Abstract
The treatment of relapsing remitting multiple sclerosis has witnessed major progress since the first effective disease modifying treatment, ß-interferon, became available in 1993. One of the most remarkable new treatments has been natalizumab. This review describes the evolution of this humanized anti-α4ß1 monoclonal antibody, from preclinical experimental research through proof-of-concept (phase 1/2) and pivotal (phase 3) clinical trials to the now extensive experience of its use in clinical practice. The future potential and challenges of natalizumab and oral therapies with a similar mechanism of action are also discussed.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-012-0171-4) contains supplementary material, which is available to authorized users.
Keywords: Natalizumab, Relapsing remitting MS, Progressive multifocal leucoencephalopathy
Introduction
Natalizumab (Tysabri® - Biogen Idec) is a humanized monoclonal antibody that is directed against the α4ß1-integrin molecule on mononuclear white blood cells, including lymphocytes. It is administered intravenously once every 4 weeks in a dose of 300 mg to adult patients with highly active relapsing remitting multiple sclerosis (MS), and has been shown in clinical trials to substantially reduce relapse rate and the accumulation of disability, making it among the most effective treatments currently available for relapsing remitting MS. However, it is also associated with a serious adverse event: progressive multifocal encephalopathy (PML), an opportunistic viral brain infection that has a high morbidity and mortality.
In this review, we will first discuss the rationale for, and development of, natalizumab, through preclinical and nontrial clinical studies. Then, we will review the experience of early clinical trials of natalizumab, followed by a discussion of the pivotal (phase 3) trials with attention to clinical and imaging findings. Subsequently, we will subsequently consider the phase 4 (i.e., postmarketing and clinical practice) experience, including the development of PML, and the implications for using natalizumab in current practice. Lastly, the review will consider possible future directions with natalizumab treatment for MS and/or other agents with a similar mechanism of action.
Preclinical Experimental Studies
As the molecular mechanisms that enable lymphocyte trafficking from the blood to tissues were elucidated during the 1980s and early 1990s, it was shown that a variety of molecules, including integrins, selectins, and cell adhesion molecules, played a part in the process. An important publication in 1990 showed that binding of the vascular cell adhesion molecule-1 ligand on activated endothelia to the VLA4 integrin receptor on lymphocytes facilitated the recruitment of mononuclear white cells to sites of inflammation [1].
A remarkable study was reported in Nature in 1992 [2], in which the molecular mechanisms of mononuclear white cell trafficking in to the central nervous system (CNS) were investigated in animals with experimental allergic encephalitis (EAE), an autoimmune CNS inflammatory disorder that is thought to have—at least in part—similar mechanisms of inflammation as those involved in MS. The investigators reported in vitro studies in which antibodies to the ß1 and α4 integrins blocked the binding of lymphocytes to frozen EAE brain sections. They also reported that administration of α4ß1 antibodies in vivo to animals with EAE reduced the development of paralysis and decreased inflammation seen histopathologically.
At about the same time that the studies of lymphocyte adhesion and migration were taking place, serial gadolinium-enhanced magnetic resonance imaging (MRI) studies identified that gadolinium enhancement, indicative of the breakdown of the blood–brain barrier (BBB), was a consistent feature of new lesions in people with relapse onset MS [3]. Such lesions were more often seen during relapse, and when the clinically eloquent lesion causing relapse was seen it was often enhancing [4]. Correlative histopathology and MRI studies demonstrated an association of gadolinium enhancement with perivascular inflammation in both EAE [5] and MS [6]. In addition, serial monthly scanning in MS revealed that there were, on average, 10 new gadolinium-enhancing lesions for 1 clinical relapse, indicating that this imaging marker of disease activity would be a sensitive measure for use in proof-of-concept trials of treatments aimed at preventing new inflammatory lesions and associated relapses. Taken together, it was evident that BBB breakdown and associated inflammation was a consistent early event in new lesion formation in relapsing MS. When humanized anti-α4ß1 antibodies were developed for clinical trials it was therefore logical to use serial enhanced MRI as a key outcome measure.
Early Clinical Trials
A notable early clinical trial (1996–1998) was undertaken in the UK, involving approximately 35 subjects per arm receiving 2 doses of placebo or natalizumab 1 month apart, with regular MRI scans for 6 months [7]. The trial cohort was smaller and the study duration shorter than is normally undertaken for a phase 2 proof-of-concept trial in relapsing remitting MS, necessitated, in part, by a limited amount of available medication at that time The study showed a significant decrease in the number of new active and new gadolinium-enhancing lesions after 12 weeks in the natalizumab versus placebo groups, with a p-value of 0.042 for the primary outcome measure. This initial study also investigated whether natalizumab was able to suppress enhancement of lesions that were enhancing immediately prior to drug administration. It was found that there was no difference between the placebo and natalizumab arms in the proportion of such lesions still enhancing after 1 month. This imaging finding implied that natalizumab would not be effective in shortening the duration or outcome of existing MS relapses, as was, indeed, confirmed directly in another study of patients treated during relapse [8].
The definitive proof-of-concept evidence for effectiveness came in a 3-arm, placebo-controlled, double-blinded study of ~70 patients per arm with relapsing remitting or secondary progressive MS who received placebo or natalizumab 3 mg/kg or 6 mg/kg (1999–2001) [9]. The treatments were given monthly for 6 months and the primary study outcome measure was the cumulative number of new active lesions seen on serial monthly brain MRI scans over 6 months. The trial emphatically reached its primary endpoint, with a 90 % decrease in new active and enhancing lesions in both natalizumab arms versus placebo. Although not powered to investigate relapse rate, the study, nevertheless, reported a significant decrease in relapse rate in the natalizumab-treated arms. At 6 months follow-up post-treatment a return to baseline levels of new lesion activity was observed in the natalizumab-treated arms. A subsequent analysis reported that natalizumab also reduced the proportion of new gadolinium-enhancing lesions becoming persistent T1 hypointense lesions, suggesting a reduction intissue matrix damage, including axonal loss in the residual lesions [10].
