Introduction
Atherosclerosis occurs in diverse vascular beds and may result in tissue ischemia. Current understanding of atherosclerotic disease has been advanced by imaging techniques such as high-resolution magnetic resonance imaging (HRMRI) studies of the coronary and carotid arteries. In these vessels, atherosclerotic plaque components can be visualized to risk-stratify patients, select treatments and advance our understanding of atherosclerosis pathophysiology in vivo. These imaging techniques are now being applied to evaluate intracranial arterial disease, both atherosclerotic and non-atherosclerotic. This review highlights the mechanisms by which intracranial atherosclerotic disease (ICAD) causes ischemia, the potential of HRMRI for identifying intracranial arterial pathology, the limitations of HRMRI in the intracranial circulation, and future applications of HRMRI for ICAD.
High Resolution MRI of ICAD
Image quality in MRI depends on several factors (e.g. slice thickness, field-of-view, signal-to-noise ratio, matrix size, magnetic field strength, etc.), but the term ‘high-resolution MRI’ is not well defined. In this review, the operational definition of HRMRI is limited to MR acquisitions using clinically available 1.5 – 3.0 Tesla magnetic field strengths targeted to intracranial arterial pathology that are of sufficient quality to visualize the arterial wall, separate from the lumen, of the proximal circle of Willis vessels. HRMRI can be accomplished at 1.5T by limiting the field of view to focus on a single vessel or point of interest, but higher field strength at 3T has many advantages over conventional (1.5T) MRI. Image acquisition is faster1, there is increased signal-to-noise2 and contrast-to-noise ratios, with better image quality3 for black-blood imaging. The increased signal and contrast that 3T provides improves the detection of complex atherosclerotic plaque4 and can identify plaque components in larger arteries5. Two dimensionally acquired HRMRI is time-consuming and must be monitored by a Neuroradiologist to ensure adequate sampling of the lesions of interest. HRMRI using three dimensional (isotropic) acquisitions permits imaging of ICAD with shorter scan times and generates better quality images.
Mechanisms of Stroke in ICAD
The primary mechanisms by which ICAD results in ischemic stroke are: plaque rupture, occlusion of small penetrating arteries, and hypoperfusion. Plaque rupture exposes the thrombogenic core to clotting factors and the resulting thrombus either occludes the artery locally or embolizes distally. ‘Vulnerable plaques’ (those with a large lipid core, intraplaque hemorrhage, or a thin or ruptured fibrous cap) are prone to rupture and cause myocardial infarction due to coronary artery atherosclerosis6, 7. The second mechanism, unique to ICAD, is growth of plaque over the ostia of penetrating arteries resulting in occlusion, described by Caplan as ‘branch atheromatous disease’8. Lastly, high-grade narrowing or occlusion of the lumen may lead to hypoperfusion of the distal brain territory, particularly in patients with inadequate collateral flow9.
Typically, the mechanism of stroke is inferred from the clinical presentation and the pattern and size of infarction. But without detailed knowledge of plaque location, severity and morphology, determination of stroke mechanism cannot be directly determined. HRMRI may provide this information and thereby clarify the mechanism of stroke (Table 1). For example, an intracranial plaque with HRMRI features of intraplaque hemorrhage and a ruptured fibrous cap in a patient with downstream ischemia is likely associated with artery-to-artery embolism, whereas a stable plaque with a large amount of fibrous tissue and small lipid core resulting in high-grade stenosis may cause hypoperfusion. Thus, HRMRI may directly determine stroke mechanism and play a role in selecting secondary prevention therapies (e.g. patients with hypoperfusion may benefit from intracranial revascularization procedures that may not benefit patients with artery-to-artery embolism). Future studies of stroke prevention may be enhanced by patient selection based on mechanism using HRMRI.
Table 1.
