Abstract
Purinergic signaling and associated ectonucleotidases, such as CD39 and CD73, have been implicated in the pathogenesis of inflammatory bowel disease (IBD). CD39 is known to be a Treg memory cell marker, and here we determine the phenotype and function of CD73+CD4+ T lymphocytes in patients with IBD. We describe elevated levels of CD73+CD4+ T cells in the peripheral blood and intestinal lamina propria of patients with active IBD. The functional phenotype of these CD73+CD4+ T cells was further determined by gene expression, ecto-enzymatic activity, and suppressive assays. Increased numbers of CD73+CD4+ T cells in the periphery and lamina propria were noted during active inflammation, which returned to baseline levels following anti-TNF treatment. Peripheral CD73+CD4+ T cells predominantly expressed CD45RO, and were enriched with IL-17A+ cells. The CD73+CD4+ cell population expressed higher levels of RORC, IL-17A, and TNF, and lower levels of FOXP3 and/or CD25, than CD73−CD4+ T cells. Expression of CD73 by peripheral CD4+ T cells was increased by TNF, and decreased by an anti-TNF monoclonal antibody (infliximab). In vitro, these peripheral CD73+CD4+ T cells did not suppress proliferation of CD25− effector cells, and expressed higher levels of pro-inflammatory markers. We conclude that the CD73+CD4+ T-cell population in patients with active IBD are enriched with cells with a T-helper type 17 phenotype, and could be used to monitor disease activity during treatment.
Keywords: CD73, Crohn’s disease, ectonucleotidase, inflammatory bowel disease, T lymphocytes
Introduction
There is genetic and experimental evidence to support a role for purinergic pathways in the regulation of mucosal immune responses in inflammatory bowel disease (IBD) [1, 2]. Chronic intestinal inflammation leads to tissue damage and release of adenine nucleotides (adenosine triphosphate (ATP), adenosine diphosphate, and others). These extracellular nucleotides have been shown to elicit expression of TNF, IL-6, and IL-23, and induce differentiation of T-helper type 17 cells (Th17); all putative factors in the pathogenesis of IBD [3]. Recent data have also demonstrated that ATP generated by certain luminal commensal microbes turns on inflammatory Th17 responses in the intestinal mucosa [3].
In vivo, cell-surface ectonucleotidases, such as CD39, on T lymphocytes can convert extracellular ATP/adenosine diphosphate to pericellular adenosine [4]. Adenosine has been demonstrated to have potent anti-inflammatory properties in animal models of Crohn’s disease, via binding the adenosine type 2A receptor [5,6]. We have shown that the expression of CD39 on human and mouse can confer either a regulatory or memory-effector phenotype, associated with the relative expression of CD25 and FOXP3 by these cells [7]. In addition, we have reported that polymorphisms in the CD39 gene increase susceptibility to Crohn’s disease, and that mice null for CD39 exhibit severe experimental colitis [1].
CD73 is a functionally related ecto-enzyme to CD39, that has ecto-5′-nucleotidase catalytic activity, leading to the in-tandem generation of adenosine. In the acute inflammatory phase of TNBS colitis in mice, CD73 appears to have a regulatory role mediated via IFN-α [2]. However, immunological studies in mice have also reported that CD73 is expressed by both regulatory and primed precursor Th (Thpp) cell populations [8]. Stimulation of Thpp-like CD73+ cells in Th1-polarizing conditions induced differentiation into populations producing mainly IFN-γ, a key cytokine in IBD [9]. This apparent property of ectonucleotidases to denote both regulatory or memory T cells of Th1-, Th2-, or Th17-types in mouse models may indicate a role in confirming conditional plasticity on such cells [10, 11]. For example, we have previously shown that the presence of CD39, but not CD73, confers immune-suppressive properties on human Th17 cells to inhibit effector T-cell function [7]. We have also reported that murine Treg cells express both CD73 and CD39, but that CD73 is largely absent on human Treg cells [7].
Bioinformatic analysis of gene expression in PBMCs, and colonic biopsies from patients with chronic IBD suggest that CD73 expression is increased in patients with chronic active Crohn’s disease [12]. However, pathogenic roles of such alterations in CD73 activity, and nature of expression, by immune cells in chronic human IBD has been unexplored to date. We have now characterized the role of this ecto-enzyme in IBD by measuring its prevalence and activity in peripheral and lamina propria (LP) T cells in patients before and after treatment, and assessing the in vitro phenotype and characteristics of such cells.
Results
Peripheral blood and LP CD73+CD4+ T cells in patients with IBD
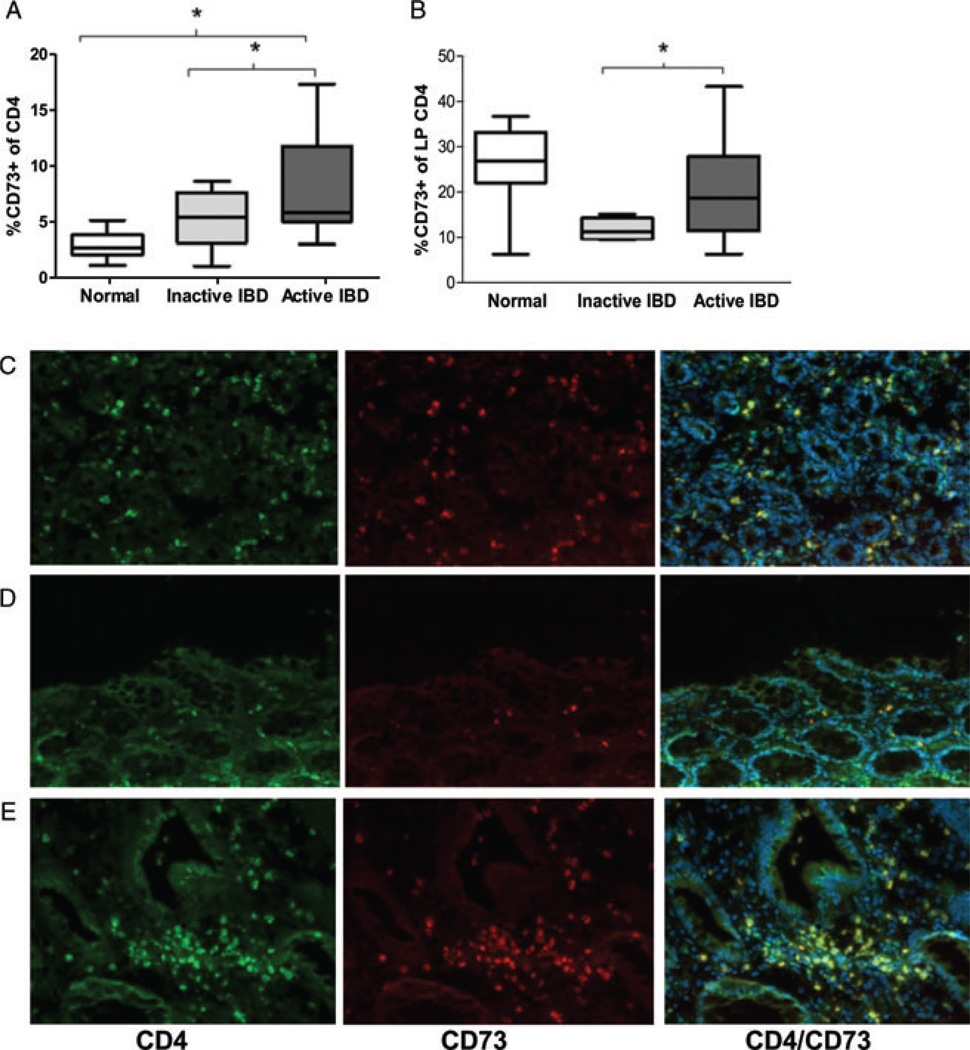
The proportions of CD4+ cells expressing CD73 represent a small component of all CD4+ cells in the peripheral blood of healthy individuals (Fig. 1A). However, in patients with active chronic intestinal inflammation (IBD), higher proportions of peripheral CD73+CD4+ T cells were noted (mean 8.2% SD 4.4%) compared to healthy controls (2.9% SD 1.3%), and patients in remission (5.5% SD 2.9%) (ANOVA p = 0.004) (Fig. 1A). There was no clear difference in the proportion of peripheral blood CD39+CD4+ T cells in patients with IBD compared to controls (data not shown).
