Abstract
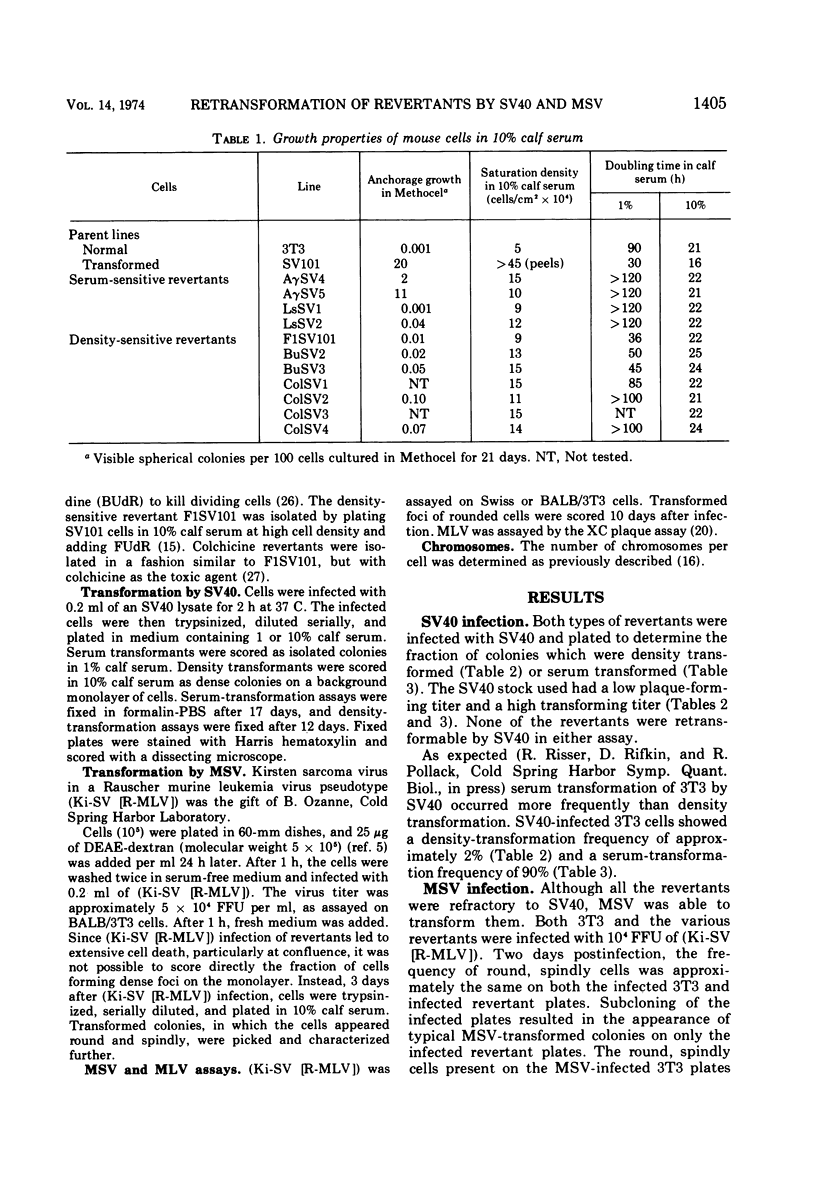
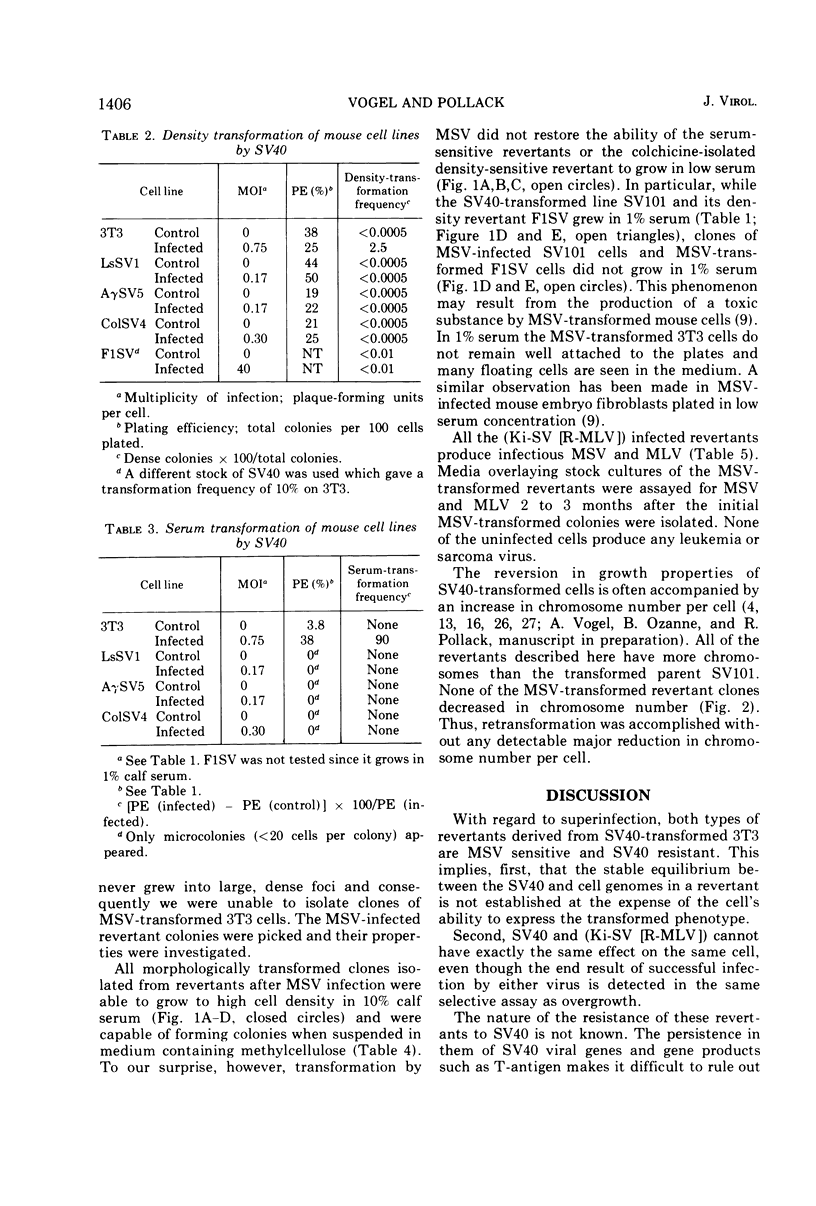
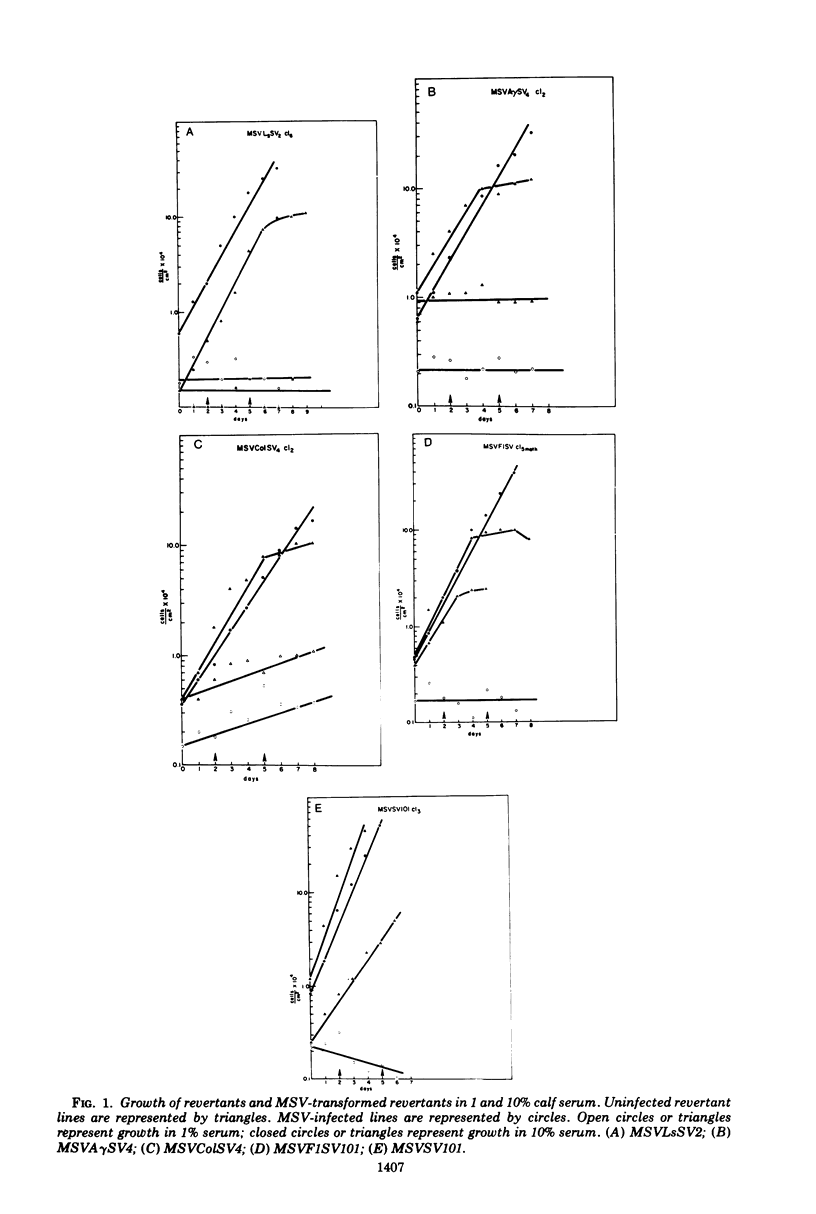
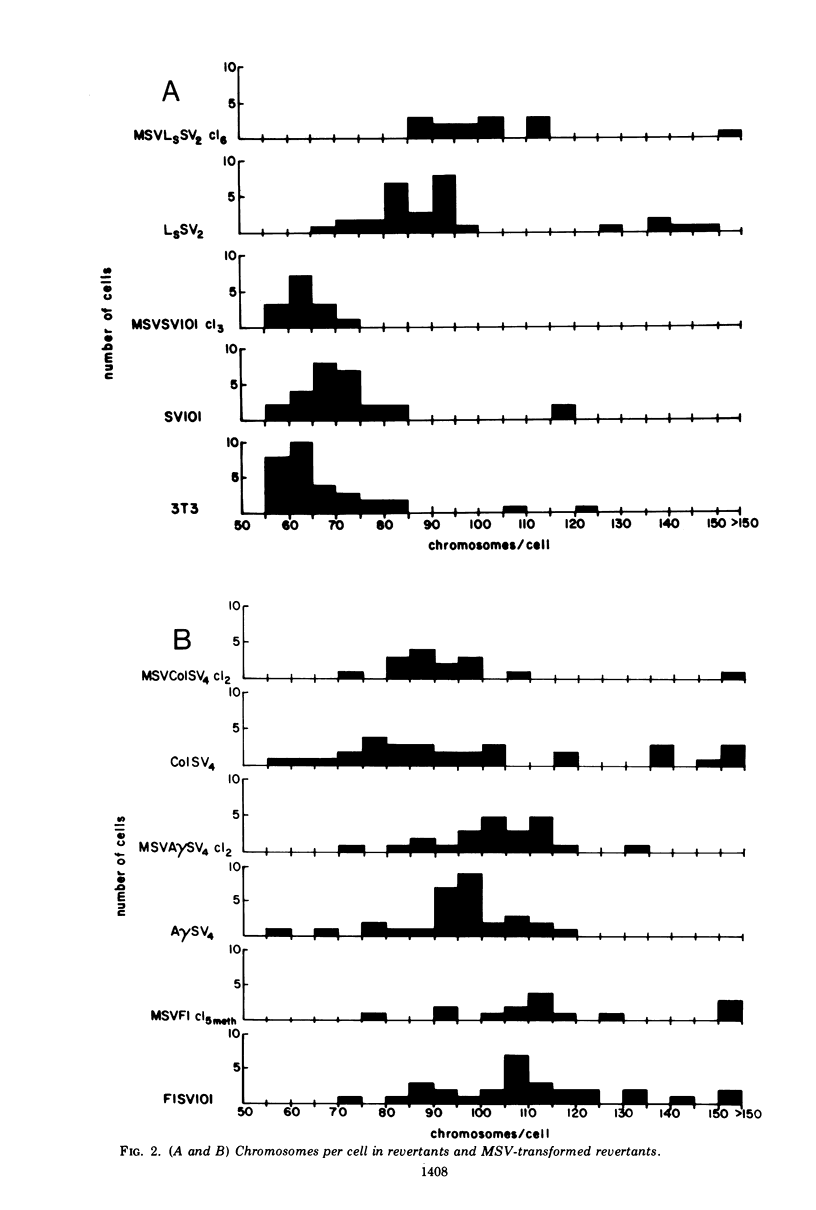
The susceptibility of two classes of revertants of Simian virus 40 (SV40)-transformed 3T3 cells to retransformation by SV40 or murine sarcoma virus (MSV) was studied. Both serum-sensitive and density-sensitive revertants are not retransformable by SV40. MSV can transform both types of revertants. The MSV-transformed revertants grow to high cell densities and form colonies when suspended in semi-solid methylcellulose medium, but are unable to grow in 1% calf serum. The MSV-transformed revertants produce infectious MSV and murine leukemia virus and possess the same number of chromosomes as the untransformed revertants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black P. H. Transformation of mouse cell line 3T3 by SV40: dose response relationship and correlation with SV40 tumor antigen production. Virology. 1966 Apr;28(4):760–763. doi: 10.1016/0042-6822(66)90262-5. [DOI] [PubMed] [Google Scholar]
- Culp L. A., Black P. H. Contact-inhibited revertant cell lines isolated from simian virus 40-transformed cells. 3. Concanavalin A-selected revertant cells. J Virol. 1972 Apr;9(4):611–620. doi: 10.1128/jvi.9.4.611-620.