Abstract
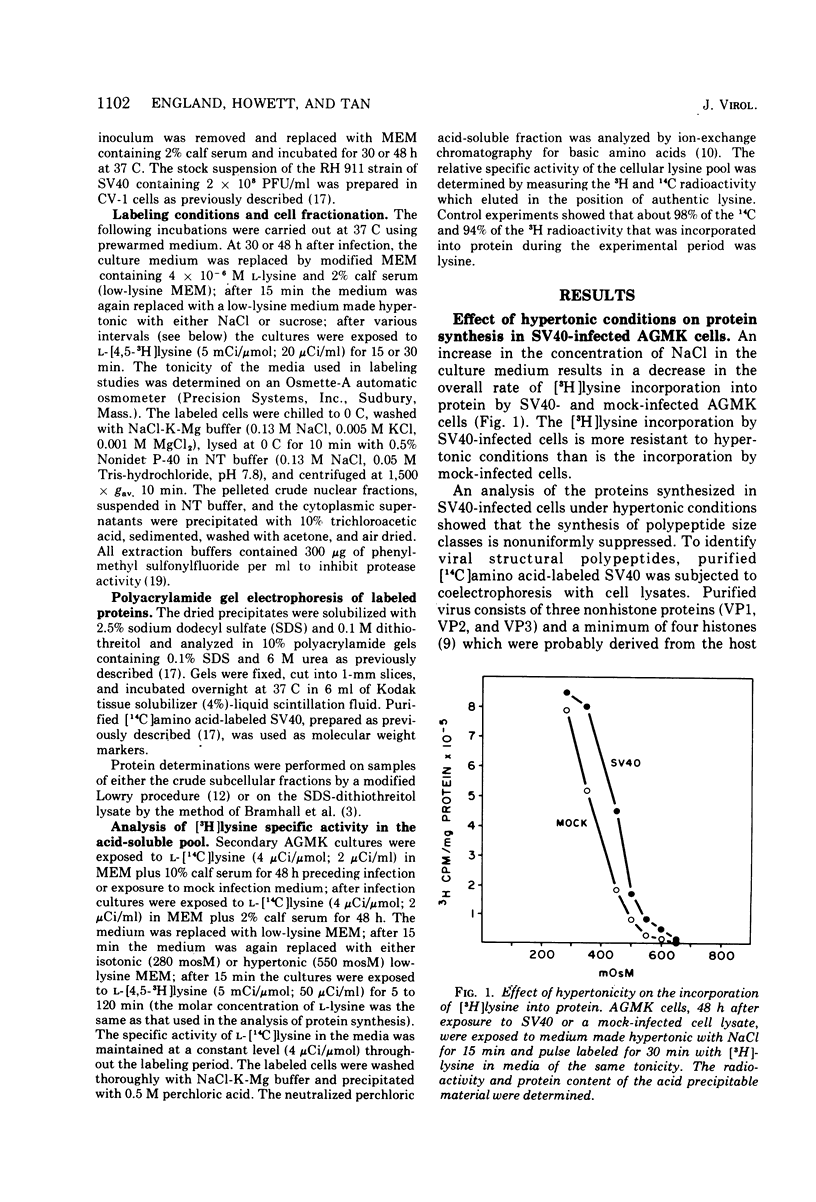
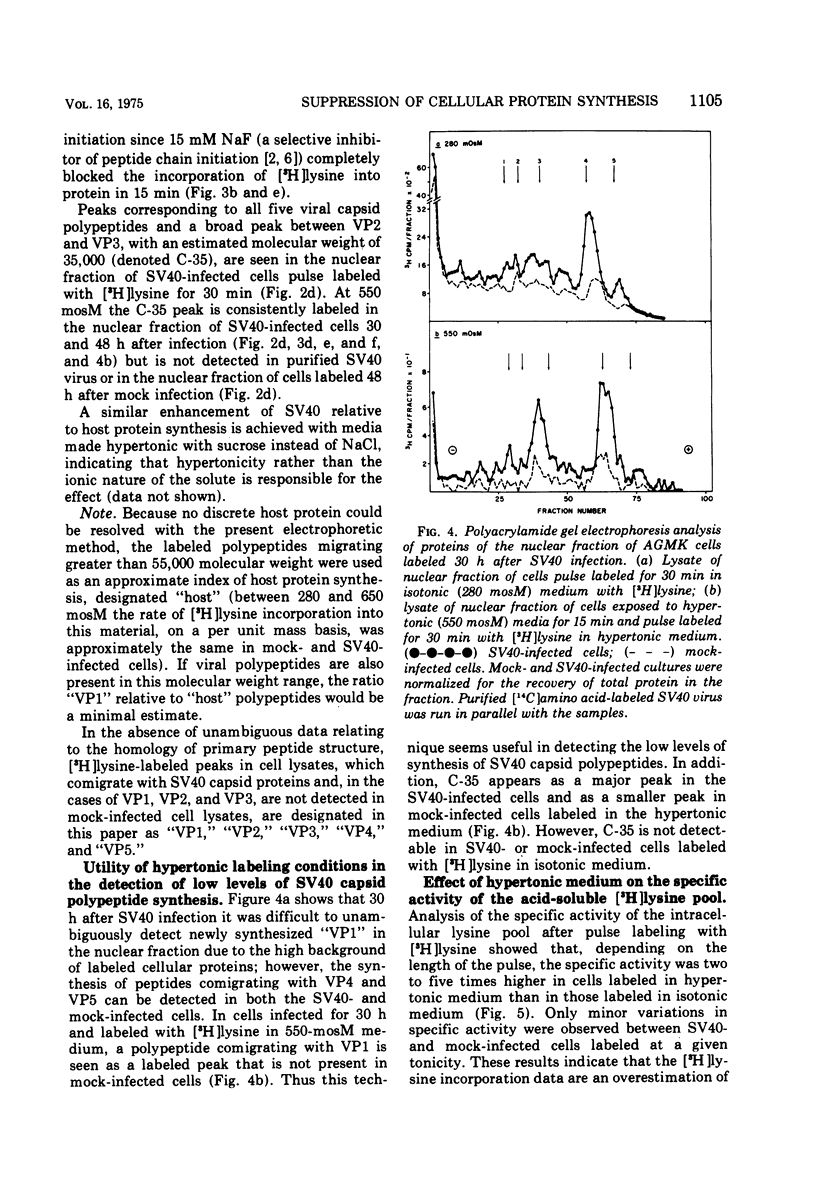
Hypertonic medium selectively suppressed the synthesis of most host cell polypeptides relative to the synthesis of simian virus 40 capsid polypeptides and a minority of cellular polypeptides, notably histones. Under optimal hypertonic conditions, the synthesis of the major capsid polypeptide (VP1) is enhanced about sevenfold relative to host polypeptide synthesis. Because of the small amounts of the other nonhistone capsid polypeptides (VP2) and VP3) present in cell lysates, it was difficult to quantitate the extent, if any, of their enhancement. The maintenance of the restricted pattern of protein synthesis caused by hypertonic medium was dependent on continual peptide chain initiations. The resistance of viral protein synthesis to hypertonic conditions provides a means of detecting relatively low levels of intracellular viral protein synthesis. Analysis of the specific activity of the acid-soluble [3H]lysine pool indicated that the rate of incorporation of [3H]lysine into protein was an overestimation of the actual rate of overall protein synthesis occurring in cells exposed to hypertonic as compared to isotonic conditions. Since it is likely that both cellular and viral protein synthesis draw lysine from a single pool, this change in pool specific activity does not affect the analysis of relative rates of protein synthesis at a given level of tonicity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Gesteland R. F. Pattern of protein synthesis in monkey cells infected by simian virus 40. J Virol. 1972 May;9(5):758–765. doi: 10.1128/jvi.9.5.758-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni C., Vesco C., Jacobs-Lorena M. The role of ribosomal subunits in mammalian cells. Cold Spring Harb Symp Quant Biol. 1969;34:555–565. doi: 10.1101/sqb.1969.034.01.063. [DOI] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Smith E. L. Histones: structure and function. Annu Rev Biochem. 1971;40:279–314. doi: 10.1146/annurev.bi.40.070171.001431. [DOI] [PubMed] [Google Scholar]
