Abstract
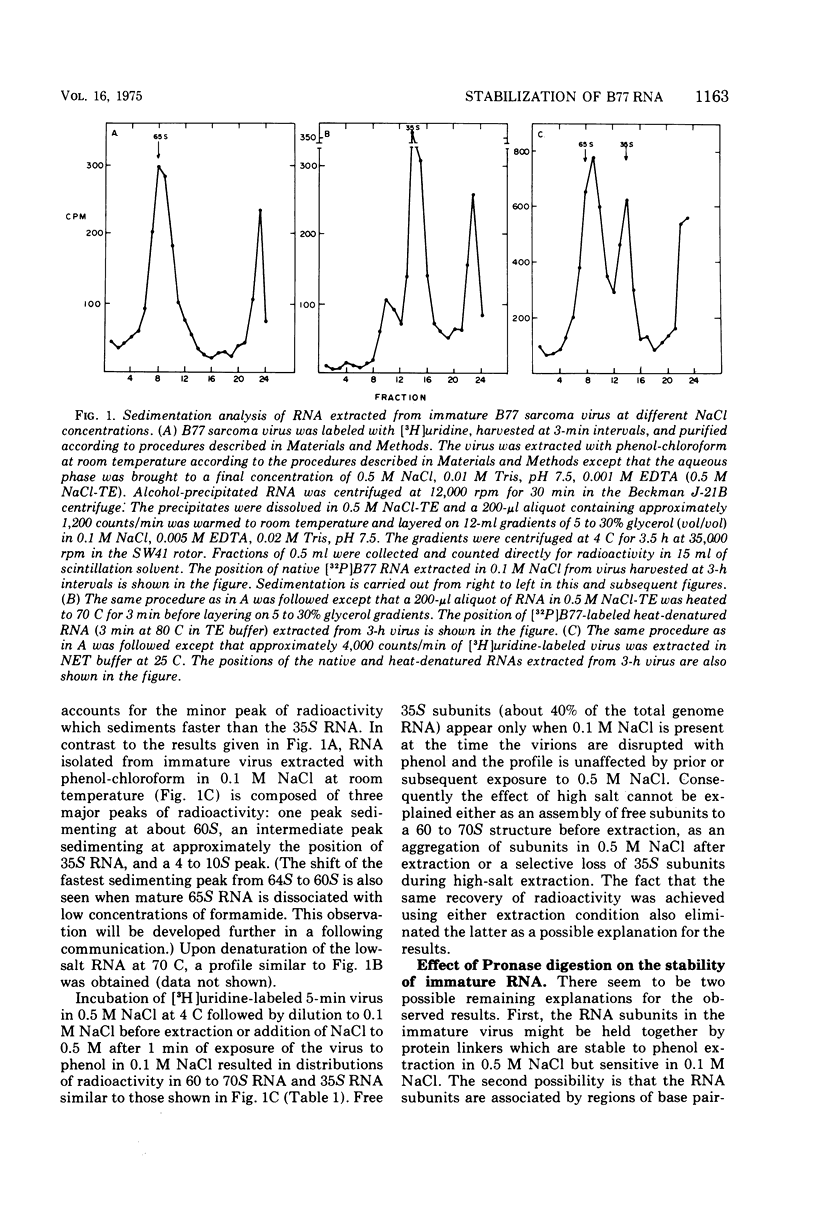
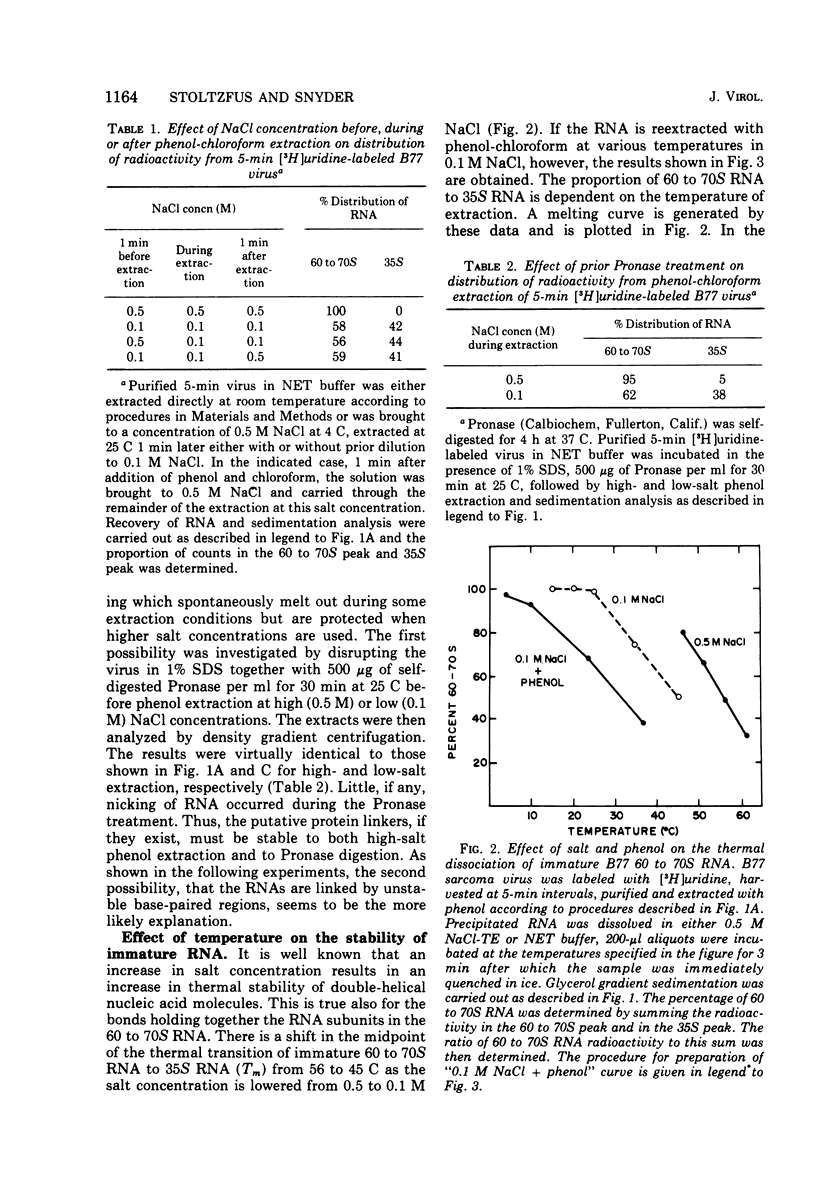
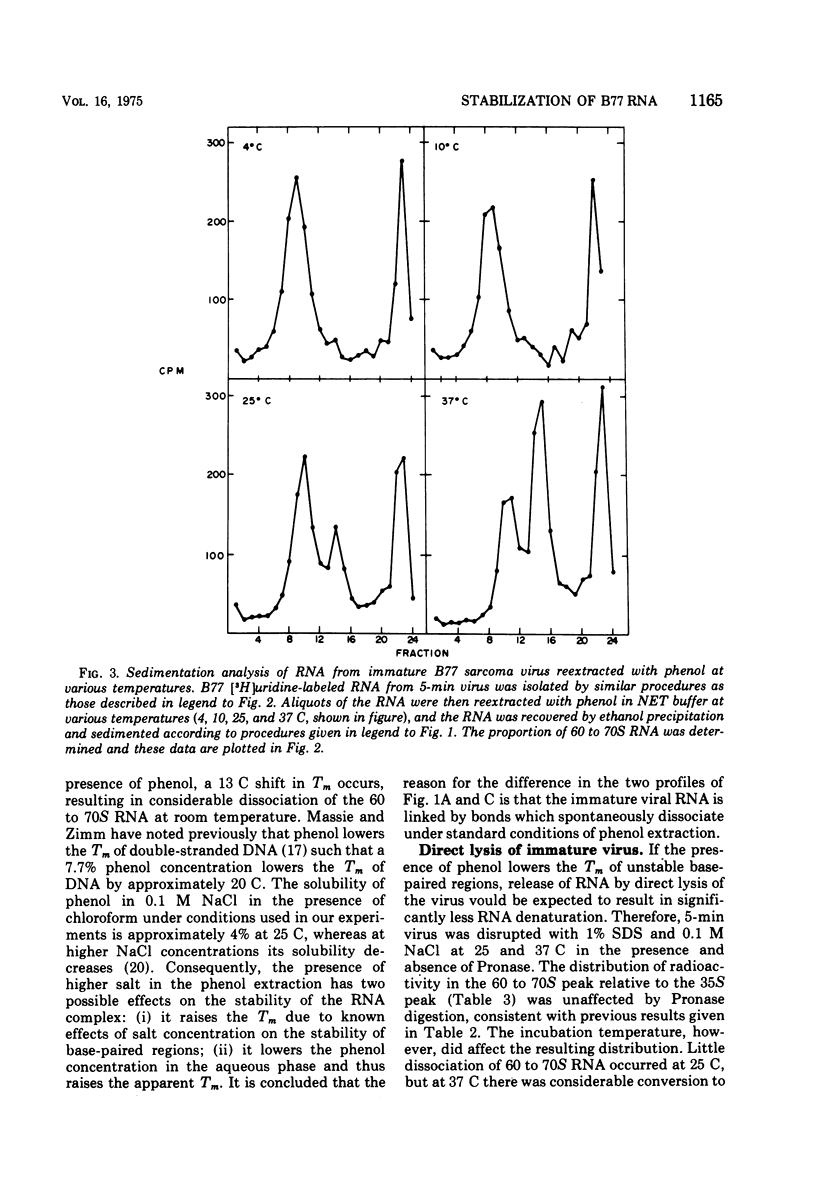
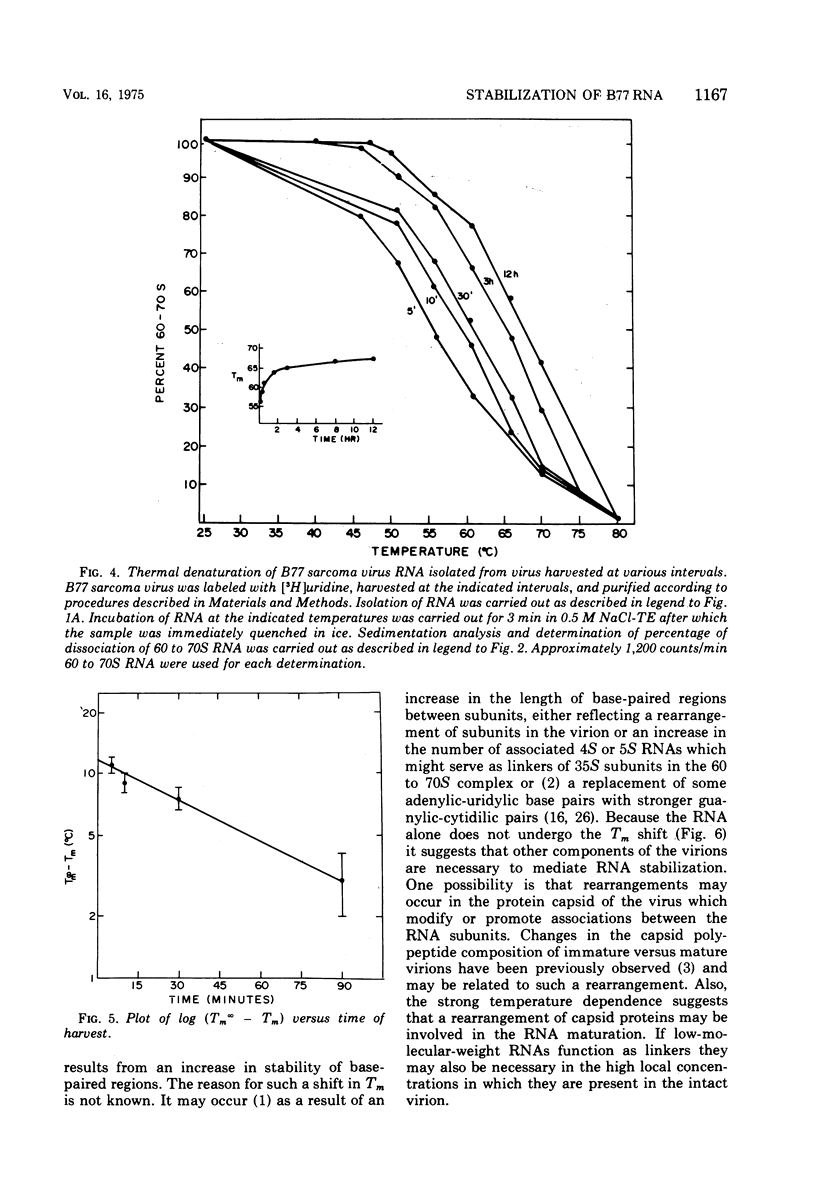
Extracellular maturation of Bratislava 77(B77) sarcoma virus RNA involves a stabilization of linkage between 35S subunits since the Tm of 60 to 70S RNA in the presence of 0.5 M NaCl increases from 56 to 67.5 C as the age of the virus increases. This stabilization process is strongly temperature dependent; the rate at 45 C is increased fourfold over the rate at 37 C. As a result of the instability of the immature RNA, denaturation to subunits occurs at room temperature during phenol extraction. This dissociation can be prevented by increasing the NaCl concentration during the extraction. The data support a model which proposes that RNA subunits of oncornaviruses exist as assembled 60 to 70S RNA even in immature virions but that the linkages between subunits are stabilized as a function of time. These linkages appear to be maintained by nucleotide base pairing rather than by protein. However, isolated RNA does not undergo stabilization, suggesting that some other component of the virion is necessary for the process to occur.