Abstract
Reports from more than 600 hematopoietic stem cell transplants (HSCT) have appeared in the medical literature for the last 1 and one-half decades. The patient’s own stem cells are harvested and stored temporarily while high doses of chemotherapy and biologics are used to destroy the auto-destructive immune system. The immune system is regenerated from the infused autologous hematopoietic stem cells. Increasing clinical experience has refined patient selection criteria and management in the peri-transplant period leading to a reduction in treatment-related complications. HSCT, when used to treat patients with aggressive highly active multiple sclerosis, can reduce or eliminate ongoing clinical relapses, halt further progression, and reduce the burden of disability in some patients, in the absence of chronic treatment with disease-modifying agents. The top 10 lessons learned from the growing experience using HSCT for the treatment of multiple sclerosis are discussed.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-012-0162-5) contains supplementary material, which is available to authorized users.
Keywords: Hematopoietic stem cell transplantation, Multiple sclerosis, Autoimmunity, Immune ablation
There are published reports of more than 600 bone marrow-based transplants performed primarily for the treatment of multiple sclerosis (MS). This review will focus on why and how the hematopoietic stem cell transplantation (HSCT) procedure is used and discuss some of the lessons that can be gleaned from the experience of using HSCT to treat MS.
MS is an organ-specific autoimmune disease that results in exclusive central nervous system (CNS) demyelination and axonal damage. Early on, multifocal localized pockets of inflammation lead to transient neurologic dysfunction manifesting as a series of relapses followed by remissions, usually with complete recovery owing to adequate CNS repair. However, as the damage accumulates, repair becomes inadequate and leads to incomplete resolution of disability, culminating in a neurodegenerative process amid a sea of smoldering and changing inflammation. This is probably the underlying state for the clinical stage of secondary progressive MS (SP-MS), a phase characterized by relentless and progressive accumulation of neurological disability with dwindling evidence of discrete relapses [1].
Targeting inflammation with interferon-β, glatiramer acetate, natalizumab, fingolimod, and other immune-modulating agents reduces the frequency of relapses, but the impact on delaying the onset, preventing or slowing the tempo of SP-MS remains uncertain [2–4]. Several other drugs are in late stage clinical trials and may provide new alternatives for some patients with relapsing-remitting MS (RR-MS). However, despite these therapeutic advances, a subset of patients seem refractory and rapidly develop severe neurological impairment, whereas other patients may have a slower progression of illness that ultimately results in marked functional disabilities. Only a single agent, mitoxantrone, has been shown to temporarily halt or slow the tempo of SP-MS [5, 6], but even then, this occurs only in SP-MS patients who have highly active inflammatory events.
HSCT was originally conceived as a method of rescuing patients from prolonged life-threatening bone marrow aplasia that results from the administration of high-dose total body irradiation or myeloablative chemotherapy. In the context of MS, a single administration of high-dose chemotherapy and total body irradiation (used individually or together) is meant to suppress or ablate the endogenous immune system with the goal of achieving long-term remission of MS immune activity without the need for ongoing treatment with disease-modifying agents. HSCT provides a source of immune and hematopoietic reconstitution.
HSCT has been used to treat patients with poor prognosis MS. Initial preclinical experimentation (performed approximately 20 years ago) demonstrated that administration of total body irradiation or high doses of cyclophosphamide followed by syngeneic HSCT prevented relapses in animals with experimental autoimmune encephalitis [7]. This finding was translated into the clinical realm in the mid 1990s. Over the ensuing two decades the European Bone Marrow Transplant (EBMT) Registry [8] has documented that 469 patients have undergone HSCT for MS, whereas another 143 patients have been reported to the Consortium for International Bone Marrow Transplant Research (CIBMTR) [9].
Clinical evidence supporting a role for HSCT in the management of certain patients with MS comes from reports of the following:
Patients undergoing HSCT for a malignancy who also have concurrent MS [10–12]
The initial prospective phases 1 and 2 cohort trials [13–17]
Reports of registry [18] based data for the treatment of MS
This information is summarized in a number of review articles [19, 20].
HSCT appears to connote a single treatment, but in reality it is composed of different steps, which can each be customized. These differences can influence the potential toxicity and effectiveness of HSCT. What are the components of HSCT? What are the variations that have been tested in HSCT for MS?
Prior to HSCT, a graft containing hematopoietic stem cells (HSCs) must be obtained. There is a large worldwide experience using autologous HSCT for MS [9, 21], however, only a few transplants have been performed using human leukocyte antigen (HLA) matched allogeneic or donor-derived HSC grafts. More than 95 % of recipients of autologous HSC have received grafts collected by peripheral vein leukopheresis. HSC may be aspirated directly from the bone marrow or mobilized from the marrow into the circulation and collected using leukopheresis. Mobilization results from the disruption of molecular mechanisms that retain HSC in their bone marrow niche. The 2 most commonly used stem cell mobilization regimens used to collect HSC from MS patients are granulocyte-colony stimulating factor (G-CSF) administered concurrently with steroids and cyclophosphamide followed by G-CSF. Sole administration of G-CSF can induce an MS relapse [13, 14, 22], however, this can be prevented by concurrent steroid or chemotherapy administration. These drugs offer the additional benefit of providing transient immunosuppression, which helps control the MS until the stem cell transplant. There is no published experience using plerixafor for graft collection from patients with autoimmune diseases, although this drug, which disrupts the HSC homing interaction between CXCR4 and SDF-1, is increasingly being used for patients with malignancies. Although plerixafor is not toxic in the manner of chemotherapy, caution is warranted in MS patients, as CXCR4-SDF-1 plays a role in protecting against autoimmunity in some experimental systems [23, 24]. Natalizumab can also disrupt HSC homing mechanisms and mobilize stem cells from the marrow into the circulation [25, 26], but it also alters the type and function of stem and immune cells in the graft [27, 28]. This drug has not been clinically used for stem cell mobilization, and again caution is warranted until there is a better understanding of how CD49d blockade influences immune reconstitution after stem cell transplantation.