Efficacy in Pivotal Clinical Trials
Clinical Efficacy
A Cochrane review has summarized the randomised controlled trial evidence for the efficacy of natalizumab in MS, specifically in relapsing remitting MS, at a dose of natalizumab of >3 mg/kg, in double-blind. placebo/active comparator controlled trials using formal systematic review methodology [11]. Three studies completely satisfied the inclusion criteria [12–14] (see also table in Horga and Tintore [15]). A fourth, potentially suitable, trial was a mixed population of relapsing remitting and secondary progressive MS randomized to 2 different doses of natalizumab [9]; however, individual patient data could not be obtained on the relapsing remitting MS subgroup and the trial was excluded.
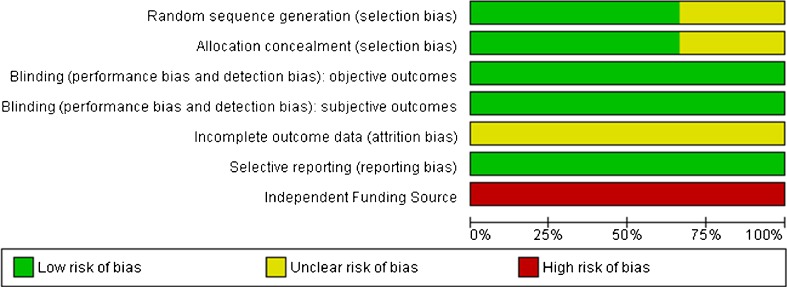
The methodological quality of the 3 trials is demonstrated on 7 indicators (Fig. 1). The risk of bias was relatively low, though all were funded by a nonindependent source.
Fig. 1.
Methodological quality graph as judged by the Cochrane review
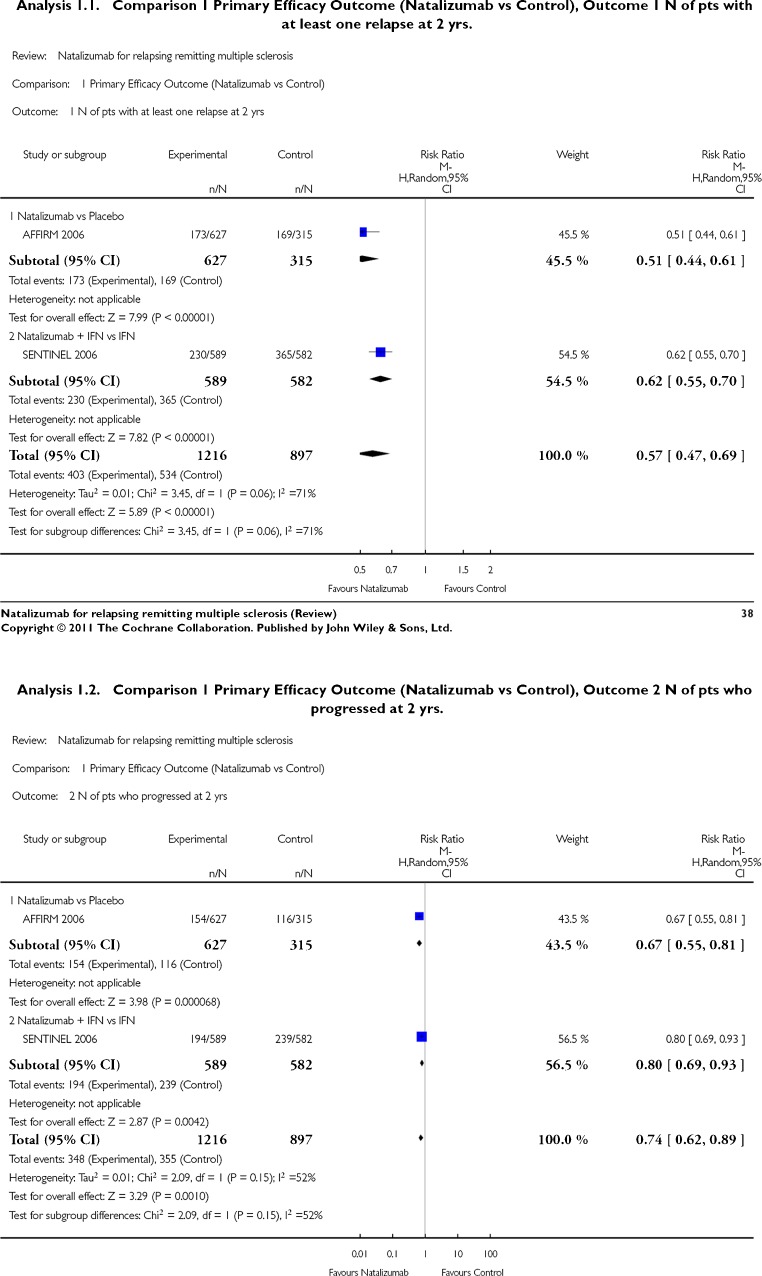
The main results of the Cochrane meta-analysis were statistically in favor of natalizumab for all the primary efficacy measures and for secondary efficacy measures for which data are available (Fig. 2—analysis 1.1 and 1.2 from the Cochrane review) [11]. It yields corresponding numbers needed to treat of 4 [95 % confidence interval (CI) 3–5] and 10 (95 % CI 7–23) for preventing relapse and disability progression respectively. The pooled estimate was relative risk = 0.57 (95 % CI 0.47–0.69) in favor of natalizumab. In the single large phase 3 trial of natalizumab versus placebo (AFFIRM study) (n = 942) there was a 67 % decrease in relapse rate and 50 % reduction in the accumulation of persistent new disability in the natalizumab-treated arm [12]. A post hoc analysis of that study looking at a stringent definition of disease-free activity (a composite of absence of activity on clinical plus absence of activity on MRI measures) yielded proportions of 37 % (natalizumab) versus (7 %) placebo. [16]
Fig. 2.
Meta-analysis of primary outcome data (Cochrane collaboration)
Health economic evaluation by the UK National Institute for Health and Clinical Excellence in 2007 [17] concluded that the incremental cost-effectiveness ratios for a rapidly evolving severe relapsing remitting MS group (defined as 2 or more disabling relapses in 1 year, and increased MRI activity or lesion load), compared with the best supportive care, ß-interferon, and glatiramer acetate were $71,000, $51,000, and $54,000 per quality-adjusted life year gained respectively.
The large phase 3 trial of natalizumab versus placebo also identified a better outcome for natalizumab-treated patients in terms of visual function [18] and health-related quality of life [19].
