Abstract
Significance: Iron and topoisomerases are abundant and essential cellular components. Iron is required for several key processes such as DNA synthesis, mitochondrial electron transport, synthesis of heme, and as a co-factor for many redox enzymes. Topoisomerases serve as critical enzymes that resolve topological problems during DNA synthesis, transcription, and repair. Neoplastic cells have higher uptake and utilization of iron, as well as elevated levels of topoisomerase family members. Separately, the chelation of iron and the cytotoxic inhibition of topoisomerase have yielded potent anticancer agents. Recent Advances: The chemotherapeutic drugs doxorubicin and dexrazoxane both chelate iron and target topoisomerase 2 alpha (top2α). Newer chelators such as di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone and thiosemicarbazone -24 have recently been identified as top2α inhibitors. The growing list of agents that appear to chelate iron and inhibit topoisomerases prompts the question of whether and how these two distinct mechanisms might interplay for a cytotoxic chemotherapeutic outcome. Critical Issues: While iron chelation and topoisomerase inhibition each represent mechanistically advantageous anticancer therapeutic strategies, dual targeting agents present an attractive multi-modal opportunity for enhanced anticancer tumor killing and overcoming drug resistance. The commonalities and caveats of dual inhibition are presented in this review. Future Directions: Gaps in knowledge, relevant biomarkers, and strategies for future in vivo studies with dual inhibitors are discussed. Antioxid. Redox Signal. 00, 000–000.
Introduction
Iron and topoisomerases are indispensable for cell proliferation. The essential nature of elemental iron is evident from its involvement in a multitude of cellular processes, including proliferation, DNA synthesis, mitochondrial electron transport, and oxygen sensing (44, 221). Iron also functions as a cofactor for many enzymes and is required for the synthesis of heme and for the activity of heme-proteins. Iron-sulfur proteins, which are characterized by the presence of iron-sulfur clusters containing sulfide-linked iron, include nicotinamide adenine dinucleotide (NADH) dehydrogenase, coenzyme Q, cytochrome c reductase, and hydrogenases. Under healthy conditions, cells balance their requirement for iron with the potential toxic effects of iron overload by regulating both iron uptake and storage. Iron is accumulated by transferrin-dependent and independent mechanisms (3, 8, 46, 216). The transferrin-dependent mechanism involves Fe3+ binding to transferrin receptors which are then endocytosed into cells where iron is released. Once inside the cell, iron exists as a labile pool that is made up of iron complexes with molecules such as amino acids, nucleotides, citrate, ascorbate, and nonheme proteins (102–104). The anthracycline group of anticancer and antibiotic drugs (e.g., doxorubicin) are among the first agents that showed both iron chelation and topoisomerase inhibitory activity (201, 216). Historically, drugs that chelate iron have been utilized to treat two life-threatening conditions: iron overload disease and iron-mediated cardiotoxicity from anthracycline chemotherapy. Recently, iron chelators have also been studied as anticancer agents, because cancer cells have a higher requirement for iron than healthy cells due to their rapid rate of proliferation. Cancer cells have a higher uptake and utilization of iron by virtue of possessing significantly higher levels of transferrin receptor 1 than healthy cells (65). The exposure of cancer cells to some iron chelators has been shown to elicit a G1-S cell cycle arrest, while others exhibit G2-M arrest (19, 296).
Topoisomerases (top) are a family of essential DNA repair enzymes that nick and religate DNA by forming a covalent enzyme-DNA intermediate between the enzyme's catalytic tyrosine residue and the end of the broken DNA (24, 200, 280). These covalent intermediates are referred to as “cleavage complexes” (200, 277, 278, 280). As a chemotherapeutic strategy, the usually transient topoisomerase cleavage complex (top cc) allows DNA to swivel during replication and repair but can be converted into a DNA lesion. Drugs such as camptothecin and etoposide trap topoisomerase by binding at the enzyme–DNA interface (23, 128, 199, 201, 202, 242). This “interfacial inhibition” effectively poisons the enzyme and converts the cleavage complex into DNA damage (201). In the continued presence of the interfacial inhibitor and unsuccessful DNA repair, cleavage complexes can be converted to DNA breaks when they are encountered by a replication fork (129). The induction of DNA breaks, stopping of DNA replication, and subsequent cell death in cancer cells are at the heart of successful antitumor activity by topoisomerase poisons.
Both iron and topoisomerase represent two distinct and mechanistically sound targets for cancer chemotherapy that have increasingly crossed paths over the past few years. Several agents identified as metal chelators have also exhibited selective topoisomerase inhibition, usually of topoisomerase 2 alpha (top2α) (Table 1). In addition, targeting both iron and topoisomerase contributed to the potent anticancer activity of these agents. Since the mechanisms of iron chelation and topoisomerase poisoning are complex, a clear reason why several structurally distinct drugs share these two targets is unclear. This review will analyze the known mechanisms of established and new iron chelators and topoisomerase inhibitors.
Table 1.
Select Known Iron Chelators with Topoisomerase Inhibitory and Anticancer Activity
| Iron chelator | Primary topoisomerase targeted | In vitro or in vivo anticancer test system | References |
|---|---|---|---|
| Doxorubicin | top2α and top2β | Human tumor xenografts in mice and breast cancer patients | (52, 72, 119) |
| ICRF-187 (Dexrazoxane) | top2α and top2β | Transgenic top2α mice and patients with various solid malignancies | (119, 163, 255) |
| ICRF-193 | top2α and top2β | Human tumor cell lines | (97, 265) |
| Dp44mT | top2α | Human tumor cell lines and breast cancer xenograft in mice | (209, 286) |
| ICRF-159 (Razoxane) | top2α | Human lung cancer cell line xenografts in mice | (42, 80, 159, 160) |
| ICRF-154 | top2α | Human lung cancer cell line xenografts in mice | (82, 159) |
| TSC-24 | top2α | Human hepatocellular carcinoma xenografts in mice | (123) |
| Triapine (3-AP) | top1 | Patients with various malignancies | (25, 179) |
| Curcumin | top2α and top2β | Human leukemic cell lines and breast cancer xenografts in mice | (137, 163) |
| Silybin (Silibinin) | top1 and top2α | Human colorectal and prostrate cell line xenografts in mice | (68, 113, 114, 257, 283) |
| Mitoxantrone, piroxantrone and isoxantrone | top2α | Human tumor cell lines | (85, 262) |
| Ferrocene | top2α and top2β | Human lymphoma cell line | (130) |
Dp44mT, di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone; top1, topoisomerase 1; top2α, topoisomerase 2 alpha; top2β, topoisomerase 2 beta; TSC, thiosemicarbazone
An agent that inhibits both DNA topoisomerase activity and iron metabolism is referred to as a dual inhibitor. This review is organized into three broad sections. The first section introduces iron and its chelation in cancer, the effects of iron-mediated cardiotoxicity, and the targeting of iron metabolism for cardioprotection and chemotherapy. The second section discusses topoisomerases, their activity in cancer, and their targeting by chemotherapeutic agents. The third section explores the overlapping mechanisms of dual inhibitors and potential biomarkers using doxorubicin, dexrazoxane, thiosemicarbazone (TSC)-24, and di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone (Dp44mT) as primary examples.
Iron Chelation in Cancer
Iron-mediated cardiac toxicity by anthracyclines
Cardiotoxicity induced by the anthracyclines is one of the most expected and monitored adverse events associated with an oncology agent (225). Anthracycline-induced cardiotoxicity is more prominent in female pediatric patients for reasons that are not fully understood. There is evidence that pediatric patients exposed to cardiotoxic chemotherapy early in life will, even if the cancer is completely eradicated, suffer from chronic cardiovascular disease later in life due to the cumulative adverse effects of anthracyclines (150, 261). Among the anthracyclines, doxorubicin is the most well-characterized agent due to its anticancer and cardiotoxic potential. Though preclinical studies did not detect cardiac toxicity, some early clinical studies as well as a retrospective evaluation of clinical studies of doxorubicin and daunorubicin established that cumulative doses of anthracyclines served as the main risk factor for dose-limiting cardiotoxicity (16, 66, 91, 144, 250, 271–274).
Anthracyline cardiotoxicity is exhibited in two clinically significant forms: acute and chronic (66). Acute toxicity occurs during or immediately after anthracycline administration. It is related to rapid intravenous administration of the drug and results in vasodilation, hypotension, and transient cardiac rhythm disturbances (66). Chronic toxicity, which can be early, delayed, or late-onset, can manifest itself decades after completion of the anticancer treatment. Chronic toxicity is marked by myofibrillar loss and cytoplasmic vacuolization in cardiac myocytes, and occurs in survivors of childhood cancers who were treated with doxorubicin without immediate adverse effects (63, 66, 237, 274). According to data from in vitro studies, the damaging effects of reactive oxygen species (ROS), generated by the interaction of doxorubicin with iron, plays a critical role in the pathogenesis of the chronic cardiotoxicity (66, 237). Sub-acute and sub-chronic toxicities are uncommon (133).
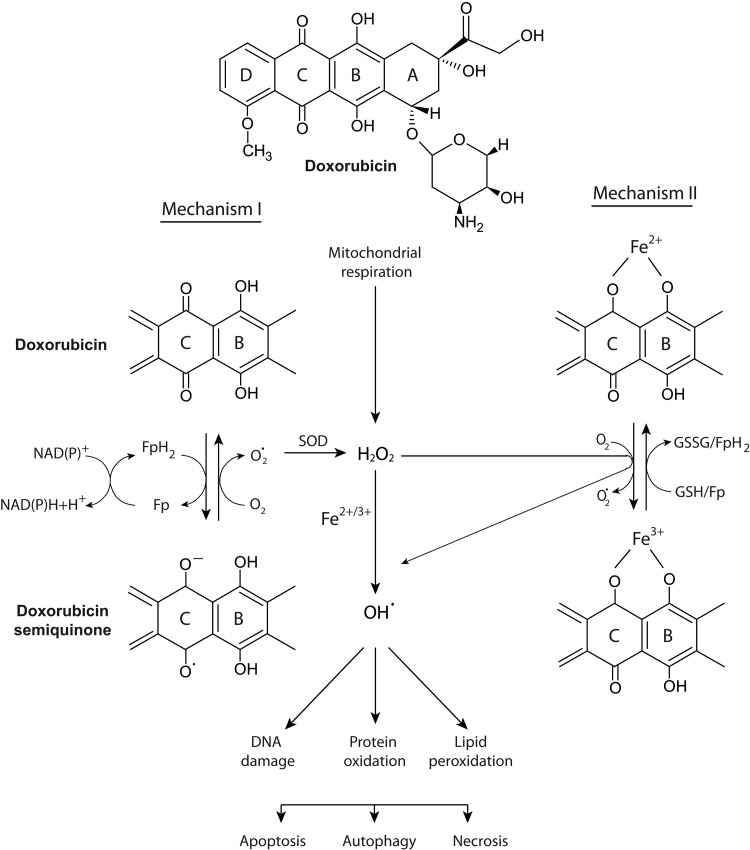
The mechanism through which doxorubicin increases ROS levels and thereby induces cardiovascular changes and toxicity is reasonably well understood (56, 71, 111, 180, 237). The DNA damaging activity of doxorubicin likely plays a limited role in cardiac tissue due to the slower rate of proliferation of cardiac cells compared with tumor cells, and because of the lack of top2α in cardiac cells (21). The currently understood mechanism through which iron-mediated ROS are elevated by doxorubicin is presented in Figure 1. Being cationic, doxorubicin is preferentially taken up by mitochondria through negative membrane potential. Inside the mitochondria, doxorubicin interacts with anionic phospholipids such as cardiolipin or phosphatidylserine that are present on the inner membrane (27, 61, 74, 75, 145). Upon binding cardiolipin, doxorubicin interferes with a number of essential mitochondrial proteins such as pyruvate and cytochrome oxidase (145). Due to cardiolipin's role in the unfolding of proteins for transport across the inner membrane, its complexation with doxorubicin also indirectly inhibits the accumulation of proteins from the cytosol into the mitochondrial matrix (61). Doxorubicin is metabolized in cardiac mitochondria to a semiquinone that undergoes futile cycles of reduction and oxidation in the mitochondrial electron transport chain, leading to excess production of hydrogen peroxide (H2O2) and other oxidizing species (41, 55, 180). The quinone moiety in doxorubicin and other anthracyclines is also known for its ability to undergo iron-mediated redox cycling and produce oxygen free radicals (73, 180). Similar to other quinones, anthracyclines can be reduced enzymatically by one or two electron-transfer reactions. Two major pathways for the generation of ROS from anthracycline exposure have been proposed, one involving the Haber-Weiss and Fenton reactions and the second through the formation of anthracycline-iron complexes (77, 237). A one-electron reduction of the C-ring of doxorubicin leads to the formation of a doxorubicin semiquinone free radical. Flavoproteins, such as complex I NADH dehydrogenase, catalyze the formation of reduced semiquinone radicals by accepting electrons from NADP or NADPH and donating them to the anthracycline (labeled as mechanism I in Fig. 1). The doxorubicin thus “redox cycled” leads to the formation of superoxide free radicals (O2•−). Superoxide dismutase catalyzes the conversion of the superoxide to H2O2. While H2O2 is stable by itself, the combination of H2O2 and O2•− can lead to the formation of toxic hydroxyl radicals (OH•). As the first step in the Haber–Weiss reaction, ferric ion (Fe3+) is reduced to ferrous ion (Fe2+) by superoxide (47, 77). The second Fenton step between ferrous ion and H2O2 leads to the production of hydroxyl radicals. The short half-life hydroxyl radical is extremely reactive with all molecules in the cell and, hence, deleterious to the cell (47, 67, 77, 180).
FIG. 1.
Currently understood mechanism for the iron-mediated generation of ROS by doxorubicin. The anthracycline doxorubicin undergoes a one-electron reduction of the C ring, leading to the formation of a semiquinone free radical metabolite. In the presence of oxygen, its unpaired electron is donated to oxygen forming superoxide radicals. Flavoproteins and glutathione (GSH/GSSG) catalyze the formation of a reduced semiquinone by accepting electrons from NADH or NADPH. SOD can catalyze the dismutation of superoxide into oxygen and H2O2 and provide an antioxidant defense, along with catalase and other antioxidant enzymes. A detailed description of the Fenton and Haber–Weiss reactions and the pathways labeled as Mechanism I or II are provided in the text. The iron-mediated generation of hydroxyl radicals can damage lipids, proteins, and DNA. The outcome of oxidative damage on critical cellular components could include apoptosis, autophagy, and/or necrosis. Modified with permission from Thomas Simunek (Charles University in Prague) (237). GSH, glutathione; GSSG, oxidized glutathione; H2O2, hydrogen peroxide; NADH, nicotinamide adenine dinucleotide; ROS, reactive oxygen species; SOD, superoxide dismutase.