Typically, only 3 to 5 % of the cells in a graft are HSCs. Peripheral blood HSC grafts are rich in peripheral blood cells, including granulocytes, monocytes, and lymphocytes. Immune reconstitution occurs by de novo lymphopoiesis of newly engrafted stem cells and by homeostatic expansion of mature lymphocytes contained in the graft [29]. In theory, expansion of autoreactive lymphocytes may result in ongoing MS activity after HSCT. Methods to reduce the immune cell load in the graft may, at least in theory, improve outcomes. The effect of lymphocyte depletion of the graft would only be apparent in HSCT using immune ablative conditioning regimens, otherwise homeostatic expansion of mature lymphocytes in the patient, not eliminated by the conditioning regimen, might confound the outcome. Cyclophosphamide administered for stem cell mobilization has the additional benefit of reducing the number of circulating lymphocytes during collection, which results in HSC grafts with lower immune cell load. Graft cell composition can be altered using ex vivo cell selection or depletion technology. Immunomagnetic CD34 cell selection rigorously depletes grafts of immune cells to prevent reintroduction of autoreactive cells [16, 17, 30–33]. The in vivo purging of lymphocytes could be accomplished by treating the patient with anti-thymocyte globulin, rituximab, or alemtuzumab prior to leukopheresis. Although this has been effective at reducing the amount of malignant T or B cells collected in HSC grafts of patients with lymphoma, there is no experience using this strategy to reduce the burden of autoreactive lymphocytes in an HSC graft collected from patients with autoimmune diseases [34, 35].
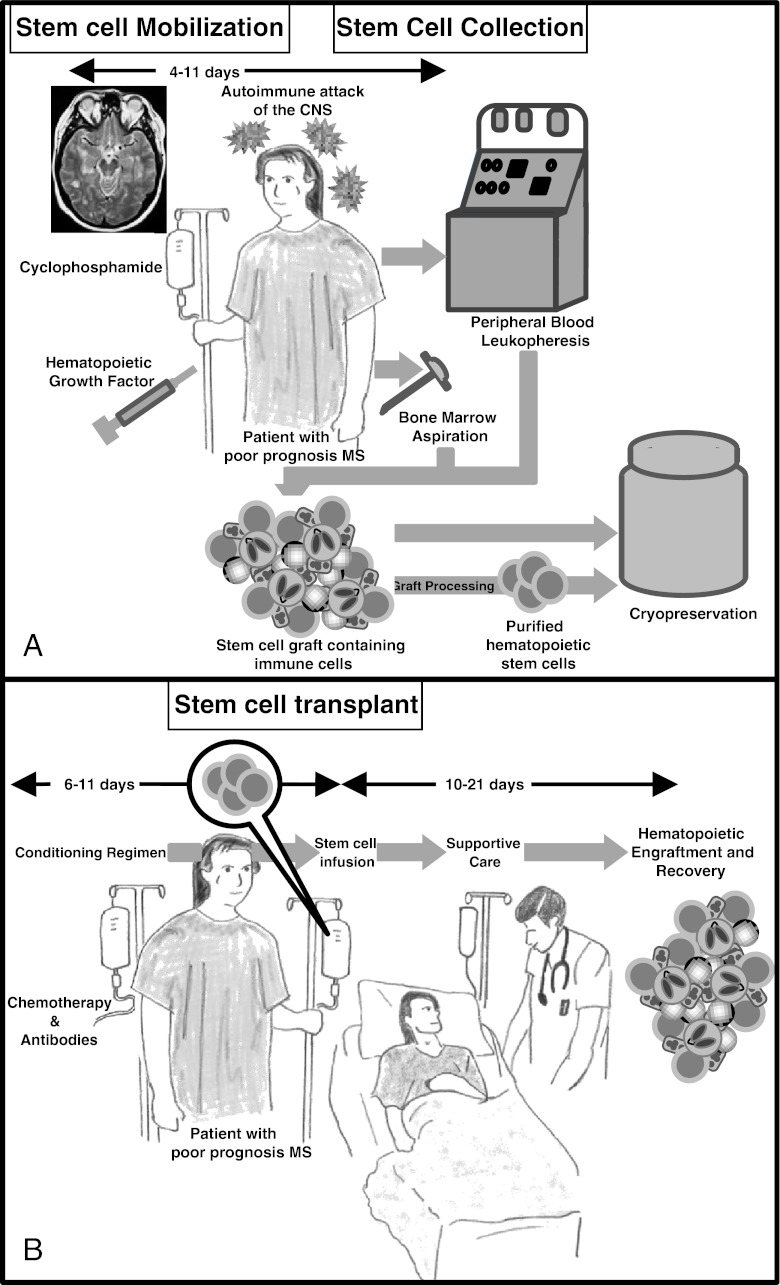
Finally, whether manipulated or not, the collected grafts are cryopreserved in liquid nitrogen until use (Fig. 1a).
Fig. 1.