Figure 1.
CD73 expression by CD4+ T lymphocytes in patients with IBD. (A) Box and whisker plots showing proportion (by flow cytometry) of peripheral blood CD4+ T cells expressing CD73 in healthy donors (white) and patients with clinically quiescent IBD (light gray) and clinically active IBD (dark gray). Data are shown as median, interquartile range, and the range of ten patients/controls per group and are pooled from 30 experiments performed. *p < 0.05 by Student’s t-test. (B) Box and whisker plots showing proportion (by flow cytometry) of LP CD4+ T cells expressing CD73 in healthy donors (white) and patients with inactive IBD (light gray) and active IBD (dark gray). Data are shown as median, interquartile range, and the range of ten patients/controls per group and are pooled from 30 experiments performed. *p < 0.05 by Student’s t-test. (C–E) Colonic biopsies were subjected to immunofluorescent staining for CD4 (bright green), CD73 (red), and co-localized CD4/CD73 (yellow), with Hoechst nuclear counterstain (blue). Representative sections from biopsied tissue from (C) an apparently healthy individual, (D) a patient with inactive IBD, and (E) a patient with active IBD. Magnification 20×. Data shown are representative of three samples examined from six experiments performed.
Since the intestinal LP is the site of ongoing lymphocytic infiltration and tissue destruction in patients with IBD, we next sought to determine whether there were differences in CD73 expression on the CD4+ T cells present in this cellular compartment. Patients with active IBD had a significantly higher proportion of CD73+CD4+ T cells (mean 20.6% SD 13.9%), than those with inactive disease (11.7% SD 2.4%) (p = 0.04 by t-test). There was no significant difference in the percentage of CD73+CD4+ T cells in the LP of patients with active IBD, when compared to healthy controls (25.8% SD 10.4%) (Fig. 1B). However, the phenotype of LP CD73+CD4+ T cells did differ substantially between healthy controls and patients with active IBD with respect to their expression of CD39, but not CD161 (Supporting Information Fig. 1A–C). Immunohistochemical stains confirmed CD73+CD4+ T cells in the LP of both controls and patients with IBD, with diffuse staining patterns in healthy controls (Fig. 1C), low levels in patients in remission on maintenance therapy (Fig. 1D), but intense pericryptal clustering of positive-staining cells in untreated active IBD (Fig. 1E).
Peripheral and LP CD73+CD4+ T cells exhibit a memory phenotype
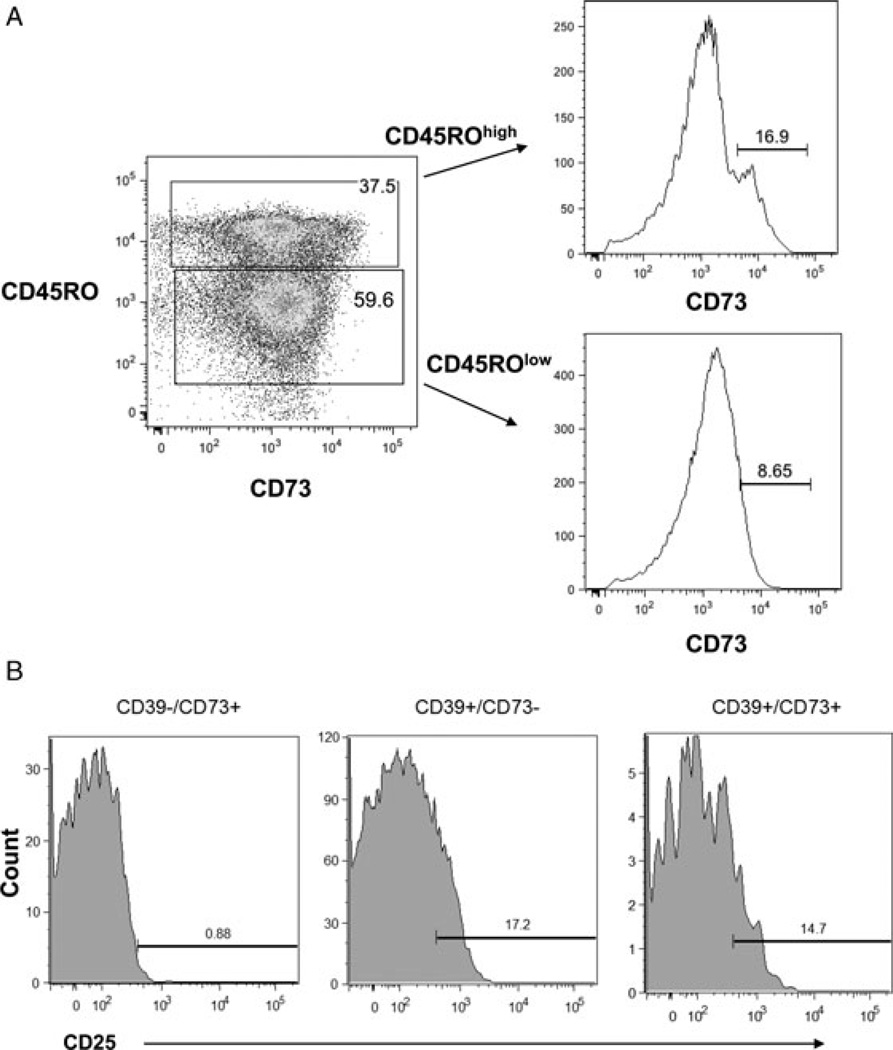
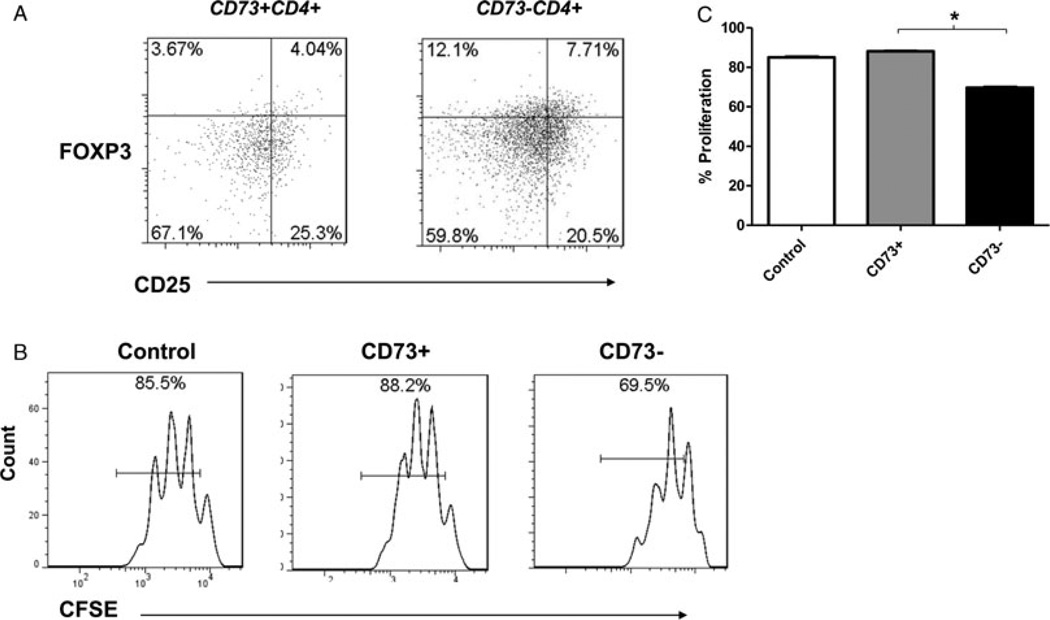
We next sought to characterize the phenotype of these CD73+CD4+ T cells in the peripheral blood of patients with active IBD. In patients with active CD, more CD73+CD4+ T cells are CD45ROhigh (memory marker) than CD45ROlow, consistent with a memory cell phenotype (Fig. 2A and Supporting Information Fig. 2A). In addition, the majority of circulating CD4+ T cells expressing high levels of CD73 lack classic Treg cell markers such as CD25, in contrast to CD39+ Treg cells (Fig. 2B). While CD4+ cells that are both CD25bright and express FOXP3 (both Treg cell markers) had significant enrichment for CD39 expression, only minor relative increases in CD73 expression were noted in human Treg cells (Supporting Information Fig. 2B), in keeping with our prior data [13]. Similar nonregulatory profiles were seen in isolated LP CD4+ cells from patients with IBD; only 4% of CD73+CD4+ T cells in the LP were CD39+CD25hiFOXP3+, compared with 7.7% of CD39+CD73−CD4+ T cells (Fig. 3A and Supporting Information Fig. 3A, p < 0.05 by t-test).