The second mechanism involves the formation of an anthracycline-iron complex that could occur in the presence or absence of a reducing system. In the presence of NADH cytochrome P450 reductase or the thiols of cysteine or glutathione, anthracycline-Fe3+ is reduced to anthracycline-Fe2+ (labeled as mechanism II in Fig. 1) The ferrous ion conjugated anthracycline can react with O2 to form O2•−, which is subsequently converted to H2O2 and could enter the Haber–Weiss reaction and produce highly reactive hydroxyl OH• radicals. Alternatively, the ferric ion-conjugated anthracyline can reduce its ferric component by either oxidation of the side chain on C9 or of the hydroquinone moiety at ring C. This results in an anthracycline free radical that is chelated with Fe2+. In the presence of O2, the anthracycline free radical can be oxidized to generate O2•− and can react with H2O2 to yield OH•.
The iron-mediated mitochondrial generation of oxidative stress by doxorubicin damages proteins, lipids, and DNA (58, 182, 190) (Fig. 1). Cardiolipin, a major component of the inner mitochondrial membrane, is rich in polyunsaturated fatty acids, making it more susceptible to peroxidative injury by the free radicals generated by doxorubicin metabolites (94). The heart is especially sensitive to this oxidative stress because it is a mitochondria-rich organ and has lower defenses in terms of endogenous levels of superoxide dismutase and catalase than other organs such as the liver or lung (57). In addition, doxorubicin has been shown to cause a reduction in cardiac glutathione peroxidase expression (238). This enzyme functions to eliminate free radicals in the heart. The heart-specific NADH-oxidoreductase is also, at least in part, responsible for cardiac toxicity of anthracyclines (56, 186, 187). Cardiac, but not liver, mitochondria can reduce anthracyclines to semiquinones (186, 187). Thus, the heart is exquisitely sensitive to oxidative stress (111). Excess ROS produced by doxorubicin also induces protein oxidation via the carbonylation of apolipoprotein A1 in mice (5). The oxidized apolipoprotein A1 increases macrophage tumor necrosis factor alpha (TNF-α) release and is suggested to contribute to the toxicity of doxorubicin. Doxorubicin redox cycling also causes mitochondrial DNA damage that is manifested as mitochondria DNA (mtDNA) depletion, mtDNA mutations, and respiratory effects (141, 142). In addition to the known cellular consequences of excessive oxidative stress including apoptosis and necrosis, a pro-survival stress response known as autophagy also occurs as an early response to doxorubicin. This is discussed in greater detail in subsequent sections of this review (125, 267, 289).
Despite lower levels of antioxidant enzymes, cardiac mitochondria do attempt to mitigate the formation of excessive oxygen free radicals by the two-electron transfer enzyme DT diaphorase [NAD(P)H:(quinone acceptor) oxidoreductase] (26, 177). The other factors that affect the extent of anthracycline toxicity from excessive ROS are the glutathione system, low levels of labile iron, low levels of superoxide dismutase, and oxygen tension (177). Based on this information, it was proposed that a sound cardioprotective strategy might be the addition of an exogenous free radical scavenger (73).
Iron chelators as cardioprotective agents
General antioxidant strategies such as with coenzyme Q, vitamin A, carotenoids, vitamin C, vitamin E, flavonoids, polyphenols, n-acetyl cysteine, catalase, or superoxide dismutase gene therapies have been tested for their ability to ameliorate anthracycline-induced cardiotoxicity (1, 2, 11, 151, 181, 206, 218, 253, 282). Early in vitro and preclinical studies with several antioxidants showed promising cardioprotection that did not translate into clinical efficacy (31, 151, 230, 282). In some instances, the serum levels of the antioxidants required for cardioprotection were not pharmacologically achievable (253), while in others the cardioprotectants yielded a reduction in the anticancer efficacy of doxorubicin (30, 31, 230). Tissue uptake of the antioxidants, whether small molecule or gene therapy based, has also limited the clinical development of general free radical scavengers (1, 2, 151, 253).
The discovery that cardiac toxicity of anthracyclines involves iron-mediated redox cycling and cytotoxic generation of ROS spawned the investigation and development of new iron chelators, including siderophore analogs and synthetic ligands. Iron chelators have been tested for their ability as cardioprotective and/or chemotherapeutic agents. For a broader understanding of the history and chemistry of iron chelators for iron overload disorders and cancer chemotherapy, the reader is referred to other review articles (86, 132, 214, 215, 269). This section of the review will focus on the iron chelators that have shown in vivo activity as cardioprotective agents and possess some activity against topoisomerases, including dexrazoxane (ICRF-187), the TSCs, 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone (311), and triapine.
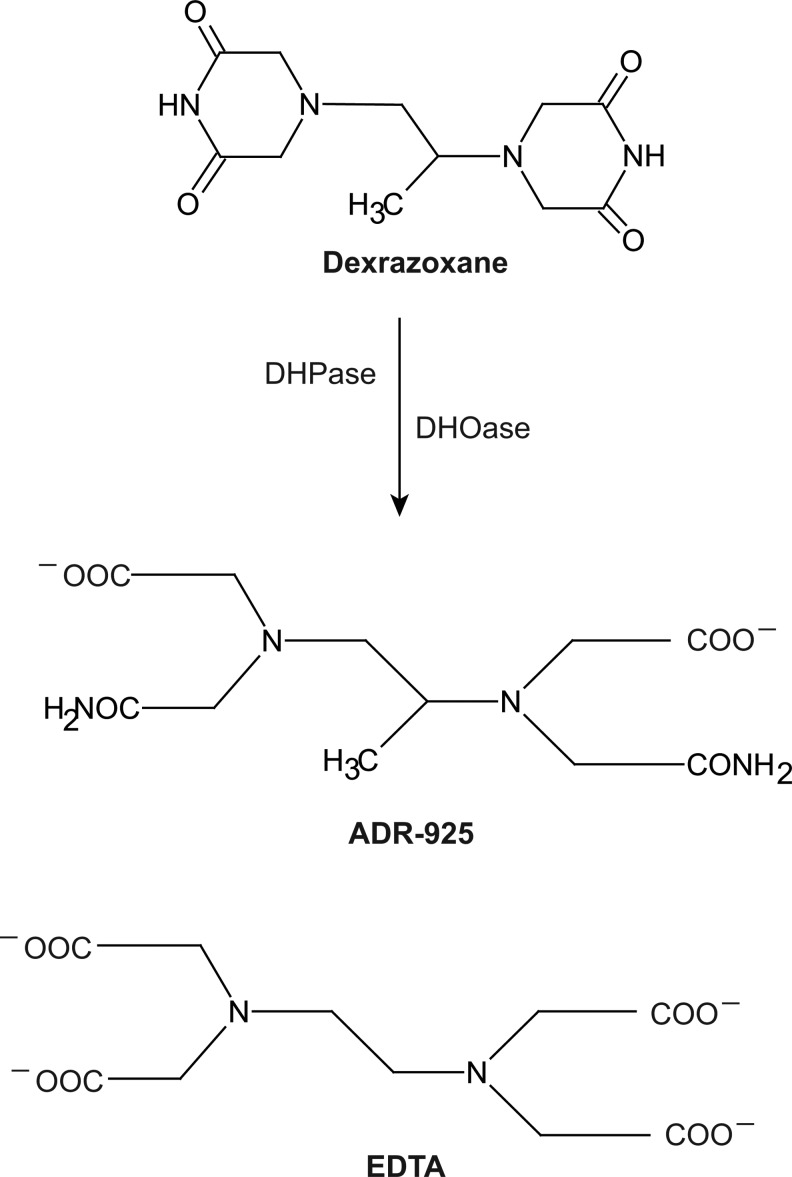
One iron chelator that has consistently shown cardioprotective ability in in vitro and in vivo test systems is dexrazoxane. Dexrazoxane is a bisdioxopiperazine that is orally active as a prodrug which is hydrolyzed to an ethylenediaminetetraacetic acid (EDTA)-like molecule, ADR-925, with iron chelating ability (Fig. 2). ADR-925 can rapidly displace iron from anthracyclines, suggesting that it has stronger affinity for iron than anthracylines. In vivo dexrazoxane has shown significant protection against doxorubicin-induced cardiotoxicity in numerous preclinical models such as mouse, rat, hamster, rabbit, and dog (81, 88–93). In addition, the cardioprotective effects were evident in both acute and chronic models of doxorubicin-induced cardiomyopathy (90, 211).
FIG. 2.
Chemical structures of dexrazoxane (ICRF-187), its iron-chelating metabolite ADR-925, and EDTA. After diffusing into cells, dexrazoxane is hydrolyzed by sequential ring openings via the catalytic actions of DHPase and DHOase enzymes. DHPase acts specifically on dexrazoxane as the first step. The structure of iron-chelating EDTA is provided as a comparison to ADR-925. DHOase, dihydroorotase; DHPase, dihydropyrimidinase; EDTA, ethylenediaminetetraacetic acid.
Dexrazoxane has provided long-term cardioprotection without compromising anticancer efficacy in doxorubicin-treated children with high-risk acute lymphoblastic leukemia (62, 152, 153). The effect was greater in girls than in boys (153). Protection from anthracycline cardiotoxicity was also documented in pediatric patients with solid tumors (28, 62). Cardioprotection was accomplished in the presence of sustained anticancer activity by the combination. While there was some concern that the addition of dexrazoxane could lower the anticancer efficacy of doxorubicin (163, 248), there has so far not been any directly supportive clinical study that suggests anything but that dexrazoxane would be a pragmatic choice for sustained chemotherapy and cardioprotection (203).
The underlying mechanism by which dexrazoxane exerts its cardioprotective effect is by chelating iron, displacing iron from doxorubicin, reducing the levels of H2O2, and aiding in the up-regulation of pro-survival Akt and Erk phosphorylation pathways (84, 288). Dexrazoxane also induces protective hypoxia inducible factor (HIF)-1α and HIF-2α protein levels and activation in the cardiac cell line H9c2 (244). HIF proteins are transcription factors that are activated in response to low oxygen and regulate genes which overcome hypoxia. The anti-apoptotic effects of dexrazoxane which are observed in a rat model suggest that the overall outcome of iron chelation and prevention of oxidative damage rescues cardiac cells from the cytotoxic effects of doxorubicin (301).
Iron chelators as chemotherapeutic agents
Historically, the development of several newer generations of cardioprotective iron chelators was a pharmacological intervention in response to the cardiotoxicity by doxorubicin, which is an iron chelator with topoisomerase-inhibitory and DNA-damaging activity (291). While iron chelators were originally designed to be ROS-scavenging antioxidants and chemoprotectants in heart cells, some have been shown to induce excess ROS generation in cancer cells. As anticancer agents, iron chelators have shown marked and selective activity in several in vitro and in vivo test systems (131, 209). Doxorubicin targets top2α and topoisomerase 2 beta (top2β). top2α is absent in cardiac tissue, whereas top2β is present in most tissue, including tumors (21, 36). While the chemistry of bispiperazinedones (such as dexrazoxane) and newer TSCs (such as Dp44mT; Fig. 3) were designed and developed for iron chelation and cardioprotective ability, our understanding of these compounds in recent years has shed more light on their anticancer ability, either as single agents or in combination with anthracyclines.
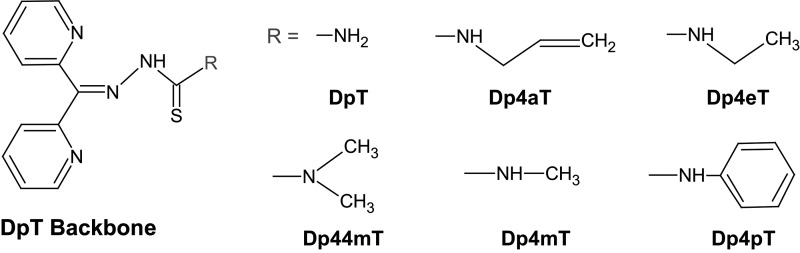
FIG. 3.
Chemical structures of the DpT family of iron chelators. The structural development of the pyridoxal isonicotinoyl hydrazone iron chelators led to the development of the DpT analogs. Among the analogs, Dp44mT showed iron chelating, top2α poisoning, and anticancer activity. DpT, di-2-pyridylketone thiosemicarbazone; Dp44mT, di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone.
Iron-chelating TSCs were first synthesized and evaluated for their anticancer activity in the late 1950s (18, 122, 123). Since then, several generations of TSCs have been tested for their chemotherapeutic potential (96, 108). In early mechanistic studies, the anticancer activity of TSCs was attributed to their ability to inhibit ribonucleotide reductase, an enzyme involved in DNA synthesis and repair (18). This inhibitory activity on ribonucleotide reductase is thought to result from the inhibition of the diferric iron core that is needed to stabilize the tyrosyl radical essential for enzyme activity (256). Recently, several TSCs have been shown to inhibit or poison topoisomerase 1 (top1), top2α, or top2β. 3-Aminopyridine-2-carboxyaldehyde thiosemicarbazone (3-AP or triapine) reduces topoisomerase I activity (25). Among those known to chelate iron and target topoisomerase IIα are dexrazoxane, (E)-N,N-dimethyl-2-(quinolin-2-ylmethylene)hydrazinecarbothioamide (TSC-24), and Dp44mT (Fig. 3) (18, 96, 108, 209). TSC-24 is considered a catalytic top2α inhibitor due to a direct interaction with the ATPase domain of top2α, which leads to a blockade of ATP hydrolysis. Interestingly, TSC-24 did not cause appreciable DNA damage as measured by neutral comet assay but rather reduced the DNA damage by the top2 inhibitor etoposide (96). Our laboratory demonstrated that Dp44mT causes DNA damage, apoptosis, and selectively poisons top2α, but not topoisomerase I or top2β, in Nalm-6 leukemic cells (209).
Topoisomerase Effects in Cancer
Types of topoisomerases and their enzymatic activity
DNA topoisomerases are a group of enzymes that resolve topological problems in the double-helical structure of DNA (24, 184, 201, 280). Topoisomerases are among some of the most well-studied enzymes for at least two reasons. First, there have been major advances in drug discovery of agents targeting topoisomerases that have successfully translated to the clinic and yielded some of the most widely used anticancer agents. Second, the crystal structures of a number of topoisomerase fragments representing nearly all known classes of topoisomerase have been solved, thereby providing molecular insights on these unique catalytic machines.
The fundamental need for this group of enzymes stems from the fact that DNA is a double-helical structure which lacks the inherent ability to rotate freely. Topoisomerases function in nearly all elements of DNA metabolism, including replication, repair, transcription, chromosome condensation and segregation, and the maintenance of genome stability (24, 201, 280). In general, these enzymes relax the positive and negative supercoiling to facilitate protein interactions with DNA and to prevent the deleterious consequences of supercoiled or unwound DNA duplexes. The structural features of all topoisomerases include hinged clamps that open and close to bind DNA, a DNA binding cavity for temporary placement of DNA fragments, and coupling of protein conformational changes to DNA rotation (24). Mechanistically, topoisomerases work by passing one strand of DNA through a break in the opposing strand (type I topoisomerases), or by passing a region of duplex DNA from the same or different DNA molecule through a double-stranded gap generated in that DNA (type II topoisomerases). All topoisomerases cleave the DNA phosphodiester backbone by nucleophilic attack from a catalytic tyrosine residue, which becomes linked to the phosphate end of the DNA break (279). These reactions do not change the DNA sequence and are reversible (201). The following section discusses therapeutically relevant classes of topoisomerases.
