Abstract
The molecular effects of obesity are mediated by alterations in the levels of adipocytokines. High leptin level associated with obese state is a major cause of breast cancer progression and metastasis, whereas adiponectin is considered a “guardian angel adipocytokine” for its protective role against various obesity-related pathogenesis including breast cancer. In the present study, investigating the role of adiponectin as a potential inhibitor of leptin, we show that adiponectin treatment inhibits leptin-induced clonogenicity and anchorage-independent growth. Leptin-stimulated migration and invasion of breast cancer cells is also effectively inhibited by adiponectin. Analyses of the underlying molecular mechanisms reveal that adiponectin suppresses activation of two canonical signaling molecules of leptin signaling axis: extracellular signal-regulated kinase (ERK) and Akt. Pretreatment of breast cancer cells with adiponectin protects against leptin-induced activation of ERK and Akt. Adiponectin increases expression and activity of the physiological inhibitor of leptin signaling, protein tyrosine phosphatase 1B (PTP1B), which is found to be integral to leptin-antagonist function of adiponectin. Inhibition of PTP1B blocks adiponectin-mediated inhibition of leptin-induced breast cancer growth. Our in vivo studies show that adenovirus-mediated adiponectin treatment substantially reduces leptin-induced mammary tumorigenesis in nude mice. Exploring therapeutic strategies, we demonstrate that treatment of breast cancer cells with rosiglitazone results in increased adiponectin expression and inhibition of migration and invasion. Rosiglitazone treatment also inhibits leptin-induced growth of breast cancer cells. Taken together, these data show that adiponectin treatment can inhibit the oncogenic actions of leptin through blocking its downstream signaling molecules and raising adiponectin levels could be a rational therapeutic strategy for breast carcinoma in obese patients with high leptin levels.
Introduction
A vast number of epidemiological studies suggest that obesity is a pandemic condition that greatly influences risk, prognosis, and progression of various cancers such as colon, prostate, endometrium, hepatocellular, and breast. Investigating the relationship of obesity with mortality from breast cancer, many studies show that obese women in the highest quintile of body mass index have double the death rate from breast cancer when compared with women in the lowest quintile [1–4], hence providing one of the few preventive interventions capable of making a significant effect on associated disease conditions. Obesity is associated with an increase in number and size of adipocytes that greatly alters the local and systemic secretion of biologically active polypeptides, adipocytokines such as leptin and adiponectin. Acting by endocrine, paracrine, and autocrine mechanisms, adipocytokines affect various biologic processes [5,6].
Several epidemiological studies have linked high levels of plasma leptin with increased risk and poor prognosis for breast carcinogenesis [7–11]. Circulating as a 16-kD protein, partially bound to plasma proteins, leptin exerts its biologic actions through specific cell surface receptors [leptin receptors (LRs)] present in a variety of tissues [12]. Breast carcinoma cells express higher levels of leptin and LR in comparison to normal mammary epithelial cells. In fact, overexpression of leptin is observed in 92%of breast tumors and LRs are overexpressed in 83% breast tumors, whereas no or very low expression of leptin and LRs is found in normal mammary epithelial cells [13]. Using loss-of-function mutants for leptin and LR, in vivo studies show that leptin or LR-deficient mouse mammary tumor virus (MMTV)-transforming growth factor-α mice do not develop oncogene-induced mammary tumors [14,15], hence providing direct evidence for the involvement of leptin in breast carcinogenesis. Hypothalamic LR-reconstituted db/db (LR-null) mice [16] crossed with MMTV-PyMT mice exhibit that LR-mediated signaling promotes breast carcinogenesis [17]. In addition, diet-induced obese MMTV-transforming growth factor-α mice show higher levels of leptin as well as increased breast tumor growth [18]. Xenografts of MMTV-Wnt1 tumors grow faster in diet-induced obese mice in comparison with lean counterparts and exhibit stunted growth when transplanted in leptin-deficient (Ob/Ob) mice [19]. In recent years, many laboratories including ours have shown that leptin increases proliferation of breast, endometrial, hepatocellular, and many other cancer cells through multiple signaling pathways including Stat3/extracellular signal-regulated kinase (ERK)/Akt signaling [20–30]. Our recent research has shown the direct stimulatory effect of leptin on breast cancer cell migration, invasion, and epithelial-mesenchymal transition (EMT) [20,21,24]. The therapeutic potential of inhibition of leptin has been evaluated to some extent in diseases associated with metabolic syndrome [31,32], but the importance of inhibition of leptin signaling in carcinogenesis is still elusive and is an active area of research.
Adiponectin (also known as ACRP30, apM1, adipoQ, and GBP28) [33–36], first identified in the mid-1990s, is an important adipocytokine that is known for its protective role against obesity-related disorders and the metabolic syndrome, particularly in the pathogenesis of type 2 diabetes and cardiovascular disease [37–39]. Multiple functions of adiponectin include suppression of proliferation and activation of immune cells, down-regulation of vascular adhesion molecules in endothelial cells, and inhibition of smooth muscle migration [40]. Adiponectin is reported to directly bind certain growth factors to control their bioavailability [41]. Cellular functions of adiponectin are mainly mediated through two adiponectin receptors, AdipoR1 and AdipoR2 [42]. Recently, T-cadherin has also been identified as AdipoR [43]. Combination of interactions between adiponectin and its receptors mediate the cellular functions of adiponectin in a tissue-dependent manner. Several recent studies evaluated and established a role for adiponectin in carcinogenesis [44–46]. Epidemiological evidences have put forth an inverse connection between obesity-associated low plasma levels of adiponectin with incidence as well as progression of many common forms of cancer [47,48]. Low-serum adiponectin levels are associated with increased risk of breast cancer in both postmenopausal and premenopausal women, independent of age, menopause status, hormone receptor status, lymph node metastasis, and status of estrogen receptor (ER) and Her2/neu. It is also suggested that tumors arising in patients with low-serum adiponectin levels may have a more aggressive phenotype (large size of tumor, high histologic grade, and increased metastasis) [47,48]. Providing molecular evidence, several recent studies show adiponectin-mediated antiproliferative response in breast cancer cells [49–53]. Investigating upstream regulatory nodes capable of orchestrating the downstream signaling axes of adiponectin, we recently show that adiponectin inhibits metastatic properties of breast cancer through activation of master upstream kinase and tumor suppressor, LKB1 [54].
In the present study, we specifically investigated if adiponectin can inhibit the oncogenic actions of leptin. Intriguingly, we found that adiponectin inhibits the effect of leptin on malignant properties of cancer cells including migration and invasion and also inhibits important downstream molecules of leptin signaling while activating physiological inhibitor of leptin signaling. In agreement with our in vitro data, we found that adiponectin treatment inhibits leptin-induced breast tumorigenesis in vivo. Thus, raising adiponectin might be an attractive goal for breast cancer prevention and therapy, particularly for patients with hyperleptinemic condition. Using thiazolidinedione drugs to raise adiponectin levels, we provide evidence that rosiglitazone treatment is capable of inhibiting leptin-induced migration and invasion of breast cancer cells.
Materials and Methods
Antibodies
Antibodies for phosphorylated Akt (pAkt), Akt, phosphorylated ERK (pERK), ERK, phosphorylated Stat3 (pStat3), Stat3, phosphorylated AMPK (pAMPK), 5′ adenosine monophosphate-activated protein kinase (AMPK), and LKB1 were purchased from Cell Signaling Technology (Danvers, MA). Anti-protein tyrosine phosphatase 1B (PTP1B) antibody (Ab) was procured from BD Biosciences (San Jose, CA). Ab for β-actin was purchased from Sigma-Aldrich (St Louis, MO).
Cell Culture, Reagents, and Treatments
The human breast cancer cell lines MCF-7, T47D, MDA-MB-231, and MDA-MB-468 were obtained from the American Type Culture Collection (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS (Gemini Bioproducts, Woodland, CA) and 2 µM l-glutamine (Invitrogen, Carlsbad, CA). Cell line authentication was done by analysis of known genetic markers or response (e.g., expression of estrogen receptor and p53 and estrogen responsiveness) [21,24]. For treatment, cells were seeded at a density of 1 x 106 per 100-mm tissue culture dish. After 24 hours of serum starvation, the culture media were changed to serum-free media containing treatments as indicated. Cultures were treated with human recombinant leptin (Sigma-Aldrich) at 100 ng/ml [24] and/or human recombinant full-length adiponectin (Biovendor, Candler, NC) at 10 µg/ml for indicated durations [54]. PTP1B inhibitor PR-129 (6-methyl-2-(oxalylamino)-4, 5, 6, 7-tetrahydrothieno [2, 3-c] pyridine-3-carboxylic acid trifluoroacetic acid salt) was procured from Enzo Life Sciences (Famingdale, NY). Rosiglitazone was purchased from Cayman Chemical Company (Ann Arbor, MI). Clinical studies examining the effect of rosiglitazone therapy in women with breast cancer showed that rosiglitazone at 8 mg yields a maximum serum concentration (Cmax ± SD) of 598 ± 117 mg/ml or 1.67 µmol/l and is well tolerated [55,56]. This concentration is within the 1 to 10 µmol/l range used to induce antiproliferative effects on mammary epithelial cells in vitro [57,58]. Additionally, higher doses of rosiglitazone have also been used in vitro [59,60]. We examined the effect of various concentrations of rosiglitazone in this study.