MRI Endpoints
In the 2-year AFFIRM study, natalizumab significantly reduced the number of new or enlarging T2-hyperintense lesions by 83 % (mean 11.0 vs 1.9; p < 0.001), gadolinium-enhancing lesions by 92 % (2.4 vs 0.2; p < 0.001), and new T1-hypointense lesions by 76 % (4.6 vs 1.1; p < 0.001) [20]. Median T2-hyperintense lesion volume increased by 8.8 % in the placebo group and decreased by 9.4 % in the natalizumab group (p < 0.001); median T1-hypointense lesion volume decreased by 1.5 % in the placebo group and decreased by 23.5 % in the natalizumab group (p < 0.001).
Brain atrophy was measured using the brain parenchymal fraction (BPF). The mean BPF reduction was greater in natalizumab-treated patients in year 1 (0.56 % vs 0.40 %, p = 0.002) and was greater in placebo-treated patients in year 2 (0.43 % vs 0.24 %, p = 0.004) [20]. Whereas the rate of brain atrophy was—as expected—constant over both years in the placebo arm, there was greater tissue volume loss in year 1 and less in year 2 in natalizumab-treated patients. A plausible explanation is that the year 1 finding reflects a marked resolution of inflammatory disease in natalizumab-treated patients with consequent decrease in brain volume, whereas in year 2 the decrease in volume loss reflects a neuroprotective effect secondary to the prevention of new inflammatory demyelinating lesions. Subsequent work suggests that the initially greater volume loss in natalizumab-treated patients comes from the white matter compartment, where inflammatory disease is most evident [21].
A substudy of the AFFIRM trial investigated for evidence of subtle BBB leakage in nonenhancing—and generally more longstanding—MRI lesions by measuring the change in signal intensity seen in such lesions on T1-weighted images following administration of triple dose (0.3 mmol/kg) of a gadolinium chelate contrast agent [22]. Signal Intensity change was greater in nonenhancing T2 lesions than paired contralateral normal-appearing white matter, consistent with low grade BBB leakage in lesions. No significant difference in the inferred BBB leakage was observed between treatment arms for the lesions. The study suggested that a subtle BBB leakage occurs within visibly nonenhancing lesions in relapsing remitting MS that is not modified by a4 integrin blockade.
In the other large pivotal trial (called SENTINEL), which compared the combination of natalizumab and β-interferon1a with β-interferon1a alone, the combined treatments significantly reduced the number of new or enlarging T2-hyperintense lesions and T1-hypointense lesions over the course of the 2-year trial. There was also a decrease in the extent of brain atrophy in year 2 of the combination treatment compared with β-interferon1a alone (0.31 % vs 0.40 %; p = 0.020) [23].
Adverse Effects
In day-to-day practice, the administration of natalizumab is uncomplicated, given at a dose of 300 mg every 4 weeks as an intravenous infusion over 1 h. Minor symptoms, such as pharyngitis, headache, and nausea, are well described. Hypersensitivity reactions in the AFFIRM study occurred in 5 % of patients, with severe reactions seen in 0.8 % (anaphylactic or anaphylactoid) [24]. Generally, they took place during the infusion or the hour afterwards, manifesting as hives with or without other symptoms. The greatest at-risk period was the interval between doses 1 and 7, particularly dose 2 [24]. They responded well to standard anti-anaphylaxis management. A smaller group of delayed hypersensitivity reactions occurred a few hours or days later, corresponding to a type III hypersensitivity phenomenon, e.g., fever, pruritis, and malaise. Apart from conventional anti-allergic measures, increasing the infusion interval or slowing the infusion rate have been suggested as ways to moderate the problem.
Natalizumab is not a conventional immunosuppressant, and standard bacterial and tuberculosis infections are not seen with increased frequency. In clinical trials herpes infections (zoster and simplex) seemed to occur a little more frequently in the natalizumab arms. The postmarketing experience has been of very rare events [25]: 1 fatal case of herpes simplex virus (HSV) encephalitis, 1 nonfatal case of herpes meningitis, and, recently, 1 non-fatal case of HSV encephalitis has been reported [26]. A case of HSV-2 meningitis has been described [27] and cutaneous HSV reactivation seems several times more common in patients receiving natalizumab [28]. Other infections reported include ocular toxoplasmosis [29] and cutaneous candidiasis [30].
Overall, the data, both during and after the trial, are insufficient to support any increased frequency of neoplasm, with 1 case of metastatic melanoma and 2 cases of lymphoma reported [31]. Monitoring of liver function is recommended, with rare cases of liver damage reported [15].
Progressive Multifocal Leukoencephalopathy
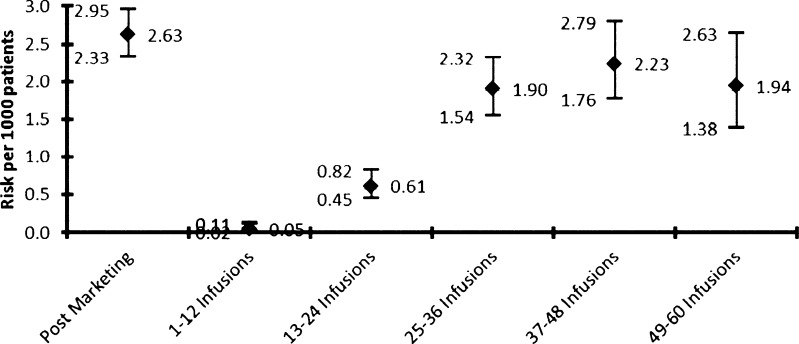
In 2005, the first 3 reports of progressive multifocal leukoencephalopathy (PML) were described: 2 in the extension phase of the SENTINEL study, where the patients were treated with both natalizumab and ß-interferon [32, 33], and 1 treated with natalizumab for Crohn’s disease who had received prior immunosuppression [34]. The drug was withdrawn and the analysis of all accumulated trial data (n = 3000) calculated an estimated incidence of 1/1000 patients after 18 months treatment [35]. After the establishment of intensive surveillance regimens—Tysabri Global Observational Programme in Safety Study (TYGRIS-US, NCT00477113), TYGRIS-Rest of the World (NCT00483847), and Tysabri Outreach: Unified Commitment to Health—natalizumab was reintroduced with long-term, real-time, safety data reporting. Figure 3 shows the PML risk estimates by treatment epoch with 285 confirmed cases of PML, as of 5 September 2012.