Type I topoisomerases work as monomers and form either 3′- or 5′-phosphotyrosyl covalent bonds. They are further divided into types IA and IB. Type IA includes the enzymes top3α and 3β and are the only enzymes that relax exclusively negative supercoiling. They form 5′-phosphotyrosyl covalent bonds with DNA. Type IB includes top1 and mitochondrial topoisomerase and form 3′-phosphotyrosyl covalent bonds. Type IIA topoisomerases work as dimers and cleave through a covalent attachment of each sub-unit of the dimer to the 5-end of the DNA through a phosphotyrosine bond (see top2 schematic in Fig. 4). In contrast to type I, the reactions of type II topoisomerases require Mg2+ and ATP hydrolysis for enzyme turnover and rapid kinetics; only one cycle of relaxation or decatenation/catenation can occur in the presence of the nonhydrolyzable analog of ATP, 5′-adenylyl-β,γ-imidodiphosphate. Type IIA topoisomerases include top2α and top2β (13). top2β has been less extensively studied compared with top2α, though they have some distinct structural features and different patterns of tissue-specific expression (13, 138, 241). The expression level of top2β is constant throughout the cell cycle, in contrast to the expression of top2α, which is low in resting cells and enhanced during proliferation (138).
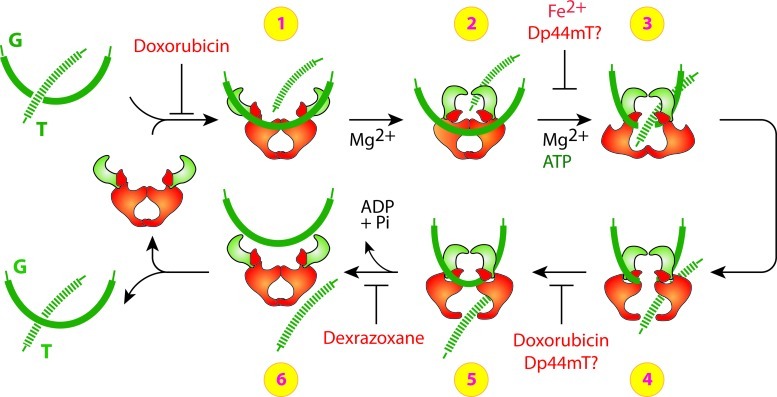
FIG. 4.
Potential sites of top2 inhibition by doxorubicin, dexrazoxane and Dp44mT. This schematic shows the currently understood processing of double-stranded DNA by the dimeric top2. Doxorubicin can block the catalytic cycle of the topoisomerase at either DNA ligation (before step 1) and/or DNA binding by interfering with top2 binding to DNA (between steps 4 and 5). Dexrazoxane blocks ATP hydrolysis and inhibits the reopening of the ATPase domain, thereby trapping the topoisomerase complex on DNA (between steps 5 and 6). While Dp44mT can trap top2α complex on DNA, further studies are needed to confirm the role of Fe2+ at other sites either on the ATPase domain or during ligation. Modified with permission from Yves Pommier (NIH/NCI) (201). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Topoisomerase as a target for chemotherapy
top1, top2α, and top3α are essential in multi-cellular organisms, as their deletion is lethal in animals (4, 149, 176), which is in contrast to the other type I and IIA topoisomerases (136). top2β deletion results in developmental and neurological defects but viable embryos (164, 165). top3α is involved in resolving Holiday junctions during homologous recombination repair to prevent sister chromatid exchanges, but its application as a therapeutic target has not yet been fully explored and will not be discussed in this review (208). The essential nature of top1 and top2α is one reason that makes these enzymes attractive therapeutic targets for cancer.
Agents that target top2 can work as catalytic inhibitors or poisons (139, 185). Catalytic inhibitors, such as dexrazoxane, can trap the top cc on DNA and inhibit its catalytic activity but do not result in a DNA strand break after a short exposure. Classical topoisomerase poisons, such as doxorubicin, increase levels of covalently bound topoisomerase-DNA complexes and generate lesions that result in a DNA double-strand break.
The mechanism through which doxorubicin and dexrazoxane target top2 is better understood than most top2 inhibitors or poisons. As illustrated in Figure 4, doxorubicin can block the catalytic cycle in two different steps. At low concentrations, doxorubicin can block DNA religation, whereas at higher concentrations, doxorubicin can interfere with top2 binding to DNA (before step 1 and between steps 4 and 5, Fig. 4) (201). The attempted DNA repair and subsequent conversion of unrepaired top2 cleavage complexes to DNA breaks by top2 poisons is discussed in the next section.
While doxorubicin can also chelate iron, its primary mechanism for anticancer cytotoxicity might not be iron mediated (163, 203, 248) and is likely due to its top2α-mediated and DNA damaging activities. Pretreatment with iron chelators dexrazoxane, desferrioximine, pyridoxal isonicotinoyl hydrazone, and salicylaldehyde isonicotinoyl hydrazone were used to show that the iron-mediated oxidative stress may not play a significant role in the cytotoxicity by doxorubicin in cancer cells.
Dexrazoxane can block ATP hydrolysis and inhibit the reopening of the ATPase domain, thereby trapping the topoisomerase complex on DNA and blocking enzyme turnover (185). The bisdioxiopiperazines dexrazoxane, ICRF-159 (rozoxane), ICRF-193, and ICRF-194 are iron chelators that enhance the formation of catalytic cleavage complexes with top2, but not top1 (7, 79, 251). Catalytic inhibitors such as dexrazoxane leave top2 trapped on DNA and interfere with DNA metabolism similar to top2 poisons but may not result in a DNA strand break in a short-term exposure (70, 100, 101, 185, 281). They act by trapping the enzyme in the form of a closed ATP-modulated protein clamp, thereby preventing the completion of the catalytic cycle (35, 82, 220). These agents are considered catalytic inhibitors and not traditional topoisomerase poisons, because not all have been shown to result in DNA double-strand breaks after the trapping of a complex with topoisomerase complex (7, 35, 79). Dexrazoxane and other bisdioxiopiperazines (ICRF-159, ICRF-193, and IRCF-194) have shown cytotoxicity in leukemic cells and cause DNA damage and apoptosis in various hematological cell lines at clinically achievable (4–5 μM) concentrations (163, 249, 293). The important role of top2α in the anticancer activity was confirmed in a transgenic mouse model with mutant TOP2A gene (76). Dexrazoxane has some clinical anticancer activity as a single agent (249, 270). Work from our laboratory and others has shown that dexrazoxane can induce DNA double-strand breaks as measured by the formation of the phosphorylated forms of the histone H2AX (termed serine 139 phosphorylated histone H2A [γ-H2AX]) in cancer cells (209, 211, 293). The DNA damage from dexrazoxane requires longer exposure times than classical DNA damaging agents such as γ-irradiation or etoposide, which is consistent with the hypothesis that dexrazoxane may function as a catalytic inhibitor of topoisomerase (185).
Expression levels of top1 and top2α are also high in cancer cells, and these expression levels correlate with the chemotherapeutic outcome from topoisomerase-targeting agents (17, 20, 33, 183, 196, 226). Levels of top1 in the NCI-60 cancer cell line panel correlate with sensitivity to the top1 poisons indenoisoquinoline and camptothecin (196, 198, 201). top2α expression is elevated in solid tumors and predicts responsiveness to anthracycline-based chemotherapy in women with primary breast cancer (33, 183, 226). Similarly, top2β levels in hematological cells correlates with sensitivity to and apoptosis by doxorubicin (117). The relationship between top2α and top2β and drug sensitivity in cancer is complex due to the role of the drug resistance proteins p-glycoprotein and multidrug resistance protein, changes in subcellular localization of top2 proteins, shared homology and catalytic activity between top2α and top2β, and phosphorylation/mutations in top2 (185).
Targeting top2β has recently been associated with increased incidences of secondary malignancies. Treatment-related acute myelocytic leukemia and myelodysplastic syndrome that progress to acute myelocytic leukemia have been reported for etoposide (158, 193). The occurrence of MLL gene transloctions has been related to the trapping of top2 cleavage complexes in the MLL gene (157, 158). The exact mechanism by which top2 cleavage complexes result in the 11q23 or 21q22 translocation in the MLL gene required for acute myelocytic leukemia is not yet clear. However, this raises the need to identify isoform-specific topoisomerase-inhibitory activity of iron chelators with potential anticancer use and the need to develop top2α-specific agents, when possible, to reduce the risk of secondary malignancies.
DNA repair responses to topoisomerase inhibition
As introduced in a previous section, the normally transient top cc can be converted to DNA lesions. Stabilization of the cleavage complex usually results from misalignment of the 5′-phosphate DNA end (200). Stabilization and misalignment can be generated by drugs bound at the interface of the enzyme and broken DNA or by alterations of the DNA substrate (such as abasic sites, mismatches, oxidized bases, and carcinogenic DNA adducts). Both top1 and top2 poisons exhibit such interfacial inhibition. For top1 poisons, the trapped enzyme generates a reversible single-strand break that is converted to a double-strand break when it is encountered by a replication fork (200). However, for top2 poisons, the two subunits of top2 can be degraded off the broken strands, yielding a double-strand break without the requirement of ongoing DNA replication (185, 254).
The repair mechanisms for top1-induced DNA damage are better understood than for top2 damage. For top1-associated DNA damage, three repair mechanisms are possible: (i) reversal of the covalent top1–DNA complex by 5′-end religation, (ii) top1 excision by tyrosyl DNA phosphodiesterase (Tdp1), and (iii) top1 excision by endonucleases. Since most iron chelators do not have an appreciable effect on top1, this review will not discuss top1 repair in greater detail. The reader is referred to other sources that discuss top1-mediated DNA damage repair in greater detail (24, 200).
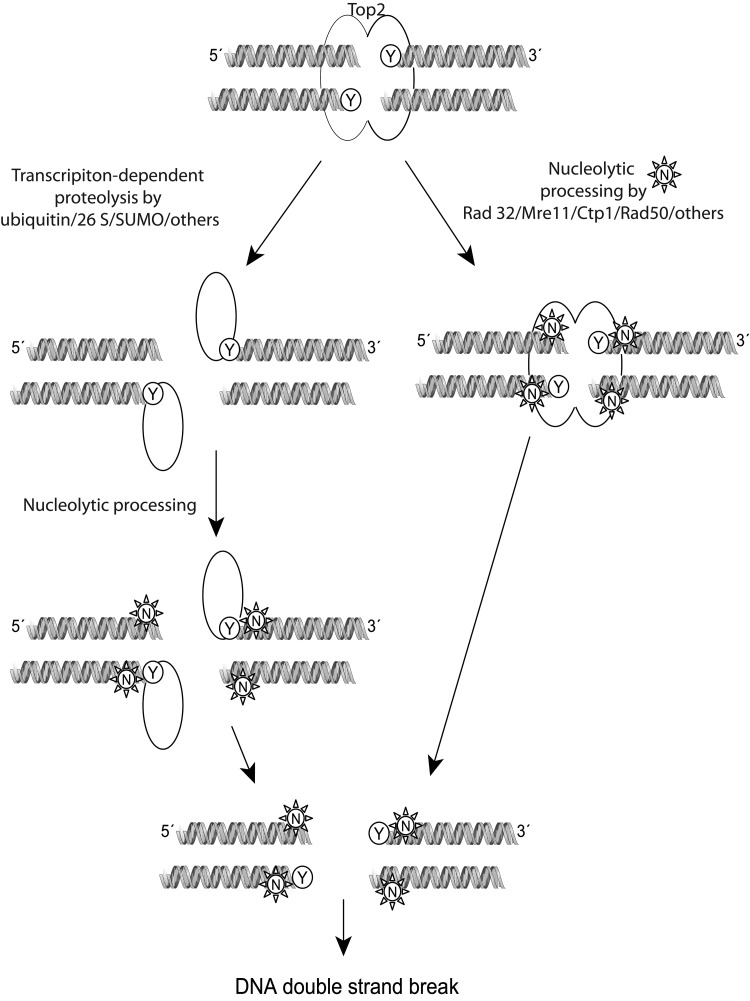
The repair of top2 DNA damage requires the sensing of the top2 complex as an abnormality, the removal of the protein bound to DNA, and the repair of the DNA strand breaks. Before recognition as DNA damage, top2 covalent complexes can be reversed without any harmful cellular effects. As shown in the schematic in Figure 5, after being sensed and once repair is initiated, the top2 complex is probably irreversible, and the cells should either repair the damage or undergo cell death (185). Proteosomal degradation (26 S) or SUMOylated degradation of covalently linked top2 has been proposed to allow access of DNA repair enzymes to the broken DNA. A proteasome inhibitor, MG132, reduces the DNA double-strand breaks induced by etoposide (300). The role of the proteasome is important for the repair of both top2α and top2β damage by etoposide, as confirmed in top2β knock-out and in knock-down cells exposed to MG132 (300). Similar to the excision of top1 by Tdp1, a 5′-tyrosine phosphodiesterase Tdp2 (or TRAF and TNF receptor-associated protein, TTRAP) is capable of excising top2-DNA adducts (37). Cells depleted of Tdp2 are hypersensitive to top2 but not top1 poisons (299). Endonucleolytic cleavage can also remove top2 trapped on DNA by cleavage of DNA near the point of attachment of top2 at the 5′-DNA end. Rad27FEN1, a 5′-endonuclease, and the nucleases Rad52, Mre11, Sae2, and CtIP have been suggested to function by cleaving the top2-DNA site. Yeast strains carrying mutations in Mre11 and Rad52 orthologs are hypersensitive to top2 poisons (166).
FIG. 5.
Mechanism of processing of the top2 cleavage complex with DNA by topoisomerase inhibitors. Either by encountering a replication fork or being sensed by RNA/DNA polymerases, the top2 covalent complexes are recognized and processed for repair by at least two pathways. Proteolytic processing of the top2 dimer could occur using the ubiquitin/26 S proteosome, or by SUMOylation. The enzyme is partially detached from the DNA but not completely removed. Other enzymes that are capable of cleaving remaining phosphotyrosyl bonds to DNA such as Tdp1/2 could assist in removal from DNA. Alternatively, nucleases such as members of the Rad family of nucleases can sever the link with DNA, generating a single- or double-stranded break. The single-stranded break could be converted to a double-stranded break during further processing or by encountering a replication fork. Homologous recombination or nonhomologous end joining can attempt to repair the double-strand breaks generated, but the exact mechanism is still being investigated. Modified with permission from John Nitiss (University of Illinois) (185). Tdp, tyrosyl DNA phosphodiesterase.