Clonogenicity Assay
To perform colony formation assay, we plated breast cancer cells (single-cell suspension) in 12-well plates at a density of 250 cells per well overnight [21]. The following day, cells were treated with 100 ng/ml human recombinant full-length leptin and 10 µg/ml human recombinant full-length adiponectin alone or in combination, and the medium was replaced with fresh medium containing treatments every 3 days. After a 10-day treatment period, the medium was removed and colonies were stained with crystal violet (0.1% in 20% methanol). Colony numbers were assessed visually and colonies containing >50 normal-appearing cells were counted. Pictures were taken using a digital camera.
Anchorage-Independent Growth Assay
Anchorage-independent growth of breast cancer cells was assayed by colony formation in soft agar [21]. Briefly, equal volumes of agar (1.2%) and complete medium were mixed to make 0.6% agar growth medium solution in six-well tissue culture plates. Cells (2 x 103 cells/well) were suspended in media with or without treatment followed by mixing with equal volume of agar (0.6%). Cell suspension agar mix (2 ml) was then added to each well. Plates were incubated at 37°C with 5% CO2 in a humidified incubator for 3 weeks, and media with or without treatment were added every 3 days. Colonies were stained with 0.005% crystal violet in phosphate-buffered saline (PBS) for 1 hour at room temperature and observed using Olympus IX50 inverted microscope. Colonies were counted in five randomly selected fields at 10x magnification. Results are expressed as average number of colonies counted per microfield.
Migration Assay
To perform migration assays [24,26], we plated cells into the 24-well cell culture plate, precoated with human fibronectin (5 µg/cm2; Sigma, St Louis, MO). Cells were allowed to grow in DMEM-containing 10% FBS to confluence and then were washed with serum-free medium and serum starved for 16 hours. A 1-mm-wide scratch was made across the cell layer using a sterile pipette tip. After washing with serum-free medium twice, DMEM containing 10 µg/ml human fibronectin was added to replace matrix depleted with the cells. Plates were photographed immediately after scratching. Cells were treated with human recombinant leptin at 100 ng/ml and/or adiponectin at 10 µg/ml alone and in combination. Plates were photographed after 24 and 48 hours at the identical location of the initial image.
Tumor Cell Invasion Assay
For an in vitro model system for metastasis, a matrigel invasion assay was performed by using a Matrigel invasion chamber from BD Biocoat Cellware (San Jose, CA) [24]. Cells were seeded at a density of 1 x 105 cells per insert and cultured overnight. After 16 hours of serum starvation, the culture media were changed to serum-free media containing treatments as indicated. Triplicate wells were used for each treatment. Cells were treated with human recombinant leptin at 100 ng/ml and/or adiponectin at 10 µg/ml alone and in combination. After 24-hour incubation, cells remaining above the insert membrane were removed by gentle scraping with a sterile cotton swab. Cells that had invaded through the matrigel to the bottom of the insert were fixed in methanol for 10 minutes. After being washed in PBS, the cells were stained with hematoxylin-eosin. The inserts were subsequently washed in PBS and briefly air-dried and mounted. The slides were coded to prevent counting bias, and the number of invaded cells on representative sections of each membrane were counted with a light microscope. The number of invaded cells for each experimental sample represents the average of triplicate wells.
Western Blot
Whole-cell lysates were prepared by scraping cells in 250 µl of ice-cold modified RIPA buffer [50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% Na deoxycholate, 1 mM PMSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 1 mM Na3VO4, and 1 mM NaF] [26]. The lysate was rotated 360° for 1 hour at 4°C followed by centrifugation at 12,000g for 10 minutes at 4°C to clear the cellular debris. Proteins were quantified using the Bradford Protein Assay Kit (Bio-Rad, Hercules, CA). Equal amounts of proteins were resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes, and Western blot analyses were performed using the previously described antibodies. Immunodetection was performed using enhanced chemiluminescence (ECL System; Amersham Pharmacia Biotech Inc, Arlington Heights, IL) according to manufacturer's instructions.
Mitogen-Activated Protein Kinase and Akt Activity Assay
Mitogen-activated protein kinase (MAPK) and Akt were immunoprecipitated with the specific antibodies following our previously published immunoprecipitation procedure [24]. For immunoprecipitation, whole-cell lysate from breast cancer cells was incubated with specific antibodies for ERK and Akt and the mixture was rotated slowly at 4°C for 16 hours. A total of 20 µl of packed protein A/G agarose beads was added, and the mixture was incubated at 4°C for 1 hour with rotation. The beads were collected by gentle centrifugation and washed twice with 1.5 ml of ice-cold buffer [50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% Na deoxycholate, 1 mM PMSF, 10 µg/ml aprotinin, and 10 µg/ml leupeptin]. After the final wash, the precipitated protein-bead complexes were resuspended in elution buffer. MAPK and Akt activities were measured using MAPK Activity Assay Kit (Chemicon International, Temecula, CA) and Akt Activity Assay Kit (Calbiochem, EMD Millipore, Billerica, MA) following the manufacturers' instructions.
PTP1B Activity Assay
Breast cancer cells were treated with 10 µg/ml adiponectin alone or in combination with 50 µM PTP1B inhibitor PR-129 for 2 hours. PTP1B activity assay was performed using PTP1B Assay Kit (Calbiochem) following manufacturer's instructions. Purified PTP1B and Suramin inhibitor available in the assay kit were used as positive and negative controls, respectively.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
Reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed following previously published protocol [21], using specific primers for adiponectin. Total cellular RNA was extracted using the TRIzol Reagent Kit (Life Technologies, Inc, Rockville, MD) and quantified by UV absorption. RT-PCR was carried out using specific sense and antisense PCR primers for amplification. PCR products were resolved by 2% agarose gel electrophoresis and visualized by ethidium bromide staining.
Breast Tumorigenesis Assay
MDA-MB-231 (5 x 106) cells in 0.1 ml of Hank's balanced salt solution were injected subcutaneously into the right gluteal region of 4- to 6-week-old female athymic nude mice [21], procured from Harlan Laboratories Inc (Indianapolis, IN). Two weeks after initial implantation, animals were grouped in five experimental groups (eight mice per group). Animals were treated with intratumoral injections of 1) recombinant adenovirus [108 plaque-forming units (pfu)] expressing adiponectin (Ad-Adn) or 2) luciferase (Ad-Luc) (kind gift from Dr Yu Wang, Assistant Professor of Pharmacology and Pharmacy, University of Hong Kong) [53] or 3) saline or 4) intraperitoneal injections of leptin (dosage of 5 mg/kg) [21] or 5) leptin and Ad-Adn together, every 36 hours for the duration of the experiment. Tumors were measured using digital vernier calipers, with tumor volume calculated using the formula (V = a/2 x b2), where V is the tumor volume in cubic millimeters and a and b are the largest and smallest diameters in millimeters, respectively. All animals were sacrificed after 4 weeks of treatment. Tumors were collected, weighed, fixed in 10% neutral-buffered formalin, and subjected to further analysis by Western and immunohistochemistry. All animal studies were in accordance with the guidelines of Emory University, Institutional Animal Care and Use Committee (IACUC).
Statistical Analysis
All experiments were independently performed three times in triplicates. Statistical analysis was done using Microsoft Excel software. Significant differences were analyzed using Student's t test and two-tailed distribution. Data were considered to be statistically significant if P < .001. Data are expressed as means ± SE between triplicate experiments. For animal studies, analysis of variance with repeated measurements was carried out to compare the mean tumor volume between the five different groups. The overall P value for testing for differences between at least two groups is <.0001.
Results
Adiponectin Inhibits Leptin-Induced Malignant Properties of Breast Cancer Cells
Mounting epidemiological and clinical evidence has put forth the role of adipocytokines on the center stage to explain the molecular connection between obesity and carcinogenesis. Recently, we and others have shown that leptin increases proliferation and growth of breast, endometrial, and hepatocellular cancers through activation of multiple downstream signaling pathways [20–30]. However, low adiponectin levels are significantly associated with an increased tumor growth and metastasis [44,47,48,61,62] indicating an anti-oncogenic role for adiponectin. We have recently shown that adiponectin inhibits growth and migration potential of breast cancer cells [54]. Here, we specifically examined if adiponectin can inhibit the pro-cancerous actions of leptin using various breast cancer cell lines. We found that adiponectin not only inhibited anchorage-dependent and anchorage-independent growth of breast cancer cells alone, but it also prevailed over the stimulatory effects of leptin. Adiponectin decreased leptin-induced clonogenicity and soft-agar colony formation of MCF7 and MDA-MB-231 breast cancer cells (Figure 1, A and B). MCF10A cells are nontumorigenic in athymic nude mice and have been used extensively as representative normal mammary epithelial cells. Adiponectin treatment did not inhibit growth of MCF10A cells, whereas leptin elicit a slight increase in clonogenicity (Figure W1). Cancer progression is a multistep process that involves invasion of basement membrane by tumor cells and migration to points far from a given primary tumor mass leading to metastasis [63]. We examined the effect of adiponectin treatment on leptin-induced invasion and migration properties of breast carcinoma cells using matrigel invasion and scratch migration assays. As expected, leptin increased migration of breast carcinoma cells, whereas adiponectin inhibited migration in a conventional scratch migration assay. Importantly, adiponectin treatment inhibited migration of MCF7, T47D, MDA-MB-231, and MDA-MB-468 breast cancer cells in the presence of leptin overcoming its strong pro-migratory potential (Figure 1C). Next, we performed matrigel invasion assay to examine the effect of adiponectin on leptin-induced invasion potential of breast carcinoma cells. As evident from Figure 1D, leptin treatment increased invasion of cancer cells through matrigel in comparison to untreated cells, whereas adiponectin treatment inhibited invasion of breast cancer (MCF7 and MDA-MB-231) cells. Leptin-mediated increased invasion of cancer cells was effectively inhibited by adiponectin (Figure 1D). Collectively, these results show that adiponectin treatment can effectively inhibit leptin-induced clonogenicity, anchorage-independent three-dimensional (3D) colony formation, and migration and invasion of breast cancer cells.