Once an appropriate candidate is identified, autologous hematopoietic stem cells (HSC) are collected (a). HSC may be collected directly from the bone marrow through multiple aspirations performed under regional or general anesthesia. Alternatively, HSC may be mobilized from the bone marrow into the circulation using chemotherapy and/or hematopoietic growth factors. HSC are then collected by leukopheresis. The product can be processed to remove contaminating immune cells and them be cryopreserved or it can be cryopreserved without further manipulation. Two to 6 weeks later, the patient may undergo HSCT (b). Chemotherapy with or without immune depleting biologic agents are administered to destroy the immune system. The cryopreserved HSC are rapidly thawed in a water bath and infused intravenously through a central venous catheter. The patient is receives supportive care during the acute phase of the transplant, when potentially the most serious side-effects may occur. Hematopoietic reconstitution generally occurs 10–14 days after HSCT. Immune reconstitution and full recovery from the chemotherapy may take 3 to 6 months
The transplant procedure begins with the administration of a conditioning regimen comprised of 1 or more chemotherapy drugs, immune-depleting biological agents, and/or radiation therapy. In this instance, the agents are given to reduce or eliminate the auto-destructive effector and memory cells of the immune system. After metabolism and elimination of the chemotherapy agents, the HSC graft is thawed and infused intravenously via a central venous catheter. The HSC circulate and home to the marrow space, where they seed the bone marrow and proliferate. They mature into circulating blood cells and contribute to de novo lymphopoiesis. After HSCT, patients are nursed through the expected toxicities resulting from the conditioning regimen (Fig. 1b). Increasing the dose intensity of the conditioning regimen results in greater immune suppression, and at high enough doses this can ablate the recipient’s immune system. However increasing dose intensity is associated with more myelotoxicity (resulting in deeper and more prolonged cytopenias), a greater requirement for HSC replacement, and increasing nonhematopoietic organ toxicity. These vary in severity and include febrile neutropenia, bacterial, fungal and viral infections, oral and gastrointestinal irritation, and other drug-specific organ toxicities. The risk of rare idiosyncratic toxicities (such as veno-occlusive disease of the liver, hemorrhagic cystitis, pulmonary toxicity, or renal failure) depends on the conditioning regimen administered. Supportive care measures include prophylactic antimicrobial agents, anti-emetics, anti-diarrheal agents, blood product transfusion, nutritional supplementation, narcotic analgesia, and cytokine stimulation of hematopoiesis. Generally, the toxicity is most severe during the first 2 weeks after HSCT.
Initially some groups used conditioning regimens containing total body irradiation [13, 14], however, most centers have subsequently adopted multi-drug chemotherapy regimens. Thus, experience with total body irradiation is limited to less than 100 MS patients. There are regional differences in the use of total body irradiation; 30 % of patients undergoing HSCT for an autoimmune disease reported to the CIBMTR [9] whereas less than 5 % of patients reported to the EBMT registry [21] received total body irradiation.
No single conditioning regimen has demonstrated a marked superiority in comparison with another, although attributing differences in outcome to a conditioning regimen is complicated (given the lack of randomized trials) by other variables such as; patient selection, graft composition, and the methodology for ongoing MS evaluation. Chemotherapy drugs that have been used for HSCT include agents that disrupt DNA replication, such as the alkylating agents (cyclophosphamide, busulphan, BCNU, melphalan), antimetabolites (cytarabine), or topoisomerase inhibitors (etoposide). Some agents, such as cyclophosphamide and busulphan, cross the blood–brain barrier [36, 37] and are able to target the CNS immune system. Immune-depleting antibodies, including anti-thymocyte globulins (ATG), alemtuzumab, or rituximab, are frequently combined with chemotherapy to further destroy the immune system and reduce the burden of lymphocytes infused in the HSC graft. Again, there are regional differences in the selection of a conditioning regimen. For instance, almost half of HSCT reported to EBMT [18] used the BEAM regimen (composed of BCNU, etoposide, cytarabine, and melphalan) combined with ATG while cyclophosphamide either given alone or in combination with other chemotherapy agents is used most often by centers reporting to the CIBMTR [9].
Although much of the know-how of performing HSCT for patients with MS is extrapolated from the hematology–oncology world, there are unique aspects surrounding the use of HSCT for a chronic refractory autoimmune disease indication. Patients with MS usually have little comorbidity, but challenges in their care arise from disabilities and functional impairment. The unique aspects of HSCT for patients with MS are considered in the lessons learned through the monitoring and reporting of the outcomes of HSCT for patients with MS.
Lesson 1: While MS Patients Generally Behave like Other Patients Undergoing HSCT, They Experience Unique Issues
MS patients undergoing HSCT experience the same regimen-related complications and roughly at the same frequency as patients undergoing autologous HSCT for lymphoma. A few complications occur more often or are unique to MS transplant recipients. Urinary tract infections are more commonly seen [13] because of the frequent use of bladder catheterization. Indwelling urinary catheters are used to minimize the risk of hemorrhagic cystitis from cyclophosphamide toxicity, given the frequency of bladder dysfunction in the MS population. Recipients of CD34 selected grafts or those treated with anti-thymocyte globulin are at risk for illnesses associated with reactivation of human herpes viruses including shingles, Epstein-Barr virus lymphoproliferation [38], human herpes virus 6, and cytomegalovirus viremia. Active surveillance and/or antiviral prophylaxis are warranted. Febrile neutropenia and infections may lead to the transient worsening of the MS symptoms and neurologic dysfunction. Awareness may minimize extraneous tests, avoid inadvertent pulse steroid treatment in an already immune compromised patient, and allow the transplant team to reassure the patient. Usually those MS patients who have a greater degree of disability prior to transplantation are at risk of developing further loss of mobility due to the chemotherapy-induced cachexia and myopathy. Access to physiotherapy during the HSCT admission may help mitigate this problem, although some patients require admission to a specialized rehabilitation unit to foster their recovery. Cognitive, as well as physical disabilities may worsen in the acute peri-transplant period, due to conditioning regimen effects on the CNS [39]. Those with impairment prior to HSCT may have a greater susceptibility to further decline after HSCT [40], although recovery may occur with time [41].
Lesson 2: Increasing Experience Using HSCT for Patients with MS Reduces Morbidity and Mortality
The mortality for HSCT performed for any disease has lessened across the years. Treatment-related mortality ranged from 4 [30] to 20 % [42] during the first decade of HSCT for MS. This may in part reflect the selection of patients with advanced disability, as well as the use of high-intensity conditioning regimens. Treatment-related mortality was mainly due to infectious complications. With time, there has been a marked reduction in transplant-related mortality. Indeed, the treatment-related mortality has decreased from 7.3 % for the period between 1995 and 2000 to 1.3 % during the period from 2000 to 2007 [20]. The reduction in mortality can be attributed to the selection of patients with better overall performance status for HSCT, a variable known to correlate with regimen-related mortality [43], combined with improvements in supportive care and the increasing experience of caring for MS patients at transplant centers.