Proteolytic degradation of the top2 enzyme itself is also a potential pathway by which the cells respond to top2 DNA damage (167). This pathway is especially relevant because the top2-specific iron chelator dexrazoxane has been shown to induce the degradation of top2 (33, 167, 300). Degradation by the classical top2 poisons etoposide leads to proteolytic degradation of the enzyme by the ubiquitin/26 S proteasome pathway (167, 300). In one study, the top2β enzyme was preferentially degraded by this pathway over the top2α isozymes, while other studies using breast and lung cancer cell lines showed that both isozymes could be efficiently degraded by the proteosome in response to teniposide (167, 285). The proteolytic removal of top2 contributes to the processing of the top2 cleavage complexes into protein-free DNA breaks for subsequent activation of repair molecules, such as Chk1, Chk2, RPA, and γ-H2AX. The degradation of top2 and subsequent DNA damage signaling is attenuated, but not completely blocked, by pretreatment with proteosome and transcriptional inhibitors (64). Thus, transcription-, proteosomal-, and replication-dependent pathways may play a role in processing the top2 damage toward DNA repair.
Iron Chelators That Target Topoisomerase
Examples and significance of dual targeting anticancer agents
Several established and investigational iron chelators have been shown to act on topoisomerases (Table 1). The next section summarizes specific examples of dual targeting agents found in the literature and in our laboratory to exhibit some cytotoxic anticancer activity. It should be pointed out that in early papers the distinction between specific activity against top2α or top2β was not clear either due to experimention in Saccharomyces cerevisiae, which does not express the two isozymes, or because the top2 enzyme used was primarily top2α due to higher expression than top2β (J. Dickey [FDA], Personal Communication).
The compounds listed in Table 1 as being iron chelators that also act on topoisomerase (referred to as dual inhibitors) show varying degrees of anticancer activity in preclinical or clinical settings, with doxorubicin being the most promising as front-line therapy for breast cancer. The overall outcome from the combined activity of targeting both iron and topoisomerase appears to be a cytotoxic effect on cancer cells both in vitro and in animal models. In the case of doxorubicin, dexrazoxane, and triapine, promising results from safety testing in cancer patients warrants further studies that show efficacy in specific cancer types. Mechanistically, attacking rapidly proliferating cancer cells at two targets that are selectively up-regulated in tumors could be advantageous. Cancer cells exhibit higher levels of iron metabolism and elevated expression of topoisomerases. Hence, dual inhibitors might have novel benefits as anticancer agents. The use of dual inhibitors in combination with other anticancer agents, including other topoisomerase poisons, warrants particular consideration. In one study that compared the cardioprotective effects of different schedules of administration, dogs were exposed to dexraxozane 2 h before doxorubicin or simultaneously (90). The observations indicated that the timing of administration of dexrazoxane with regard to that of doxorubicin was an important factor, and the degree of protection was most evident when the agents were administered simultaneously. In vitro studies have suggested that the combined effect of doxorubicin and dexrazoxane is enhanced cytotoxicity in cancer cells (275, 276). A clinical study would address whether the iron chelation capabilities and metabolism of either agent interferes with the combined anticancer efficacy. Catalytic inhibitors of topoisomerase have been suggested to interfere with the DNA damaging activity of classical topoisomerase poisons, suggesting that iron chelators which are catalytic inhibitors but not poisons might benefit from being administered after the administration of a classical topoisomerase poison. This may allow the topoisomerase-induced DNA damage to occur before the iron chelation also exerts its antiproliferative effect. It is important to note that not all iron chelators interact with DNA and/or topoisomerases. Conversely, not all topoisomerase inhibitors can chelate iron to a physiologically relevant degree. Studies that have directly tested desferrioxamine and iron chelators of the pyridine-2-carboxaldehyde isonicotinoyl hydrazone class show high iron chelation potential but weak DNA binding capacity and no in vitro topoisomerase inhibitory activity (25). These agents also exhibit weaker cytotoxic effects compared with Dp44mT and doxorubicin and would not necessarily be expected to serve as anticancer agents. Some agents such as desferal are also limited in their bioavailability after oral administration and by a short half life on systemic administration. Studies designed to specially address the importance of the timing of inhibiting iron metabolism and poisoning topoisomerase are needed to fully understand the benefits of dual targeting drugs.
TSCs chelated with other divalent metal ions have also shown to inhibit topoisomerase II activity and kill cancer cells. These include Cu(II) complexed 2-acetyl-pyridyl-4N-substituted TSCs (171) and 1,2-naphthoquinone-2-thiosemicarbazone derivatives (234). Their ability to inhibit proliferation in breast cancer cell lines overexpressing top2α was greater than in cells with lower levels of top2α (171). A similar correlation between top2α levels and the ability of other copper-complexed TSCs to inhibit cancer cell proliferation has been shown in mice-bearing cancer cells with either high or low levels of top2α (284). Ruthenium(II) metal complexed TSCs have also been shown to inhibit top2α (12). Thus, TSCs and other chelators complexed with iron or other divalent metal ions have shown activity against topoisomerases that is associated with anticancer cell killing.
Possible mechanisms and caveats of dual inhibition
In this section, some possible mechanisms by which dual targeting agents may function as anti-cancer agents will be discussed. For the purpose of this review, the agents are discussed within the two broad categories: (i) iron chelators that are catalytic topoisomerase inhibitors and (ii) iron chelators that are poisons for topoisomerases and cause DNA damage. As with most pharmacological agents, this categorization is not complete and does not account for other possible mechanisms of action. Some of the agents classified as catalytic inhibitors would benefit from further investigation on their ability to directly intercalate with DNA or cause DNA-strand breaks; for instance, by using methods such as alkaline elution or induction of γ-H2AX foci (127, 197).
Iron chelators that are catalytic topoisomerase inhibitors, such as ICRF-159 or ICRF-193, could function by primarily targeting the high iron metabolism of cancer cells. These agents might have greater utility in cancer cell types where levels of iron or iron-regulatory proteins are high, in cell types that are not necessarily as high in their cell proliferation rates, or where targeting DNA synthesis has not been therapeutically beneficial. For example, an iron regulatory gene signature has been suggested to predict outcomes in breast cancer patients (170). In this study, the ferroportin-hepcidin regulatory axis was correlated to the breast cancer outcome; specifically, a decrease in ferroportin protein levels and an increase in hepcidin levels were reported in malignant breast cancer cells compared with normal mammary epithelial cells. In other studies, the levels of transferin receptor, uptake of iron, and the ratio of placental-like isoferritin to normal ferritin were higher in breast cancer cells than in healthy mammary epithelial cells (233, 295). Similarly, high concentrations of ferritin and iron metabolism have been noted in neuroblastoma cells (43). top1 activity was higher in immature neuroblastomas than in adrenal glands (266). Using desferoxamine, which does not target topoisomerase, some studies in neuroblastoma cancer models have found no benefit from chelating iron (235, 236), while the use of top1 or 2α poisons have shown antineoplastic effects in neuroblastoma patients with low tumor burden (135, 266). Future studies may benefit from identifying the contribution of DNA damage and topoisomerase poisoning in the success or failures of iron chelators in cancers with high iron metabolism. The timing of administration of iron chelating catalytic topoisomerase inhibitors with topoisomerase poisons (e.g., dexrazoxane with doxorubicin or TSC24 with doxorubicin) is also important because catalytic inhibitors could competitively interfere with the cytotoxic effects of topoisomerase poisons.
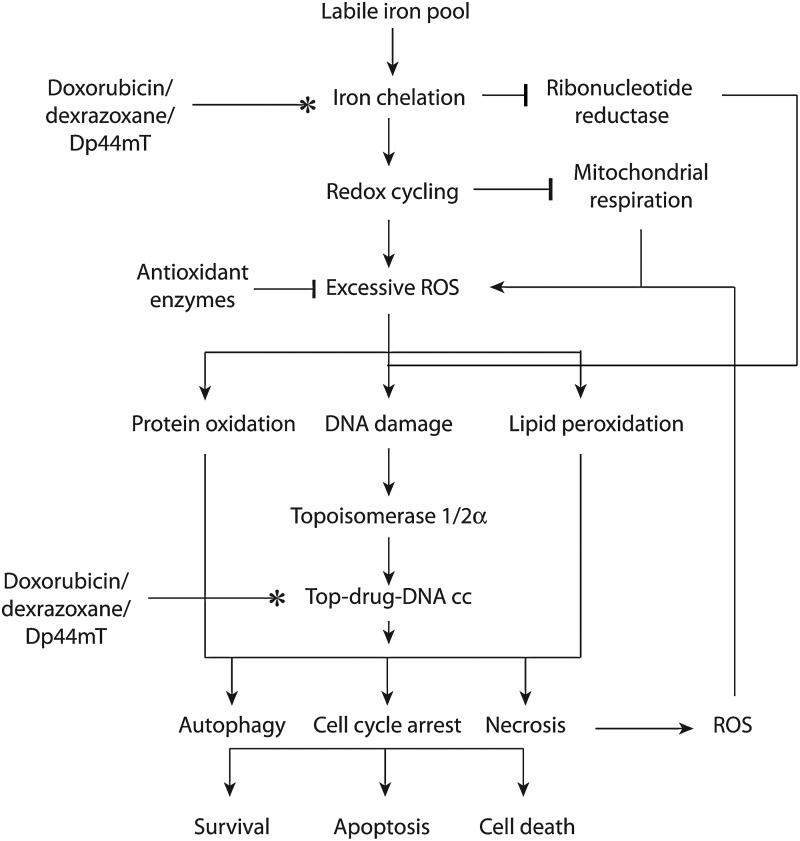
Iron chelators that are poisons for topoisomerase, such as doxorubicin and Dp44mT, could target cancer cells by working on their separate targets or by accentuating the activity of topoisomerase (Fig. 6). In the simplest scenario, dual targeting agents chelate iron and poison topoisomerase separately to stop DNA synthesis and cause DNA strand breaks and cell death. The two mechanisms may work completely independently to elicit the convergent goal of growth arrest and cell death. Such a multi-modal mechanism is likely to be beneficial in cancer cells that are resistant to one or more of these therapeutic interventions. For instance, specific populations of breast or lung cancer cells with decreased levels of top2 expression and resistance to top2 poisons might still benefit from iron chelation by dual inhibitors (155, 228).
FIG. 6.
Possible mechanisms of action by iron chelators that poison topoisomerases. Agents such as doxorubicin, dexrazoxane, and Dp44mT likely exert their anticancer activity by a combination of biochemical effects on the labile iron pool and on topoisomerase-mediated DNA damage. The generation of excess ROS by chelating iron could accentuate the DNA damaging effects by the agent. The oxidative damage of proteins and lipids also likely contributes to the cytotoxicity by excess ROS. Please refer to the text for an explanation of the possible pathways by which chelating iron and DNA damage could interplay. Top-DNA cc, topoisomerase DNA cleavage complex.
The indirect effects of chelating iron on the activity of ribonucleotide reductase could also contribute toward DNA synthesis inhibition. The indirect effects of leaching iron away from critical DNA repair enzymes also affects other proteins with iron-sulfer clusters such as the helicases XPD and FancJ, excision repair glycosylases, frataxin, and DNA methylation enzyme AlkB, each of which function to repair genotoxic DNA damage (15, 162, 212, 222).
Ironically, two critical antioxidant enzymes also contain iron or other divalent metal ions as cofactor that could, hypothetically, be leached by iron chelators and lead to decreased enzyme activity and excess ROS formation (118, 169). Both superoxide dismutase and catalase contain active site metal ions that are critical for their function (106, 264, 298). In eukaryotes, copper and zinc-dependent superoxide dismutase are most common, whereas iron-dependent superoxide dismutases are mainly found in Escherichia coli, Mycobacterium tuberculosis, and plants (34, 178, 217).
The possible contribution of other factors in drug resistance to the activity of dual inhibitors, such as the expression of multidrug resistance (MDR) genes, is still being investigated. However, it is important to note that we and others have observed sustained anticancer killing by Dp44mT in breast cancer cells that are resistant to etoposide (209, 287). Dp44mT is thought not to act as a substrate of ABC transporters such as P-glycoprotein and MDR-associated protein 1, through which cells sometimes gain resistance to the top2 poison etoposide (287).
Given the cytotoxic topoisomerase-poisoning activity of doxorubicin, the primary mechanism of action for doxorubicin in cancer cells may or may not be dependent on iron chelation. It is noteworthy that, unlike traditional iron chelators such as EDTA, doxorubicin does not mobilize iron but rather induces iron accumulation in ferritin (290). The lack of an effect of pretreatment with iron chelators such as dexrazoxane suggests that iron-mediated oxidative stress may not play a significant role in the cytotoxicity by doxorubicin in cancer cells (163). In our laboratory, the silencing of top2α significantly reduced, but did not abolish, tumor cell growth inhibition by Dp44mT (209). Dissecting the precise role of iron chelation in the cytotoxic effects of these agents needs to be addressed in vivo using side-by-side comparisons with synthetically modified drug analogs that lack the ability to bind to iron.
Another possibility is that dual targeting may accentuate topoisomerase inhibitory action. This may be possible when the iron-chelated drug can (i) act as a stronger interfacial inhibitor of topoisomerase due to conformational changes brought about by the presence of chelated iron, (ii) act as a carrier of Fe2+ and present an alternate divalent cation for mis-incorporation into the metal-binding pocket of topoisomerase, (iii) act as a carrier of Fe2+ and allow a direct interaction of iron with the DNA, which could produce DNA breaks that are sensed by topoisomerases, or (iv) undergo redox cycling and generate excess ROS and 8-oxoguanine lesions on DNA that can increase levels of top cc (see schematic in Fig. 6).
The in vitro study of agents chelated to iron and their effects on topoisomerase and DNA cleavage has been technically challenging because of the rapid degradation of plasmid DNA in the presence of iron; plasmid DNA is often used as the target substrate for these kinds of studies. Molecular docking simulations have shown that dual agents such as TSC-24 and dexrazoxane can bind to the ATP-binding site of top2α (82, 96, 163). These theoretical simulations were confirmed by competitive inhibition assays (96). Dexrazoxane itself can form a tight complex with the ATPase domain of both top2α and top2β. The amino acid side chains involved in the interactions between dexrazoxane and both top2 isozymes are identical (163). In a seminal study, Hasinoff et al. (82) compared the ability of the bisdioxopiperazines (including dexrazoxane) to inhibit topoisomerase catalytic activity, and a two-log range in the inhibitory concentration 50 values for inhibiting topoisomerase was reported. It was suggested that the bisdioxopiperazine binding site on topoisomerase II is large enough or flexible enough to accommodate the methyl group in either enantiomer of racemic ICRF-159. The cytotoxicity of the bisdioxipiperazines toward Chinese hamster ovary cells is highly correlated with their inhibition of the catalytic activity of type II topoisomerase. In our laboratory, in vivo studies with Dp44mT used an assay that extracted topoisomerase-drug-DNA complexes from whole cells, separated the DNA containing fractions, and probed the complexes with an anti-topoisomerase antibody. In this experimental setup, it can be assumed that some fraction of the drug is chelated to iron in the cell, but we could not estimate what fraction of the cellular drug-iron chelates was complexed with topoisomerase on the DNA. Cell-free studies with iron-complexed dexrazoxane, doxorubicin, or Dp44mT are needed to compare their ability to interface with topoisomerase and to iron-free drug.