Use of HR MRI to determine mechanism of ischemia in ICAD
| Mechanism of ischemia | HR MRI identification of mechanism | Impact on stroke care |
|---|---|---|
|
| ||
| Plaque rupture with local occlusion or artery-to-artery embolization |
|
|
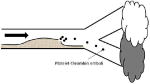
| ||
|
| ||
| Plaque overgrowth of perforator artery ostia |
|
|
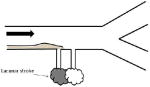
| ||
|
| ||
| Hypoperfusion through stenotic artery |
|
|
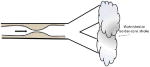
| ||
Conventional ICAD Imaging Compared to HRMRI
Degree of Stenosis
Conventional imaging of ICAD focuses on the vessel lumen. Digital subtraction angiography (DSA), computerized axial tomography angiography (CTA), and magnetic resonance angiography (MRA) identify luminal patency and stenosis10 while TCD indirectly estimates luminal stenosis by measuring blood flow velocity11. In contrast to these methods, HRMRI allows direct visualization of the vessel wall, permitting assessment of both luminal stenosis and features of vessel wall pathology12, 13, which can aid diagnostic specificity and sensitivity.
Determining Etiology of Stenosis
Intracranial stenosis can be caused by diverse pathologies (e.g. atherosclerosis, inflammation, vasospasm), with diverse treatment implications. While conventional imaging detects luminal narrowing, different pathologies can result in similar patterns of stenosis. Currently, differentiating causes of intracranial stenosis require invasive testing such as a spinal tap or brain biopsy. However, HRMRI may non-invasively differentiate between etiologies of intracranial stenosis by identifying plaque components or unique enhancement patterns. Swartz et al. studied post-contrast HRMRI images in 37 patients with intracranial stenosis and found that symptomatic atherosclerotic vasculopathies (ICAD) had eccentric wall thickening and enhancement, but inflammatory vasculopathies had concentric thickening and enhancement patterns14. Along those lines, idiopathic Moya-moya disease was associated with luminal narrowing with neither wall thickening nor enhancement14 and vasospasm due to non-inflammatory processes (Reversible cerebral vasoconstriction syndrome) was associated with concentric wall thickening without enhancement15. HRMRI has identified a Moya-moya phenomenon due to atherosclerosis16 and distinguished between atherosclerosis and basilar artery hypoplasia17 by identifying plaque components.
Detection of Non-stenotic Plaques
While conventional imaging can detect luminal narrowing, lumen diameter may be maintained in atherosclerotic arteries through compensatory vascular remodeling that results in minimal stenosis detectable by conventional imaging18. This process of ‘positive’ or ‘outward’ remodeling (Figure 1) results in plaque rupture in acute coronary syndromes19 and has been reported in ICAD20-23. HRMRI can identify remodeling without stenosis that may be clinically important, but missed by conventional imaging. For example, HRMRI showed plaque in MCA and basilar arteries that appeared normal on MRA, but resulted in stroke due to occlusion of penetrating arteries24-26. In some cases, ICAD was identified in the setting of small lacunar strokes that would previously have been attributed to lipohyalinosis of the penetrating arteries26-28.
Figure 1. Illustration of positive (outward) and negative (inward) remodeling in ICAD.
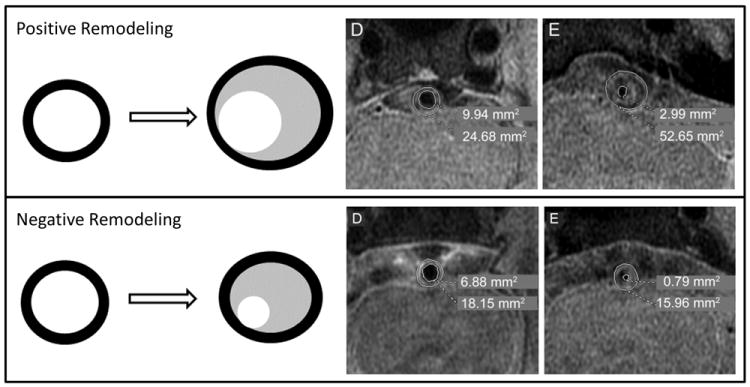
Positive Remodeling: Top panel (left) shows diagram of positive remodeling: the vessel wall (black) expands outward with accumulation of lipid (gray) and may or may not decrease the lumen (white). Top panel (right) shows an example of positive remodeling in a case of severe basilar stenosis (reproduced from Ma et al. Neurology, 201020). At the level of maximal stenosis (E), the wall area is increased and the lumen area is decreased relative to a distal basilar segment (D). Negative remodeling: Bottom panel (left) shows diagram of negative remodeling: the vessel wall (black) does not expand outward with accumulation of lipid (gray) and results in decrease in the lumen (white). Bottom right panel shows an example of negative remodeling in a case of severe basilar stenosis (reproduced from Ma et al. Neurology, 201020). At the level of maximal stenosis (E), the wall area is slightly decreased and the lumen is decreased relative to a distal basilar segment (D).