Lesson 3: CNS Toxicity of Very High-Dose Regimens may Adversely Influence Outcomes
The intensity of the conditioning regimen must strike a balance between adequate immune ablation and regimen-related morbidity and mortality. The choice of the condition regimen must also take into account toxicity to the CNS. Neurotoxicity has been associated with agents such as busulphan (seizures, atrophy) and radiation [44]. Either agent may cause dose-dependent neurotoxicity, aggravate MS associated CNS damage or prevent the ability of the CNS to recover from an insult by damaging the endogenous regenerative mechanism in the brain. HSCT after total body irradiation has been associated with poorer MS outcomes [14, 45] and this could be attributed to these mechanisms. In the future, a risk-adapted treatment approach could tailor the optimum conditioning regimen dose intensity to the risks of neurotoxicity and to the aggressiveness of the MS.
Lesson 4: HSCT is More Likely to be Effective for MS Patients with Active CNS Inflammation
Initially, patients with SP-MS and advanced disability were selected for HSCT. However, it was observed that these patients (with an Expanded Disability Status Scale [EDSS] score ≥ 6.0) had poor outcomes compared with patients who entered HSCT with less disability [14]. A review of data from the EBMT registry that stratified patients by age and duration of MS, showed markedly better outcomes for recipients who were younger than 40 years of age and had been diagnosed within the last 5 years compared with older patients who had longstanding MS [18]. But these factors, EDSS, age, and time since diagnosis are surrogate endpoints that separate patients on the basis of their dominant neuropathophysiology. Younger and less disabled patients who have been recently diagnosed are more likely to have active neuroinflammation and are more likely to benefit from HSCT. Some patients with very advanced functional impairment due to the brisk neuroinflammation associated with malignant MS have had (at times) dramatic responses to transplantation [46–48], supporting the idea that it is neuropathology rather than disability that determines the outcome to HSCT.
Lesson 5: HSCT is Effective at Controlling Inflammatory MS Activity, with Higher Response Rate Observed with More Intense Conditioning Regimens
Less intensive regimens, such as cyclophosphamide with ATG, have resulted in the reduction of signs of CNS inflammation; however, some of these patients breakthrough and experience ongoing relapses after HSCT. Clinical relapses or magnetic resonance imaging evidence of ongoing CNS relapse were seen in 38 % of recipients during the 3 years after HSCT [15, 49]. The use of higher intensity conditioning regimens, such as BEAM with ATG or busulphan/cyclophosphamide with ATG, is effective at suppressing episodic CNS inflammation. Among patients having relapses until the time of HSCT, more than 85 % who received a conditioning regimen of BEAM plus ATG were free from clinical relapses in the absence of ongoing treatment with other disease-modifying agents [50, 51]. Similarly, gadolinium-enhancing CNS lesions or new lesions on serial magnetic resonance imaging were uncommon [52, 53]. Although randomized comparisons of different intensity conditioning regimens have not been performed, within the limitations of available information, it appears that dose-intensity influences the control of CNS inflammation. However, intense regimens come with potentially higher treatment-related morbidity, and (as previously discussed) patient- and disease-factor based risk-adapted strategies might be helpful in the selection of optimal conditioning regimen intensity.
Lesson 6: HSCT can Result in Long-Term Progression-Free Outcomes
Several cohort studies and registry reports have demonstrated that HSCT can result in the cessation of progressive accumulation of disabilities and long-term stability of the EDSS in patients with SP-MS, without the need for ongoing disease-modifying agents. Approximately 45 % of patients have a stable EDSS score at 5 to 7 years after HSCT [21, 51, 54]. Although the accumulated outcome data of HSCT for MS is largely from cohort studies that lack a randomized control group, most of the patients selected for HSCT had ongoing progression and had previously exhausted all other therapeutic options. The duration of stability in objective disability measures has been remarkable and appears to represent a marked change in outcome when compared to historical and concurrent MS populations. However, caution should be exercised in interpreting these results as the progression-free survival is lower in studies with longer follow-up [50, 52].
A recently initiated joint EBMT and CIBMTR registry-based [55], long-term follow-up study [56] and a proposed phase III randomized trial of stem cell transplants versus the best available therapy for patients with highly active MS who have failed interferon-β therapy [57] will provide further information regarding the role of HSCT in MS.
Lesson 7: The Recovery of Functional Ability can Occur in HSCT Recipients
Disability, as measured by the EDSS can improve after HSCT, at least for some patients. A reduction in EDSS occurred in 27 % of HSCT recipients with more than 7 years of follow-up in an Italian cohort study [51]. However, in this instance, the improvements were sustained, but this has not always been the case. Fassas et al. [52] reported that 16 patients initially had an improvement in their EDSS score after HSCT, but only 2 of 35 patients had sustained long-term improvement in EDSS at 7 years. The transplant regimen was similar in both instances, and thus the difference in outcome is conceivably due to subtle differences in patient selection, although baseline EDSS scores, age at HSCT, and time from diagnosis were similar in the 2 cohorts. Other groups have also reported a sustained reduction in EDSS scores after HSCT. A reduction of the mean EDSS from a score of 3.5 before HSCT to 2.0 at 3 years after HSCT was seen among 90 patients in a cohort from Russia [58], whereas a reduction in the mean EDSS score by 3.1 was seen among 10 of 25 HSCT recipients in a study from China [59]. Although a few patients have had dramatic improvements after HSCT, most reports of improvement are modest (i.e., in the 0.5–1.0 range). More dramatic improvements have been reported for patients with malignant MS who have undergone HSCT. With follow-up extending to 4 years, the EDSS score dropped from a mean of 6.8 (range, 3.5–9.0) prior to HSCT to a mean of 3.1 (range, 0–6.0) among 13 patients with disability who progressed rapidly after diagnosis [46–48]. The mechanism of recovery has not been investigated and it is not clear whether the HSCT graft contributes directly or indirectly to the recovery.