Fig. 3.
PML risk by treatment epoch (as of 5 September 2012)
There is a clear inflection point occurring after 24 months of treatment. That is, overall risks (with 95 % confidence limits/1000 patients) are: 0.05 (0.02–0.11), 0.61 (0.45–0.84), 1.90 (1.54–2.32), 2.23 (1.76–2.79), and 1.94 (1.38–2.63) at epochs 1–12, 13–24, 25–36, 37–48, and 49–60 months. Data beyond 60 months are currently more limited, with wider confidence intervals [36].
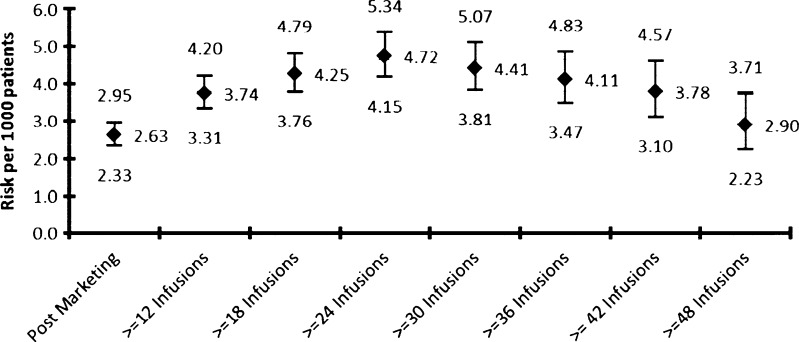
Figure 4 presents the cumulative PML risk, as of 5 September 2012, by treatment duration.
Fig. 4.
PML risk by treatment duration (as of 5 September 2012)
PML is, of course, well known from the last 3 decades of human immunodeficiency virus epidemiology, where it occurs at a rate of ~5 % [37], as well as with the use of immunosuppressive treatments, including alkylating agents (e.g., cyclophosphamide) and monoclonal antibodies (e.g., rituximab, infliximab). The cause is an active replication in the glial cells of the John Cunningham virus (JCV), a member of the polyoma family, with resultant oligodendrocyte destruction. It is estimated that half of the population naturally carry JCV, often harboring it in the kidney and shedding into the urine [38]. There is little understanding as to why such a relatively benign association between host and virus should become pathogenic. It may be explained partly by structural changes in the viral surface protein that are found in wild type (archetype) and pathological JCV, as well as the change in the host immune environment. A comparative analysis of the JCV capsid protein, VP1, has found a subset of amino acids exclusively amongst PML cases, located within the sialic binding site. It is postulated that such changes would allow JCV to avoid getting trapped outside the CNS (e.g., by receptors on red blood cells), which facilitates brain entry and therefore pathogenicity. This can be demonstrated in mouse polyomavirus models [39]. Other proposed mechanisms include mutations that increase JCV tropism and immune escape [39].
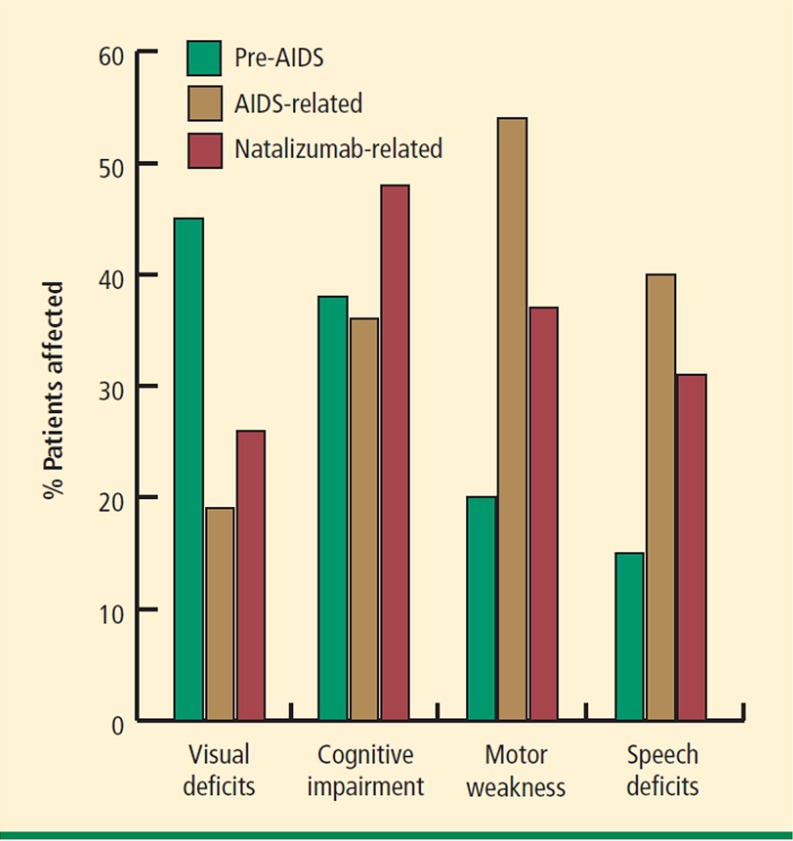
The clinical presentation of PML in patients with MS is not necessarily straightforward, with much of the same neuro-anatomical territory at risk from the two processes. Pointers include: progressive and rapid deterioration (over weeks), cognitive and behavioral issues, language disturbance, hemiparesis, and seizures. Figure 5 shows the clinical presentations in PML patients in the context of pre-acquired immune deficiency syndrome, acquired immune deficiency syndrome, and natalizumab, with the particular involvement of cognition noted in the latter (from [40]).
Fig. 5.
Progressive multifocal leukoencephalopathy presentation in the context of human immunodeficiency virus infection and natalizumab therapy. AIDS acquired immune deficiency syndrome [40]
The outcome of natalizumab-associated PML was reported recently for 242 patients [41]. Of these, there were 190 survivors (78.5 %) and 52 deaths (21.5 %). Survivors were younger and had a lower pre-PML disability. The majority of survivors appeared to sustain moderate-to-severe disability from PML and their clinical status was relatively stable after 6 months.