Both type IA and type II topoisomerases require a divalent metal ion to function (51, 227, 239). For top2α, in vivo evidence has suggested that the DNA cleavage reaction requires Mg2+ as a cofactor for DNA scission (143). The solved crystal structure of top2α also showed a single Mg2+ atom per active site. In a series of in vitro studies, the ability of several divalent ions to support DNA cleavage by topoisomerases was examined in detail (48, 227, 239). The divalent ions Ca2+, Mg2+, Co2+, and Mn2+ were examined for their ability to affect topoisomerase function, and it was reported that top2α requires two divalent metal ions for DNA cleavage (227). The two metal ions are apparently necessary for DNA cleavage/ligation activities of the enzyme (51). One metal ion makes a critical interaction with the bridging atom of the scissile phosphate, which is most likely needed to stabilize the leaving 3′-oxygen and facilitate cleavage. The second metal ion is required for DNA scission; this ion is most likely needed to stabilize the DNA transition state and/or assist with the deprotonation of an active site tyrosine [see Fig. 7, adapted with permission from refs. (51, 227)]. A separate metal ion is required for the interaction with ATP. The exact role played by the metal ions is still being explored and the importance of their interaction with other sites in the primase domain, for instance, remains unclear (143). Divalent ions Mn2+, Ca2+, and Co2+ generated higher levels of DNA cleavage than Mg2+ (49). Fe2+ was not reported in these studies, probably because of technical challenges due to direct DNA damage by iron. However, previous studies suggest that other divalent metal ions can be substituted for Mg2+ in binding to topoisomerases.
FIG. 7.
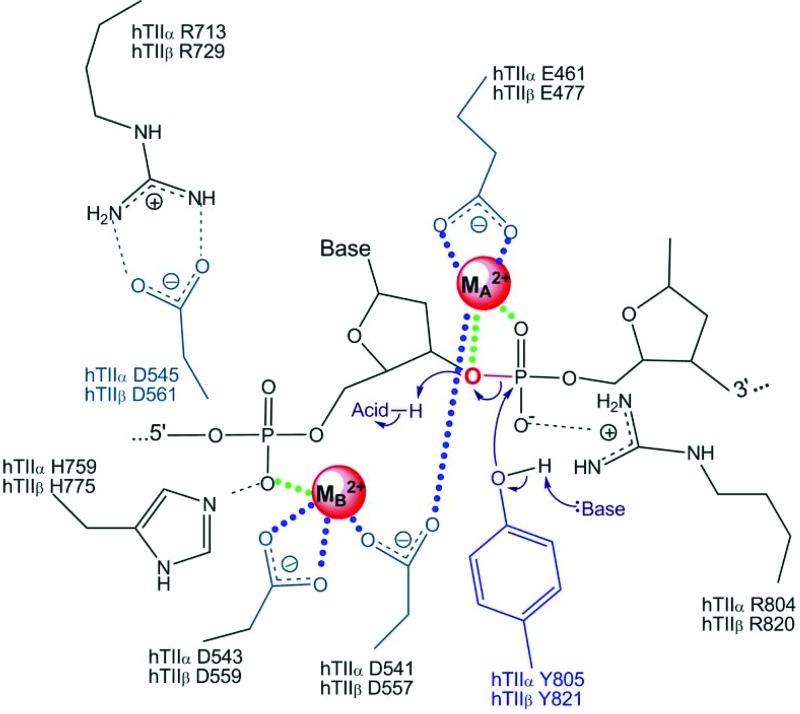
Proposed mechanism of divalent metal interaction with top2α. The amino acids on top2α (in green or blue) postulated to interact with two divalent metal ions (denoted MA2+ and MB2+) are shown (48, 51, 227). The DNA cleavage reaction has an absolute requirement for a divalent metal ion. Dewesse and Osheroff proposed that MA2+ stabilizes the transition state by making contacts with the 3′-bridging atom (red) and a nonbridging atom of the scissile phosphate (48). MB2+ is proposed to be required for DNA scission by providing anchoring and connects with the nonbridging oxygen of the phosphate that contacts the −1 and −2 bases upstream from the scissile bond (227, 239). While Mg2+ appears to fulfill this function in most studies, other metal ions such as Mn2+, Ca2+, and Co2+ can support double-stranded DNA cleavage in vitro (48, 50, 51). Please refer to the text for a discussion on the hypothetical potential for other metal ions, including Fe2+, to interact with top2α and/or DNA. Reproduced with permission from Niel Osheroff (Vanderbilt University) and Macmillan Publishers Ltd. (Nature) (51, 227). To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars.
An examination of the amino acids in top2α that are in the active metal binding sites and postulated to interact with Mg2+ (D543, D541, Y805, R804, E461, R713, and H759), and the comparative stability constants of various metal ions for binding to these amino acids (aspartic acid, tyrosine, arginine, glutamic acid, and histidine) (40, 51, 60, 98, 168, 294) suggest that it is theoretically possible for iron to form a stable complex with top2α. In most of the studies cited, stability constants were determined in cell-free systems using free amino acids, under aqueous conditions, and using the formula K=[ML]/[M][L], where K is the stability constant, and M and L are the metal and ligand concentrations. This does not necessarily correlate with how metals behave when interacting with amino acids within native protein conformations, or how they would behave when chelated to other small molecules (including free amino acids). However, it highlights the need for further studies examining how other metal complexes, such as with iron, can affect topoisomerase structure and function. To add to this argument, topoisomerase I in E. coli was recently suggested to be an iron- and zinc-binding protein (161). Using inductively coupled plasma-emission spectroscopy on purified proteins and the iron indicator Ferrozine to determine metal content, the authors found that top1 isolated from E. coli is an iron-binding protein; earlier, it had been suggested to be only a zinc-binding protein. The iron-bound top1 had little or no activity compared with the zinc-bound top1, suggesting that iron could be bound to top1 but not substituted for zinc in supporting its catalytic activity. The physical ability, but catalytic inability, of iron to substitute for zinc is also seen in estrogen receptor zinc finger protein and transcription factor for polymerase III A (32). In contrast, this metal substitution is catalytically functional in the case of neural zinc finger factor 1, tristetraprolin-2D, and GATA-1 zinc finger proteins (14, 54, 191). It may be possible that the mis-incorporated iron in eukaryotic top2α could lead to catalytic inactivity of the enzyme or DNA damage. This incorporation could also trigger metal-catalyzed oxidation of topoisomerase by the formation of hydroxyl radicals. The hydroxyl radicals generated by the redox active topoisomerase could then cause damage to adjacent DNA. The iron metal-catalyzed oxidation could also inactivate the enzymatic activity of topoisomerase and trap the enzyme on the DNA, similar to the iron metal-catalyzed oxidation and inactivation of alkaline phosphatase, glutamine synthase, or coenzyme Q1 (173, 174, 246).
The paradigm of accentuating or diminishing DNA damage is important for iron- and other metal-chelating drugs that could alter the expected responses from concurrently administered topoisomerase poisons. These include certain antibiotics that have antitumor activity and nonferrous metal-binding abilities such as 4′[(9-acridinyl)-amino]methanesulphon-m-anisidide, (mAMSA) netropsin, and tetracylines (87). For instance, mAMSA exerts copper-dependent oxidative and top2-mediated DNA cleavage (87). Hence, the role of chelated iron, its effects on topoisomerases and on the resultant cleavage complex formation, and anticancer outcomes require further investigation.
The final two possibilities for the actions of the dual inhibitors discussed here involve inducing DNA breaks. Doxorubicin is the most intensively studied example, and it is one of the few with direct proof of being an iron chelator with topoisomerase poisoning activity that can directly interact with DNA and chromatin (229, 307). The doxorubicin C-9 side chain plays an important role in determining the strength and specificity of the anthracycline–DNA interaction. The propensities of other chelators to bind directly to DNA have shown varying degrees of ability, all of which are lower than doxorubicin (25). The ability of chelators to interact directly with DNA has typically been determined by the measurement of DNA-mediated hypochromicity and hyperchromicity in the UV-vis spectra of the DNA ligand and iron complex. The decrease in absorbance (hypochromicity) or increase in absorbance (hyperchromicity) on addition of DNA to the compound was considered indicative of an interaction between the two (25, 38). Whether Dp44mT, dexrazoxane, and the newer dual inhibitors directly interact with DNA remains to be investigated.
Acting as a carrier of the metal ion to DNA, iron chelators could also damage DNA through direct hydrolysis of the phosphodiester backbone catalyzed by the metal ion (192, 232, 240, 259). Iron in its ferrous form and in an aqueous environment, can induce nonenzymatic strand cleavage and alkali-labile lesions (107, 240). Iron can incorporate into DNA and bind to it, possibly more so in the ferrous than ferric form (232, 263), although other studies suggest that the ferric form exerts the major stereochemical effect (6). The direct role of iron in mediating such DNA breaks was also documented as a part of the mechanism for the anticancer drug bleomycin, which complexes with iron (126, 194, 195, 240). It has also been proposed that superoxide promotes hydroxyl radical formation and oxidative DNA damage by releasing iron from storage proteins with enzymatic iron-sulfer clusters (118, 146). Such leaching of iron, or other mechanisms, may provide a pathway for DNA damage.
Finally, iron chelated drugs can undergo redox cycling and generate excess ROS (hydroxyl radicals) and 7′,8′-dihydro-8-oxoguanine (8-oxoG) lesions on DNA that can increase the formation of top cc (154). The mechanism for generation of excess hydroxyl radicals by doxorubicin has been previously described in this review. The iron chelator Dp44mT has been shown to induce excess ROS using 2′,7′-dichlorodihydrofluorescein dye, in one study using M109 cells and in our laboratory using MDA-MB-231 cells (unpublished results) (297). While ADR-925 has been shown to mediate hydroxyl radical formation, dexrazoxane has been primarily shown to reduce oxygen radical formation (78). Within cells, if the net result of the redox cycling of metal-bound chelators was to generate excess hydroxyl radicals, they could target DNA to produce 8-oxoG, the most abundant oxidative modifications in DNA. Using oligonucleotides containing 8-oxoG modifications, a significant increase in top1-mediate cleavage was found when the 8-oxoG was at the +1 or +2 positions relative to the cleavage site (204). The oxidative lesion 5′-hydroxycytosine also enhanced top1 cleavage by two-fold. The trapping of top1 adjacent to an 8-oxoG was enhanced when asparagine adjacent to the catalytic tyrosine was mutated to histidine, suggesting a direct interaction between the asparagine and the DNA major groove. Quenching of excess ROS by N-acetyl cysteine abrogates the formation of top1 cleavage complexes by the top1 poison staurosporine (243). Interestingly, the exposure of leukemic cells to H2O2 can also induce top1 cleavage complexes in DNA (39). In other cancer cell lines, H2O2 or 8-oxoG lesions induced top2 cleavage complexes in DNA (148, 223). ROS-induced formation of top cc is likely a secondary effect of dual inhibitors but one that would have cytotoxic consequences in the presence of sustained excess ROS. A recent report suggests a lack of cell cycle arrest, apoptosis, DNA damage, or top2α inhibition by Dp44mT at early time points and proposes that the top2α inhibition observed by 24 h could be due to iron-Dp44mT complex-induced oxidative DNA damage which can stimulate top2α-DNA complexes (292). Although cell line, technical, or reagent differences could also be probable causes for the observed lack of top2 inhibition, cell cycle arrest, or apoptosis, metal-complexed Dp44mT has been shown to have redox activity that generates hydroxyl radicals (105, 156). While we cannot presently rule out that top2α-directed activity of Dp44mT might be due to its redox-active and metal- complexed form, it nevertheless contributes to the irreversible cell killing consistently demonstrated in different cancer cells by several other groups (188, 209, 258, 286, 297).
Cellular responses to dual inhibition
Dual inhibitors may cause cell cycle arrest, apoptosis, necrosis, and/or autophagy in cancer cells. The ultimate cellular outcome most likely depends on the concentration and duration of drug exposure. Transient cell cycle arrest and autophagy could aid the cell in survival, while permanent cell cycle arrest could lead to apoptosis and/or necrosis. The targets of dual inhibitors discussed in this review trigger at least two overlapping downstream pathways that initiate a cytotoxic cellular response, namely excess ROS generation and DNA damage. Excess ROS could originate from redox cycling of iron-chelated drugs, metal-catalyzed oxidation of proteins, or from the activation of immune cells as an inflammatory response to drug treatment [e.g., formation of the oxidant taurine chloramine by from activated neutophils (121)]. Generation of excess ROS has been explored as a strategy for selective anticancer activity (121, 302). The deleterious effects of excess ROS would be via the oxidative modification of essential proteins, lipids, and DNA. DNA damage could also ensue from the unrepaired topoisomerase-DNA cleavage complex formation, or from direct DNA binding. Apoptosis induction by iron chelators or by anthracylines is covered elsewhere in this issue of the journal. Necrosis induction by iron dual inhibitors has been poorly defined, although it has been documented as one of the cellular responses to iron overloading, iron chelation, and in response to doxorubicin (260, 268). The remaining part of this section will focus on cell cycle arrest and autophagy.
Dual inhibitors have shown some variability in the phase of cell cycle arrest they elicit in cancer cells. Dp44mT and TSC-24 arrest cells in G1-S phase, which is also a feature of iron chelators with no topoisomerase-inhibitory activity such as desferal (95, 96, 188, 209). On the other hand, doxorubicin and dexrazoxane arrest cancer cells in the G2-M phase of the cell cycle, which is a feature of classical topoisomerase poisons such as etoposide (175, 275). The reason for this is not clear, although it is thought that chelation of iron inhibits DNA synthesis so that iron chelators induce an S-phase arrest. Inhibition of ribonucleotide reductase, up-regulation of the GADD family members, and increases in p53 levels are, at least in part, responsible for the S-phase arrest (224). top2 plays an essential role in sister chromatid separation at anaphase of mitosis, which could explain why top2 poisons cause an M-phase arrest. Both protein levels and catalytic activity of top2 peak during the G2-M phase of the cell cycle (99, 140). The top2 checkpoint monitors the catenation status of sister chromatids after the S-phase and prevents exit from G2 until the sister chromatids are sufficiently decatenated (59). Cell cycle arrest by dual inhibitors is likely due to the primary mechanism of action of the agent or the stage of the cell cycle in which the cells encountered the dual inhibitors. An analysis of synchronized populations of cells (for instance, in G1-S using aphidicolin or M phase using nocadazole) would be beneficial to identify the most sensitive cell cycle state for cancer cells exposed to dual inhibitors. Such studies could also help deliniate the primary target of the dual inhibitors.
