitt MM, et al. ime-Efficient Mass Localization on Automated Breast Ultrasound With Dual-Modality Information. In: Med JU, editor. AIUM Annual Convention. New York: 2009. p. S40. abstract only. [Google Scholar]
- 53.Chan HP, Wu YT, Sahiner B, et al. Characterization of masses in digital breast tomosynthesis: Comparison of machine learning in projection views and reconstructed slices. Medical Physics. 2010 Jul;37(7):3576–3586. doi: 10.1118/1.3432570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gur D, Rockette HE, Armfield DR, et al. Prevalence effect in a laboratory environment. Radiology. 2003 Jul;228(1):10–14. doi: 10.1148/radiol.2281020709. [DOI] [PubMed] [Google Scholar]