Figure 1.
Adiponectin reduces the stimulatory effect of leptin on clonogenicity, anchorage-independent growth migration and invasion potential of breast carcinoma cells. (A) Breast cancer cells (MCF7 and MDA-MB-231) were treated with 100 ng/ml leptin (L) and 10 µg/ml adiponectin (Adn) alone and in combination (L + Adn) and subjected to clonogenicity assay. Colonies containing >50 normal-appearing cells were counted. Adiponectin inhibited leptin-induced clonogenicity. (B) Breast cancer cells were subjected to soft-agar colony formation assay in the presence of leptin (L) and/or adiponectin (Adn) as in A for 3 weeks. Results are expressed as average number of colonies counted (in six microfields). *P < .005, compared with untreated controls; **P < .001, compared with untreated controls; #P < .005, compared with L treatment. Adiponectin inhibited leptin-induced anchorage-independent growth. (C) Breast cancer (MCF7, T47D, MDA-MB-231, and MDA-MB-468) cells were subjected to scratch migration assay in the presence of leptin and adiponectin treatments as described in A. The histogram shows the fold change in migration. *P < .01 and **P < .005, compared to untreated controls; #P < 0.01, compared to leptin (L)-treated cells. Adiponectin inhibited migration of breast cancer cells even in the presence of leptin. (D) MCF7 and MDA-MB-231 cells were cultured in Matrigel invasion chambers followed by treatment as in A for 24 hours. The number of cells that invaded through the matrigel was counted in five different regions. The slides were blinded to remove counting bias. The result shows mean of three independent experiments performed in triplicates. *P < .005, compared with untreated controls; #P < .001, compared to leptin-treated cells. Adiponectin treatment significantly reduced leptin-induced matrigel invasion.
Adiponectin Inhibits Phosphorylation of Key Components of Leptin Signaling in Breast Cancer Cells
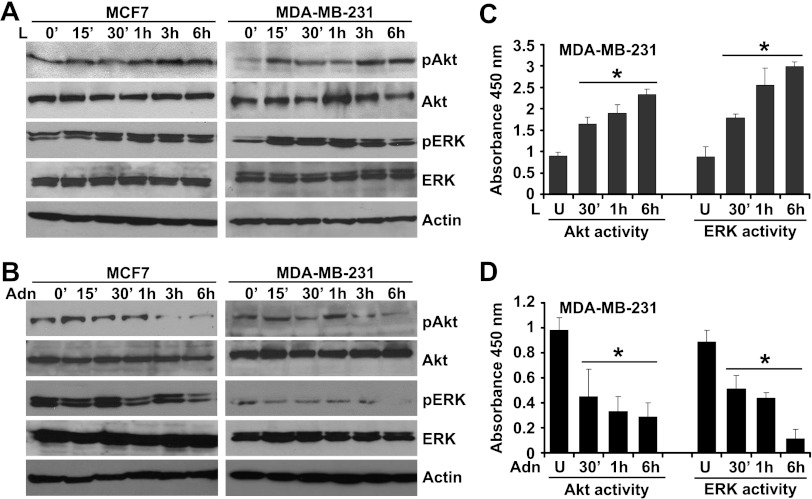
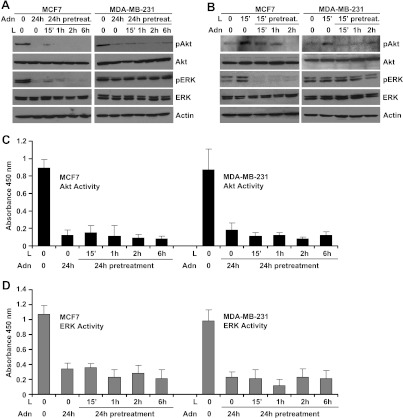
Binding of leptin to the LR (Ob-Rb) results in phosphorylation of conserved tyrosine residues [64], and these phosphorylation events are important for subsequent signaling events including Janus kinase (JAK) and Stat3 activation [64]. Canonical downstream signaling of leptin involves activation of phosphatidylinositol 3-kinase/Akt and ERK signaling [24,26]. Our previous studies have shown the direct involvement of JAK/Stat3, phosphatidylinositol 3-kinase/Akt, and ERK signaling in pro-cancerous actions of leptin [25,27]. We sought to determine the underlying molecular mechanism by which adiponectin treatment inhibits oncogenic actions of leptin. Leptin increased phosphorylation of Ser473 on Akt and Thr202 and Tyr204 on p42 ERK and p44 ERK within 15 to 30 minutes after leptin treatment, which remain elevated for the course of the experiment, whereas no change was observed in total protein levels (Figure 2A). An increase in ERK and Akt activity was also observed within 30 minutes of leptin treatment (Figure 2C). However, adiponectin treatment inhibited phosphorylation of Akt and ERK (Figure 2B) as well as Akt and ERK activity (Figure 2D). To examine the antagonistic effect of adiponectin on leptin-induced phosphorylation of Akt and ERK, we pretreated breast cancer cells with adiponectin for 24 hours followed by leptin treatment for various intervals of time. Pretreatment with adiponectin rendered breast cancer cells largely unresponsive to stimulatory effects of leptin showing that adiponectin pretreatment could protect cells against the oncogenic actions of leptin. Leptin failed to increase Akt and ERK phosphorylation (Figure 3A) as well as Akt and ERK activity (Figure 3, C and D) in adiponectin-pretreated breast cancer cells. In a reverse experiment, cells were pretreated with leptin followed by adiponectin treatment for various intervals of time. Adiponectin treatment successfully inhibited leptin-induced phosphorylation of Akt and ERK (Figure 3B), exhibiting that adiponectin treatment could override the biologic effects of leptin.
Figure 2.
Evidence of adiponectin-mediated inhibition of leptin signaling in breast cancer cells. (A) Breast cancer cells were treated with 100 ng/ml leptin (L) for various intervals of time. (B) Breast cancer cells were treated with 10 µg/ml adiponectin (Adn) for various intervals of time. Total protein was isolated and equal amounts of proteins were resolved by SDS-PAGE and subjected to immunoblot analysis using antibodies for pERK and pAkt. The membranes were reblotted using total ERK and Akt antibody. Anti-actin antibody was used as a control. The blots are representative of multiple independent experiments. MDA-MB-231 cells were treated with leptin (C) or adiponectin (D) for various intervals of time and subjected to ERK and Akt activity assay. Leptin increases phosphorylation and activity of ERK and Akt. *P < .005, compared with untreated controls. Adiponectin treatment inhibited phosphorylation and activity of ERK and Akt.
Figure 3.
Adiponectin inhibits key nodes of leptin signaling in breast cancer cells. (A) Breast cancer cells were pretreated with 10 µg/ml adiponectin (Adn) for 24 hours followed by 100 ng/ml leptin (L) treatment for various intervals of time. (B) Breast cancer cells were pretreated with 100 ng/ml leptin (L) followed by 10 µg/ml adiponectin (Adn) treatment for various intervals of time. Total protein was isolated and equal amounts of proteins were resolved by SDS-PAGE and subjected to immunoblot analysis using antibodies for pERK and pAkt. The membranes were reblotted using total ERK and Akt antibody. Anti-actin antibody was used as a control. The blots are representative of multiple independent experiments. Adiponectin treatment decreased leptin-induced phosphorylation of ERK and Akt in breast cancer cells. (C, D) Breast cancer cells were pretreated with 10 µg/ml adiponectin (Adn) for 24 hours followed by 100 ng/ml leptin (Lpn) treatment for various intervals of time and subjected to ERK and Akt activity assay. Adiponectin pretreatment decreased leptin-induced Akt and ERK activity.