The induction of autophagy by dual inhibitors can occur as a survival mechanism to overcome oxidative or chromatin damage. The exact role of iron in the formation of autophagosomes is unclear; however, iron chelators do induce the formation of catabolic autophagosomes (45). Interestingly, since lysosomes contain most of the cellular supply of labile iron, iron binding proteins can be sequestered in autophagosomes as a strategy to minimize the damage from free iron (134). In some circumstances, iron chelators can prevent autophagy by sequestering the iron used in the formation of autophagosomes in response to H2O2 (22, 134). Dp44mT can also cause autophagy, which is one pathway proposed to explain selective toxicity to cancer cells versus healthy cells (156, 209). Lovejoy et al. have proposed that since autophagic pathways are abnormal in cancer cells by virtue of monoallelic deletion of the autophagic regulator, beclin1, apoptosis, and cell death would be the favored pathway in cancer cells (156). Doxorubicin also induces autophagy as one of its cellular responses (205). This has been exploited with the autophagy inhibitor, resveratol, to attenuate the cardiotoxic effects of doxorubicin (289). We have observed increased autophagy in response to both doxorubicin and dexrazoxane without an appreciable decrease in the levels of the autophagy-associated protein myosin-binding light chain protein (LC3)-II in cancer cells (unpublished observations).
Future Directions
Potential biomarkers for iron chelators and topoisomerase inhibitors
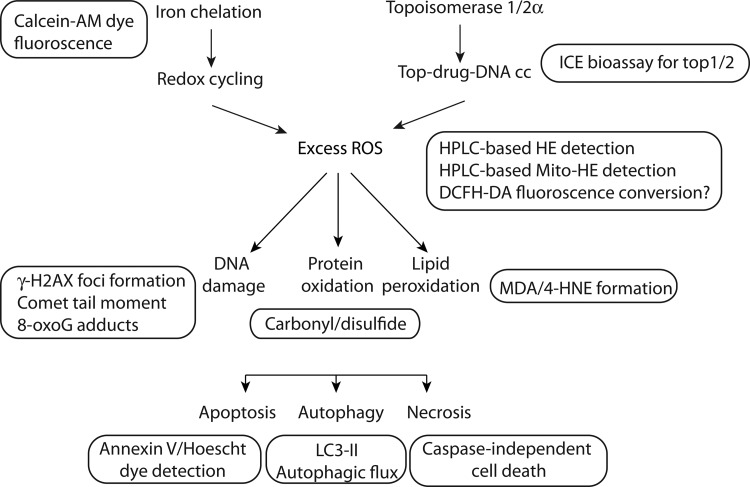
Future studies that utilize dual inhibitors as tools for research or for development of therapeutic interventions in cancer could benefit from selecting a set of biomarkers which reflect the known mechanisms of such dual targeting agents. This section summarizes characteristics that can be objectively measured and evaluated as indicators of biological response to dual inhibitors. The list of biomarkers discussed here is not suggested to be absolute, but rather a starting point for exploratory studies. The assays listed were considered physiologically relevant, technically feasible, and translatable across multiple laboratories. The endpoints are also illustrated in Figure 8 alongside the relevant mechanism.
FIG. 8.
Biomarkers for further studies on iron chelation and topoisomerase inhibition. Some of the biochemical causes and cellular consequences of iron-mediated ROS increase and DNA damage in cancer cells could be probed as illustrated in the diagram. The protein, lipid, and DNA mediators of cellular damage and the cellular outcomes of apoptosis, autophagy, and necrosis can be assayed using the endpoints that are shown adjacent to each step in the mechanism. A brief description of each of these assays is provided in the accompanying text. 8-oxoG, 7′,8′-dihydro-8-oxoguanine; AM, acetomethoxy derivative; DCFH-DA, 2′,7′-dichlorfluorescein-diacetate; HPLC, high performance liquid chromatography; ICE, immune complex with enzyme; LC3, microtubule-associated light chain protein; γ-H2AX, serine 139 phosphorylated histone H2A.
The unknown iron chelating capacity of known topoisomerase inhibitors could be probed using the fluorescent dye calcein-acetomethoxy derivative (10). The displacement of preloaded iron from calcein, as indicated by a change in the floruorescence of trapped intracellular calcein-iron complex over time, can be used as a marker of iron chelation. The iron chelation abilities of Dp44mT and other molecules have been shown using this method and appear to correlate with electron paramagnetic resonance spin trapping experiments with ferric and ferrous ion complexed with a chelator (83).
Endpoints of excess free radical formation are most frequently studied using redox-sensitive probes. The high performance liquid chromatography (HPLC)-based detection of superoxide-generated products of hydroethidine (HE) can be used to measure excess ROS (306). The quantification of 2-OH-E(+), the product of the reaction between HE and superoxide, can be measured in cell lysates as well as cell-free systems (210, 303). Mitochondrially targeted Mito-HE is capable of undergoing similar redox chemistry and may be useful in identifying mitochondrial ROS (109, 219). 2′,7′-Dichlorfluorescein-diacetate (DCFH-DA) is another fluorescent probe that is used for measuring intracellular H2O2 and oxidative stress (109). However, this probe may not be specific for H2O2 and could give misleading results due to oxidation by lipid hydroperoxides and H2O2, as mediated by cellular iron-sensing molecules (304, 305). Oxidant signaling by redox-active iron, heme proteins, or cytosolic, enzymatically active cytochrome C had been implicated in the changes observed in DCFH-DA (305). Karlsson et al. reported that the oxidation of this dye was neither dependent on Fenton-type reactions nor on unspecific enzymatic oxidation by cytochrome c, because neither superoxide nor H2O2 directly oxidizes the dye (112). Hence, the conversion of this dye might be indicative of lysosomal membrane permeabilization and the relocation of intracellular, lysosomal iron, and of mitochondrial cytochrome c rather than H2O2/ROS levels or general ‘oxidative stress’. Since doxorubicin itself is fluorescent, the analysis of any fluorescent probe should account for the background and specific wavelength of fluorescence emission of the redox dye as well as doxorubicin itself. Hence, results with DCFH-DA, especially those using fluorescent iron chelators, should be interpreted with caution. For comprehensive reviews of other redox dyes and their chemistry, the reader is referred to other publications (110, 308).
Robust quantitative measurements of protein oxidation and lipid peroxidation are still being optimized, though several assay platforms provide a good starting point (53, 147, 231). Protein carbonylation and disulfide-bridged dimer formation are two of the major types of protein oxidation that can be initiated by ROS. Protein carbonylation refers to the formation of reactive ketones or aldehydes on side chains of lysine, arginine, proline, and threonine residues; these react with 2,3-dinitrophenylhydrazine (DNPH) to generate hydrazones (DNP) (147, 231). Antibodies against DNP allow the detection of oxidized proteins via immunological methods (53, 231). Disufilde-bridge linked dimers can be formed between highly reactive cysteine residues. The oxidized protein dimers can be detected using a gel-shift assay under reducing and nonreducing conditions (210). Lipid peroxidation can be detected via the formation of lipid adducts such as malondialdehyde and 4-hydroxynonenal using mass spectrometry or antibody-based detection (213, 245).
The appearance of top cc using the immune complex with enzyme bioassay method indicates in vivo topoisomerase-directed poisoning activity (247). This method utilizes the collection of DNA-containing fractions from sarkosyl-lysed cells separated by a cesium chloride gradient. The fractions are loaded on a slot blot and probed with top1, top2α, or top2β antibody to indicate complex formation between the DNA, drug, and topoisomerase.
Two widely used assays for measuring single- or double-strand DNA breaks are the single-cell gel electrophoresis (Comet) assay and the phosphorylated histone H2AX (γ-H2AX) nuclear foci assay. The comet assay measures DNA damage as a “tail moment” or “comet” formed by the broken DNA of cells that are electrophoresed under alkaline or neutral conditions (189). Under alkaline conditions, structures in addition to single-stranded breaks such as double-strand breaks and abasic sites are also detected. The induction of γ-H2AX can be measured using antibodies which are specifically raised against the phosphorylated form of histone H2AX that are formed after DNA double-strand breaks (197). Microscopic and high-throughput formats of the assay have been developed and used for testing the DNA damaging actions of topoisomerase poisons (9, 120, 207). To measure oxidative modifications associated with DNA breaks, the comet assay has been modified to detect oxidatively clustered DNA lesions that might be informative for dual inhibitors which cause excess ROS (69). 8-oxoG oxidative DNA lesions have also been measured with antibodies or sensitive HPLC coupled with electrochemical detectors (115).
The cellular outcomes of cell cycle arrest and apoptosis have been measured as populations of cells in specific cell cycle phases or by annexin-V-positivity (124). While the activation of a noncaspase-mediated cell killing is probably suggestive of necrosis, a definitive biochemical marker of necrosis has not emerged. Studies are ongoing to define specific markers of necrosis, such as cyclophilin A, that could be utilized in drug discovery studies (29, 116). Future studies could benefit from identifying the contribution of autophagy toward resistance or sensitivity to dual inhibitors. Autophagy is a catabolic process that utilizes members of the Atg family and other proteins to engulf cellular contents for recycling. During autophagic flux, the cyctoplasmic form of LC3-I (Atg8) is lipidated and proteolysed to LC3-II during autophagosome formation. An increase in LC3-II can be measured by Western blotting and is indicative of the number of autophagosomes. When measuring autophagic flux by LC3-II, it is important to interpret the levels of LC3-II with caution because LC3-II itself is degraded during autophagy (124, 172). Hence, the levels of LC3-II should be measured in the absence and presence of lysosomal protease inhibitors such as E64d and pepstatin A (124, 210). The LC3-II, thus, measured would be indicative of autophagic flux and lysosomal turnover of LC3-II, rather than a transient increase in LC3-II (252).
Conclusions
The rapid evolution of stronger iron chelators, topoisomerase inhibitors, and a growing list of agents that target both has prompted an examination of the commonalities, opportunities, and caveats of developing dual inhibitors as anticancer drugs. Doxorubicin is a front-line drug against breast cancer, while dexrazoxane serves as a cardioprotectant that can be used with doxorubicin. Newer agents such as TSC-24 and Dp44mT and their analogs are still being explored but appear promising in preclinical models. This review presents arguments addressing the possibility for enhanced therapeutic activity through the use of agents that target both labile iron pools and elevated topoisomerase levels in cancer cells. Whether the theoretical rationale for greater cytotoxicity by dual-targeting agents translates into the clinic will have to be tested. The known mechanisms of excess ROS generation and DNA damage by these agents are highlighted as opportunities for a cytotoxic therapeutic outcome. The arguments against their use as anticancer agents include the high concentrations required for cytotoxicity, no appreciable anticancer cytotoxicity by some cardioprotective iron chelators, secondary malignancies associated with top2β activity, and decreased cytotoxicity in combination with other topoisomerase poisons. In vitro biochemical studies are needed to dissect exactly how iron binding and topoisomerase cleavage-complex formation are related in rapidly dividing cells. Gaps in knowledge are identified in the mechanisms of these agents, and where possible, avenues for pragmatic drug development studies are presented for future studies.
Abbreviations Used
- 8-oxoG
7′,8′-dihydro-8-oxoguanine
- AM
acetomethoxy derivative
- DCFH-DA
2′,7′-dichlorfluorescein-diacetate
- DHOase
dihydroorotase
- DHPase
dihydropyrimidinase
- DNP
2,3-dinitrophenylhydrazone
- DNPH
2,3-dinitrophenylhydrazine
- Dp44mT
di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone
- DpT
di-2-pyridylketone thiosemicarbazone
- DT diaphorase
NAD(P)H:quinine oxidoreductase
- EDTA
ethylenediaminetetraacetic acid
- GSH
glutathione
- GSSG
oxidized glutathione
- H2O2
hydrogen peroxide
- HE
hydroethidine
- HIF
hypoxia inducible factor
- HPLC
high performance liquid chromatography
- ICE
immune complex with enzyme
- LC3
microtubule-associated light chain protein
- mAMSA
4′[(9-acridinyl)-amino] methanesulphon-m-anisidide
- MDR
multidrug resistance
- mtDNA
mitochondria DNA
- NADH
nicotinamide adenine dinucleotide
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- Tdp
tyrosyl DNA phosphodiesterase
- TNFα
tumor necrosis factor alpha
- top1
topoisomerase 1
- top2α
topoisomerase 2 alpha
- top2β
topoisomerase 2 beta
- top cc
topoisomerase cleavage complex
- Top-DNA cc
topoisomerase DNA cleavage complex
- TSC
thiosemicarbazone
- γ-H2AX
serine 139 phosphorylated histone H2A
Acknowledgments
V.A.R. is thankful to the Food and Drug Administration Critical Path Initiative and the National Cancer Institute for research support. The author wishes to thank Drs. Emily Shacter (FDA), Eugene Herman (FDA), and Yves Pommier (NCI) for insightful discussions and encouragement. Drs. John Nittis (University of Illinois), Neil Osheroff (Vanderbilt University), and Tomas Simunek (Charles University in Prague) are acknowledged for graciously agreeing to allow an adaptation of their published figures. Drs. Jennifer Dickey (FDA) and Melanie Simpson (NCI) are thanked for their critical reading of the article.
Author Disclosure Statement
No competing financial interests exist. The opinions expressed in this article are those of the author alone and do not necessarily reflect the official position or policy of the U.S. Food and Drug Administration.