Figure 2.
Characterization of CD73+ human peripheral blood CD4+ cells. (A) Flow cytometry study of CD45RO status (memory) in peripheral blood CD4+ T cells from a patient with active Crohn’s disease. The expression of CD73 in relation to the memory marker CD45RO was evaluated (left). The percentage of CD73+ cells amongst CD45ROhigh cells (top) and CD45ROlow cells (bottom) was also evaluated. Data shown are representative of three independent experiments. (B) Histogram of CD25 expression in CD39−CD73+ (left) and CD39+CD73− (center) and CD39+CD73+ (right) CD4+ T cells. Data shown are representative of three independent experiments.
Figure 3.
Phenotype of CD73+ cells. (A) Representative example of a flow cytometry density plot of CD25 and FOXP3 expression in the population of LP CD73+CD39+CD4+ T cells (left) and CD73−CD39+CD4+ T cells (right). The proportion of double-positive cells is indicated as a percentage in the top right quadrant. Data shown are representative of three independent experiments. (B) Representative histogram of CD25loCD4+ T-cell numbers in the absence of other cells (control), or in the presence of CD73+CD4+ T cells (center), or CD73−CD4+ T cells (right). CD25loCD4+ T cells (2 × 104/well) were cultured with the same amount of CD73+CD4+ T cells, or CD73−CD4+ T cells and stimulated with anti-CD3/28 beads for 3 days. Data shown are representative of three independent experiments. (C) Mean percentage proliferation of CD25loCD4+ T cells (2 × 104/well) in control medium (white column), or co-cultured with equal numbers of CD73+CD4+ T cells (gray column) or CD73−CD4+ T cells (black column) after stimulation with anti-CD3/28 beads for 3 days. Data are shown as mean + SD of nine samples pooled from three independent experiments performed. *p < 0.05 by t-test.
In order to determine whether the expression of CD73 by CD4+ T cells has regulatory properties that impair effector T-cell proliferation, we co-cultured labeled CD4+CD25lo effector T cells (1:1) in the presence of unlabeled CD73+ or CD73−CD4+ T cells and measured cell proliferation. T effector cell (CD4+CD25lo) proliferation was suppressed in the presence of CD73− cells, but not CD73+ cells (p = 0.004 by t-test, Fig. 3B and C). Although CD73+ cells expressed less FOXP3 overall than did CD73− cells, the relatively low percentage of FOXP3+ CD73− cells (8%) appears unlikely to explain these differences in suppressive properties alone (Supporting Information Fig. 3A). We next sought to determine whether this lack of suppressive effect was due to impairment of CD73 enzymatic activity in these cells. However, thin layer chromatography (TLC) analysis of products from ex vivo incubation of peripheral blood CD4+ T cells with 14C labeled AMP demonstrated AMPase-type activity in the CD73+ cell population (Supporting Information Fig. 3B), with associated generation of adenosine.