Adiponectin Modulates an Important Modifier of Leptin Signaling, PTP1B
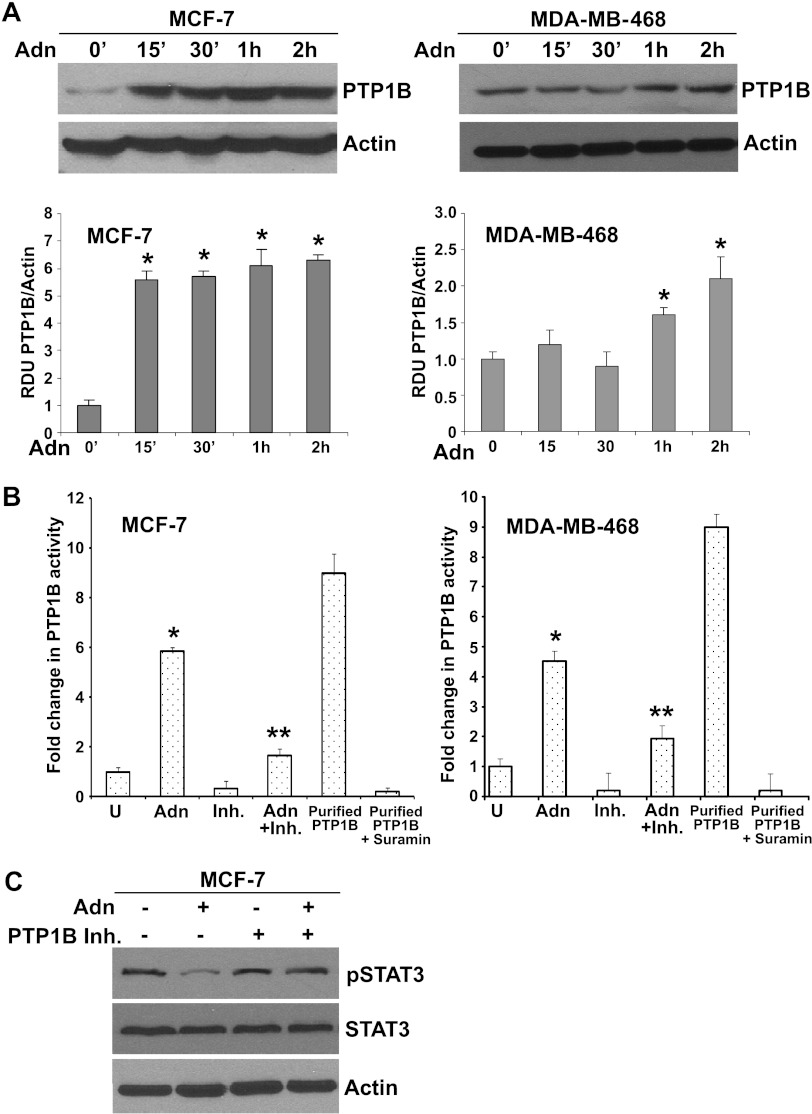
Probing the hierarchy of leptin signaling events, we previously showed that activation of JAK/Stat is upstream of the activation of ERK and Akt molecules [25,27]. Leptin signaling can be inhibited by two main inhibitory molecules: the suppressor of cytokine signaling 3 (SOCS3) and PTP1B [65,66]. SOCS proteins contain a central Src Homology 2 (SH2) domain, which allows these proteins to inhibit signaling by binding to phosphorylated JAK proteins or through direct interaction with tyrosine phosphorylated receptors. Overexpression of SOCS3 inhibits leptin-mediated tyrosine phosphorylation of JAK2 and subsequently Stat3 activation [65]. PTP1B is another significant downstream regulator of leptin signal transduction [66] that recognizes a specific substrate motif within JAK2. Overexpression of PTP1B decreases phosphorylation of JAK2 and blocks leptin signaling. We hypothesized that adiponectin may inhibit leptin signaling by upregulating these physiological inhibitors of leptin signaling. Therefore, we examined the effect of adiponectin treatment on the expression of PTP1B in breast cancer cells. Employing Western blot analysis, we found that adiponectin treatment significantly increased PTP1B expression in breast cancer cells (Figure 4A). Adiponectin treatment increased PTP1B protein expression within 15 minutes after treatment in MCF7 and within 30 minutes to 1 hour in MDA-MB-468 breast cancer cells. Next, we examined the modulation of PTP1B activity in response to adiponectin treatment. Adiponectin treatment significantly increased PTP1B activity, whereas PTP1B inhibitor PR-129 (6-methyl-2-(oxalylamino)-4, 5, 6, 7-tetrahydrothieno [2, 3-c] pyridine-3-carboxylic acid trifluoroacetic acid salt) effectively inhibited PTP1B activity. Combined treatment with PR-129 and adiponectin showed a reduction in adiponectin-induced PTP1B activity (Figure 4B). Purified PTP1B and Suramin were used as positive and negative controls for PTP1B activity assay. To investigate whether PTP1B plays a critical role in leptin-antagonist function of adiponectin, we treated breast cancer cells with adiponectin alone and in combination with PTP1B inhibitor followed by examination of phosphorylation status of Stat3, a key node of leptin-signaling network [24,25]. Adiponectin treatment reduced phosphorylation of Stat3 in breast cancer cells. Showing importance of PTP1B in leptin-antagonist function of adiponectin, PR-129 treatment inhibited adiponectin-mediated inhibition of Stat3 phosphorylation (Figure 4C). These findings suggest an important mechanistic link by which adiponectin can block leptin signaling through modulating the levels of physiological upstream inhibitor, PTP1B.
Figure 4.
Adiponectin increases PTP1B expression in breast cancer cells. (A) Breast cancer cells MCF7 and MDA-MB-468 were treated with 10 µg/ml adiponectin (Adn) for various intervals of time as indicated. Untreated cells are denoted as 0. Total protein was isolated and equal amounts of proteins were resolved by SDS-PAGE and subjected to immunoblot analysis using specific antibodies for PTP1B. The blots are representative of multiple independent experiments. Adiponectin treatment increased PTP1B expression in MCF7 and MDA-MB-468 breast cancer cells. The histogram is the mean of densitometric analysis showing relative density units (RDUs) of the Western blot signals for PTP1B normalized to actin in multiple experiments. *P < .005, compared with untreated controls. (B) Breast cancer cells were treated with 10 µg/ml adiponectin (Adn) and 50 µM of PR-129 (Inh.) alone or in combination (Adn + Inh.). Purified lysates were subjected to PTP1B activity assay. Purified PTP1B and Suramin were used as positive and negative controls, respectively. Adiponectin treatment increases PTP1B activity. (C) MCF7 cells were treated with 10 µg/ml adiponectin (Adn) and 50 µM of PR-129 (Inh.) alone or in combination (Adn + Inh.). Total protein was isolated and equal amounts of proteins were resolved by SDS-PAGE and subjected to immunoblot analysis using specific antibodies for pStat3 and Stat3. The blots are representative of multiple independent experiments. Actin was used as control. Adiponectin decreased pStat3, whereas PTP1B inhibitor abrogates adiponectin's effect.
PTP1B Plays an Important Role in Leptin-Antagonist Function of Adiponectin
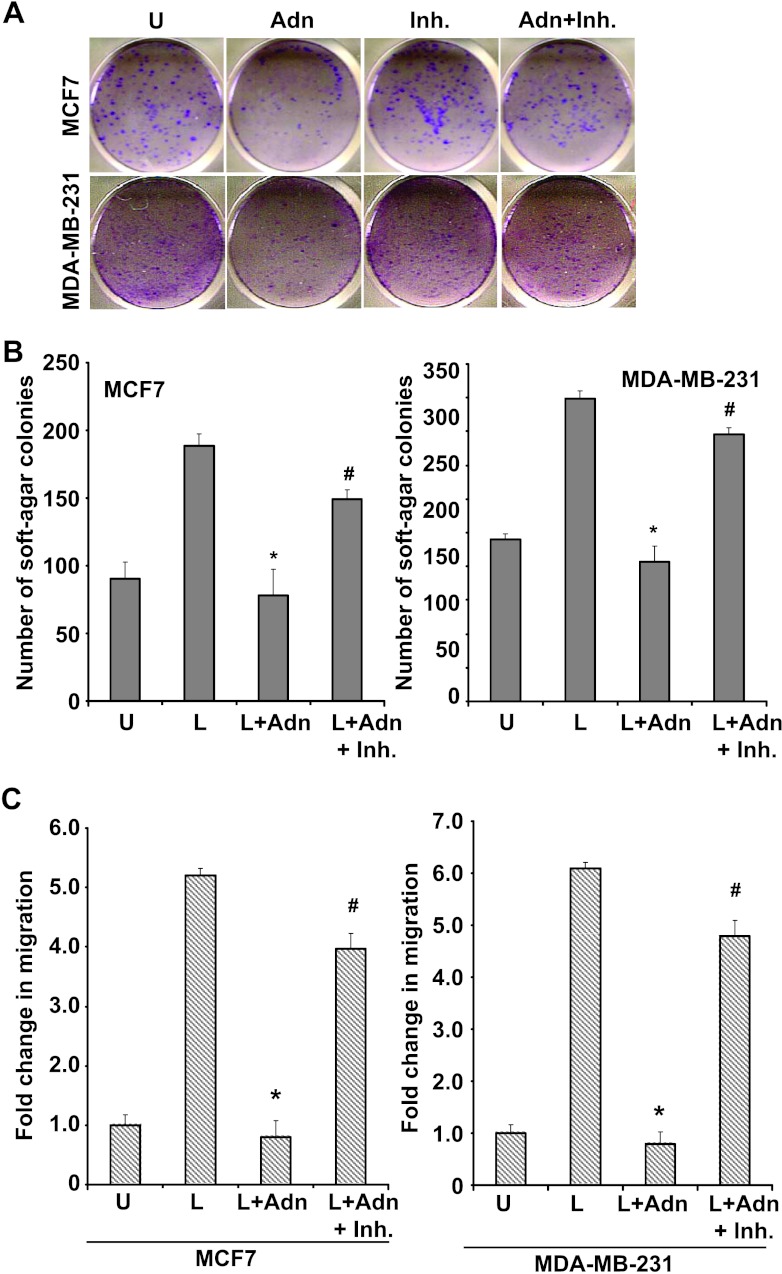
Our studies showed that PR-129 could inhibit PTP1B activity and reversed adiponectin-mediated inhibition of key leptin-signaling events (Figure 4). Next, we investigated the importance of PTP1B in adiponectin-mediated inhibition of leptin-induced malignant properties of breast cancer cells. Breast cancer cells were treated with adiponectin and PR-129 in combination with leptin and subjected to clonogenicity, anchorage-independent 3D colony formation, and migration assay. Inhibition of clonogenicity observed on adiponectin treatment was reversed in the presence of PR-129 (Figure 5A). Adiponectin treatment inhibited leptin-induced anchorage-independent colony formation of MCF7 and MDA-MB-231 cells. Combined treatment with PR-129 effectively reduced inhibitory effect of adiponectin, resulting in an increase in number of leptin-induced 3D colonies formed (Figure 5B). As observed earlier, adiponectin inhibited leptin-induced migration of both MCF7 and MDA-MB-231 breast cancer cells. Importantly, combined treatment with PTP1B inhibitor abrogated adiponectin-mediated inhibition of leptin-induced migration of breast cancer cells (Figure 5C). These results showed the importance of PTP1B in leptin-antagonist function of adiponectin, as inhibition of PTP1B activity clearly blocked adiponectin's inhibitory effect on leptin function.