Lesson 8: Tolerance can Develop Resulting in Obliteration of Autoimmunity while Preserving Protective Immunity
Treatments currently being used or being considered for use in MS, such as fingolimod, rituximab, cladribine, cyclophosphamide, and others are global immunosuppressants. They reduce active neuroinflammation, but their lack of selectivity comes with an increase in the risk of developing opportunistic infections [60, 61]. Although infectious complications occur during the neutropenic phase of HSCT, the risk of late opportunistic infections is small once immune reconstitution has occurred. Thus, HSCT is fundamentally different than the current immunomodulatory or immunosuppressive regimens in restoring tolerance and controlling the autoimmune process with a fully functional immune system.
Lesson 9: The Reconstituted Immune System Retains a Predisposition to Autoimmunity
Although the primary autoimmune disease may be suppressed after HSCT, nearly 10 % of patients undergoing HSCT for an autoimmune disease have been reported to develop a second autoimmune illness unrelated to their indication for autologous HSCT within the first 2 years after HSCT [62]. Autoimmune thyroid disease occurred in half of these cases. Secondary autoimmune disease was more common in those patients who underwent HSCT for systemic lupus erythematosus, and was less frequent in those who underwent HSCT for other illnesses, including MS. Other autoimmune phenomenon ,such as immune cytopenias, predominantly immune thrombocytopenic purpura (ITP), may occur up to several years post-transplant [63].
The occurrence of secondary autoimmune diseases may be related to the use of lymphocyte-depleting antibodies administered during HSCT conditioning [64]. A retrospective registry review reports a 9 % incidence of secondary autoimmunity among patients who received ATG during HSCT conditioning, but secondary autoimmune disease occurred in only 4 % of patients who did not receive a lymphocyte-depleting agent [62]. Alemtuzumab may be more potent at inducing secondary autoimmune phenomenon, as the incidence of secondary autoimmunity was noted to be 16 % when alemtuzumab was included in the conditioning regimen, but only 2 % with ATG in a large single-center experience [63].
Lesson 10: Information on the Economics and Health Resource Usefulness of HSCT for MS Needs to be Gathered
The first study to address the economics of HSCT for MS plugged outcome data from case-matched SPMS patients who were treated with mitoxantrone or HSCT into a Markov-based economic model. The estimated cost of HSCT was less than £3000 (or $4700) per quality-adjusted life year [65]. Assumptions regarding treatment outcomes, such as the risk of mortality, the effectiveness in producing sustained stability in the EDSS and in procedural costs, influenced the outcome of the analysis. A variety of assumptions were made that tended to be biased against HSCT, including a relatively high regimen-related mortality of 5.3 % and the exclusion of costs associated with serious mitxoantrone-related complications, such as secondary acute leukemia. Notwithstanding these factors, HSCT was cost effective, using a yardstick of other treatments considered to be cost-effective in the United Kingdom. For comparison, current MS drug therapy cost effectiveness has been estimated to be between $80,000 to $168,000 per avoided relapse [66, 67] or upwards of $73,000 per incremental quality-adjusted life year [68, 69].
Although an estimation of the direct medical costs of HSCT for MS has begun, other factors clearly need to be considered in the economic evaluation of HSCT for MS. Indirect HSCT-related costs borne by the patient, their caregivers, and their families (e.g., costs for traveling to and from the hospital, costs associated with relocation to a center that is able to perform HSCT for MS, and outpatient drug costs used for prophylaxis during the first year after HSCT, among a variety of other costs) may pose a financial barrier to HSCT. In addition, consideration must be given to nonmedical societal costs over a long follow up period, given that disability may be reduced, at least in a subset of HSCT recipients, which could result in productivity gains and a reduction in disability payouts for those able to return to work or be able to continue work.
Summary
When the overall worldwide experience is viewed together, HSCT appears most beneficial for patients with highly active MS who are progressing and who are refractory to conventional MS therapies. HSCT can be performed with acceptable treatment-related morbidity and little mortality. Selected subpopulations, such as those patients with malignant MS, may benefit even more substantially from HSCT. Further evidence regarding the role of HSCT in the treatment of MS will be provided from a long-term, follow-up study of patients reported to the 2 international registries [56].
Are further studies required, given that HSCT seems feasible and can stabilize patients who have aggressive MS once they fail conventional therapies? The answer is undoubtedly yes. The body of evidence demonstrating a role for HSCT in MS has helped to refine which questions remain unanswered. For instance, although HSCT can be used as a salvage therapy, it seems to work better earlier in the course of MS, when relapses dominate the clinical picture. Could HSCT provide better outcomes in this group of patients compared to current or new immunosuppressive agents, especially with the increasing risk of toxicity with long-term use of newer agents? A proposed international multicenter, collaborative, randomized clinical trial of HSCT compared to the best standard-of-care treatment will improve the understanding of the role that HSCT should play in the management of MS [57]. In addition, clinical experience using HSCT for patients with other CNS autoimmune demyelinating diseases (e.g., neuromyelitis optica) has just starting to be documented [70, 71]. Further detailed cohort and registry studies will help determine whether HSCT is able to fill this unmet clinical need.
HSCT performed for patients with severe MS for the last 15 years has shown us the usefulness of this treatment. More importantly, it has provided a new treatment paradigm in which the immune system is repaired rather than suppressed. Although HSCT remains a sledgehammer approach to treating MS, reserved for those with the most aggressive forms, the opportunity exists to capitalize on the growing knowledge and experience in the field to create less toxic ways of eliminating and replacing the immune system toward the goal of broader applicability of this promising therapy.