Peripheral CD73+CD4+ T cells exhibit a Th17 phenotype
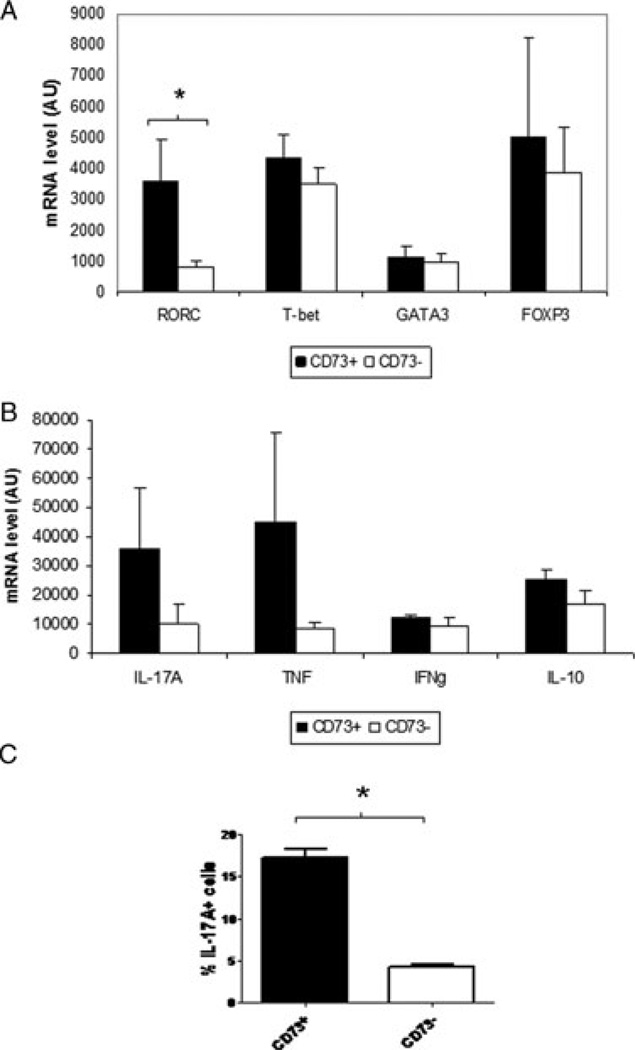
Since our results so far suggested a memory-effector phenotype for CD73+CD4+ T cells, we therefore evaluated the transcription factor and cytokine expression profiles of activated peripheral blood CD73+CD4+ T cells by quantitative RT-PCR (Fig. 4A and B). In the resting state in healthy controls, CD73+CD4+ T cells do not express TNF, IL-17A, IL-10, or FOXP3 (data not shown). However, activated CD73+CD4+ T cells exhibited greater expression of the Th17 transcription factor retinoid-related orphan receptor gamma (RORγ) relative to CD73−CD4+ T cells (Fig. 4A; p = 0.035 by t-test). In contrast, CD73+CD4+ T cells expressed the Th1 (T-bet) and Th2 (GATA3) transcription factors, and FOXP3, at similar levels to CD73−CD4+ T cells after activation (Fig. 4A).
Figure 4.
Gene expression profile of activated peripheral blood CD4+ cells clustered by CD73 expression. (A) Mean mRNA levels by PCR (AUs, y axis) of RORC, T-bet, GATA-3 and FOXP3 in activated CD73+CD4+ peripheral blood T cells (dark columns, n = 4 samples) or CD73−CD4+ T cells (clear columns, n = 4 samples) isolated by flow cytometry and sorted according to CD73 expression. Activation of CD4+ T cells was performed by 24 h of ex vivo activation with antibodies to CD3/CD28. Data are shown as mean + SD of 32 samples pooled from two independent experiments performed. *p < 0.05 by t-test. (B) Mean mRNA levels by PCR (AUs, y axis) of IL-17A, TNF, IFN-γ, and IL-10 in activated CD73+CD4+ T cells (dark columns, n = 4 samples) or CD73−CD4+ T cells (clear columns, n = 4 samples) isolated by flow cytometry and sorted according to CD73 expression. Activation of CD4+ T cells was performed by 24 h of ex vivo activation with antibodies to CD3/CD28. Data are shown as mean + SD of 32 samples pooled from two independent experiments performed. *p < 0.05 by Student’s t-test. (C) Mean percentage IL-17+ cells (by flow cytometry) amongst CD73+CD4+ T cells (dark column) or CD73−CD4+ T cells (clear column) in peripheral blood from patients with Crohn’s disease. Data are shown as mean + SD of six samples pooled from three independent experiments performed. *p < 0.05 by Student’s t-test.
Since RORC is a transcriptional regulator of Th17 cells, we also measured expression of pro- and anti-inflammatory markers by CD73+CD4+ T cells. Mean gene expression of IL-17A and TNF appeared to be higher in peripheral CD73+ than CD73− CD4+ T cells, but this trend did not reach statistical significance (Fig. 4B, p = 0.08 by t-test). Levels of IFN-γ, and IL-10 were similar regardless of CD73 status (Fig. 4B). In addition, activated peripheral CD73+CD4+ T cells included a greater proportion of IL-17A high cells (mean 17.3% ±1% of CD73+CD4+ T cells were Il-17+) than activated CD73−CD4+ T cells (4.3% ±0.2%) (p = 0.02 by t-test) (Fig. 4C). These data suggest that a proportion of CD73+CD4+ T cells exhibit an effector Th17, rather than regulatory, phenotype. In addition, in patients with intestinal inflammation due to active IBD, a greater proportion of LP CD4+ T cells were CD73+ IL-17A+, when compared to patients with inactive disease (Supporting Information Fig. 4).
Increased expression of CD73+ on peripheral TNF-activated CD4 cells
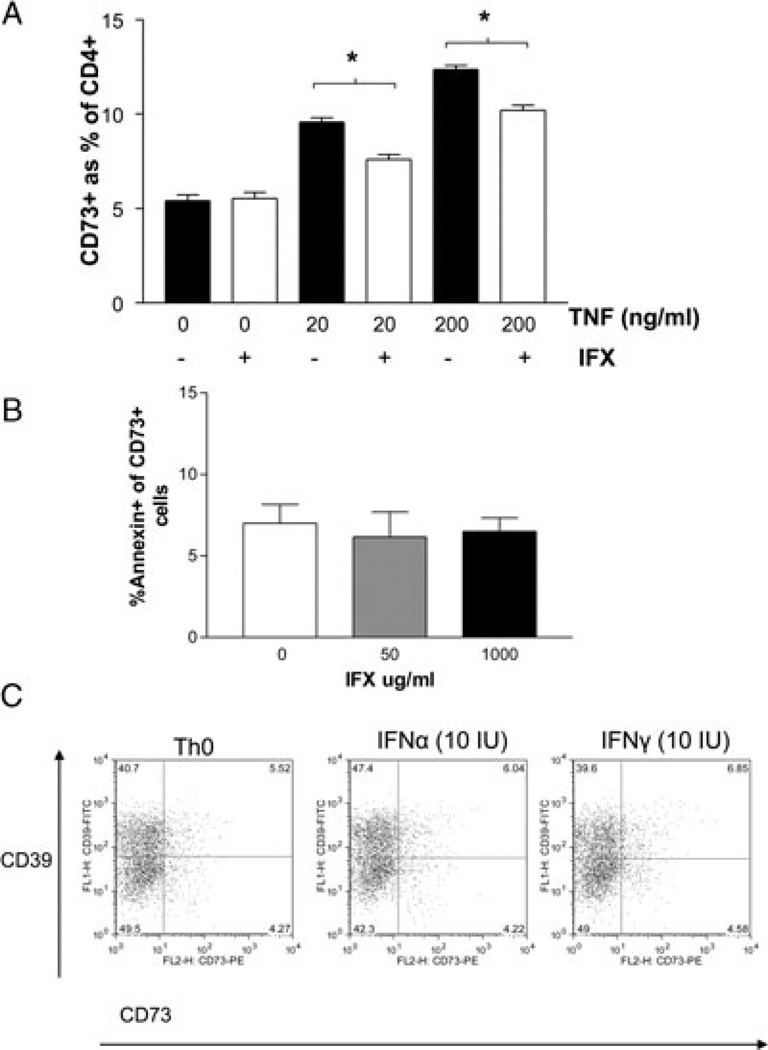
Since IBD is characterized by elevations in serum cytokines such as TNF, we further sought to determine whether such cytokines can lead to the expansion of CD73+CD4+ T-cell populations, as seen in patients with active IBD. Naïve peripheral blood CD4+ T cell cultured in the presence of TNF had increases in the number of CD73+ cells in a dose-dependent manner, to levels similar to those seen in patients with IBD (Fig. 5A, black columns). There was a dose-dependent increase in proportion of CD73+CD4+ T cells with exposure to increasing doses of TNF (p = 0.02 by ANOVA between 0, 20, 200 ng/mL). This increase in the proportion of CD73+CD4+ T cells was attenuated when CD4+ T cells were treated with TNF at increasing doses in the presence of a monoclonal antibody to TNF (infliximab, 1000 µg/mL), consistent with specific TNF-mediated