Figure 5.
Inhibition of PTP1B reduces adiponectin's inhibitory effects on clonogenicity and anchorage-independent growth of breast carcinoma cells. (A) Breast cancer cells (MCF7 and MDA-MB-231) were treated with adiponectin (Adn) and PR-129 alone (Inh.) and in combination (Adn + Inh.) and subjected to clonogenicity assay. Colonies containing >50 normal-appearing cells were counted. PR-129 inhibited adiponectin's inhibitory effect on clonogenicity. (B) Breast cancer cells were subjected to soft-agar colony formation assay in the presence of leptin, adiponectin + leptin, and adiponectin + leptin + PR-129 (Inh.) for 3 weeks. Results are expressed as average number of colonies counted (in five microfields). *P < .005, compared with leptin-treated cells; #P < .01, compared to adiponectin + leptin-treated cells. PR-129 abrogates adiponectin-mediated inhibition of leptin-induced anchorage-independent growth. (C) Breast cancer cells were grown to confluence, scratched with a pipette tip, and photographed immediately following scratching (0 hour). Culture media were replaced with media containing 100 ng/ml leptin, leptin + 10 µg/ml adiponectin (Adn), and leptin + adiponectin + 50 µM PR-129 (Inh.). The plates were photographed at the identical location of the initial image (0 hour) at 24 and 48 hours. The results shown are representative of three independent experiments performed in triplicates. PR-129 inhibits adiponectin's effect on leptin-induced migration of breast cancer cells. The histogram shows the fold change in migration. *P < .01, compared to leptin-treated cells; #P < .01, compared to leptin + adiponectin-treated cells. All the experiments were performed thrice in triplicates.
Adiponectin Inhibits Leptin-Induced Breast Tumor Progression in Athymic Nude Mice
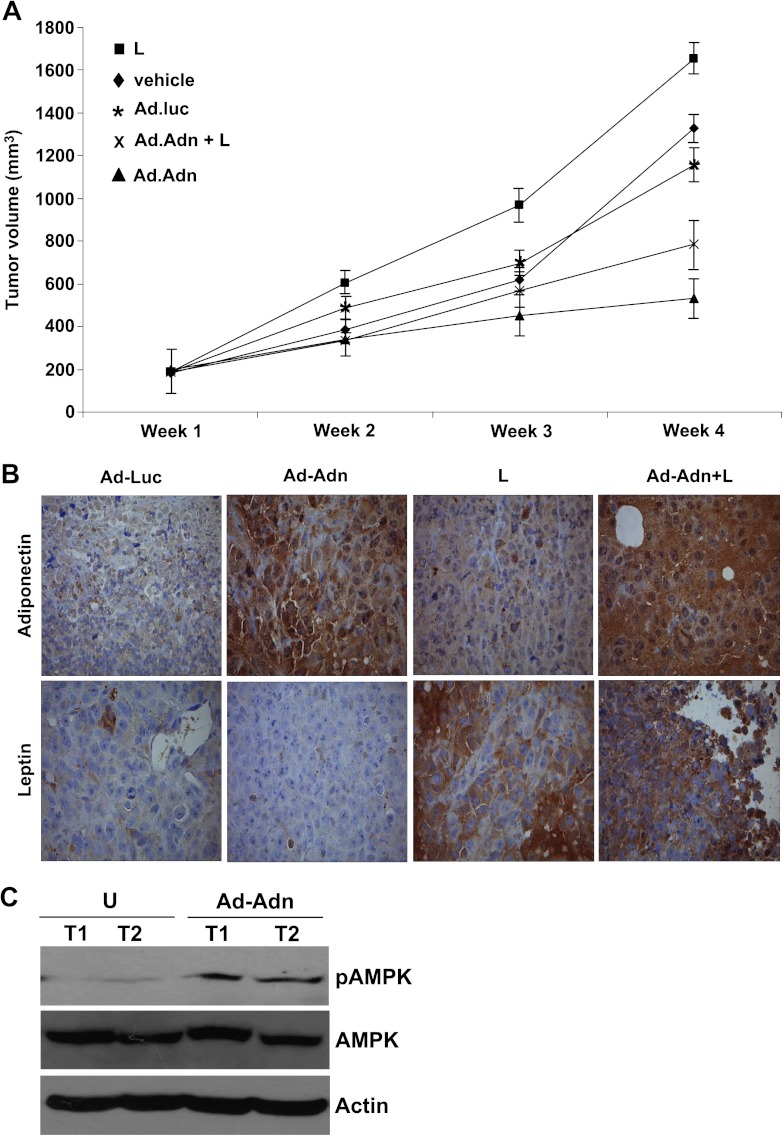
We investigated the physiological relevance of our in vitro findings by evaluating whether adiponectin has any suppressive effects on leptin-induced development of breast tumorigenesis in vivo. Leptin treatment significantly increased tumor growth as compared to the vehicle-treated group. Adiponectin treatment using adenovirus-Adn inhibited tumor growth resulting in reduced tumor size compared to vehicle and adenovirus-luciferase control. Importantly, adiponectin treatment efficiently inhibited leptin-induced breast tumor growth (Figure 6A). Previous studies from our laboratory showed adiponectin-mediated activation of the LKB1-AMPK-S6K axis in breast cancer cells [54]. Adiponectin adenovirus-treated breast cancer cells show elevated LKB1 levels indicating functionally active adiponectin (Figure W2). Adiponectin adenovirus-treated tumors showed elevated levels of adiponectin, whereas leptin-treated tumors showed increased staining for leptin as compared to control group (Figure 6B). The immunohistochemical assessment of tumor proliferation showed higher MIB1 (Ki-67 receptor) and phosphohistone H3 expression in leptin-treated group, whereas low to none MIB1 and phosphohistone H3 expression was observed in adiponectin-treated group (data not shown). We previously reported that adiponectin activates phosphorylation of AMPK, which is an important marker of biologic activity of adiponectin [54]. Here, we examined if adiponectin treatment using adenovirus-Adn increased AMPK phosphorylation, hence showing activation of adiponectin signaling. Adenovirus-adiponectin-treated tumors exhibited increased phosphorylation of AMPK in comparison to adenovirus-luciferase-treated tumors. These results confirmed that adenovirus-adiponectin treatment elevated adiponectin signaling in breast tumors. These results collectively show that adiponectin inhibits oncogenic actions of leptin including migration and invasion of breast cancer cells and inhibits components of the signaling machinery used by leptin while up-regulating an important upstream inhibitor, PTP1B.
Figure 6.
Adiponectin treatment inhibits leptin-induced breast tumor growth in nude mice. MDA-MB-231 cell-derived tumors were developed in nude mice and treated with leptin (L), vehicle (V), control-adenoviral (Ad-Luc), adiponectin-adenoviral (Ad-Adn; 108 pfu), and L + Ad-Adn. (A) Tumor growth was monitored by measuring the tumor volume for 6 weeks (n = 8 mice per group). Ad-Adn treatment reduced tumor size as compared to Ad-Luc, *P < .01. Adiponectin treatment significantly reduced leptin-induced tumor size as compared to leptin alone (P < .01) and Ad-luc (P < .01). (B) Tumor samples were subjected to immunohistochemical analysis using leptin and adiponectin antibody. Ad-Adn-treated tumors showed significant increase in adiponectin expression as compared to Ad-Luc-treated tumors, *P < .05 Ad-Adn versus Ad-Luc. Leptin-treated tumors showed significant increase in leptin expression as compared to Ad-luc, *P < .05 leptin versus Ad-Adn. (C) Tumor lysates were subjected to immunoblot analysis using pAMPK, AMPK, and actin antibodies. Adiponectin-treated tumors showed increased phosphorylation of AMPK showing increased adiponectin signaling.