Identification of Plaque Components
HRMRI can reliably identify plaque features in other vascular territories and shows promise for use in ICAD (Table 2). In other vascular beds, identification of atherosclerotic plaque components has helped risk-stratify patients and select treatments. Using imaging, clinical, and pathological correlations, studies of coronary and carotid artery disease have identified features that indicate plaque vulnerability: intraplaque hemorrhage, lipid core size, and fibrous cap thickness. These vulnerable plaque characteristics are also present in ICAD32, but are less well studied.
Table 2.
Examples of suspected plaque components identified by HR MRI in ICAD
| Intraplaque Hemorrhage | Sequential T1 sagittal images of the MCA showing intraplaque hemorrhage defined as >150% of medial pterygoid muscle signal (2nd and 3rd images). (Turan et al., J Neuroimag, 200929) | Sagittal T2 (panel C) and T1-weighted fat-suppressed (panel D) imaging of symptomatic MCA. Lower right corner shows central region magnified. IPH is defined as bright signal on T1 fat-suppressed images (arrow in panel D). (Xu et al, Annals Neuro 201230) |
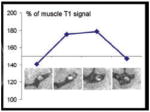
|
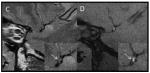
|
|
|
| ||
| Fibrous cap rupture and lipid core | T1 weighted axial images of basilar artery showing ruptured fibrous cap. Panel D is without contrast showing plaque (arrow). Panel E is post-contrast showing isointense lipid core (thin arrow) and plaque enhancement (arrow). (Vergouwen et al, Arch Neuro 201131) | Axial images of basilar artery stenosis (T1-weighted pre- and post-contrast, T2-weighted, and FLAIR) and normal volunteer FLAIR. Row A is unmarked and row B shows plaque features marked. Lipid is isodense on T1 and hypodense on T2 (white +). Ruptured fibrous cap is indicated by enhancement (black *). Artery wall to CSF boundary (white dash line) is not visualized in normal artery on FLAIR. (Turan, 2012 submitted) |
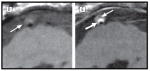
|

|
|
Intraplaque Hemorrhage
Intraplaque hemorrhage (IPH) from rupture of plaque microvessels causing accumulation of erythrocyte membranes, deposition of cholesterol, macrophage infiltration and enlargement of the necrotic core results in atheroma growth and plaque destabilization7. In patients with extracranial carotid stenosis, HRMRI-defined IPH correlates well with pathological specimens33, 34 and IPH is associated with stroke symptoms in both cross-sectional35 and prospective observational studies36-38.
While HRMRI-defined IPH is a well-described predictor of ischemic events due to carotid stenosis, it is an emerging area of research in ICAD. One case report showed HRMRI-defined IPH in a patient with symptomatic ICAD29. Another case series using HRMRI identified intracranial IPH in 2 of 13 patients with ischemic stroke22. In the first systematic attempt to associate IPH with recent symptoms in ICAD in 107 patients with HRMRI of MCA stenoses, the prevalence of IPH in symptomatic patients (19.6%) was higher than in asymptomatic patients (3.2%)30. The prevalence of IPH in other (basilar, ICA, vertebral) intracranial arteries has not been systematically studied. The timing, initial appearance, and duration that IPH is detectible on HRMRI may be critical factors that require additional study. While these data suggest an association between IPH and recent symptoms, only prospective follow-up will determine if IPH predicts high risk.