increases in CD73 expression in these studies (Fig. 5A, white columns). A lower dose of infliximab (50 µg/mL) attenuated the effects of TNF to a lesser extent, and murine IgG1 did not attenuate the percentage of CD73+CD4+ T cells, suggesting the infliximab effect is due to dose-dependent binding to TNF (Supporting Information Fig. 5). The decrease in the percentage of CD73+CD4+ T cells was not due to infliximab-induced apoptosis, as there were no increases in the percentage of annexin+ CD73+CD4+ T cells after exposure to increasing doses of infliximab (Fig. 5B). In contrast, stimulation of CD4+ T cells with IFN-α or IFN-γ had no effect on expression of CD73 by these cells (Fig. 5C). Similarly, TGFβ did not increase CD73 expression (data not shown).
Figure 5.
CD73 expression in CD4+ cells. (A) Bar chart of the percentage CD73+ expression in CD4+ T cells from healthy peripheral blood (n = 3 samples) treated with TNF (0, 20, 200 ng/mL) for 12 h without (black columns) or with (white columns) infliximab 1000 µg/mL). Data are shown as mean + SD of 18 samples pooled from three independent experiments performed. *p < 0.05 by Student’s t-test. (B) Bar chart of the percentage of annexin-positive CD73+CD4+ T cells after in vitro treatment with infliximab at concentrations of 0, 50, 1000 µg/mL. CD4+ T cells from healthy peripheral blood (n = 3 samples) were treated with infliximab for 12 h, then CD73 and annexin were detected by flow cytometry. Data are shown as mean + SD of nine samples pooled from three independent experiments performed. *p < 0.05 by Student’s t-test. (C) Representative example of a flow cytometry density plot of CD39 (y-axis) and CD73 (x-axis) expression in peripheral blood CD4+ T cells cultured alone (Th0) or in the presence of IFN-α or IFN-γ. Data shown are representative of three independent experiments.
CD73+CD4+ T cells are modulated by anti-TNF therapy
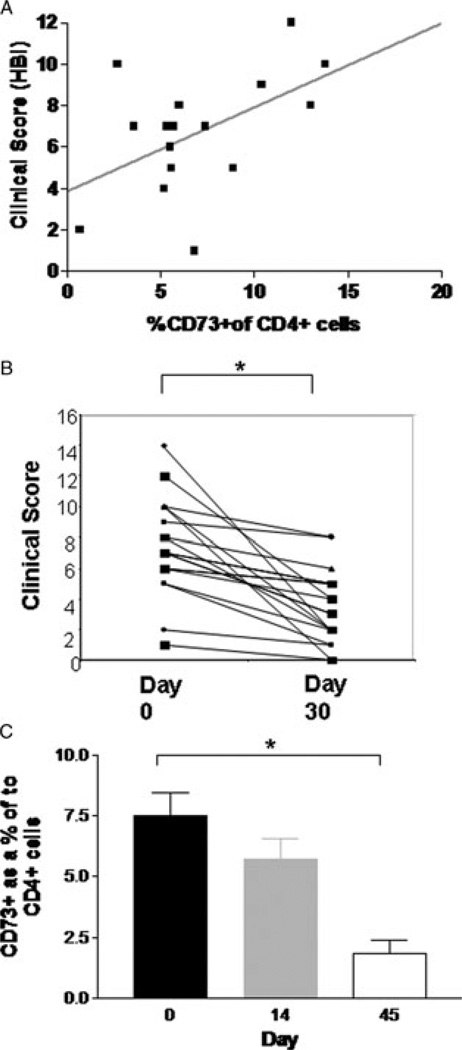
We recruited patients with active Crohn’s Disease (Supporting Information Table 1), and correlated numbers of CD73+CD4+ T cells to clinical scores (Harvey-Bradshaw index) and biochemical markers (C-reactive protein levels) in patients with active disease. There was a modest correlation between Harvey Bradshaw index (HBI) score and the percentage of CD73+CD4+ T cells (Fig. 6A, p = 0.03, R2 0.3).
Figure 6.
Influence of infliximab (IFX) on clinical outcomes and CD73+ cells (A) X–Y plot of percentage CD73+CD4+ T cells to all CD4+ T cells (x-axis), and clinical score (HBI) (y-axis) in patients with Crohn’s disease (n = 22) before treatment. Diagonal line represents linear regression line. (B) Line graph of clinical scores (HBI) for enrolled patients before (0), and 14 days (14), after an infusion of infliximab 5mg/kg. *p-value < 0.05 by Student’s t-test comparison of means, n = 13 patients. (C) Ratio of CD73+CD4+ T cells to all CD4+ T cells in peripheral blood of enrolled patients before (0), and 14 days (14) and 45 days (45), after an infusion of infliximab 5 mg/kg. Horizontal line indicates mean. * indicates p < 0.05 for ANOVA and comparison of means with Bonferroni correction, n = 13 patients.
We next sought to evaluate the relationship, if any, between CD73 expression by circulating immune cells and response to anti-TNF therapy. Serial measurements of CD73 expression by peripheral blood CD4+ T cells from Crohn’s disease patients at baseline, and at two later time-points (14 and 42 days) following treatment with infliximab, were performed. In the patients studied, both mean HBI scores (Fig. 6B) and mean CRP levels improved significantly 14 days after a single infusion of infliximab (5 mg/kg), consistent with clinical and biochemical response to therapy. There was a parallel reduction in the proportion of CD73+CD4+ T cells by day 14. This was significantly less than pretreatment levels by day 42 (Fig. 6C, ANOVA p = 0.034 with mean comparison between day 0 and day 42 with Bonferroni correction p = 0.032). Levels of CD73 expressing CD4+ T cells (in patients with active Crohn’s disease) after day 42 of treatment had returned to levels similar to those seen in healthy controls (Fig. 6C).