Rosiglitazone Increases Adiponectin Expression and Inhibits Oncogenic Effects of Leptin on Breast Cancer Cells
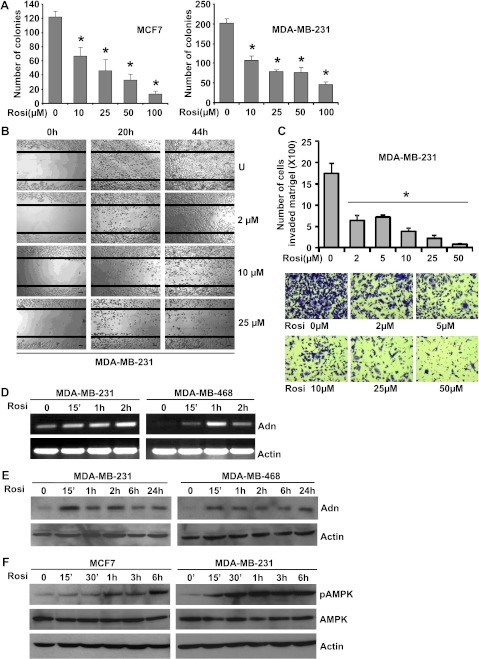
In conjunction with higher levels of leptin, obese state is associated with decreased levels of adiponectin. Our studies show that adiponectin acts as a leptin antagonist through modulating PTP1B, a physiological inhibitor of leptin signaling, resulting in inhibition of key nodes of leptin-signaling network. We hypothesize that therapeutic intervention capable of modulating adiponectin levels may prove effective in inhibiting leptin-induced breast cancer growth and metastatic potential. Thiazolidinediones, synthetic ligands for the transcription factor peroxisome proliferator-activated receptor-γ, were reported to increase serum adiponectin levels [67,68]. Several peroxisome proliferator-activated receptor-γ ligands are already in clinical use for the treatment of type 2 diabetes [69]. Different concentrations of each of these ligands (pioglitazone, troglitazone, and rosiglitazone) were tested for their effect on adiponectin expression in breast cancer cells (data not shown). Breast cancer cells were treated with various concentrations ranging from 1 to 100 µM rosiglitazone and subjected to clonogenicity (Figures 7A and W3A) and anchorage-independent growth assay (Figure W3B). Dose-dependent and statistically significant inhibition of clonogenicity and soft-agar colony formation was observed in the presence of rosiglitazone. Treatment with 10 µM rosiglitazone resulted in ∼50% to 60% inhibition in clonogenicity and soft-agar colony formation, whereas higher concentrations (25, 50, and 100 µM) were more inhibitory.
Figure 7.
Rosiglitazone increases adiponectin expression, modulates adiponectin signaling molecule, inhibits clonogenicity and migration and invasion of breast cancer cells. (A) Breast cancer cells (MCF7 and MDA-MB-231) were treated with various concentrations of rosiglitazone and subjected to clonogenicity assay. Colonies containing >50 normal-appearing cells were counted. Rosiglitazone inhibited clonogenic potential of breast cancer cells. (B) MDA-MB-231 cells were subjected to scratch migration assay in the presence of rosiglitazone treatments as indicated. Plates were photographed immediately after scratching, 20 and 44 hours after rosiglitazone treatment at the identical location of the initial image. Rosiglitazone inhibited migration of breast cancer cells. (C) MDA-MB-231 cells were cultured in Matrigel invasion chambers followed by rosiglitazone treatment as indicated. The number of cells that invaded through the matrigel was counted in five different regions. The slides were blinded to remove counting bias. *P < .005, compared with untreated controls. Rosiglitazone treatment significantly reduced matrigel invasion potential of breast cancer cells. Representative images of cells invaded through matrigel are shown. Breast cancer cells (MDA-MB-231 and MDA-MB-468) were treated with 50 µM rosiglitazone for various intervals of time as indicated. Untreated cells are denoted as 0. (D) Total RNA was isolated followed by RT-PCR to analyze the expression of adiponectin using specific primer sets. (E) Total protein was isolated and equal amounts of proteins were resolved by SDS-PAGE and subjected to immunoblot analysis using specific antibodies for adiponectin. The blots are representative of multiple independent experiments. Rosiglitazone treatment increases adiponectin expression in MDA-MB-231 and MDA-MB-468 breast cancer cells. (F) MCF7 and MDA-MB-231 cells were treated with rosiglitazone as in D. Total protein was isolated and equal amounts of proteins were resolved by SDS-PAGE and subjected to immunoblot analysis using specific antibodies for pAMPK and AMPK. Actin was used as control.
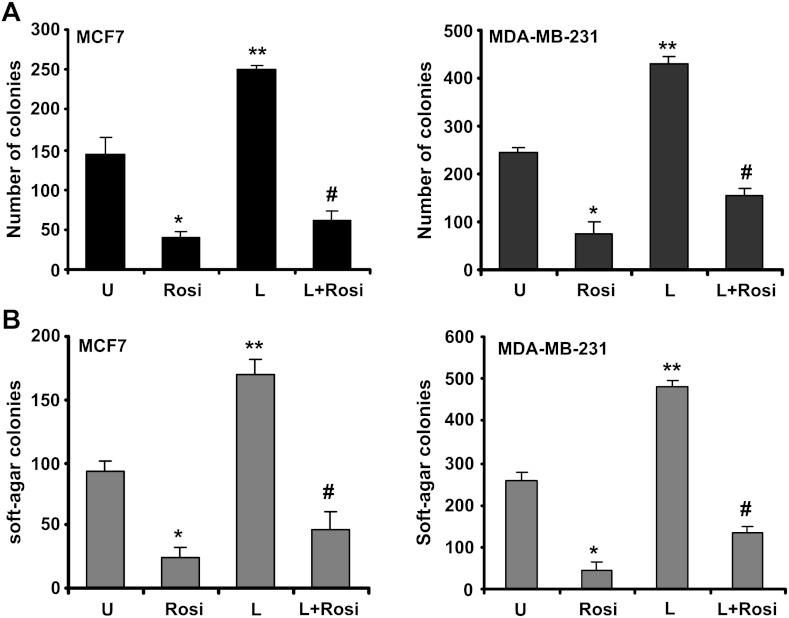
MCF10A and MCF12A cells were treated with various concentrations of rosiglitazone ranging from 5 to 100 µM in an anchorage-dependent growth assay. We found that MCF10A and MCF12A were significantly more resistant to the growth inhibition by rosiglitazone compared with MDA-MB-231 and MCF7 cells. For example, survival of MDA-MB-231 cells was decreased by ∼50% in 10 µM rosiglitazone, whereas growth of MCF10A and MCF12A remains unaffected by the similar treatment (Figure W4). These results indicate that the human breast cancer cells are significantly more sensitive to growth suppression by rosiglitazone compared with a normal mammary epithelial cell line. Selectivity toward cancer cells is highly desirable in potential cancer preventive and therapeutic agents. With invasion and migration being two important processes in cancer progression, we examined the effect of rosiglitazone on breast cancer cell migration and invasion by using scratch migration and matrigel invasion assays. Rosiglitazone treatment (as low as 2 µM) resulted in inhibition of migration of breast cancer cells (Figure 7B) in comparison with untreated cells. As evident from Figure 7C, low doses of rosiglitazone treatment decreased invasion of breast cancer cells through matrigel compared to untreated cells. Next, we determined the effect of rosiglitazone on adiponectin expression. Western blot and RT-PCR analyses showed that rosiglitazone stimulated expression of adiponectin in MDA-MB-231 and MDA-MB-468 cells within 15 minutes after treatment with a significant increase after 1 hour of treatment as compared to untreated cells (Figure 7, D and E). Adiponectin activates phosphorylation of AMPK in breast cancer cells [51,54]. Next, we examined AMPK phosphorylation levels upon rosiglitazone treatment. Rosiglitazone treatment led to increased phosphorylation of AMPK in MCF7, MDA-MB-231, and MDA-MB-468 cells within 15 minutes after treatment, whereas no change in total AMPK protein expression level was observed (Figures 7F and W5). To test our hypothesis that therapeutic intervention capable of increasing adiponectin levels in breast cancer cells may prove effective in inhibiting leptin-induced growth and metastatic potential, breast cancer cells were treated with a combination of rosiglitazone and leptin followed by analysis of clonogenic potential and anchorage-independent 3D colony growth. Rosiglitazone treatment not only inhibited anchorage-dependent and independent growth as expected, but it also effectively inhibited leptin-induced clonogenicity and soft-agar colony formation (Figure 8, A and B). Collectively, these results provide in vitro as well as in vivo evidence that adiponectin treatment can inhibit the oncogenic actions of leptin in breast cancer cells and suggest the involvement of PTP1B in blocking key nodes of leptin signaling and using rosiglitazone could be a rational therapeutic strategy for breast carcinoma in obese patients with high leptin levels.
Figure 8.
Rosiglitazone reduces the stimulatory effect of leptin on clonogenicity and anchorage-independent growth of breast carcinoma cells. (A) Breast cancer cells (MCF7 and MDA-MB-231) were treated with 100 ng/ml leptin (L) and 50 µM rosiglitazone (Rosi) alone and in combination (L + Rosi) and subjected to clonogenicity assay. Colonies containing >50 normal-appearing cells were counted. Rosiglitazone inhibited leptin-induced clonogenicity. (B) Breast cancer cells were subjected to soft-agar colony formation assay in the presence of leptin (L) and/or rosiglitazone (Rosi) as in A for 3 weeks. Results are expressed as average number of colonies counted (in six microfields). *P < .005, compared with untreated controls; **P < .001, compared with untreated controls; #P < .005, compared with L treatment. Rosiglitazone inhibited leptin-induced anchorage-independent growth.