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp L. A., Grimes W. J., Black P. H. Contact-inhibited revertant cell lines isolated from SV40-transformed cells. I. Biologic, virologic, and chemical properties. J Cell Biol. 1971 Sep;50(3):682–690. doi: 10.1083/jcb.50.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc-Nguyen H. Enhancing effect of diethylaminoethyl-dextran on the focus-forming titer of a murine sarcoma virus (Harvey strain). J Virol. 1968 Jun;2(6):643–644. doi: 10.1128/jvi.2.6.643-644.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainchill J. L., Todaro G. J. Stimulation of cell growth in vitro by serum with and without growth factor. Relation to contact inhibition and viral transformation. Exp Cell Res. 1970 Jan;59(1):137–146. doi: 10.1016/0014-4827(70)90632-4. [DOI] [PubMed] [Google Scholar]
- Kotler M. Control of multiplication of uninfected mouse embryo fibroblasts and mouse embryo fibroblasts converted by infection with murine sarcoma virus (Harvey). Cancer Res. 1970 Oct;30(10):2493–2496. [PubMed] [Google Scholar]
- Oey J., Vogel A., Pollack R. Intracellular cyclic AMP concentration responds specifically to growth regulation by serum. Proc Natl Acad Sci U S A. 1974 Mar;71(3):694–698. doi: 10.1073/pnas.71.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Sharp P. A., Sambrook J. Transcription of simian virus 40. II. Hybridization of RNA extracted from different lines of transformed cells to the separated strands of simian virus 40 DNA. J Virol. 