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahic M., Vigne R. Properties of visna virus particles harvested at short time intervals: RNA content, infectivity, and ultrastructure. J Virol. 1975 May;15(5):1222–1230. doi: 10.1128/jvi.15.5.1222-1230.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. On the structure of RNA tumor viruses. Curr Top Microbiol Immunol. 1970;51:78–104. [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- East J. L., Allen P. T., Knesek J. E., Chan J. C., Bowen J. M., Dmochowski L. Structural rearrangement and subunit composition of RNA from released Soehner-Dmochowski murine sarcoma virions. J Virol. 1973 May;11(5):709–720. doi: 10.1128/jvi.11.5.709-720.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L. Studies on the RNA from avian myeloblastosis virus. Virology. 1969 Jan;37(1):124–131. doi: 10.1016/0042-6822(69)90313-4. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Garapin A. C., Levinson W. E., Bishop J. M., Goodman H. M. Characterization of the low-molecular-weight RNAs associated with the 70S RNA of Rous sarcoma virus. J Virol. 1973 Aug;12(2):334–342. doi: 10.1128/jvi.12.2.334-342.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L., Salden M. H., Bloemendal H. Virus-specific messenger RNA on free and membrane-bound polyribosomes from cells infected with Rauscher leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1093–1097. doi: 10.1073/pnas.71.