Discussion
With epithelial and other cells accounting for only approximately 10% of human breast volume, adipocytes are the most predominant cell type in breast tumor microenvironment. Close positioning between breast tumor cells and adipocytes owing to reduction in separating connective tissue, invasion of carcinoma cells through the basement membrane leading to infiltration of fibrous tissue barriers allows increased paracrine cross talk. Adipocytes are active endocrine cells that secrete various biologically active adipocytokines, providing a potential molecular mechanism linking obesity and carcinogenesis [64]. Since obesity is a hyperleptinemic and hypoadiponectinemic state, in the present study, we investigated the effect of physiological levels of adiponectin on oncogenic effects of leptin. The following novel findings are described in this study: 1) adiponectin treatment inhibits malignant properties such as clonogenicity, anchorage-independent 3D colony formation, and invasion and migration of breast carcinoma cells; 2) adiponectin blocks oncogenic effects of leptin by inhibiting leptin-induced malignant properties; 3) adiponectin treatment inhibits key molecules of leptin signaling; 4) adiponectin treatment leads to overexpression of PTP1B, which is an upstream physiological inhibitor of leptin signaling; 5) adiponectin treatment inhibits leptin-induced breast tumorigenesis in vivo; 6) rosiglitazone increases adiponectin expression and inhibits oncogenic effects of leptin on breast cancer cells. These results show that adiponectin treatment significantly inhibits leptin-induced malignant properties of breast carcinoma cells and inhibits activation of key molecules of leptin signaling; thus, using adiponectin analogs or augmentation of its levels or activity may be a suitable therapeutic strategy for metastatic breast carcinoma.
Obese breast cancer patients exhibit a higher risk for lymph node metastasis, larger tumor burden, and mortality when compared with nonobese breast cancer patients [1,70] irrespective of the estrogen receptor status. Our studies along with others have clearly shown that leptin induces proliferation, migration, and invasion of breast carcinoma cells; hence, strategies blocking leptin activity might prove useful for breast cancer patients with elevated leptin levels. Biologic effects of leptin are mediated through active LRs; therefore, neutralization of leptin activity can be achieved with soluble LRs that bind free leptin in the circulation, leptin antagonists that bind LRs leading to their inactivation, and specific anti-LR monoclonal Abs (mAbs) that bind to the receptor preventing leptin signaling or antibodies developed against leptin. Anti-LR mAbs exhibit a long half-life in the circulation and good affinity for the receptor, but these mouse-generated mAbs need to be humanized to eliminate their potential immunogenicity. Another approach to target LR is presented by the development of recombinant, monomeric nanobodies. Nanobodies block leptin-induced conformational change of LR without interfering with the leptin-LR interaction. Nanobodies do not cross the blood-brain barrier; hence, they can selectively inhibit peripheral activity of leptin. Importantly, recent development of Allo-aca, a nine-amino acid-long peptide analog of LR binding site III of leptin, presents new possibilities of research and therapeutic strategy. Allo-aca and LR antagonists not only suppress the growth of established breast tumors in vivo but also inhibit leptin-induced angiogenesis, leptin-induced inflammatory signal transduction events, and autoimmunity-derived inflammation [32,71].
While all these agents to counteract leptin signaling are in various stages of development, we decided to investigate the potential antagonistic effect of protective adipocytokine adiponectin on leptin-induced oncogenic activities in breast carcinoma. Most of the adipocytokines are casually linked to obesity-related diseases, whereas adiponectin has shown promising insulin-sensitizing, anti-inflammatory, and anti-atherogenic activities. Adiponectin levels are decreased in obesity and various obesity-related diseases. The clinical relevance of adiponectin treatment has been suggested by studies showing that treatment with adiponectin can improve glucose/lipid homeostasis, increase insulin sensitivity, and prevent atherosclerosis in animal models [44,72,73]. Raising adiponectin level thus becomes an attractive goal for breast cancer therapeutics as well as prevention. Epidemiological data report that thiazolidinedione use is associated with reduced cancer risk [74] and rosiglitazone, a thiazolidinedione, increases plasma adiponectin levels in overweight women with polycystic ovary syndrome (PCOS) [75], subjects with type 2 diabetes mellitus and with impaired glucose tolerance [76]. Our study showed that rosiglitazone treatment increased adiponectin levels in breast cancer cells and induced the activation of adiponectin-signaling network. Of interest, rosiglitazone treatment also inhibited leptin-induced clonogenicity and growth of breast cancer cells. It is interesting to note that some anti-diabetic drugs (e.g., metformin) and bioactive-molecules (e.g., honokiol) can partially mimic adiponectin action and induce AMPK signaling in cancer cells [77,78]. Mouse models of caloric restriction and wheel running/exercise exhibit increase in adiponectin levels and protection against breast carcinogenesis [79,80], indicating alternative approaches to modulate adiponectin and its biologic effects. Preclinical development of adiponectin-based peptide compounds acting as AdipoR agonists presents another approach for adiponectin-based therapeutics. Identification of minimal adiponectin active site followed by development of pharmacologically improved analogs led to the development of ADP 355 as an optimal AdipoR agonist effectively inhibiting growth of AdipoR-positive breast cancer cells (MCF7, MDA-MB-231) and modulating adiponectin-signaling network [81]. In addition to increasing adiponectin levels using adiponectin analogs, modulating AdipoR activity, augmentation of its effectiveness [82], can potentially become a future beneficial treatment for breast carcinoma patients. Collectively, this study underscores the importance of adipocytokine levels, as they impact breast carcinogenesis and also provide mechanistic insight. Considering the high prevalence of obesity in the United States, novel therapeutic strategies to modulate leptin/adiponectin levels have the potential to significantly impact the vast majority of obese breast carcinoma patients and improving overall prognosis.
Supplementary Material
Footnotes
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) National Institute of Health (NIH) (K01DK076742 and R03DK089130; to N.K.S.), National Cancer Institute (NCI) NIH (R01CA131294), Safeway Foundation, Avon Foundation, and Breast Cancer Research Foundation (90047965; to D.S.). There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
This article refers to supplementary materials, which are designated by Figures W1 to W5 and are available online at www.neoplasia.com.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev. 2004;5:153–165. doi: 10.1111/j.1467-789X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Rose DP, Gilhooly EM, Nixon DW. Adverse effects of obesity on breast cancer prognosis, and the biological actions of leptin (review) Int J Oncol. 2002;21:1285–1292. [PubMed] [Google Scholar]
- 4.Ray A, Cleary MP. Obesity and breast cancer: a clinical biochemistry perspective. Clin Biochem. 2012;45:189–197. doi: 10.1016/j.clinbiochem.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 6.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 7.Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, Yu JC, Sun CA. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100:578–582. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–1453. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 9.Snoussi K, Strosberg AD, Bouaouina N, Ben Ahmed S, Helal AN, Chouchane L. Leptin and leptin receptor polymorphisms are associated with increased risk and poor prognosis of breast carcinoma. BMC Cancer. 2006;6:38. doi: 10.1186/1471-2407-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, Ballard-Barbash R. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14:2881–2888. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han C, Zhang HT, Du L, Liu X, Jing J, Zhao X, Yang X, Tian B. Serum levels of leptin, insulin, and lipids in relation to breast cancer in China. Endocrine. 2005;26:19–24. doi: 10.1385/ENDO:26:1:019. [DOI] [PubMed] [Google Scholar]
- 12.Houseknecht KL, Mantzoros CS, Kuliawat R, Hadro E, Flier JS, Kahn BB. Evidence for leptin binding to proteins in serum of rodents and humans: modulation with obesity. Diabetes. 1996;45:1638–1643. doi: 10.2337/diab.45.11.1638. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–4331. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 14.Cleary MP, Juneja SC, Phillips FC, Hu X, Grande JP, Maihle NJ. Leptin receptor-deficient MMTV-TGF-α/LeprdbLeprdb female mice do not develop oncogene-induced mammary tumors. Exp Biol Med (Maywood) 2004;229:182–193. doi: 10.1177/153537020422900207. [DOI] [PubMed] [Google Scholar]
- 15.Cleary MP, Phillips FC, Getzin SC, Jacobson TL, Jacobson MK, Christensen TA, Juneja SC, Grande JP, Maihle NJ. Genetically obese MMTV-TGF-α/LepobLepob female mice do not develop mammary tumors. Breast Cancer Res Treat. 2003;77:205–215. doi: 10.1023/a:1021891825399. [DOI] [PubMed] [Google Scholar]
- 16.Chua SC, Jr, Liu SM, Li Q, Sun A, DeNino WF, Heymsfield SB, Guo XE. Transgenic complementation of leptin receptor deficiency. II. Increased leptin receptor transgene dose effects on obesity/diabetes and fertility/lactation in lepr-db/db mice. Am J Physiol Endocrinol Metab. 2004;286:E384–E392. doi: 10.1152/ajpendo.00349.2003. [DOI] [PubMed] [Google Scholar]
- 17.Park J, Kusminski CM, Chua SC, Scherer PE. Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. Am J Pathol. 2010;177:3133–3144. doi: 10.2353/ajpath.2010.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dogan S, Hu X, Zhang Y, Maihle NJ, Grande JP, Cleary MP. Effects of high-fat diet and/or body weight on mammary tumor leptin and apoptosis signaling pathways in MMTV-TGF-α mice. Breast Cancer Res. 2007;9:R91. doi: 10.1186/bcr1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunez NP, Perkins SN, Smith NC, Berrigan D, Berendes DM, Varticovski L, Barrett JC, Hursting SD. Obesity accelerates mouse mammary tumor growth in the absence of ovarian hormones. Nutr Cancer. 2008;60:534–541. doi: 10.1080/01635580801966195. [DOI] [PubMed] [Google Scholar]