Electronic Supplementary Material
(PDF 492 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in Multiple sclerosis. Brain. 2010;133:1900–1913. doi: 10.1093/brain/awq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shirani A, Zhao Y, Karim ME, et al. Association between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2012;308:247–256. doi: 10.1001/jama.2012.13573. [DOI] [PubMed] [Google Scholar]
- 3.Trojano M, Paolicelli D, Tortorella C, et al. Natural history of multiple sclerosis: have available therapies impacted long-term prognosis? Neurol Clin. 2011;29:309–321. doi: 10.1016/j.ncl.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Bergamaschi R, Quaglini S, Tavazzi E, et al. Immunomodulatory therapies delay disease progression in multiple sclerosis. Mult Scler 2012. doi:10.1177/1352458512445941. [DOI] [PubMed]
- 5.Hartung HP, Gonsette R, König N, et al. Mitoxantrone in Multiple Sclerosis Study Group (MIMS). Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–2025. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- 6.Marriott JJ, Miyasaki JM, Gronseth G, O’Connor PW. Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence Report: The efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;74:1463–1470. doi: 10.1212/WNL.0b013e3181dc1ae0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karussis DM, Vourka-Karussis U, Lehmann D, et al. Prevention and reversal of adoptively transferred, chronic relapsing experimental autoimmune encephalomyelitis with a single high dose cytoreductive treatment followed by syngeneic bone marrow transplantation. J Clin Invest. 1993;92:765–772. doi: 10.1172/JCI116648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snowden JA, Saccardi R, Allez M, et al. Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47:770–790. doi: 10.1038/bmt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquini MC, Voltarelli J, Atkins HL, et al. Transplantation for autoimmune diseases in north and south america: a report of the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2012;18:1471–1478. doi: 10.1016/j.bbmt.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marmont AM. Stem cell transplantation for severe autoimmune diseases: progress and problems. Haematologica. 1998;83:733–743. [PubMed] [Google Scholar]
- 11.Meloni G, Capria S, Salvetti M, et al. Autologous peripheral blood stem cell transplantation in a patient with multiple sclerosis and concomitant Ph+ acute leukemia. Haematologica. 1999;84:665–667. [PubMed] [Google Scholar]
- 12.McAllister LD, Beatty PG, Rose J. Allogeneic bone marrow transplant for chronic myelogenous leukemia in a patient with multiple sclerosis. Bone Marrow Transplant. 1997;19:395–397. doi: 10.1038/sj.bmt.1700666. [DOI] [PubMed] [Google Scholar]
- 13.Nash RA, Bowen JD, McSweeney PA, et al. High-dose immunosuppressive therapy and autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Blood. 2003;102:2364–2372. doi: 10.1182/blood-2002-12-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burt RK, Cohen BA, Russell E, et al. Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood. 2003;102:2373–2378. doi: 10.1182/blood-2003-03-0877. [DOI] [PubMed] [Google Scholar]
- 15.Carreras E, Saiz A, Marín P, et al. CD34+ selected autologous peripheral blood stem cell transplantation for multiple sclerosis: report of toxicity and treatment results at one year of follow-up in 15 patients. Haematologica. 2003;88:306–314. [PubMed] [Google Scholar]
- 16.Kozák T, Havrdová E, Pit’ha J, et al. High-dose immunosuppressive therapy with PBPC support in the treatment of poor risk multiple sclerosis. Bone Marrow Transplant. 2000;25:525–531. doi: 10.1038/sj.bmt.1702180. [DOI] [PubMed] [Google Scholar]
- 17.Fassas A, Anagnostopoulos A, Kazis A, et al. Autologous stem cell transplantation in progressive multiple sclerosis—an interim analysis of efficacy. J Clin Immunol. 2000;20:24–30. doi: 10.1023/A:1006686426090. [DOI] [PubMed] [Google Scholar]
- 18.Saccardi R, Kozak T, Bocelli-Tyndall C, et al. Autoimmune Diseases Working Party of EBMT. Autologous stem cell transplantation for progressive multiple sclerosis: update of the European Group for Blood and Marrow Transplantation autoimmune diseases working party database. Mult Scler. 2006;12:814–823. doi: 10.1177/1352458506071301. [DOI] [PubMed] [Google Scholar]
- 19.Atkins H. Hematopoietic SCT for the treatment of multiple sclerosis. Bone Marrow Transplant. 2010;45:1671–1681. doi: 10.1038/bmt.2010.168. [DOI] [PubMed] [Google Scholar]
- 20.Mancardi G, Saccardi R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008;7:626–636. doi: 10.1016/S1474-4422(08)70138-8. [DOI] [PubMed] [Google Scholar]
- 21.Farge D, Labopin M, Tyndall A, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years’ experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica. 2010;95:284–292. doi: 10.3324/haematol.2009.013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Openshaw H, Stuve O, Antel JP, et al. Multiple sclerosis flares associated with recombinant granulocyte colony-stimulating factor. Neurology. 2000;54:2147–2150. doi: 10.1212/WNL.54.11.2147. [DOI] [PubMed] [Google Scholar]
- 23.Aboumrad E, Madec AM, Thivolet C. The CXCR4/CXCL12 (SDF-1) signalling pathway protects non-obese diabetic mouse from autoimmune diabetes. Clin Exp Immunol. 2007;148:432–439. doi: 10.1111/j.1365-2249.2007.03370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miljković D, Stanojević Z, Momcilović M, et al. CXCL12 expression within the CNS contributes to the resistance against experimental autoimmune encephalomyelitis in Albino Oxford rats. Immunobiology. 2011;216:979–987. doi: 10.1016/j.imbio.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Zohren F, Toutzaris D, Klärner V, Hartung HP, Kieseier B, Haas R. The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood. 2008;111:3893–3895. doi: 10.1182/blood-2007-10-120329. [DOI] [PubMed] [Google Scholar]