- Fischer H., Sauer G. Identification of virus-induced proteins in cells productively infected with simian virus 40. J Virol. 1972 Jan;9(1):1–9. doi: 10.1128/jvi.9.1.1-9.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerz W., McCarty K. S. Evidence for a proposed initiation complex for protein synthesis in reticulocyte polyribosome profiles. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1206–1213. doi: 10.1073/pnas.63.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn E. D. Protein metabolism in SV40-infected cells. Virology. 1973 Nov;56(1):313–333. doi: 10.1016/0042-6822(73)90309-7. [DOI] [PubMed] [Google Scholar]
- Lake R. S., Barban S., Salzman N. P. Resolutions and identification of the core deoxynucleoproteins of the simian virus 40. Biochem Biophys Res Commun. 1973 Sep 18;54(2):640–647. doi: 10.1016/0006-291x(73)91471-x. [DOI] [PubMed] [Google Scholar]
- Madore H. P., England J. M. Selective suppression of cellular protein synthesis in BHK-21 cells infected with rabies virus. J Virol. 1975 Nov;16(5):1351–1354. doi: 10.1128/jvi.16.5.1351-1354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The molecular weights of vertebrate histones exploiting a modified sodium dodecyl sulfate electrophoretic method. J Biol Chem. 1971 Dec 25;246(24):7557–7560. [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Pederson T., Klein P. Comparison of mitotic phenomena and effects induced by hypertonic solutions in HeLa cells. J Cell Biol. 1970 Feb;44(2):400–416. doi: 10.1083/jcb.44.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Enancement of uptake of Simian virus 40 by nuclei of permissive cells. Proc Soc Exp Biol Med. 1973 Dec;144(3):802–807. doi: 10.3181/00379727-144-37686. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Structural proteins of simian virus 40: phosphoproteins. J Virol. 1972 Nov;10(5):985–994. doi: 10.1128/jvi.10.5.985-994.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Walter G., Roblin R., Dulbecco R. Protein synthesis in Simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1972 Apr;69(4):921–924. doi: 10.1073/pnas.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Medium hypertonicity and polyribosome structure in Hela cells. The influence of hypertonicity of the growth medium on polyribosomes in Hela cells. Eur J Biochem. 1972 May;27(1):162–173. doi: 10.1111/j.1432-1033.1972.tb01822.x. [DOI] [PubMed] [Google Scholar]