References
- 1.Abou El Hassan MA. Heijn M. Rabelink MJ. van der Vijgh WJ. Bast A. Hoeben RC. The protective effect of cardiac gene transfer of CuZn-sod in comparison with the cardioprotector monohydroxyethylrutoside against doxorubicin-induced cardiotoxicity in cultured cells. Cancer Gene Ther. 2003;10:270–277. doi: 10.1038/sj.cgt.7700564. [DOI] [PubMed] [Google Scholar]
- 2.Abou-El-Hassan MA. Rabelink MJ. van der Vijgh WJ. Bast A. Hoeben RC. A comparative study between catalase gene therapy and the cardioprotector monohydroxyethylrutoside (MonoHER) in protecting against doxorubicin-induced cardiotoxicity in vitro. Br J Cancer. 2003;89:2140–2146. doi: 10.1038/sj.bjc.6601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aisen P. Wessling-Resnick M. Leibold EA. Iron metabolism. Curr Opin Chem Biol. 1999;3:200–206. doi: 10.1016/S1367-5931(99)80033-7. [DOI] [PubMed] [Google Scholar]
- 4.Akimitsu N. Adachi N. Hirai H. Hossain MS. Hamamoto H. Kobayashi M. Aratani Y. Koyama H. Sekimizu K. Enforced cytokinesis without complete nuclear division in embryonic cells depleting the activity of DNA topoisomerase IIalpha. Genes Cells. 2003;8:393–402. doi: 10.1046/j.1365-2443.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- 5.Aluise CD. Miriyala S. Noel T. Sultana R. Jungsuwadee P. Taylor TJ. Cai J. Pierce WM. Vore M. Moscow JA. St. Clair DK. Butterfield DA. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic Biol Med. 2011;50:1630–1638. doi: 10.1016/j.freeradbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Ambroz HB. Kemp TJ. Rodger A. Przybytniak Gy. Ferric and ferrous ions: binding to DNA and influence on radiation-induced processes. Radiat Phys Chem. 2004;71:1023–1030. [Google Scholar]
- 7.Andoh T. Ishida R. Catalytic inhibitors of DNA topoisomerase II. Biochim Biophys Acta. 1998;1400:155–171. doi: 10.1016/s0167-4781(98)00133-x. [DOI] [PubMed] [Google Scholar]
- 8.Andrews NC. Levy JE. Iron is hot: an update on the pathophysiology of hemochromatosis. Blood. 1998;92:1845–1851. [PubMed] [Google Scholar]
- 9.Avondoglio D. Scott T. Kil WJ. Sproull M. Tofilon PJ. Camphausen K. High throughput evaluation of gamma-H2AX. Radiat Oncol. 2009;4:31. doi: 10.1186/1748-717X-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnabe N. Zastre JA. Venkataram S. Hasinoff BB. Deferiprone protects against doxorubicin-induced myocyte cytotoxicity. Free Radic Biol Med. 2002;33:266–275. doi: 10.1016/s0891-5849(02)00873-0. [DOI] [PubMed] [Google Scholar]
- 11.Bast A. Haenen GR. Bruynzeel AM. Van der Vijgh WJ. Protection by flavonoids against anthracycline cardiotoxicity: from chemistry to clinical trials. Cardiovasc Toxicol. 2007;7:154–159. doi: 10.1007/s12012-007-0018-0. [DOI] [PubMed] [Google Scholar]
- 12.Beckford FA. Thessing J. Shaloski M., Jr. Mbarushimana PC. Brock A. Didion J. Woods J. Gonzalez-Sarrias A. Seeram NP. Synthesis and characterization of mixed-ligand diimine-piperonal thiosemicarbazone complexes of ruthenium(II): biophysical investigations and biological evaluation as anticancer and antibacterial agents. J Mol Struct. 2011;992:39–47. doi: 10.1016/j.molstruc.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger JM. Gamblin SJ. Harrison SC. Wang JC. Structure and mechanism of DNA topoisomerase II. Nature. 1996;379:225–232. doi: 10.1038/379225a0. [DOI] [PubMed] [Google Scholar]
- 14.Besold AN. Lee SJ. Michel SL. Sue NL. Cymet HJ. Functional characterization of iron-substituted neural zinc finger factor 1: metal and DNA binding. J Biol Inorg Chem. 2010;15:583–590. doi: 10.1007/s00775-010-0626-1. [DOI] [PubMed] [Google Scholar]
- 15.Bleijlevens B. Shivarattan T. Flashman E. Yang Y. Simpson PJ. Koivisto P. Sedgwick B. Schofield CJ. Matthews SJ. Dynamic states of the DNA repair enzyme AlkB regulate product release. EMBO Rep. 2008;9:872–877. doi: 10.1038/embor.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonadonna G. Monfardini S. De Lena M. Fossati-Bellani F. Beretta G. Phase I and preliminary phase II evaluation of adriamycin (NSC 123127) Cancer Res. 1970;30:2572–2582. [PubMed] [Google Scholar]
- 17.Boonsong A. Marsh S. Rooney PH. Stevenson DA. Cassidy J. McLeod HL. Characterization of the topoisomerase I locus in human colorectal cancer. Cancer Genet Cytogenet. 2000;121:56–60. doi: 10.1016/s0165-4608(00)00242-9. [DOI] [PubMed] [Google Scholar]
- 18.Brockman RW. Thomson JR. Bell MJ. Skipper HE. Observations on the antileukemic activity of pyridine-2-carboxaldehyde thiosemicarbazone and thiocarbohydrazone. Cancer Res. 1956;16:167–170. [PubMed] [Google Scholar]
- 19.Brodie C. Siriwardana G. Lucas J. Schleicher R. Terada N. Szepesi A. Gelfand E. Seligman P. Neuroblastoma sensitivity to growth inhibition by deferrioxamine: evidence for a block in G1 phase of the cell cycle. Cancer Res. 1993;53:3968–3975. [PubMed] [Google Scholar]
- 20.Burgess DJ. Doles J. Zender L. Xue W. Ma B. McCombie WR. Hannon GJ. Lowe SW. Hemann MT. Topoisomerase levels determine chemotherapy response in vitro and in vivo. Proc Natl Acad Sci U S A. 2008;105:9053–9058. doi: 10.1073/pnas.0803513105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capranico G. Tinelli S. Austin CA. Fisher ML. Zunino F. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim Biophys Acta. 1992;1132:43–48. doi: 10.1016/0167-4781(92)90050-a. [DOI] [PubMed] [Google Scholar]
- 22.Castino R. Fiorentino I. Cagnin M. Giovia A. Isidoro C. Chelation of lysosomal iron protects dopaminergic SH-SY5Y neuroblastoma cells from hydrogen peroxide toxicity by precluding autophagy and Akt dephosphorylation. Toxicol Sci. 2011;123:523–541. doi: 10.1093/toxsci/kfr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Champoux JJ. Structure-based analysis of the effects of camptothecin on the activities of human topoisomerase I. Ann N Y Acad Sci. 2000;922:56–64. doi: 10.1111/j.1749-6632.2000.tb07025.x. [DOI] [PubMed] [Google Scholar]
- 24.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 25.Chaston TB. Richardson DR. Interactions of the pyridine-2-carboxaldehyde isonicotinoyl hydrazone class of chelators with iron and DNA: implications for toxicity in the treatment of iron overload disease. J Biol Inorg Chem. 2003;8:427–438. doi: 10.1007/s00775-002-0434-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen S. Deng PS. Bailey JM. Swiderek KM. A two-domain structure for the two subunits of NAD(P)H:quinone acceptor oxidoreductase. Protein Sci. 1994;3:51–57. doi: 10.1002/pro.5560030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheneval D. Muller M. Toni R. Ruetz S. Carafoli E. Adriamycin as a probe for the transversal distribution of cardiolipin in the inner mitochondrial membrane. J Biol Chem. 1985;260:13003–13007. [PubMed] [Google Scholar]
- 28.Choi HS. Park ES. Kang HJ. Shin HY. Noh CI. Yun YS. Ahn HS. Choi JY. Dexrazoxane for preventing anthracycline cardiotoxicity in children with solid tumors. J Korean Med Sci. 2010;25:1336–1342. doi: 10.3346/jkms.2010.25.9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christofferson DE. Yuan J. Cyclophilin A release as a biomarker of necrotic cell death. Cell Death Differ. 2010;17:1942–1943. doi: 10.1038/cdd.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conklin KA. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr Cancer. 2000;37:1–18. doi: 10.1207/S15327914NC3701_1. [DOI] [PubMed] [Google Scholar]
- 31.Conklin KA. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 32.Conte D. Narindrasorasak S. Sarkar B. In vivo and in vitro iron-replaced zinc finger generates free radicals and causes DNA damage. J Biol Chem. 1996;271:5125–5130. doi: 10.1074/jbc.271.9.5125. [DOI] [PubMed] [Google Scholar]
- 33.Coon JS. Marcus E. Gupta-Burt S. Seelig S. Jacobson K. Chen S. Renta V. Fronda G. Preisler HD. Amplification and overexpression of topoisomerase IIalpha predict response to anthracycline-based therapy in locally advanced breast cancer. Clin Cancer Res. 2002;8:1061–1067. [PubMed] [Google Scholar]
- 34.Cooper JB. McIntyre K. Badasso MO. Wood SP. Zhang Y. Garbe TR. Young D. X-ray structure analysis of the iron-dependent superoxide dismutase from Mycobacterium tuberculosis at 2.0 Angstroms resolution reveals novel dimer-dimer interactions. J Mol Biol. 1995;246:531–544. doi: 10.1006/jmbi.1994.0105. [DOI] [PubMed] [Google Scholar]
- 35.Corbett AH. Osheroff N. When good enzymes go bad: conversion of topoisomerase II to a cellular toxin by antineoplastic drugs. Chem Res Toxicol. 1993;6:585–597. doi: 10.1021/tx00035a001. [DOI] [PubMed] [Google Scholar]
- 36.Cornarotti M. Tinelli S. Willmore E. Zunino F. Fisher LM. Austin CA. Capranico G. Drug sensitivity and sequence specificity of human recombinant DNA topoisomerases IIalpha (p170) and IIbeta (p180) Mol Pharmacol. 1996;50:1463–1471. [PubMed] [Google Scholar]
- 37.Cortes Ledesma F. El Khamisy SF. Zuma MC. Osborn K. Caldecott KW. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461:674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- 38.Darnell G. Richardson DR. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents III: the effect of the ligands on molecular targets involved in proliferation. Blood. 1999;94:781–792. [PubMed] [Google Scholar]
- 39.Daroui P. Desai SD. Li TK. Liu AA. Liu LF. Hydrogen peroxide induces topoisomerase I-mediated DNA damage and cell death. J Biol Chem. 2004;279:14587–14594. doi: 10.1074/jbc.M311370200. [DOI] [PubMed] [Google Scholar]
- 40.Dauter Z. Wilson KS. Sieker LC. Moulis JM. Meyer J. Zinc- and iron-rubredoxins from Clostridium pasteurianum at atomic resolution: a high-precision model of a ZnS4 coordination unit in a protein. Proc Natl Acad Sci U S A. 1996;93:8836–8840. doi: 10.1073/pnas.93.17.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies KJ. Doroshow JH. Hochstein P. Mitochondrial NADH dehydrogenase-catalyzed oxygen radical production by adriamycin, and the relative inactivity of 5-iminodaunorubicin. FEBS Lett. 1983;153:227–230. doi: 10.1016/0014-5793(83)80153-7. [DOI] [PubMed] [Google Scholar]
- 42.Davies SL. Bergh J. Harris AL. Hickson ID. Response to ICRF-159 in cell lines resistant to cleavable complex-forming topoisomerase II inhibitors. Br J Cancer. 1997;75:816–821. doi: 10.1038/bjc.1997.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dayani PN. Bishop MC. Black K. Zeltzer PM. Desferoxamine (DFO)—mediated iron chelation: rationale for a novel approach to therapy for brain cancer. J Neurooncol. 2004;67:367–377. doi: 10.1023/b:neon.0000024238.21349.37. [DOI] [PubMed] [Google Scholar]
- 44.De Domenico I. McVey Ward D. Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 45.De Domenico I. Ward DM. Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546–4551. doi: 10.1182/blood-2009-05-224188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Silva DM. Askwith CC. Kaplan J. Molecular mechanisms of iron uptake in eukaryotes. Physiol Rev. 1996;76:31–47. doi: 10.1152/physrev.1996.76.1.31. [DOI] [PubMed] [Google Scholar]
- 47.Dean RT. Fu S. Stocker R. Davies MJ. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324(Pt 1):1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deweese JE. Burch AM. Burgin AB. Osheroff N. Use of divalent metal ions in the DNA cleavage reaction of human type II topoisomerases. Biochemistry. 2009;48:1862–1869. doi: 10.1021/bi8023256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deweese JE. Burgin AB. Osheroff N. Human topoisomerase IIalpha uses a two-metal-ion mechanism for DNA cleavage. Nucleic Acids Res. 2008;36:4883–4893. doi: 10.1093/nar/gkn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deweese JE. Guengerich FP. Burgin AB. Osheroff N. Metal ion interactions in the DNA cleavage/ligation active site of human topoisomerase IIalpha. Biochemistry. 2009;48:8940–8947. doi: 10.1021/bi900875c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deweese JE. Osheroff N. The use of divalent metal ions by type II topoisomerases. Metallomics. 2010;2:450–459. doi: 10.1039/c003759a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Leo A. Larsimont D. Gancberg D. Jarvinen T. Beauduin M. Vindevoghel A. Michel J. Focan CH. Ries F. Gobert PH. Closon-Dejardin MT. Dolci S. Rouas G. Paesmans M. Lobelle JP. Isola J. Piccart MJ. HER-2 and topo-isomerase IIalpha as predictive markers in a population of node-positive breast cancer patients randomly treated with adjuvant CMF or epirubicin plus cyclophosphamide. Ann Oncol. 2001;12:1081–1089. doi: 10.1023/a:1011669223035. [DOI] [PubMed] [Google Scholar]
- 53.Dickey JS. Rao VA. Current and proposed biomarkers of anthracycline cardiotoxicity in cancer: emerging opportunities in oxidative damage and autophagy. Curr Mol Med. 2012;12:763–771. doi: 10.2174/156652412800792561. [DOI] [PubMed] [Google Scholar]
- 54.diTargiani RC. Lee SJ. Wassink S. Michel SL. Functional characterization of iron-substituted tristetraprolin-2D (TTP-2D, NUP475-2D): RNA binding affinity and selectivity. Biochemistry. 2006;45:13641–13649. doi: 10.1021/bi060747n. [DOI] [PubMed] [Google Scholar]
- 55.Doroshow JH. Davies KJ. Comparative cardiac oxygen radical metabolism by anthracycline antibiotics, mitoxantrone, bisantrene, 4′-(9-acridinylamino)-methanesulfon-m-anisidide, and neocarzinostatin. Biochem Pharmacol. 1983;32:2935–2939. doi: 10.1016/0006-2952(83)90399-4. [DOI] [PubMed] [Google Scholar]
- 56.Doroshow JH. Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–3074. [PubMed] [Google Scholar]
- 57.Doroshow JH. Locker GY. Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980;65:128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doroshow JH. Synold TW. Somlo G. Akman SA. Gajewski E. Oxidative DNA base modifications in peripheral blood mononuclear cells of patients treated with high-dose infusional doxorubicin. Blood. 2001;97:2839–2845. doi: 10.1182/blood.v97.9.2839. [DOI] [PubMed] [Google Scholar]
- 59.Downes CS. Clarke DJ. Mullinger AM. Gimenez-Abian JF. Creighton AM. Johnson RT. A topoisomerase II-dependent G2 cycle checkpoint in mammalian cells. Nature. 1994;372:467–470. doi: 10.1038/372467a0. [DOI] [PubMed] [Google Scholar]