If a patient is felt to have PML, the most important step is to stop and, ideally, remove the natalizumab. Attempts to treat PML have centred on antiviral and immunomodulatory strategies. A recently reported strategy (n = 6), with early encouraging results, was the use of combination filgrastim (to restore lymphocyte adhesion), oral maraviroc (modulation of T cell recruitment), and mefloquin/mirtazapine (derived from in vitro drug testing for possible anti-JCV effects) coupled with plasma exchange to remove the natalizumab [42]. Plasma exchange is required as natalizumab is detectable in the circulation for up to 12 weeks, and mean α4-integrin saturation levels remain at >70 % for 4 weeks after infusion. In a different study, three 1.5-volume plasma exchange sessions over 5 or 8 days produces a reduction in mean natalizumab concentration of 92 % from baseline, and when the level is <1 μ/ml, the α4-integrin reduces to <50 % [43].
Unfortunately, the necessary sudden removal of natalizumab exposes the patients to an immune reconstitution inflammatory syndrome (IRIS) characterized by an intense lymphocytic infiltration and inflammatory brain damage, with can occur in days or weeks, with mortalities of 20–30 % reported. The only useful anti-IRIS treatment appears to be high-dose corticosteroids [44]. IRIS has also been reported in a patient who did not receive plasma exchange and merely stopped the natalizumab [45].
From the discussion, it can be seen that being able to stratify PML risk would be very useful. Pivotal to this is the JCV antibody sero-status of an individual because if negative, PML is unlikely (though not impossible) to occur. There is a number of assays in operation, with most data from a sensitive two-step enzyme-linked immunosorbent assay and supplemental test developed by Gorelik et al. [46]. Nearly 6000 patients from the AFFIRM, TYGRIS-US, STRATIFY-1, and the Swedish MS registry had baseline blood samples available for anti-JCV antibody testing. The prevalence increased with age, was lower among women than men, and gave an overall prevalence of 54.9 % (95 % CI 53.7–56.2) [36]. Data from pre-infection archived blood samples from 54 natalizumab-PML patients with MS showed a 100 % seropositive rate. The derived PML risks (with 95 % confidence limits/1000 patients) for JCV positive and negative patients are 3.87 (2.91–5.05) and 0.00 (0.00–0.32) respectively [37]. As, at the time of the study, no patient who went on to develop PML had tested negative for JCV antibody without testing positive at a later date, and the assay has a false-negative rate of ~2.5 % [47, 48], a sensitivity analysis was carried out assuming 1 hypothetical case of PML with JCV positivity at testing. This gave a PML risk of 0.09 (0.00–0.48) [36]. Additional risk factors for PML are previous immunosuppression and treatment duration, which allows for overall risk scenario estimates to be derived and which are being updated continually. For example, based on 212 reported PML cases in February 2012, Bloomgren et al. [37] reported PML risks per 1000 patients for JCV-positive patients as follows: (i) treatment duration <24 months and no prior immunosuppression [0.56 (0.36–0.83)]; (ii) treatment duration <24 months and had prior immunosuppression [1.6 (0.91–2.6)]; (iii) treatment duration >24 months and no prior immunosuppression [4.6 (3.7–5.6)]; and (iv) treatment duration >24 months and had prior immunosuppression [11.1 (8.3–14.5)].
Other routes to monitor JCV activity are clearly DNA polymerase chain reaction (PCR) assays in blood, urine, and cerebrospinal fluid (CSF). Unfortunately, at present, this provides no additional help to the stratification algorithm [49].
How should this information be used in clinical practice? First, it should be remembered that natalizumab is a very effective treatment in preventing relapses, with good evidence that it also reduces relapse-related irreversible disability. Often, patients who start natalizumab have experienced on-going active relapsing disease whilst being treated with first-generation disease modifying treatments. The use of natalizumab varies around the world; it can be used as a first- or second-line agent, depending on local policy. The decision to start natalizumab is a product of the aggressiveness of the disease combined with the JCV antibody serostatus parameters described earlier. The risk calculation would vary across the spectrum from a patient with an occasional sensory relapse who was JCV positive (unlikely to use), to a patient with frequent disabling motor relapses and who was JCV negative (likely to use).
It is appropriate to document the baseline JCV and MRI statuses, and update these parameters on a yearly basis. Clinical vigilance for PML remains paramount. However, according to currently available data, the risk of natalizumab-related PML is very low if the JCV titre remains negative. The relatively high PML-risk in JCV positive patients after 24 months of treatment warrants careful review of the clinical treatment decision at that time point, balancing disease state and natalizumab response against the higher PML risk. On one hand, if the second aforementioned scenario is reconsidered in the face of JCV positive status at baseline or seroconversion within 24 months, then a reasonable policy might be to treat to 24 months and reconsider the options at the end of that time period, which would include a decision to continue with natalizumab (depending on response) or to switch to another disease-modifying agent—the latter is discussed in the following section. If, on the other hand, the patient remains JCV negative then continuation of treatment seems appropriate.
If a patient’s clinical status changes, and PML is considered to be possible clinically and/or radiologically (see next section), a reasonable strategy is to retest the JCV serostatus (if previously negative) and perform JCV CSF PCR. A negative result from the CSF PCR is helpful in moving the risk away from PML, although PCR-negative cases have been observed [49]. Finally, of course, brain biopsy can be carried out.