Interestingly, absolute decreases in typical markers of systemic inflammation (such as CRP) paralleled these progressive decreases in CD73+CD4+ T cells. Mean decreases in CRP of 33 (SEM 9.5) and 13.9 mg/dL (SEM 10.5), respectively were observed in responders and nonresponders to infliximab therapy.
Discussion
Recent studies have highlighted the importance of ectonucleotidase expression by T cells in the pathogenesis of inflammation [7, 14, 15]. In humans, single nucleotide polymorphisms associated with decreased CD39 expression are enriched in patients with Crohn’s disease over healthy controls [1]. We have noted CD39 to be chiefly expressed by human Treg cells [4]. However, the expression of CD39 by CD25lo T cells also reflects a memory-effector type T-cell [7].
CD73 is the linked ectonucleotidase responsible for scavenging of extracellular nucleotides that is required for adenosine generation. In addition to its function as an ectonucleotidase, CD73 co-ordinates T-cell activation via TCR [16]. A protective role for CD73 in experimental colitis has previously been proposed based on a TNBS model [2]. However, computational analyses have reported increased relative expression of CD73 in peripheral blood and LP in patients with active CD [12]. Prior human data from our group has shown that CD73 expression was not linked to the Treg-cell phenotype [7].
We report here on the presence of an expanded CD4+ T-cell population expressing CD73 in both the circulation and mucosal compartments of patients with active Crohn’s disease. The expression of CD73 by these cells is increased by TNF in vitro, which may account for the expansion of peripheral and intestinal CD73+CD4+ T cells in patients with active Crohn’s disease. We have noted dynamic changes in circulating levels of these cells with anti-TNF treatment that parallel changes in disease activity.
Contrary to our initial expectations, these CD73+CD4+ T cells express surface markers suggestive of an effector-memory phenotype (CD45RO+ and CD45RA−), and lack classic Treg-cell markers (CD25, FOXP3). Once activated, a proportion of CD73+CD4+ T cells further express RORC, TNF, and IL-17A, also consistent with Th17-type pro-inflammatory memory-type phenotypes. Importantly, CD73 on these cells remains functionally active, with an ability to generate adenosine from AMP, although the lack of CD39 co-expression might be expected to provide a relative paucity of substrate for CD73 AMPase bioactivity.
Since prior studies from animal models have suggested a predominant regulatory or anti-inflammatory phenotype in CD4 cells expressing ectonucleotidases [2, 11, 17], the expansion of CD73+CD4+ T cells in humans with chronic Crohn’s disease raises a number of interesting questions regarding their immunological role. We propose that CD73 expression by the effector-memory population might be of great importance in autocrine regulation of cell activation, rather than by the paracrine mechanisms, associated with the Treg-cell phenotype [18]. Although these cells exhibit a tendency to differentiate toward Th17 phenotype, plasticity of Th-type cell phenotypes in intestinal inflammation has been increasingly recognized of late [19].
In particular, duality and plasticity between the Treg/Th17 adaptive responses are now accepted [20, 21]. Observations from animal studies suggest that colitogenic effector-memory T cells are generated on an ongoing basis [22], may be located in extra-intestinal sites [23] but can then re-circulate to access the intestinal LP [24] and may with time convert to a suppressive phenotype, capable of attenuating colitis [19].
Our recent observations in clinical human transplantation, and by Fletcher et al. in multiple sclerosis, suggest that CD39 may play a key role in precluding Th17 differentiation by T cells, by the regulated phosphohydrolysis of ATP [7, 14]. How CD73 impacts this process is unclear, but clearance of AMP could play a secondary role in allowing memory-effector cells to access sites of inflammation in the intestine. Adenosine generated by CD73 and CD39 components might play an important role in the resolution of inflammation and in the promotion of healing.
CD73 may also have other distinct functions, perhaps also playing an important role in immune cell trafficking, with recent suggestions that CD73 expression may be necessary for T cells to access specific sites of inflammation [15]. Clearly, in cell transfer models, adenosine augmentation or supplementation is beneficial in experimental/T-cell-mediated colitis [25,26]. T-cell transfer studies to study role of immune cell ectonucleotidases in experimental colitis have been done (not shown here), using adoptive transfers as we have already published in avascular skin transplant studies [11]. Cell-targeted deletions of ectonucleotidases on Foxp3+ Treg cells and/or on endothelial cells are now being undertaken to develop these studies further.
From a translational perspective, the correlation between Crohn’s disease activity and the expression of CD73 by CD45RO+ cells further supports a role for the targeting of purinergic pathways in the treatment of IBD. It has been noted by other groups that the therapeutic efficacy of methotrexate, which is effective in treating Crohn’s disease, may occur secondary to induction of heightened levels of extracellular adenosine in patients with rheumatoid arthritis [27, 28]. Similarly, the anti-inflammatory effects of azathioprine, a purine-based anti-metabolite, have been ascribed to induce apoptosis of predominantly CD45RO+ memory T cells [29].
In our study, infliximab appeared to block upregulation of CD73 on CD4+ T cells in response to TNF, but did not induce apoptosis in cells expressing high levels of CD73 ab initio. We speculate that the resolution of the inflammatory response induced by infliximab in the LP results in downstream reduction of the inflammatory mediators of CD73 expression.
In conclusion, we propose that the expression of CD73 by CD4+ T cells in blood represents a novel memory-effector cell population. This pattern of CD73 expression on CD4+ T cells further reflects disease activity in patients with Crohn’s disease, and could be used as a surrogate marker for this. This cell population is enriched with memory-effector Th17 cells that are considered important in Crohn’s disease pathogenesis. Our results have implications for novel therapies for patients with Crohn’s disease targeting purinergic pathways.
Materials and methods
Human subjects
Patients with active CD prior to initiation of infliximab (monoclonal antibody to TNF-α) therapy were recruited and consented at the Center for Inflammatory Bowel Disease at Beth Israel Deaconess Medical Center, Boston, MA, USA under a protocol approved by the hospital institutional review board. EDTA blood samples were obtained prior to infliximab infusion at the initiation of therapy (day 0) and at the time of attendance for the two subsequent infusions (day 14 and 42) of induction therapy. Infliximab was given at a dose of 5 mg/kg intravenously at day 0, 14, and 42. Questionnaires were administered at baseline and day 30 and HBI was calculated for each patient.
In addition, blood samples from healthy controls were obtained at the Blood Donor Center at Children’s Hospital, Boston. As an inflammatory control, blood samples were also obtained from patients with ulcerative colitis in remission, as per an institutional review board approved protocol. Tissue samples taken from areas of normal and inflamed mucosa from patients with IBD were frozen in optimal cutting temperature medium (Sakura Finetek, Torrance, CA, USA).