- 26.Bonig H, Wundes A, Chang KH, Lucas S, Papayannopoulou T. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111:3439–3441. doi: 10.1182/blood-2007-09-112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jing D, Oelschlaegel U, Ordemann R, et al. CD49d blockade by natalizumab in patients with multiple sclerosis affects steady-state hematopoiesis and mobilizes progenitors with a distinct phenotype and function. Bone Marrow Transplant. 2010;45:1489–1496. doi: 10.1038/bmt.2009.381. [DOI] [PubMed] [Google Scholar]
- 28.Lesesve JF, Debouverie M. Decarvalho Bittencourt M, Béné MC. CD49d blockade by natalizumab therapy in patients with multiple sclerosis increases immature B-lymphocytes. Bone Marrow Transplant. 2011;46:1489–1491. doi: 10.1038/bmt.2010.328. [DOI] [PubMed] [Google Scholar]
- 29.Gress RE, Emerson SG, Drobyski WR. Immune reconstitution: how it should work, what’s broken, and why it matters. Biol Blood Marrow Transplant. 2010;16:S133–S137. doi: 10.1016/j.bbmt.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni XS, Ouyang J, Zhu WH, Wang C, Chen B. Autologous hematopoietic stem cell transplantation for progressive multiple sclerosis: report of efficacy and safety at three year of follow up in 21 patients. Clin Transplant. 2006;20:485–489. doi: 10.1111/j.1399-0012.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 31.Carreras E, Saiz A, Marín P, et al. CD34+ selected autologous peripheral blood stem cell transplantation for multiple sclerosis: report of toxicity and treatment results at one year of follow-up in 15 patients. Haematologica. 2003;88:306–314. [PubMed] [Google Scholar]
- 32.Su L, Xu J, Ji BX, et al. Autologous peripheral blood stem cell transplantation for severe multiple sclerosis. Int J Hematol. 2006;84:276–281. doi: 10.1532/IJH97.A10516. [DOI] [PubMed] [Google Scholar]
- 33.Atkins H, Freedman M. Immunoablative therapy as a treatment aggressive multiple sclerosis. Neurol Clin. 2005;23:273–300. doi: 10.1016/j.ncl.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 34.van Heeckeren WJ, Vollweiler J, Fu P, et al. Randomised comparison of two B-cell purging protocols for patients with B-cell non-Hodgkin lymphoma: in vivo purging with rituximab versus ex vivo purging with CliniMACS CD34 cell enrichment device. Br J Haematol. 2006;132:42–55. doi: 10.1111/j.1365-2141.2005.05827.x. [DOI] [PubMed] [Google Scholar]
- 35.Shustov A, Cherian S, Shaw A, et al. In-vivo purging of the circulating clonal T-cells with Alemtuzumab prior to autologous peripheral blood hematopoietic cell mobilization and transplantation. Br J Haematol. 2011;154:415–418. doi: 10.1111/j.1365-2141.2011.08619.x. [DOI] [PubMed] [Google Scholar]
- 36.Bahr U, Schulten HR, Hommes OR, Aerts F. Determination of cyclophosphamide in urine, serum and cerebrospinal fluid of multiple sclerosis patients by field desorption mass spectrometry. Clin Chim Acta. 1980;103:183–192. doi: 10.1016/0009-8981(80)90212-0. [DOI] [PubMed] [Google Scholar]
- 37.Hassan M, Oberg G, Ehrsson H, et al. Pharmacokinetic and metabolic studies of high-dose busulphan in adults. Eur J Clin Pharmacol. 1989;36:525–530. doi: 10.1007/BF00558081. [DOI] [PubMed] [Google Scholar]
- 38.Nash RA, Dansey R, Storek J, et al. Epstein-Barr virus-associated posttransplantation lymphoproliferative disorder after high-dose immunosuppressive therapy and autologous CD34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Biol Blood Marrow Transplant. 2003;9:583–591. doi: 10.1016/S1083-8791(03)00228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JT, Collins DL, Atkins HL, et al. Brain atrophy after immunoablation and stem cell transplantation in multiple sclerosis. Neurology. 2006;66:1935–1937. doi: 10.1212/01.wnl.0000219816.44094.f8. [DOI] [PubMed] [Google Scholar]
- 40.Friedman MA, Fernandez M, Wefel JS, Myszka KA, Champlin RE, Meyers CA. Course of cognitive decline in hematopoietic stem cell transplantation: a within-subjects design. Arch Clin Neuropsychol. 2009;24:689–698. doi: 10.1093/arclin/acp060. [DOI] [PubMed] [Google Scholar]
- 41.Syrjala KL, Artherholt SB, Kurland BF, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol. 2011;29:2397–2404. doi: 10.1200/JCO.2010.33.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Openshaw H, Lund BT, Kashyap A, et al. Peripheral blood stem cell transplantation in multiple sclerosis with busulphan and cyclophosphamide conditioning: report of toxicity and immunological monitoring. Biol Blood Marrow Transplant. 2000;6:563–575. doi: 10.1016/S1083-8791(00)70066-8. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg SL, Klumpp TR, Magdalinski AJ, Mangan KF. Value of the pretransplant evaluation in predicting toxic day-100 mortality among blood stem-cell and bone marrow transplant recipients. J Clin Oncol. 1998;16:3796–3802. doi: 10.1200/JCO.1998.16.12.3796. [DOI] [PubMed] [Google Scholar]
- 44.Chamberlain MC. Neurotoxicity of cancer treatment. Curr Oncol Rep. 2010;12:60–67. doi: 10.1007/s11912-009-0072-9. [DOI] [PubMed] [Google Scholar]
- 45.Reston JT, Uhl S, Treadwell JR, Nash RA, Schoelles K. Autologous hematopoietic cell transplantation for multiple sclerosis: a systematic review. Mult Scler. 2011;17:204–213. doi: 10.1177/1352458510383609. [DOI] [PubMed] [Google Scholar]
- 46.Mancardi GL, Murialdo A, Rossi P, et al. Autologous stem cell transplantation as rescue therapy in malignant forms of multiple sclerosis. Mult Scler. 2005;11:367–371. doi: 10.1191/1352458505ms1181cr. [DOI] [PubMed] [Google Scholar]
- 47.Kimiskidis V, Sakellari I, Tsimourtou V, et al. Autologous stem-cell transplantation in malignant multiple sclerosis: a case with a favorable long-term outcome. Mult Scler. 2008;14:278–283. doi: 10.1177/1352458507082604. [DOI] [PubMed] [Google Scholar]