Lipid Core
A large amount of lipid within the necrotic core of a plaque is another sign of plaque vulnerability6, 35. HRMRI measurement of lipid-necrotic core area in carotid plaques correlates well with pathology39. A large lipid core area relative to the plaque area on HRMRI has also been associated with stroke symptoms in cross-sectional35 and prospective studies38 of patients with carotid stenosis.
In patients with ICAD, the presence and amount of lipid core within plaque is not well reported. Turan et al. presented a case series of 8 intracranial plaques (MCA and basilar) evaluated using HRMRI, wherein the presence of lipid core was visualized in 75%40. However, the relationship between the presence or amount of lipid within the plaque and prognosis requires study.
Fibrous Cap
The fibrous cap is a layer of connective tissue covering the lipid-necrotic core41. In patients with carotid stenosis, pathology specimens show that a thin fibrous cap overlying a lipid core is a feature of plaque vulnerability and that subsequent rupture of the fibrous cap exposes the thrombogenic lipid core to circulating blood, resulting in thromboembolism42. Thick fibrous caps are less prone to rupture6. HRMRI can identify fibrous cap characteristics (thin, thick, or ruptured) in carotid arteries43-45. HRMRI of carotid plaques showed patients with recent stroke symptoms had significantly more ruptured caps (70%) and thin caps (50%) than thick caps (9%), suggesting fibrous cap status may also be a predictor of stroke risk46.
While more difficult to visualize, fibrous caps have also been identified in patients with ICAD31, 40, but there has been no systematic study of the relationship between fibrous cap status and recent stroke symptoms.
Limitations of HRMRI
Despite the advantages of HRMRI ICAD imaging, this technique currently has limitations. Imaging characteristics in ICAD have not yet been correlated with pathological specimens because, while HRMRI of the carotids can be correlated with endarterectomy specimens, intracranial vessels are not accessible to pathology sampling in live patients. Therefore, the signal characteristics of intracranial plaque components can only be extrapolated from carotid HRMRI studies at present. Pathological correlation with HRMRI signal characteristics will be a key step in validating this technique in ICAD.
Additional challenges in HRMRI ICAD imaging are due to the tortuosity and variable course of the intracranial arteries. 3D image acquisition and the ability to reconstruct images in multiple planes may be used to ‘straighten’ tortuous or angled arteries to provide a more accurate representation of the lesion. Another challenge is the small size (2.0-5.0 mm) and depth of the intracranial vessels, which require relatively long acquisition times, making HRMRI imaging difficult due to patient motion artifact and limitations in resolution. In the future, motion-correction algorithms may be used to evaluate challenging patients.
HRMRI is limited by its cost and availability. The average Medicare reimbursement for a brain MRI is $53347, but as ICAD imaging is not standard of care there is currently no reimbursement for the extra scan time. A recent survey reported that MRI was available at 66% of hospitals, with small and rural hospitals having less access48. On the other hand, 3T MRI is available at most large academic centers and is the fastest growing MR market segment49.
Finally, a disadvantage of higher field strength is the increased specific absorption rate, which results in the potential for more tissue heating, raising safety concerns. While not as much of a concern with 3T, clinical imaging at 7T is performed in a few centers and its use is strictly limited to patients without any possibility of metal implants, such as cardiac stents or metal clips, to prevent tissue injury50.
Future Directions of Research
The use of HRMRI to characterize ICAD is an emerging field and has built upon cardiac and carotid studies. IPH, fibrous cap status, and large lipid core area are plaque components that can be detected in ICAD and have been associated with stroke in extracranial carotid plaques. Rigorous study of the prognostic value of HRMRI in ICAD will need to establish the reliability of HRMRI for detection of plaque features, the prevalence of plaque features, and describe the temporal relationship between the appearance and/or resolution of imaging abnormalities and ischemic symptoms.