MRI Aspects of PML
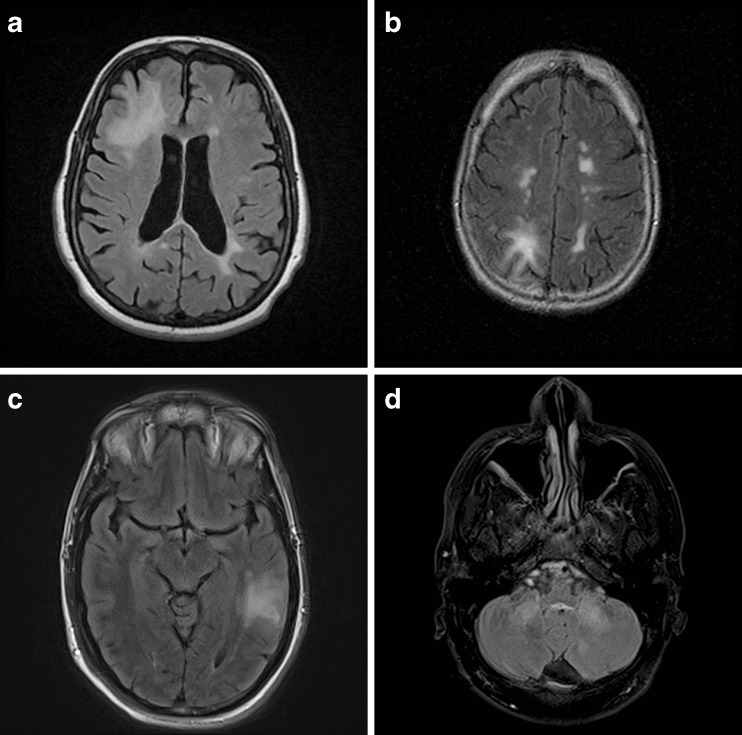
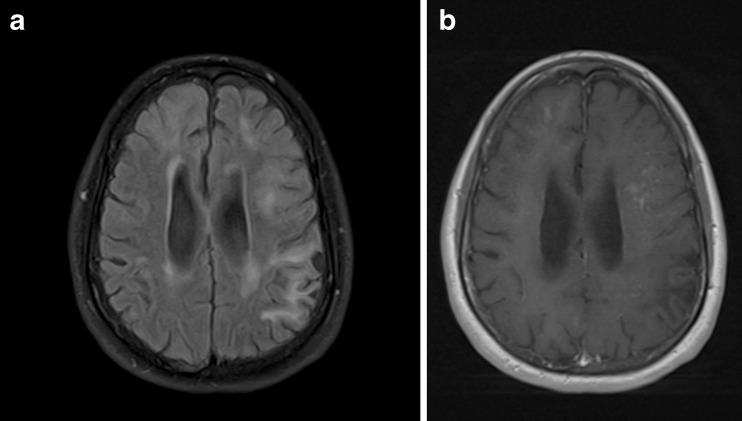
A detailed radiological analysis has been reported recently in 22 subjects who developed PML whilst being treated with natalizumab in the postmarketing setting [50]. This analysis identified several common and characteristic features of PML lesions. These included large (>3 cm) subcortical (including U-fibers) white matter lesions that were hyperintense on T2-weighted spin echo and fluid attenuated inversion recovery (FLAIR) images (Figs. 6a–d), hypointense on T1-weighted spin echo images and hyperintense on diffusion-weighted images. Lesions typically showed a sharp border towards the grey matter and a poorly-defined border towards white matter. Contrast enhancement after gadolinium chelate administration was observed in about 40 % of cases (Figs. 7a, b). In spite of the white matter lesions often being large there is usually no mass effect. Two other distinct features were noticed in some cases: (i) the contrast enhancement had a punctate pattern—in contrast, ring enhancement is more suggestive and typical for MS; (ii) areas of punctate “cyst-like” T2 hyperintensity adjacent to typical PML white matter lesions.
Fig. 6.
a PML lesion R frontal lobe b PML lesion R occipital lobe c PML lesion L temporal lobe d PML lesion cerebellum. Image courtesy of Dr Nancy Richert, Biogen Idec
Fig. 7.
a multifocal PML lesions on T2-weighted MRI b puntate gadolinium enhancement of some of the PML lesions. Image courtesy of Dr Nancy Richert, Biogen Idec
Seven cases were observed in which acute PML-IRIS developed. In all 7 cases the lesions were subcortical, confluent, and T1 hypointense without mass effect. In 5/7 lesions (70 %) there was hyperintensity on diffusion-weighted imaging and gadolinium enhancement.
A review of the radiological features of 66 cases of natalizumab-associated PML was presented at the ECTRIMS congress in October 2012 [51]. The most common location of PML lesions was the frontal lobe (48 %), but lesions could also be seen in the occipital (20 %), parietal (12 %), and temporal (10 %) lobes, and in the cerebellum (~10 %) (Figs. 6a–d). MRI can show a single lesion that is uni- or multilobar, or multiple noncontiguous lesions (Fig. 7a). Survival was noted in 80–85 % of patients with a single lesion and 66 % with multiple noncontiguous lesions. Involvement of the cortical ribbon was also observed in some cases and, in rare instances, a thalamic lesion with the appearance of a lacunar infarct was observed initially.
Taken together, these studies highlight a number of characteristic radiological features that suggest PML. Familiarity with such findings and expert neuroradiological review may help enable an early diagnosis of PML in some cases, with the potential for a better outcome as a result.
Considerations for Stopping Natalizumab
If the decision has been made to stop natalizumab and/or switch to another disease-modifying agent, what evidence is there to guide the process? The voluntary suspension of natalizumab that occurred in 2005, following the initial reports of PML from the pivotal trials, provides a large dataset to analyse MS disease kinetics in this circumstance. Relapses were analyzed in 1866 patients and gadolinium-enhancing lesions in 341 patients [52]. Both relapse and MRI lesion activity recurred in the months after stopping the natalizumab, peaking at 4–7 months. There was no rebound above baseline placebo arm levels in this series. This is contrary to some other (smaller) cohorts, which have described clinical exacerbations 3–4 months after natalizumab cessation [52].
The RESTORE study (ClinTrials.gov NCT01071083) attempts to answer what would be the next step of treatment if the decision is made to stop natalizumab. Theoretically, 3 options could be considered: to stop all treatment and assess the current basal activity level, move back to a first-generation disease-modifying treatment (β-interferon and glatiramer acetate), or move to emerging second-generation treatment (e.g., fingolimod, dimethylfumarate). In the RESTORE study, patients with stable MRI and clinical status have their natalizumab treatment interrupted, and are randomized 1:1:2 to natalizumab:placebo:open-label β-interferon1a or glatiramer acetate. In another small study (n = 19), patients were randomized to continue natalizumab or switch to β-interferon. Early results indicated some nonsignificant recurrence of clinical activity and increased new T2 lesion load on the β-interferon arm. [53]. It can be seen, therefore, that the data to fully inform this difficult decision are not currently available. Apart from these options, corticosteroids can be used empirically to provide a bridge in the first few months after natalizumab cessation.