Blood mononuclear cell isolation
Peripheral blood CD4+ T cells from healthy donors were isolated by Human CD4+ T cells Enrichment Cocktail kit (StemCell Technologies, Vancouver, BC, Canada), followed by density gradient isolation using Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) for subsequent analysis. Isolated CD4+ cells were confirmed as T lymphocytes by assessment of CD3 status by flow cytometry (more than 99% CD4+CD3+). Subtype CD4+ T cells were subsequently sorted by FACScan instrument (BD Biosciences).
Following surface labeling performed as outlined below, CD73 positive and negative CD4+ cells were isolated by sorting in an FACS Aria II cell sorter (Becton Dickinson, Mountain View, CA, USA). In vitro activation was performed for 24 h using anti-human CD3/CD28 coated dynabeads (Invitrogen, Carlsbad, CA, USA) in culture media; RPMI 1640 with L-glutamine was supplemented with 1% nonessential amino acids, 10% FCS, 50 U/mL penicillin and 50 µg/mL streptomycin (all from Gibco BRL, Maryland, USA) at 37°C/5% CO2. For gene expression studies, harvested cells were pelleted and collected in RLT (RNA lysis) buffer (Qiagen, Valencia, CA, USA) and frozen at −80°C until use.
LP cell isolation
Lamina propria mononuclear cells (LPMCs) were isolated from freshly obtained colonic biopsies from healthy controls, and patients with IBD, obtained during colonoscopy (different cohort to PBMC samples). In brief, the colonic biopsies were washed in HBSS-calcium-magnesium-free solution and then were incubated in HBSS containing 0.75 mM EDTA (Sigma-Aldrich) and 1 mM dithiothreitol (Sigma-Aldrich) at 37°C for about 30 min to remove the epithelium. The tissues were digested further in RPMI 1640 medium (Cellgro, Mediatech, Inc.) containing 400 U/mL collagenase IV (Sigma-Aldrich) and 0.01 mg/mL DNase I (Sigma-Aldrich) in a shaking incubator at 37°C. This step was repeated three to five times. LPMCs released from the tissues were purified by a 40 to 100% Percoll (GE Healthcare) gradient. LPMCs were cultured in complete RPMI 1640 medium containing 10% fetal bovine serum, 2 mM glutamine, 25 mM HEPES, 100 U/mL penicillin, and 100 µg/mL streptomycin.
Th-cell differentiation
Sorted CD4+ T cells were in vitro cultured in RPMI1640 supplemented with 10% (vol/vol) FCS and antibiotics. A total of 5 × 105/mL T cells were stimulated with anti-CD3/CD28 antibodies coated beads (the ratio of bead and cell is 1:1, Invitrogen) for 48 or 72 h. For Th17 condition, cultures were added with Th17-driving cytokines including 30 ng/mL IL-6, 30 ng/mL IL-1β, 10 ng/mL IL-23, and 1 ng/mL TGF-β (R&D System). For Th1 condition, cultures were introduced with 10 ng/mL IL-12 (R&D System). For Treg condition, cells were treated with 10 ng/mL IL-2 and 10 ng/mL TGF-β. To induce IL-17 expression, CD4+ T cells were treated for 5 h with 50 ng/mL phorbol 12-myristate 13-acetate and 500 ng/mL ionomycin in the presence of 10 µg/mL brefeldin A (Sigma-Aldrich).
Flow cytometry
For cell-surface staining, single-cell suspensions were prepared and labeled for 15 min at 4°C with optimal dilutions of each mAb. The following anti-human monoclonal antibodies were used; FITC-mouse anti-human CD39 (BU-61; Ancell Corp. Bayport MN, USA); PE- mouse anti-human CD73 (AD2; BD Pharmingen, San Jose, CA, USA); Allophycocyanin-mouse anti-human CD25 (BC96; E-bioscience, San Diego, CA, USA); Pacific Blue (PB)-mouse anti-human CD127 (eBioRDR5; E-bioscience); phycoerythrin-Cychrome 7 (PE-Cy7)-mouse anti-human CD4 (RPA-T4; E-bioscience), For intra-cellular staining, cells were stained with Pacific Blue or Alexa-Fluor 647 mouse-anti-human FoxP3 (259D; BioLegend, San Diego, CA, USA), anti-IL-17 (BL168, Biolegend), anti-IFN-γ (4S.B3, Biolegend), or anti-IL-10 (JES3–19F1, Biolegend), according to the manufacturer’s instructions for fixation, permeabilization, and staining. An isotype-matched control mAb (murine IgG1) was used to determine nonspecific staining for gating. Expression of cell surface or intracellular markers was assessed using a flow cytometer (LSRII; Becton Dickinson). After gating on live cells determined by scatter characteristics, data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA). The number of CD73+ cells in the CD4+ population was expressed as a percentage based on flow cytometry cell numbers.
Cell suppression assays
A total of 4 × 105/mL CD4+CD25− T cells as effecter cells were labeled with 2.5 µM carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen), and stimulated with anti-CD3/CD28 antibodies coated beads (the ratio of bead and cell is 1:1, Invitrogen) in the presence or absence of Th17 driving cytokines including 30 ng/mL IL-6, 30 ng/mL IL-1β, 10 ng/mL IL-23, and 1 ng/mL TGF-β (R&D System) for 72 to 120 h. For suppression assay, 2 × 104/mL CFSE (2.5 µM) labeled effector cells (CD25lo) were co cultured with 2 × 104/mL FACS-sorted CD4+CD73+ or CD4+CD73− T cells, and stimulated with anti-CD3/CD28 antibodies coated beads for 72 to 120 h. Cell recruitment and division were analyzed by labeling the cells with CFSE.
Tissue immunofluorescence
Sections (6 µm) of frozen human colon embedded in optimal cutting temperature were cut with a cryostat and fixed in freshly prepared 2% paraformaldehyde. Sections were treated with 0.5% Triton X-100 for 4 min followed by blocking with 7% normal horse serum for 30 min. Sections were next incubated with primary antibodies; FITC mouse anti human CD4 (BD Pharmingen cat# 550369 1:30), and rabbit anti-human CD73-NT5E (Abgent cat#AP2014a 1:250) at 4°C overnight. Tissues were rinsed with phosphate buffered saline and then exposed to secondary antibody, donkey anti-rabbit Alexa Flour 594 (Invitrogen), at room temperature for 1 h. Counter staining was performed with Hoechst 33258 (Molecular Probes) and sections were mounted with polyvinyl alcohol mounting medium (Fluka cat#10981). Sections were imaged with a ZEISS Axiovert 200 microscope (Carl-Zeiss, Germany).
Thin-layer chromatography
The patterns of nucleotide hydrolysis to distinct metabolites were determined by TLC using the substrate (14C)AMP (PerkinElmer, Boston, MA, USA). Cells were isolated by cell sorting based on expression of CD4 and CD73 as outlined. Then 0.3 × 104 cells per sample were washed in incubation buffer containing calcium and magnesium in excess. Adenosine uptake and deamination were blocked with dipyridamole 10 mol/L. 2 µCi/mL [14C]AMP was added to cells in suspension; aliquots were removed at 5 min intervals and the reaction stopped with EDTA. Samples were analyzed for the presence of [14C]AMP hydrolysis by TLC. Chromatography plates were exposed to a phosphor-imaging plate for 24 h and imaging was performed using a STORM860 scanner (GE Healthcare, Piscataway, NJ, USA) and ImageQuant™ software (version 8.1).