Demonstrating that HRMRI ICAD plaque features are predictors of stroke could lead to changes in clinical management. Currently, conventional angiographic imaging is used to identify ICAD patients at highest risk of recurrent stroke, such as those with severe (70-99%) stenosis51 and poor collateral flow9. However, HRMRI may non-invasively identify other predictors of recurrent symptoms. For example, a patient with 60% stenosis and thin fibrous cap may be at higher risk of recurrent stroke than a patient with 80% stenosis and an intact thick fibrous cap. While the association between stroke risk and HRMRI plaque features is not yet proven, the ability to identify plaque characteristics may enhance future risk stratification and individualization of treatment. Jiang et al. have already described cases of ICAD patients in which HRMRI plaque imaging helped guide the endovascular treatment52. In addition, the HRMRI identification of plaque components in arteries appearing normal using conventional imaging techniques may reclassify patients previously thought to have cryptogenic or lacunar stroke and thereby change their management.
Demonstrating that HRMRI ICAD plaque features are predictors of stroke could lead to changes in patient selection for future clinical trials that evaluate new prevention treatments, such as using IPH as an entry criterion rather than severe stenosis. Establishing that ICAD plaque features are predictors of atherosclerotic progression could also lead to the use of plaque features as surrogate markers to evaluate therapies targeted at preventing progression of atherosclerosis, such as statins, as has been done with carotid plaque features53.
In the future, HRMRI in ICAD may also lead to new understanding of the mechanisms of atherosclerotic pathology. The use of novel, targeted MRI contrast agents may allow visualization of biological processes such as angiogenesis or inflammation within the plaque wall to identify ‘vulnerable’ plaques using HRMRI. For example, Gadolinium-loaded paramagnetic nanoparticles54 have been used to identify neovascularization in animal models. As these novel technologies continue to be developed and studied in other vascular territories, their application in ICAD will also warrant study.
Conclusion
HRMRI of intracranial arteries is an emerging tool that can help identify stroke mechanisms, determine the degree and etiology of stenoses, identify non-stenotic plaques, and identify potentially high-risk plaque components. These plaque characteristics are not visualized with conventional luminal imaging and may be important predictors of stroke. Additional research is needed to establish the reliability of HRMRI for detection of high-risk plaque features in ICAD, which may set the stage for prospective studies to determine the predictive power of HRMRI in ICAD and for future therapeutic trials to investigate new treatments for this high-risk disease.
Acknowledgments
Sources of Funding:
Dr. Turan receives funding from the NIH (K23NS069668). This research was also supported by the Medical University of South Carolina (MUSC) Center for Advanced Imaging Research and the South Carolina Clinical & Translational Research Institute (CTSA) Award UL1RR029882. Dr. Swartz is supported by the Heart and Stroke Foundation of Canada for HRMRI research in intracranial vascular diseases.
RHS- funded by the Heart and Stroke Foundation of Canada for HRMRI research; TNT – funded by NIH for HRMRI research.