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett O., Pitts J. D., Whalley J. M., Clason A. E., Hay J. Isolation of the nucleic acid of feline leukemia virus. Virology. 1971 Jan;43(1):317–320. doi: 10.1016/0042-6822(71)90252-2. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Properties of maedi nucleic acid and the presence of ribonucleic acid- and deoxyribonucleic acid-dependent deoxyribonucleic acid polymerase in the virions. J Virol. 1972 Aug;10(2):228–233. doi: 10.1128/jvi.10.2.228-233.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Uhlenbeck O. C., Doty P. Self-complementary oligoribonucleotides: adenylic acid-uridylic acid block copolymers. J Mol Biol. 1971 Apr 28;57(2):201–215. doi: 10.1016/0022-2836(71)90341-x. [DOI] [PubMed] [Google Scholar]
- Massie H. R., Zimm B. H. THE USE OF HOT PHENOL IN PREPARING DNA. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1641–1643. doi: 10.1073/pnas.54.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quade K., Smith R. E., Nichols J. L. Poly(riboadenylic acid) and adjacent nucleotides in Rous sarcoma virus RNA. Virology. 1974 Nov;62(1):60–70. doi: 10.1016/0042-6822(74)90303-1. [DOI] [PubMed] [Google Scholar]
- Riggin C. H., Bondurant M. C., Mitchell W. M. Differences between murine leukemia virus and murine sarcoma virus: effects of virion age and multiplicity of infection on viral RNA. Intervirology. 1974;2(4):209–221. doi: 10.1159/000149426. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Viruses with segmented ribonucleic acid genomes: multiplication of influenza versus reovirus. Bacteriol Rev. 1971 Sep;35(3):250–266. doi: 10.1128/br.35.3.250-266.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E. High specific infectivity avian RNA tumor viruses. Virology. 1974 Aug;60(2):543–547. doi: 10.1016/0042-6822(74)90348-1. [DOI] [PubMed] [Google Scholar]
- Stone L. B., Takemoto K. K., Martin M. A. Physical and biochemical properties of progressive pneumonia virus. J Virol. 1971 Oct;8(4):573–578. doi: 10.1128/jvi.8.4.573-578.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Robin M. S., Green M. Viral RNA subunits in cells transformed by RNA tumor viruses. Science. 1972 Jun 30;176(4042):1418–1420. doi: 10.1126/science.176.4042.1418. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Martin F. H., Doty P. Self-complementary oligoribonucleotides: effects of helix defects and guanylic acid-cytidylic acid base pairs. J Mol Biol. 1971 Apr 28;57(2):217–229. doi: 10.1016/0022-2836(71)90342-1. [DOI] [PubMed] [Google Scholar]