Future Prospects
Long-term studies will be needed to determine the long-term course of MS following natalizumab treatment of relapsing remitting MS. As yet, it is not known whether long-term disability (over 5–10 years or longer) is deferred, or whether the developments of secondary progressive MS is delayed or prevented. The efficacy of natalizumab in secondary progressive MS is now being investigated in a large-phase 3 placebo-controlled trial.
A key challenge for the future is to identify ways of more accurately predicting the occurrence of PML so that treatment of individual patients can be given with a minimization of the risk for this complication. The development of an effective treatment for PML itself would also be welcome; thus far, trials of putative anti-JCV agents have been small and unsuccessful.
An alternative strategy for providing α4ß1 receptor blockade is the use of a small-molecule oral therapy. Such an approach may have the attraction of oral administration and a short half-life that would allow almost immediate restoration of α4ß1-mediated cell trafficking when treatment is stopped. The latter property might be valuable in cases that develop PML, where rapid restoration of the immune response at an early stage may improve the final outcome. It is also possible that subtle differences in the mechanism of receptor blockade with a small oral molecule than with a large humanized antibody might favorably modify PML risk, although this is speculative.
A phase 2 proof-of-concept trial of firategrast an oral anti-α4βintegrin agent in relapsing remitting MS was reported recently [54]. Three-dose arms of firategrast were compared with placebo. Because of a short half-life, firategrast was administered twice daily. The highest firategrast dose arm (1200 mg for males and 900 mg for females twice a day) reduced the cumulative number of new gadolinium-enhancing lesions by 49 % compared with placebo. There was also a significant reduction in the number of new T2 lesions and a nonsignificant (27 %) decrease in relapse rate. Further investigation revealed evidence of an exposure–response relationship, but decreased lesion activity was significant only in the quartile of participants with the highest average concentrations of firategrast during the 6 h after an oral dose. In future, an improved pharmacokinetic profile that increases systemic exposure and/or reduces between-patient variability within a dose level could provide greater efficacy for patients with relapsing remitting MS treated with oral short-acting α4βintegrin blockade therapies.
Natalizumab has changed the way relapsing remitting MS can be treated. It provides a superior option for β-interferon/glatiramer acetate failure or aggressive disease management. However, with the emergence of PML, the decision to use natalizumab is difficult and one that will continue to evolve over time.
Electronic supplementary material
(PDF 510 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Elices MJ, Osborn L, Takada Y, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-W. [DOI] [PubMed] [Google Scholar]
- 2.Yednock TA, Cannon C, Fritz LC, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 3.Miller DH, Rudge P, Johnson G, et al. Serial gadolinium enhanced magnetic resonance imaging in multiple sclerosis. Brain. 1988;111:927–939. doi: 10.1093/brain/111.4.927. [DOI] [PubMed] [Google Scholar]
- 4.Youl BD, Turano G, Miller DH, et al. The pathophysiology of acute optic neuritis: an association of gadolinium leakage with clinical and electrophysiological deficits. Brain. 1991;114:2437–2450. doi: 10.1093/brain/114.6.2437. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins CP, Munro PM, MacKenzie F, et al. Duration and selectivity of blood-brain barrier breakdown in chronic relapsing experimental allergic encephalomyelitis studied by gadolinium-DTPA and protein markers. Brain. 1990;113:365–378. doi: 10.1093/brain/113.2.365. [DOI] [PubMed] [Google Scholar]
- 6.Katz D, Taubenberger JK, Cannella B, McFarlin DE, Raine CS, McFarland HF. Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann Neurol. 1993;34:661–669. doi: 10.1002/ana.410340507. [DOI] [PubMed] [Google Scholar]
- 7.Tubridy N, Behan PO, Capildeo R, et al. The effect of anti-alpha4 integrin antibody on brain lesion activity in MS. The UK Antegren Study Group. Neurology. 1999;53:466–472. doi: 10.1212/WNL.53.3.466. [DOI] [PubMed] [Google Scholar]
- 8.O'Connor PW, Goodman A, Willmer-Hulme AJ, et al. Randomized multicenter trial of natalizumab in acute MS relapses: clinical and MRI effects. Neurology. 2004;62:2038–2043. doi: 10.1212/01.WNL.0000128136.79044.D6. [DOI] [PubMed] [Google Scholar]
- 9.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 10.Dalton CM, Miszkiel KA, Barker GJ, et al. Effect of natalizumab on conversion of gadolinium enhancing lesions to T1 hypointense lesions in relapsing multiple sclerosis. J Neurol. 2004;251:407–413. doi: 10.1007/s00415-004-0332-4. [DOI] [PubMed] [Google Scholar]
- 11.Giuliani P, Solari A, Simi S, et al. Natalizumab for relapsing remitting multiple sclerosis. Cochrane Database Syst Rev 2011, Issue 10. Art. No.CD007621. [DOI] [PubMed]
- 12.Polman CH, O'Connor PW, Havrdová E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 13.Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- 14.Goodman AD, Rossman H, Bar-Or A, et al. GLANCE: Results of a phase 2, randomized, double-blind, placebo-controlled study. Neurology. 2009;72:806–812. doi: 10.1212/01.wnl.0000343880.13764.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horga A, Tintore M. Natalizumab for relapsing-remitting multiple sclerosis. Neurologia. 2011;26:357–368. doi: 10.1016/j.nrl.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Havadrova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the natalizumab safety and efficacy in relapsing-remitting multiple sclerosis (AFFIRM) study. Lancet Neurol. 2009;8:254–260. doi: 10.1016/S1474-4422(09)70021-3. [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Clinical Excellence. Natalizumab for the treatment of adults with highly active relapsing–remitting multiple sclerosis, 2007. Available at: www.nice.org.uk/TA127. Accessed 7 Sept 2012.
- 18.Balcer LJ, Galetta SL, Calabresi PA, et al. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology. 2007;68:1299–1304. doi: 10.1212/01.wnl.0000259521.14704.a8. [DOI] [PubMed] [Google Scholar]
- 19.Rudick RA, Miller D, Hass S, et al. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62:355–346. doi: 10.1002/ana.21163. [DOI] [PubMed] [Google Scholar]
- 20.Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68:1390–1401. doi: 10.1212/01.wnl.0000260064.77700.fd. [DOI] [PubMed] [Google Scholar]
- 21.Soon D. A study of grey and white matter atrophy in a placebo-controlled trial of natalizumab in relapsing remitting multiple sclerosis. In: Soon D MRI evaluation of the anti-adhesion molecule antibody Natalizumab and the blood-brain barrier in Multiple Sclerosis PhD Thesis. UCL. 2010;Pg 117–149.