Footnotes
Disclosures:
JDB, EF, ZR, TB have no relevant disclosures
References
- 1.Bammer R, Hope TA, Aksoy M, Alley MT. Time-resolved 3d quantitative flow mri of the major intracranial vessels: Initial experience and comparative evaluation at 1.5t and 3.0t in combination with parallel imaging. Magnetic resonance in medicine. 2007;57:127–140. doi: 10.1002/mrm.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anumula S, Song HK, Wright AC, Wehrli FW. High-resolution black-blood mri of the carotid vessel wall using phased-array coils at 1.5 and 3 tesla. Academic radiology. 2005;12:1521–1526. doi: 10.1016/j.acra.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarnykh VL, Terashima M, Hayes CE, Shimakawa A, Takaya N, Nguyen PK, et al. Multicontrast black-blood mri of carotid arteries: Comparison between 1.5 and 3 tesla magnetic field strengths. Journal of magnetic resonance imaging. 2006;23:691–698. doi: 10.1002/jmri.20562. [DOI] [PubMed] [Google Scholar]
- 4.Koops A, Ittrich H, Petri S, Priest A, Stork A, Lockemann U, et al. Multicontrast-weighted magnetic resonance imaging of atherosclerotic plaques at 3.0 and 1.5 tesla: Ex-vivo comparison with histopathologic correlation. European radiology. 2007;17:279–286. doi: 10.1007/s00330-006-0265-7. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann LV, Liddell RP, Eng J, Wasserman BA, Arepally A, Lee DS, et al. Human peripheral arteries: Feasibility of transvenous intravascular mr imaging of the arterial wall1. Radiology. 2005;235:617–622. doi: 10.1148/radiol.2352040340. [DOI] [PubMed] [Google Scholar]
- 6.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 7.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, et al. Intraplaque hemorrhage and progression of coronary atheroma. NEJM. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 8.Caplan LR. Intracranial branch atheromatous disease: A neglected, understudied, and underused concept. Neurology. 1989;39:1246–1250. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- 9.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, Chimowitz MI. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Annals of neurology. 2011;69:963–974. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. American Journal of Neuroradiology. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 11.Newell DW, Aaslid R. Transcranial doppler: Clinical and experimental uses. Cerebrovascular and brain metabolism reviews. 1992;4:122–143. [PubMed] [Google Scholar]
- 12.Klein I, Lavallee P, Oouboul J, Schouman-Claeys E, Amarenco P. In vivo middle cerebral artery plaque imaging by high resolution mri. Neurology. 2006;67:327–329. doi: 10.1212/01.wnl.0000225074.47396.71. [DOI] [PubMed] [Google Scholar]
- 13.Ryu CW, Jahng GH, Kim EJ, Choi WS, Yang DM. High resolution wall and lumen mri of the middle cerebral arteries at 3 tesla. Cerebrovascular Diseases. 2009;27:433–442. doi: 10.1159/000209238. [DOI] [PubMed] [Google Scholar]
- 14.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced mri. Neurology. 2009;72:627–634. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 15.Mandell DM, Matouk CC, Farb RI, Krings T, Agid R, terBrugge K, et al. Vessel wall mri to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: Preliminary results. Stroke. 2012;43:860–862. doi: 10.1161/STROKEAHA.111.626184. [DOI] [PubMed] [Google Scholar]
- 16.Ashley WW, Jr, Zipfel GJ, Moran CJ, Zheng J, Derdeyn CP. Moyamoya phenomenon secondary to intracranial atherosclerotic disease: Diagnosis by 3t magnetic resonance imaging. Journal of neuroimaging. 2009;19:381–384. doi: 10.1111/j.1552-6569.2008.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariani LL, Klein I, Pico F. Hypoplasia or stenosis: Usefulness of high-resolution mri. Revue Neurologique. 2011;167:619–621. doi: 10.1016/j.neurol.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. NEJM. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 19.Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes : An intravascular ultrasound study. Circulation. 2000;101:598–603. doi: 10.1161/01.cir.101.6.598. [DOI] [PubMed] [Google Scholar]