- 20.Yan D, Avtanski D, Saxena NK, Sharma D. Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem. 2012;287:8598–8612. doi: 10.1074/jbc.M111.322800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight BB, Oprea-Ilies GM, Nagalingam A, Yang L, Cohen C, Saxena NK, Sharma D. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr Relat Cancer. 2011;18:413–428. doi: 10.1530/ERC-11-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Nagalingam A, Saxena NK, Singh SV, Sharma D. Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis. 2011;32:359–367. doi: 10.1093/carcin/bgq267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma D, Wang J, Fu PP, Sharma S, Nagalingam A, Mells J, Handy J, Page AJ, Cohen C, Anania FA, et al. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology. 2010;52:1713–1722. doi: 10.1002/hep.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxena NK, Taliaferro-Smith L, Knight BB, Merlin D, Anania FA, O'Regan RM, Sharma D. Bidirectional crosstalk between leptin and insulin-like growth factor-I signaling promotes invasion and migration of breast cancer cells via transactivation of epidermal growth factor receptor. Cancer Res. 2008;68:9712–9722. doi: 10.1158/0008-5472.CAN-08-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, Anania FA. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxena NK, Vertino PM, Anania FA, Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282:13316–13325. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–640. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Q, Hursting SD, Reizes O. Leptin regulates cyclin D1 in luminal epithelial cells of mouse MMTV-Wnt-1 mammary tumors. J Cancer Res Clin Oncol. 2012;138:1607–1612. doi: 10.1007/s00432-012-1252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo S, Liu M, Wang G, Torroella-Kouri M, Gonzalez-Perez RR. Oncogenic role and therapeutic target of leptin signaling in breast cancer and cancer stem cells. Biochim Biophys Acta. 2012;1825:207–222. doi: 10.1016/j.bbcan.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barone I, Catalano S, Gelsomino L, Marsico S, Giordano C, Panza S, Bonofiglio D, Bossi G, Covington KR, Fuqua SA, et al. Leptin mediates tumor-stromal interactions that promote the invasive growth of breast cancer cells. Cancer Res. 2012;72:1416–1427. doi: 10.1158/0008-5472.CAN-11-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorden P, Gavrilova O. The clinical uses of leptin. Curr Opin Pharmacol. 2003;3:655–659. doi: 10.1016/j.coph.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Otvos L, Jr, Surmacz E. Targeting the leptin receptor: a potential new mode of treatment for breast cancer. Expert Rev Anticancer Ther. 2011;11:1147–1150. doi: 10.1586/era.11.109. [DOI] [PubMed] [Google Scholar]
- 33.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 34.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 35.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 36.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 37.Maahs DM, Ogden LG, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson JE, Ehrlich J, Eckel RH, Rewers M. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 39.Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein BJ, Scalia R. Adiponectin: a novel adipokine linking adipocytes and vascular function. J Clin Endocrinol Metab. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 43.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28:4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Wang Y. Adiponectin and breast cancer. Med Oncol. 2011;28:1288–1295. doi: 10.1007/s12032-010-9617-x. [DOI] [PubMed] [Google Scholar]
- 47.Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, Papadiamantis Y, Markopoulos C, Spanos E, Chrousos G, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 48.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, Noguchi S. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 49.Arditi JD, Venihaki M, Karalis KP, Chrousos GP. Antiproliferative effect of adiponectin on MCF7 breast cancer cells: a potential hormonal link between obesity and cancer. Horm Metab Res. 2007;39:9–13. doi: 10.1055/s-2007-956518. [DOI] [PubMed] [Google Scholar]
- 50.Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, Pecquery R. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008;20:971–977. [PubMed] [Google Scholar]
- 51.Kim KY, Baek A, Hwang JE, Choi YA, Jeong J, Lee MS, Cho DH, Lim JS, Kim KI, Yang Y. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009;69:4018–4026. doi: 10.1158/0008-5472.CAN-08-2641. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Lam JB, Chow KH, Xu A, Lam KS, Moon RT, Wang Y. Adiponectin stimulates Wnt inhibitory factor-1 expression through epigenetic regulations involving the transcription factor specificity protein 1. Carcinogenesis. 2008;29:2195–2202. doi: 10.1093/carcin/bgn194. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, Wu D, Cooper GJ, Xu A. Adiponectin modulates the glycogen synthase kinase-3β/β-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66:11462–11470. doi: 10.1158/0008-5472.CAN-06-1969. [DOI] [PubMed] [Google Scholar]
- 54.Taliaferro-Smith L, Nagalingam A, Zhong D, Zhou W, Saxena NK, Sharma D. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene. 2009;28:2621–2633. doi: 10.1038/onc.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yee LD, Williams N, Wen P, Young DC, Lester J, Johnson MV, Farrar WB, Walker MJ, Povoski SP, Suster S, et al. Pilot study of rosiglitazone therapy in women with breast cancer: effects of short-term therapy on tumor tissue and serum markers. Clin Cancer Res. 2007;13:246–252. doi: 10.1158/1078-0432.CCR-06-1947. [DOI] [PubMed] [Google Scholar]
- 56.Esteva FJ, Moulder SL, Gonzalez-Angulo AM, Ensor J, Murray JL, Green MC, Koenig KB, Lee MH, Hortobagyi GN, Yeung SC. Phase I trial of exemestane in combination with metformin and rosiglitazone in nondiabetic obese postmenopausal women with hormone receptor-positive metastatic breast cancer. Cancer Chemother Pharmacol. 2012 doi: 10.1007/s00280-012-1977-9. [Epub ahead of print 28 September] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yee LD, Guo Y, Bradbury J, Suster S, Clinton SK, Seewaldt VL. The antiproliferative effects of PPARγ ligands in normal human mammary epithelial cells. Breast Cancer Res Treat. 2003;78:179–192. doi: 10.1023/a:1022978608125. [DOI] [PubMed] [Google Scholar]
- 58.Patel L, Pass I, Coxon P, Downes CP, Smith SA, Macphee CH. Tumor suppressor and anti-inflammatory actions of PPARγ agonists are mediated via upregulation of PTEN. Curr Biol. 2001;11:764–768. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 59.Feng YH, Velazquez-Torres G, Gully C, Chen J, Lee MH, Yeung SC. The impact of type 2 diabetes and antidiabetic drugs on cancer cell growth. J Cell Mol Med. 2011;15:825–836. doi: 10.1111/j.1582-4934.2010.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mossner R, Schulz U, Kruger U, Middel P, Schinner S, Fuzesi L, Neumann C, Reich K. Agonists of peroxisome proliferator-activated receptor γ inhibit cell growth in malignant melanoma. J Invest Dermatol. 2002;119:576–582. doi: 10.1046/j.1523-1747.2002.01861.x. [DOI] [PubMed] [Google Scholar]
- 61.Barb D, Pazaitou-Panayiotou K, Mantzoros CS. Adiponectin: a link between obesity and cancer. Expert Opin Investig Drugs. 2006;15:917–931. doi: 10.1517/13543784.15.8.917. [DOI] [PubMed] [Google Scholar]
- 62.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, Chen HY, Hou MF, Yuan SS. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 63.Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81:223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 65.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 66.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 67.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 68.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 69.Galli A, Mello T, Ceni E, Surrenti E, Surrenti C. The potential of antidiabetic thiazolidinediones for anticancer therapy. Expert Opin Investig Drugs. 2006;15:1039–1049. doi: 10.1517/13543784.15.9.1039. [DOI] [PubMed] [Google Scholar]
- 70.Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA. Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2005;14:2009–2014. doi: 10.1158/1055-9965.EPI-05-0106. [DOI] [PubMed] [Google Scholar]
- 71.Otvos L, Jr, Kovalszky I, Riolfi M, Ferla R, Olah J, Sztodola A, Nama K, Molino A, Piubello Q, Wade JD, et al. Efficacy of a leptin receptor antagonist peptide in a mouse model of triple-negative breast cancer. Eur J Cancer. 2011;47:1578–1584. doi: 10.1016/j.ejca.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 72.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 73.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 74.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25:1476–1481. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 75.Majuri A, Santaniemi M, Rautio K, Kunnari A, Vartiainen J, Ruokonen A, Kesaniemi YA, Tapanainen JS, Ukkola O, Morin-Papunen L. Rosiglitazone treatment increases plasma levels of adiponectin and decreases levels of resistin in overweight women with PCOS: a randomized placebo-controlled study. Eur J Endocrinol. 2007;156:263–269. doi: 10.1530/eje.1.02331. [DOI] [PubMed] [Google Scholar]
- 76.Osei K, Gaillard T, Kaplow J, Bullock M, Schuster D. Effects of rosglitazone on plasma adiponectin, insulin sensitivity, and insulin secretion in high-risk African Americans with impaired glucose tolerance test and type 2 diabetes. Metabolism. 2004;53:1552–1557. doi: 10.1016/j.metabol.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res. 2010;16:1695–1700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagalingam A, Arbiser JL, Bonner MY, Saxena NK, Sharma D. Honokiol activates AMP-activated protein kinase in breast cancer cells via an LKB1-dependent pathway and inhibits breast carcinogenesis. Breast Cancer Res. 2012;14:R35. doi: 10.1186/bcr3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu Z, Jiang W, Zacher JH, Neil ES, McGinley JN, Thompson HJ. Effects of energy restriction and wheel running on mammary carcinogenesis and host systemic factors in a rat model. Cancer Prev Res (Phila) 2012;5:414–422. doi: 10.1158/1940-6207.CAPR-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Lorenzo MS, Baljinnyam E, Vatner DE, Abarzua P, Vatner SF, Rabson AB. Caloric restriction reduces growth of mammary tumors and metastases. Carcinogenesis. 2011;32:1381–1387. doi: 10.1093/carcin/bgr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Otvos L, Jr, Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, Lovas S, Nama K, Hoffmann R, Knappe D, et al. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol. 2011;11:90. doi: 10.1186/1472-6750-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.