- 22.Soon D, Altmann DR, Fernando KT, et al. A study of subtle blood brain barrier disruption in a placebo-controlled trial of natalizumab in relapsing remitting multiple sclerosis. J Neurol. 2007;254:306–314. doi: 10.1007/s00415-006-0356-z. [DOI] [PubMed] [Google Scholar]
- 23.Radue EW, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a reduces lesion formation in relapsing multiple sclerosis. J Neurol Sci. 2010;292:28–35. doi: 10.1016/j.jns.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Philips J, O’Connor P, Havrdova E, et al. Infusion-related hypersensitivity reactions during natalizumab treatment. Neurology. 2006;67:1717–1718. doi: 10.1212/01.wnl.0000242629.66372.33. [DOI] [PubMed] [Google Scholar]
- 25.Ransohoff R. Natalizumab for multiple sclerosis. NEJM. 2007;356:2622–2629. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- 26.Kwiatkowski A, Gallois J, Bilbault N, et al. Herpes encephalitis during natalizumab treatment in multiple sclerosis. Multiple Sclerosis. 2012;18:909–911. doi: 10.1177/1352458511428082. [DOI] [PubMed] [Google Scholar]
- 27.Seiguer Shenoy E, Mylonakis E, Hurtado RM, et al. Natalizumab and HSV meningitis. J Neurovirol 2011; 17: 288–290. [DOI] [PMC free article] [PubMed]
- 28.Scheiss N, Zong J, Hayward G, et al. Reactivation of herpes virus in multiple sclerosis patients on natalizumab therapy. Poster Presentation at the American Academy of Neurology 61st Annual Meeting, Seattle, WA, April 28 2009, P03.163.
- 29.Zecca C, Nessi F, Bernasconi E, et al. Ocular toxoplasmosis during natalizumab treatment. Neurology. 2009;73:1418–1419. doi: 10.1212/WNL.0b013e3181bd114f. [DOI] [PubMed] [Google Scholar]
- 30.Gutwinski S, Eerbe S, Munch C, et al. Severe cutaneous Candida infection during natalizumab therapy in multiple sclerosis. Neurology. 2010;74:521–523. doi: 10.1212/WNL.0b013e3181cef810. [DOI] [PubMed] [Google Scholar]
- 31.Schweikert A, Kremer M, Ringel F, et al. Primary central nervous system lymphoma in a patient treated with natalizumab. Ann Neurol. 2009;66:403–406. doi: 10.1002/ana.21782. [DOI] [PubMed] [Google Scholar]
- 32.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 33.Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 34.Van Assche G, Van RM, Sciot R, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 35.Yousry TA, Major EO, Ryschkewitsch C, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354:924–933. doi: 10.1056/NEJMoa054693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 37.Cinque P, Koralnik IJ, Gerevini S, et al. Progressive multifocal leukoencephalopathy in HIV-1 infection. Lancet Infect Dis. 2009;9:625–636. doi: 10.1016/S1473-3099(09)70226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kean JM, Rao S, Wang M, et al. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunyaev S, Lugovskoy A, Simon A, et al. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML) PLoS Genet. 2009;5:e1000368. doi: 10.1371/journal.pgen.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger J. The clinical features of PML. Cleveland Clin J Med 2011;78(Suppl.2):S8-S12. [DOI] [PubMed]
- 41.Dong-Si T, Richman S, Gangadharan A, et al. Functional disability after natalizumab-associated PML in a large cohort of survivors. MS Journal 2012;18(suppl 4):516
- 42.Stefoski D, Ko M, Javed A, et al. Novel interventions with favorable resolution of natalizumab-induced progressive multifocal leukoencephalopathy (PML). Neurology April 22, 2012 78:P07.062
- 43.Khatri B, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72:402–409. doi: 10.1212/01.wnl.0000341766.59028.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan I, McArthur J, Clifford D, et al. Immune reconstitution inflammation syndrome in natalizumab-associated PML. Neurology. 2011;77:1061–1067. doi: 10.1212/WNL.0b013e31822e55e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clifford DB, De LA, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- 46.Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol. 2010;68:295–303. doi: 10.1002/ana.22128. [DOI] [PubMed] [Google Scholar]
- 47.Bozic C, Richman S, Plavina T, et al. Anti-JCV antibody prevalence in multiple sclerosis patients: baseline results of STRATIFY-1. Ann Neurol. 2011;70:742–750. doi: 10.1002/ana.22606. [DOI] [PubMed] [Google Scholar]
- 48.Hunt D, Giovannoni G. Natalizumab-associated progressive multifocal leucoencephalopathy: a practical approach to risk profiling and monitoring. Pract Neurol. 2012;12:25–35. doi: 10.1136/practneurol-2011-000092. [DOI] [PubMed] [Google Scholar]
- 49.Ayzenberg I, Lukas C, Trampe N, et al. Value of MRI as a surrogate marker for PML in natalizumab long-term therapy. J Neurol. 2012;259:1732–1733. doi: 10.1007/s00415-012-6426-5. [DOI] [PubMed] [Google Scholar]
- 50.Yousry T,Pelletier D, Cadavid D, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2012 (in press). [DOI] [PubMed]
- 51.Richert N, Bloomgren G, Cadavid D, et al. Imaging findings for PML in natalizumab-treated MS patients. MS Journal. 2012;18(Suppl. 4):27–28. [Google Scholar]
- 52.O'Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology. 2011;76:1858–1865. doi: 10.1212/WNL.0b013e31821e7c8a. [DOI] [PubMed] [Google Scholar]
- 53.Zecca C, Meier S, Tschuor S, et al. Natalizumab de-escalation to interferon-b-1b in multiple sclerosis: a randomised, controlled, pilot trial. MS Journal. 2012;18(Suppl. 4):463. [Google Scholar]
- 54.Miller DH, Weber T, Groves R, et al. Firategrast for relapsing remitting multiple sclerosis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012;11:131–139. doi: 10.1016/S1474-4422(11)70299-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)