- 20.Ma N, Jiang WJ, Lou X, Ma L, Du B, Cai JF, et al. Arterial remodeling of advanced basilar atherosclerosis: A 3-tesla mri study. Neurology. 2010;75:253–258. doi: 10.1212/WNL.0b013e3181e8e714. [DOI] [PubMed] [Google Scholar]
- 21.Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. In vivo high-resolution mr imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. 2010;212:507–511. doi: 10.1016/j.atherosclerosis.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 22.Park JK, Kim SH, Kim BS, Choi G, Jeong SY, Choi JC. Imaging of intracranial plaques with black-blood double inversion recovery mr imaging and ct. Journal of neuroimaging. 2011;21:e64–68. doi: 10.1111/j.1552-6569.2010.00499.x. [DOI] [PubMed] [Google Scholar]
- 23.Shi MC, Wang SC, Zhou HW, Xing YQ, Cheng YH, Feng JC, et al. Compensatory remodeling in symptomatic middle cerebral artery atherosclerotic stenosis: A high-resolution mri and microemboli monitoring study. Neurol Res. 2012;34:153–158. doi: 10.1179/1743132811Y.0000000065. [DOI] [PubMed] [Google Scholar]
- 24.Li ML, Xu WH, Song L, Feng F, You H, Ni J, et al. Atherosclerosis of middle cerebral artery: Evaluation with high-resolution mr imaging at 3t. Atherosclerosis. 2009;204:447–452. doi: 10.1016/j.atherosclerosis.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Klein I, Lavallee P, Schouman-Claeys E, Amarenco P. High-resolution mri identifies basilar artery plaques in paramedian pontine infarct. Neurology. 2005;64:551–552. doi: 10.1212/01.WNL.0000150543.61244.06. [DOI] [PubMed] [Google Scholar]
- 26.Klein IF, Lavallee PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: A high-resolution mri study. Stroke. 2010;41:1405–1409. doi: 10.1161/STROKEAHA.110.583534. [DOI] [PubMed] [Google Scholar]
- 27.Niizuma K, Shimizu H, Takada S, Tominaga T. Middle cerebral artery plaque imaging using 3-tesla high-resolution mri. Journal of Clinical Neuroscience. 2008;15:1137–1141. doi: 10.1016/j.jocn.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. Plaque distribution of stenotic middle cerebral artery and its clinical relevance. Stroke. 2011;42:2957–2959. doi: 10.1161/STROKEAHA.111.618132. [DOI] [PubMed] [Google Scholar]
- 29.Turan TN, Bonilha L, Morgan PS, Adams RJ, Chimowitz MI. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. Journal of Neuroimaging. 2011;21:e159–61. doi: 10.1111/j.1552-6569.2009.00442.x. [DOI] [PubMed] [Google Scholar]
- 30.Xu WH, Li ML, Gao S, Ni J, Yao M, Zhou LX, et al. Middle cerebral artery intraplaque hemorrhage: Prevalence and clinical relevance. Annals of neurology. 2012;71:195–198. doi: 10.1002/ana.22626. [DOI] [PubMed] [Google Scholar]
- 31.Vergouwen MD, Silver FL, Mandell DM, Mikulis DJ, Krings T, Swartz RH. Fibrous cap enhancement in symptomatic atherosclerotic basilar artery stenosis. Archives of neurology. 2011;68:676. doi: 10.1001/archneurol.2011.89. [DOI] [PubMed] [Google Scholar]
- 32.Chen XY, Wong KS, Lam WW, Zhao HL, Ng HK. Middle cerebral artery atherosclerosis: Histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovascular diseases. 2008;25:74–80. doi: 10.1159/000111525. [DOI] [PubMed] [Google Scholar]
- 33.Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O’Brien KD, et al. Hemorrhage in the atherosclerotic carotid plaque: A high-resolution mri study. Stroke. 2004;35:1079–1084. doi: 10.1161/01.STR.0000125856.25309.86. [DOI] [PubMed] [Google Scholar]
- 34.Moody AR, Murphy RE, Morgan PS, Martel AL, Delay GS, Allder S, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation. 2003;107:3047–3052. doi: 10.1161/01.CIR.0000074222.61572.44. [DOI] [PubMed] [Google Scholar]
- 35.U King-Im J, Tang TY, Patterson A, Graves MJ, Howarth S, Li ZY, et al. Characterisation of carotid atheroma in symptomatic and asymptomatic patients using high resolution mri. J Neurol Neurosurg Psychiatry. 2008;79:905–912. doi: 10.1136/jnnp.2007.127969. [DOI] [PubMed] [Google Scholar]