1973 Jul;12(1):90–98. doi: 10.1128/jvi.12.1.90-98.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B. Variants of simian virus 40-transformed 3T3 cells that are resistant to concanavalin A. J Virol. 1973 Jul;12(1):79–89. doi: 10.1128/jvi.12.1.79-89.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Vogel A. Isolation and characterization of revertant cell lines. II. Growth control of a polyploid revertant line derived from SV40-transformed 3T3 mouse cells. J Cell Physiol. 1973 Aug;82(1):93–100. doi: 10.1002/jcp.1040820111. [DOI] [PubMed] [Google Scholar]
- Pollack R., Wolman S., Vogel A. Reversion of virus-transformed cell lines: hyperploidy accompanies retention of viral genes. Nature. 1970 Dec 5;228(5275):938–passim. doi: 10.1038/228938a0. [DOI] [PubMed] [Google Scholar]
- Renger H. C., Basilico C. Temperature-sensitive simian virus 40-transformed cells: phenomena accompanying transition from the transformed to the "normal" state. J Virol. 1973 May;11(5):702–708. doi: 10.1128/jvi.11.5.702-708.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renger H. C. Retransformation of ts SV40 transformants by murine sarcoma virus at non-permissive temperature. Nat New Biol. 1972 Nov 1;240(96):19–21. doi: 10.1038/newbio240019a0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Nelson-Rees W. A. Direct isolation and characterization of "flat" SV40-transformed cells. Nat New Biol. 1971 Oct 27;233(43):263–265. doi: 10.1038/newbio233263a0. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Scher C. D., Todaro G. J. Induction of cell division in medium lacking serum growth factor by SV40. Virology. 1971 May;44(2):359–370. doi: 10.1016/0042-6822(71)90267-4. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H., GOLDBERG B. D. TRANSFORMATION OF PROPERTIES OF AN ESTABLISHED CELL LINE BY SV40 AND POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:66–73. doi: 10.1073/pnas.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G., Matsuya Y., Bloom S., Robbins A., Green H. Stimulation of RNA synthesis and cell division in resting cells by a factor present in serum. Wistar Inst Symp Monogr. 1967;7:87–101. [PubMed] [Google Scholar]
- Vogel A., Pollack R. Isolation and characterization of revertant cell lines. IV. Direct selection of serum-revertant sublines of SV40-transformed 3T3 mouse cells. J Cell Physiol. 1973 Oct;82(2):189–198. doi: 10.1002/jcp.1040820207. [DOI] [PubMed] [Google Scholar]
- Vogel A., Risser R., Pollack R. Isolation and characterization of revertant cell lines. 3. Isolation of density-revertants of SV40-transformed 3T3 cells using colchicine. J Cell Physiol. 1973 Oct;82(2):181–188. doi: 10.1002/jcp.1040820206. [DOI] [PubMed] [Google Scholar]