- 48.Fagius J, Lundgren J, Oberg G. Early highly aggressive MS successfully treated by hematopoietic stem cell transplantation. Mult Scler. 2009;15:229–237. doi: 10.1177/1352458508096875. [DOI] [PubMed] [Google Scholar]
- 49.Burt RK, Loh Y, Cohen B, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8:244–253. doi: 10.1016/S1474-4422(09)70017-1. [DOI] [PubMed] [Google Scholar]
- 50.Krasulová E, Trneny M, Kozák T, et al. High-dose immunoablation with autologous haematopoietic stem cell transplantation in aggressive multiple sclerosis: a single centre 10-year experience. Mult Scler. 2010;16:685–693. doi: 10.1177/1352458510364538. [DOI] [PubMed] [Google Scholar]
- 51.Mancardi GL, Sormani MP, Di Gioia M, et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-centre experience. Mult Scler. 2012;18:835–842. doi: 10.1177/1352458511429320. [DOI] [PubMed] [Google Scholar]
- 52.Fassas A, Kimiskidis VK, Sakellari I, et al. Long-term results of stem cell transplantation for MS: a single-center experience. Neurology. 2011;76:1066–1070. doi: 10.1212/WNL.0b013e318211c537. [DOI] [PubMed] [Google Scholar]
- 53.Saccardi R, Mancardi GL, Solari A, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105:2601–2607. doi: 10.1182/blood-2004-08-3205. [DOI] [PubMed] [Google Scholar]
- 54.Bowen JD, Kraft GH, Wundes A, et al. Autologous hematopoietic cell transplantation following high-dose immunosuppressive therapy for advanced multiple sclerosis: long-term results. Bone Marrow Transplant. 2012;47:946–951. doi: 10.1038/bmt.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pasquini MC, Griffith LM, Arnold DL, et al. Hematopoietic stem cell transplantation for multiple sclerosis: collaboration of the CIBMTR and EBMT to facilitate international clinical studies. Biol Blood Marrow Transplant. 2010;16:1076–1083. doi: 10.1016/j.bbmt.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muraro P. Long-term outcomes after autologous HCT for severe multiple sclerosis. In: Center for International Blood and Marrow Transplant Research. Available at: www.cibmtr.org/Studies/Observational/StudyLists/pages/ObservationalStudy.aspx?OSID=34. Accessed August 5, 2012.
- 57.Saccardi R, Freedman MS, Sormani MP, et al. A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a position paper. Mult Scler. 2012;18:825–834. doi: 10.1177/1352458512438454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shevchenko JL, Kuznetsov AN, Ionova TI, et al. Autologous haematopoietic stem cell transplantation with reduced intensity conditioning in multiple sclerosis. Exp Hematol. 2012;40:892–898. doi: 10.1016/j.exphem.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Chen B, Zhou M, Ouyang J, et al. Long-term efficacy of autologous haematopoietic stem cell transplantation in multiple sclerosis at a single institution in China. Neurol Sci. 2012;33:881–886. doi: 10.1007/s10072-011-0859-y. [DOI] [PubMed] [Google Scholar]
- 60.Tavazzi E, Ferrante P, Khalili K. Progressive multifocal leukoencephalopathy: an unexpected complication of modern therapeutic monoclonal antibody therapies. Clin Microbiol Infect. 2011;17:1776–1780. doi: 10.1111/j.1469-0691.2011.03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 62.Daikeler T, Labopin M, Di Gioia M, et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood. 2011;118:1693–1698. doi: 10.1182/blood-2011-02-336156. [DOI] [PubMed] [Google Scholar]
- 63.Loh Y, Oyama Y, Statkute L, et al. Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases: role of conditioning regimen used. Blood. 2007;109:2643–2548. doi: 10.1182/blood-2006-07-035766. [DOI] [PubMed] [Google Scholar]
- 64.Cuker A, Coles AJ, Sullivan H, et al. A distinctive form of immune thrombocytopenia in a phase 2 study of alemtuzumab for the treatment of relapsing-remitting multiple sclerosis. Blood. 2011;118:6299–6305. doi: 10.1182/blood-2011-08-371138. [DOI] [PubMed] [Google Scholar]
- 65.Tappenden P, Saccardi R, Confavreux C, et al. Autologous haematopoietic stem cell transplantation for secondary progressive multiple sclerosis: an exploratory cost-effectiveness analysis. Bone Marrow Transplant. 2010;45:1014–1021. doi: 10.1038/bmt.2009.305. [DOI] [PubMed] [Google Scholar]
- 66.O’Day K, Meyer K, Miller RM, Agarwal S, Franklin M. Cost-effectiveness of natalizumab versus fingolimod for the treatment of relapsing multiple sclerosis. J Med Econ. 2011;14:617–627. doi: 10.3111/13696998.2011.602444. [DOI] [PubMed] [Google Scholar]
- 67.Goldberg LD, Edwards NC, Fincher C, Doan QV, Al-Sabbagh A, Meletiche DM. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm. 2009;15:543–555. doi: 10.18553/jmcp.2009.15.7.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S, Baxter DC, Limone B, Roberts MS, Coleman CI. Cost-effectiveness of fingolimod versus interferon beta-1a for relapsing remitting multiple sclerosis in the United States. J Med Econ 2012;15:1088–1096. [DOI] [PubMed]
- 69.Noyes K, Bajorska A, Chappel A, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based study. Neurology. 2011;77:355–363. doi: 10.1212/WNL.0b013e3182270402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng F, Qiu W, Li J, et al. A preliminary result of treatment of neuromyelitis optica with autologous peripheral hematopoietic stem cell transplantation. Neurologist. 2010;16:375–378. doi: 10.1097/NRL.0b013e3181b126e3. [DOI] [PubMed] [Google Scholar]
- 71.Xu J, Ji BX, Su L, et al. Clinical outcome of autologous peripheral blood stem cell transplantation in opticospinal and conventional forms of secondary progressive multiple sclerosis in a Chinese population. Ann Hematol. 2011;90:343–348. doi: 10.1007/s00277-010-1071-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 492 kb)