- 36.Altaf N, Daniels L, Morgan PS, Auer D, MacSweeney ST, Moody AR, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. Journal of vascular surgery. 2008;47:337–342. doi: 10.1016/j.jvs.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 37.Altaf N, MacSweeney ST, Gladman J, Auer DP. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke. 2007;38:1633–1635. doi: 10.1161/STROKEAHA.106.473066. [DOI] [PubMed] [Google Scholar]
- 38.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: A prospective assessment with mri--initial results. Stroke. 2006;37:818–823. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 39.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, et al. Quantitative evaluation of carotid plaque composition by in vivo mri. ATVB. 2005;25:234–239. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 40.Turan TN, Bonilha L, Morgan PS, Adams RJ, Chimowitz MI. Characterization of intracranial atherosclerosis using high-resolution mri [abstract] ATVB. 2010;30:e317. [Google Scholar]
- 41.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. ATVB. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Ortiz A, Badimon JJ, Falk E, Fuster V, Meyer B, Mailhac A, et al. Characterization of the relative thrombogenicity of atherosclerotic plaque components: Implications for consequences of plaque rupture. Journal American College Cardiology. 1994;23:1562–1569. doi: 10.1016/0735-1097(94)90657-2. [DOI] [PubMed] [Google Scholar]
- 43.Trivedi RA, U King Im J, Graves MJ, Horsley J, Goddard M, Kirkpatrick PJ, et al. Multi-sequence in vivo mri can quantify fibrous cap and lipid core components in human carotid atherosclerotic plaques. Eur J Vasc Endovasc Surg. 2004;28:207–213. doi: 10.1016/j.ejvs.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Mitsumori LM, Hatsukami TS, Ferguson MS, Kerwin WS, Cai J, Yuan C. In vivo accuracy of multisequence mr imaging for identifying unstable fibrous caps in advanced human carotid plaques. Journal of magnetic resonance imaging. 2003;17:410–420. doi: 10.1002/jmri.10264. [DOI] [PubMed] [Google Scholar]
- 45.Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation. 2000;102:959–964. doi: 10.1161/01.cir.102.9.959. [DOI] [PubMed] [Google Scholar]
- 46.Yuan C, Zhang S, Polissar NL, Echelard D, Ortiz G, Davis JW, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation. 2002;105:181–185. doi: 10.1161/hc0202.102121. [DOI] [PubMed] [Google Scholar]
- 47.Siemens Healthcare. 2011 medicare physician fee schedule payment rates: Magnetic resonance imaging. [August 18 2012];2011 webiste: http://www.medical.siemens.com/siemens/en_US/rg_marcom_FBAs/files/Reimbursement/MR_2011_Final_MPFS_Payment_Rates.pdf.
- 48.Ginde AA, Foianini A, Renner DM, Valley M, Camargo CA., Jr Availability and quality of computed tomography and magnetic resonance imaging equipment in U.S. Emergency departments. Academic emergency medicine. 2008;15:780–783. doi: 10.1111/j.1553-2712.2008.00192.x. [DOI] [PubMed] [Google Scholar]
- 49.Global Industry Analysts. [Accessed August 18 2012];Mri equipment market to reach 5.5 billion dollars by 2010, according to new report by global industry analysts, inc. Medical News Today. 2008 [Google Scholar]
- 50.van der Kolk AG, Hendrikse J, Zwanenburg JJM, Visser F, Luijten PR. Clinical applications of 7t mri in the brain. European Journal of Radiology. 2011 doi: 10.1016/j.ejrad.2011.07.007. Epub Sep 19 2011. [DOI] [PubMed] [Google Scholar]
- 51.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 52.Jiang WJ, Yu W, Ma N, Du B, Lou X, Rasmussen PA. High resolution mri guided endovascular intervention of basilar artery disease. J Neurointerv Surg. 2011 doi: 10.1136/jnis.2010.004291. Epub Mar 4 2011. [DOI] [PubMed] [Google Scholar]
- 53.Schneck DW, Knopp RH, Ballantyne CM, McPherson R, Chitra RR, Simonson SG. Comparative effects of rosuvastatin and atorvastatin across their dose ranges in patients with hypercholesterolemia and without active arterial disease. Am J Cardiol. 2003;91:33–41. doi: 10.1016/s0002-9149(02)02994-6. [DOI] [PubMed] [Google Scholar]
- 54.Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant hdl-like nanoparticles: A specific contrast agent for mri of atherosclerotic plaques. Journal of American Chemical Society. 2004;126:16316–16317. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]