Quantification of gene expression
Total RNA was isolated from sorted cells using RNAeasy Mini kit (Qiagen) according to the manufacturer’s instructions, and the concentration was measured on Nanodrop ND 1000 spectrophotometer (Wilmington, DE, USA). RNA samples were treated with DNAse I to remove contaminating genomic DNA and reverse transcribed with Superscript II (Invitrogen).
Quantitative real-time PCR (qt-RT-PCR) analysis was performed by a two-step process, a 10-cycle preamplification step (AmpliTaq® DNA Polymerase Kit; Applied Biosystems, Inc.) followed by measurement of mRNA with an ABI PRISM 7900HT Sequence Detection System. For the measurement of mRNA levels of Tbet, GATA3, RORC, FOXP3, IFN-γ, IL-4, IL-10, IL-17A, TNF-α, CD39, and CD73 custom-designed primer-probe (P&P) sets were used. QuantiFast Probe PCR Master Mix was purchased from Qiagen (Hilden, Germany) and qt-RT-PCR was performed. Amplification was carried out in a total volume of 20 µL for 40 cycles of 3 s at 95°C, 30 s at 60°C. Initial enzyme activation was performed for 3 min at 95°C. For normalizing expression of genes-of-interest, 18s rRNA (housekeeping gene expression was used.
Statistical analysis
Results were generally expressed as mean values ± SE of the mean. Comparison between two groups was performed by two-way Student’s t-test and between three or more groups by ANOVA with comparison of means with Bonferroni correction. p-Values less than 0.05 were considered significant. Statistical analysis was performed using Stata Software (version 11.0; Statacorp, College Station, TX, USA).
Supplementary Material
Acknowledgements
We would like to acknowledge the assistance of David Friedman, Yan Wu, Xiaofeng Sun, Nielsen Fernandez-Becker, and Keiichi Enjyoji and the contributions of staff at the Center for Inflammatory Bowel Disease, BIDMC (especially Judy Bloom) for their assistance with patient recruitment. The kind assistance of the flow cytometry core facility at BIDMC is also acknowledged. We offer special thanks to Terry Strom for his support and insights. SCR supported by NIH grant R01 HL094400. ACM is supported by NIH grant K23DK084338. We also wish to acknowledge support by Harvard Clinical and Translational Science Center, from the National Center for Research Resources (Grant Number UL1 RR025758) and by Doris Toby Axelrod & Lawrence J. Marks.
Abbreviations
- HBI
Harvey Bradshaw index
- IBD
inflammatory bowel disease
- LP
lamina propria
- LPMC
lamina propria mononuclear cell
- TLC
thin layer chromatography
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Friedman DJ, Kunzli BM, Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, et al. From the cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl. Acad. Sci. USA. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J. Immunol. 2008;180:4246–4255. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 3.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 4.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J. Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 7.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am. J. Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Kobie JJ, Mosmann TR. CD73 and Ly-6A/E distinguish in vivo primed but uncommitted mouse CD4 T cells from type 1 or type 2 effector cells. J. Immunol. 2005;175:6458–6464. doi: 10.4049/jimmunol.175.10.6458. [DOI] [PubMed] [Google Scholar]
- 9.Sarra M, Monteleone I, Stolfi C, Fantini MC, Sileri P, Sica G, Tersigni R, et al. Interferon-gamma-expressing cells are a major source of interleukin-21 in inflammatory bowel diseases. Inflamm. Bowel. Dis. 2010;16:1332–1339. doi: 10.1002/ibd.21238. [DOI] [PubMed] [Google Scholar]
- 10.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, et al. Molecular antagonism and plasticity of regulatory and inflammatory T-cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, Tigges J, et al. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am. J. Transplant. 2009;9:2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman B, Wunderlich JE, Huang K, et al. New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm. Bowel. Dis. 2009;15:971–984. doi: 10.1002/ibd.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic. Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farrelly C, Tubridy N, et al. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J. Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 15.Mills JH, Thompson LF, Mueller C, Waickman AT, Jalkanen S, Niemela J, Airas L, et al. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol. Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 17.Alam MS, Kurtz CC, Rowlett RM, Reuter BK, Wiznerowicz E, Das S, Linden J, et al. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J. Infect. Dis. 2009;199:494–504. doi: 10.1086/596205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Adv. Pharmacol. 2011;61:301–332. doi: 10.1016/B978-0-12-385526-8.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Totsuka T, Kanai T, Nemoto Y, Tomita T, Tsuchiya K, Sakamoto N, Okamoto R, et al. Immunosenescent colitogenic CD4(+) T cells convert to regulatory cells and suppress colitis. Eur. J. Immunol. 2008;38:1275–1286. doi: 10.1002/eji.200737914. [DOI] [PubMed] [Google Scholar]
- 20.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 22.Tomita T, Kanai T, Fujii T, Nemoto Y, Okamoto R, Tsuchiya K, Totsuka T, et al. Continuous generation of colitogenic CD4(+) T cells in persistent colitis. Eur. J. Immunol. 2008;38:1264–1274. doi: 10.1002/eji.200737745. [DOI] [PubMed] [Google Scholar]
- 23.Nemoto Y, Kanai T, Kameyama K, Shinohara T, Sakamoto N, Totsuka T, Okamoto R, et al. Long-lived colitogenic CD4+ memory T cells residing outside the intestine participate in the perpetuation of chronic colitis. J. Immunol. 2009;183:5059–5068. doi: 10.4049/jimmunol.0803684. [DOI] [PubMed] [Google Scholar]
- 24.Tomita T, Kanai T, Nemoto Y, Fujii T, Nozaki K, Okamoto R, Tsuchiya K, et al. Colitogenic CD4 +effector-memory T cells actively recirculate in chronic colitic mice. Inflamm. Bowel. Dis. 2008;14:1630–1640. doi: 10.1002/ibd.20636. [DOI] [PubMed] [Google Scholar]
- 25.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for A2A adenosine receptors in the T-cell-mediated regulation of colitis. J. Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 26.Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, et al. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5’-nucleotidase-mediated conversion of adenine nucleotides. J. Clin. Invest. 1998;101:295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riksen NP, Barrera P, van den Broek PH, van Riel PL, Smits P, Rongen GA. Methotrexate modulates the kinetics of adenosine in humans in vivo. Ann. Rheum. Dis. 2006;65:465–470. doi: 10.1136/ard.2005.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atreya I, Neurath MF. Understanding the delayed onset of action of azathioprine in IBD: are we there yet? Gut. 2009;58:325–326. doi: 10.1136/gut.2008.163485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.