- 60.Durrani A. The effect of solvent on stability constant of mixed ligand complexes. Rasayan J Chem. 2011;4:554–556. [Google Scholar]
- 61.Eilers M. Endo T. Schatz G. Adriamycin, a drug interacting with acidic phospholipids, blocks import of precursor proteins by isolated yeast mitochondria. J Biol Chem. 1989;264:2945–2950. [PubMed] [Google Scholar]
- 62.Elbl L. Hrstkova H. Tomaskova I. Michalek J. Late anthracycline cardiotoxicity protection by dexrazoxane (ICRF-187) in pediatric patients: echocardiographic follow-up. Support Care Cancer. 2006;14:128–136. doi: 10.1007/s00520-005-0858-8. [DOI] [PubMed] [Google Scholar]
- 63.Ewer MS. Von Hoff DD. Benjamin RS. A historical perspective of anthracycline cardiotoxicity. Heart Fail Clin. 2011;7:363–372. doi: 10.1016/j.hfc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Fan JR. Peng AL. Chen HC. Lo SC. Huang TH. Li TK. Cellular processing pathways contribute to the activation of etoposide-induced DNA damage responses. DNA Repair (Amst) 2008;7:452–463. doi: 10.1016/j.dnarep.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Faulk WP. Hsi BL. Stevens PJ. Transferrin and transferrin receptors in carcinoma of the breast. Lancet. 1980;2:390–392. doi: 10.1016/s0140-6736(80)90440-7. [DOI] [PubMed] [Google Scholar]
- 66.Ferrans VJ. Clark JR. Zhang J. Yu ZX. Herman EH. Pathogenesis and prevention of doxorubicin cardiomyopathy. Tsitologiia. 1997;39:928–937. [PubMed] [Google Scholar]
- 67.Fu S. Davies MJ. Stocker R. Dean RT. Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem J. 1998;333(Pt 3):519–525. doi: 10.1042/bj3330519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gazak R. Svobodova A. Psotova J. Sedmera P. Prikrylova V. Walterova D. Kren V. Oxidised derivatives of silybin and their antiradical and antioxidant activity. Bioorg Med Chem. 2004;12:5677–5687. doi: 10.1016/j.bmc.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 69.Georgakilas AG. Panayiotidis M. Biomarkers of oxidative stress and cancer. from chemistry, biology to clinical applications and personalized therapy. Curr Mol Med. 2012;12:653–654. doi: 10.2174/156652412800792552. [DOI] [PubMed] [Google Scholar]
- 70.Germe T. Hyrien O. Topoisomerase II-DNA complexes trapped by ICRF-193 perturb chromatin structure. EMBO Rep. 2005;6:729–735. doi: 10.1038/sj.embor.7400465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gervasi PG. Agrillo MR. Lippi A. Bernardini N. Danesi R. Del Tacca M. Superoxide anion production by doxorubicin analogs in heart sarcosomes and by mitochondrial NADH dehydrogenase. Res Commun Chem Pathol Pharmacol. 1990;67:101–115. [PubMed] [Google Scholar]
- 72.Gomez HL. Pinto JA. Olivera M. Vidaurre T. Doimi FD. Vigil CE. Velarde RG. Abugattas JE. Alarcon E. Vallejos CS. Topoisomerase II-alpha as a predictive factor of response to therapy with anthracyclines in locally advanced breast cancer. Breast. 2011;20:39–45. doi: 10.1016/j.breast.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 73.Goodman J. Hochstein P. Generation of free radicals and lipid peroxidation by redox cycling of adriamycin and daunomycin. Biochem Biophys Res Commun. 1977;77:797–803. doi: 10.1016/s0006-291x(77)80048-x. [DOI] [PubMed] [Google Scholar]
- 74.Goormaghtigh E. Huart P. Brasseur R. Ruysschaert JM. Mechanism of inhibition of mitochondrial enzymatic complex I-III by adriamycin derivatives. Biochim Biophys Acta. 1986;861:83–94. doi: 10.1016/0005-2736(86)90374-3. [DOI] [PubMed] [Google Scholar]
- 75.Goormaghtigh E. Huart P. Praet M. Brasseur R. Ruysschaert JM. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys Chem. 1990;35:247–257. doi: 10.1016/0301-4622(90)80012-v. [DOI] [PubMed] [Google Scholar]
- 76.Grauslund M. Thougaard AV. Fuchtbauer A. Hofland KF. Hjorth PH. Jensen PB. Sehested M. Fuchtbauer EM. Jensen LH. A mouse model for studying the interaction of bisdioxopiperazines with topoisomerase IIalpha in vivo. Mol Pharmacol. 2007;72:1003–1014. doi: 10.1124/mol.107.036970. [DOI] [PubMed] [Google Scholar]
- 77.Halliwell B. Gutteridge JM. Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-y. [DOI] [PubMed] [Google Scholar]
- 78.Hasinoff BB. Chemistry of dexrazoxane and analogues. Semin Oncol. 1998;25:3–9. [PubMed] [Google Scholar]
- 79.Hasinoff BB. Abram ME. Barnabe N. Khelifa T. Allan WP. Yalowich JC. The catalytic DNA topoisomerase II inhibitor dexrazoxane (ICRF-187) induces differentiation and apoptosis in human leukemia K562 cells. Mol Pharmacol. 2001;59:453–461. doi: 10.1124/mol.59.3.453. [DOI] [PubMed] [Google Scholar]
- 80.Hasinoff BB. Hellmann K. Herman EH. Ferrans VJ. Chemical, biological and clinical aspects of dexrazoxane and other bisdioxopiperazines. Curr Med Chem. 1998;5:1–28. [PubMed] [Google Scholar]
- 81.Hasinoff BB. Herman EH. Dexrazoxane: how it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc Toxicol. 2007;7:140–144. doi: 10.1007/s12012-007-0023-3. [DOI] [PubMed] [Google Scholar]
- 82.Hasinoff BB. Kuschak TI. Yalowich JC. Creighton AM. A QSAR study comparing the cytotoxicity and DNA topoisomerase II inhibitory effects of bisdioxopiperazine analogs of ICRF-187 (dexrazoxane) Biochem Pharmacol. 1995;50:953–958. doi: 10.1016/0006-2952(95)00218-o. [DOI] [PubMed] [Google Scholar]
- 83.Hasinoff BB. Patel D. The iron chelator Dp44mT does not protect myocytes against doxorubicin. J Inorg Biochem. 2009;103:1093–1101. doi: 10.1016/j.jinorgbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 84.Hasinoff BB. Schnabl KL. Marusak RA. Patel D. Huebner E. Dexrazoxane (ICRF-187) protects cardiac myocytes against doxorubicin by preventing damage to mitochondria. Cardiovasc Toxicol. 2003;3:89–99. doi: 10.1385/ct:3:2:89. [DOI] [PubMed] [Google Scholar]
- 85.Hasinoff BB. Tran KT. The displacement of iron(III) from its complexes with the anticancer drugs piroxantrone and losoxantrone by the hydrolyzed form of the cardioprotective agent dexrazoxane. J Inorg Biochem. 1999;77:257–259. doi: 10.1016/s0162-0134(99)00194-4. [DOI] [PubMed] [Google Scholar]
- 86.Hatcher HC. Singh RN. Torti FM. Torti SV. Synthetic and natural iron chelators: therapeutic potential and clinical use. Future Med Chem. 2009;1:1643–1670. doi: 10.4155/fmc.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henichart JP. Waring MJ. Riou JF. Denny WA. Bailly C. Copper-dependent oxidative and topoisomerase II-mediated DNA cleavage by a netropsin/4′-(9-acridinylamino)methanesulfon-m-anisidide combilexin. Mol Pharmacol. 1997;51:448–461. [PubMed] [Google Scholar]
- 88.Herman EH. el-Hage A. Ferrans VJ. Protective effect of ICRF-187 on doxorubicin-induced cardiac and renal toxicity in spontaneously hypertensive (SHR) and normotensive (WKY) rats. Toxicol Appl Pharmacol. 1988;92:42–53. doi: 10.1016/0041-008x(88)90226-8. [DOI] [PubMed] [Google Scholar]
- 89.Herman EH. el-Hage AN. Creighton AM. Witiak DT. Ferrans VJ. Comparison of the protective effect of ICRF-187 and structurally related analogues against acute daunorubicin toxicity in Syrian golden hamsters. Res Commun Chem Pathol Pharmacol. 1985;48:39–55. [PubMed] [Google Scholar]
- 90.Herman EH. Ferrans VJ. Timing of treatment with ICRF-187 and its effect on chronic doxorubicin cardiotoxicity. Cancer Chemother Pharmacol. 1993;32:445–449. doi: 10.1007/BF00685888. [DOI] [PubMed] [Google Scholar]
- 91.Herman EH. Ferrans VJ. Preclinical animal models of cardiac protection from anthracycline-induced cardiotoxicity. Semin Oncol. 1998;25:15–21. [PubMed] [Google Scholar]
- 92.Herman EH. Ferrans VJ. Young RS. Hamlin RL. Pretreatment with ICRF-187 allows a marked increase in the total cumulative dose of doxorubicin tolerated by beagle dogs. Drugs Exp Clin Res. 1988;14:563–570. [PubMed] [Google Scholar]
- 93.Herman EH. Zhang J. Chadwick DP. Ferrans VJ. Comparison of the protective effects of amifostine and dexrazoxane against the toxicity of doxorubicin in spontaneously hypertensive rats. Cancer Chemother Pharmacol. 2000;45:329–334. doi: 10.1007/s002800050048. [DOI] [PubMed] [Google Scholar]
- 94.Hershko C. Pinson A. Link G. Prevention of anthracycline cardiotoxicity by iron chelation. Acta Haematol. 1996;95:87–92. doi: 10.1159/000203954. [DOI] [PubMed] [Google Scholar]
- 95.Hoke EM. Maylock CA. Shacter E. Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radic Biol Med. 2005;39:403–411. doi: 10.1016/j.freeradbiomed.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 96.Huang H. Chen Q. Ku X. Meng L. Lin L. Wang X. Zhu C. Wang Y. Chen Z. Li M. Jiang H. Chen K. Ding J. Liu H. A series of alpha-heterocyclic carboxaldehyde thiosemicarbazones inhibit topoisomerase IIalpha catalytic activity. J Med Chem. 2010;53:3048–3064. doi: 10.1021/jm9014394. [DOI] [PubMed] [Google Scholar]
- 97.Huang KC. Gao H. Yamasaki EF. Grabowski DR. Liu S. Shen LL. Chan KK. Ganapathi R. Snapka RM. Topoisomerase II poisoning by ICRF-193. J Biol Chem. 2001;276:44488–44494. doi: 10.1074/jbc.M104383200. [DOI] [PubMed] [Google Scholar]
- 98.Hutcheson RM. Engelmann MD. Cheng IF. Voltammetric studies of Zn and Fe complexes of EDTA: evidence for the push mechanism. Biometals. 2005;18:43–51. doi: 10.1007/s10534-004-5769-5. [DOI] [PubMed] [Google Scholar]
- 99.Isaacs RJ. Davies SL. Sandri MI. Redwood C. Wells NJ. Hickson ID. Physiological regulation of eukaryotic topoisomerase II. Biochim Biophys Acta. 1998;1400:121–137. doi: 10.1016/s0167-4781(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 100.Ishida R. Miki T. Narita T. Yui R. Sato M. Utsumi KR. Tanabe K. Andoh T. Inhibition of intracellular topoisomerase II by antitumor bis(2,6-dioxopiperazine) derivatives: mode of cell growth inhibition distinct from that of cleavable complex-forming type inhibitors. Cancer Res. 1991;51:4909–4916. [PubMed] [Google Scholar]
- 101.Ishida R. Sato M. Narita T. Utsumi KR. Nishimoto T. Morita T. Nagata H. Andoh T. Inhibition of DNA topoisomerase II by ICRF-193 induces polyploidization by uncoupling chromosome dynamics from other cell cycle events. J Cell Biol. 1994;126:1341–1351. doi: 10.1083/jcb.126.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jacobs A. Serum ferritin. Birth Defects Orig Artic Ser. 1976;12:97–103. [PubMed] [Google Scholar]
- 103.Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977;50:433–439. [PubMed] [Google Scholar]
- 104.Jacobs A. Serum ferritin and iron stores. Fed Proc. 1977;36:2024–2027. [PubMed] [Google Scholar]
- 105.Jansson PJ. Hawkins CL. Lovejoy DB. Richardson DR. The iron complex of Dp44mT is redox-active and induces hydroxyl radical formation: an EPR study. J Inorg Biochem. 2010;104:1224–1228. doi: 10.1016/j.jinorgbio.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 106.Jomova K. Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 107.Joyner JC. Reichfield J. Cowan JA. Factors influencing the DNA nuclease activity of iron, cobalt, nickel, and copper chelates. J Am Chem Soc. 2011;133:15613–15626. doi: 10.1021/ja2052599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kalinowski DS. Quach P. Richardson DR. Thiosemicarbazones: the new wave in cancer treatment. Future Med Chem. 2009;1:1143–1151. doi: 10.4155/fmc.09.80. [DOI] [PubMed] [Google Scholar]
- 109.Kalyanaraman B. Oxidative chemistry of fluorescent dyes: implications in the detection of reactive oxygen and nitrogen species. Biochem Soc Trans. 2011;39:1221–1225. doi: 10.1042/BST0391221. [DOI] [PubMed] [Google Scholar]
- 110.Kalyanaraman B. Darley-Usmar V. Davies KJ. Dennery PA. Forman HJ. Grisham MB. Mann GE. Moore K. Roberts LJ., 2nd Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kanter MM. Hamlin RL. Unverferth DV. Davis HW. Merola AJ. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol. 1985;59:1298–1303. doi: 10.1152/jappl.1985.59.4.1298. [DOI] [PubMed] [Google Scholar]
- 112.Karlsson M. Kurz T. Brunk UT. Nilsson SE. Frennesson CI. What does the commonly used DCF test for oxidative stress really show? Biochem J. 2010;428:183–190. doi: 10.1042/BJ20100208. [DOI] [PubMed] [Google Scholar]
- 113.Kaur M. Velmurugan B. Tyagi A. Agarwal C. Singh RP. Agarwal R. Silibinin suppresses growth of human colorectal carcinoma SW480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling. Neoplasia. 2010;12:415–424. doi: 10.1593/neo.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaur M. Velmurugan B. Tyagi A. Deep G. Katiyar S. Agarwal C. Agarwal R. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma LoVo cells in culture and tumor xenograft. Mol Cancer Ther. 2009;8:2366–2374. doi: 10.1158/1535-7163.MCT-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kennedy LJ. Moore K., Jr. Caulfield JL. Tannenbaum SR. Dedon PC. Quantitation of 8-oxoguanine and strand breaks produced by four oxidizing agents. Chem Res Toxicol. 1997;10:386–392. doi: 10.1021/tx960102w. [DOI] [PubMed] [Google Scholar]
- 116.Kepp O. Galluzzi L. Lipinski M. Yuan J. Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov. 2011;10:221–237. doi: 10.1038/nrd3373. [DOI] [PubMed] [Google Scholar]
- 117.Kersting G. Tzvetkov MV. Huse K. Kulle B. Hafner V. Brockmoller J. Wojnowski L. Topoisomerase II beta expression level correlates with doxorubicin-induced apoptosis in peripheral blood cells. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:21–30. doi: 10.1007/s00210-006-0091-0. [DOI] [PubMed] [Google Scholar]
- 118.Keyer K. Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci U S A. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim R. Hirabayashi N. Nishiyama M. Jinushi K. Toge T. Okada K. Experimental studies on biochemical modulation targeting topoisomerase I and II in human tumor xenografts in nude mice. Int J Cancer. 1992;50:760–766. doi: 10.1002/ijc.2910500516. [DOI] [PubMed] [Google Scholar]
- 120.Kinders RJ. Hollingshead M. Lawrence S. Ji J. Tabb B. Bonner WM. Pommier Y. Rubinstein L. Evrard YA. Parchment RE. Tomaszewski J. Doroshow JH. Development of a validated immunofluorescence assay for gammaH2AX as a pharmacodynamic marker of topoisomerase I inhibitor activity. Clin Cancer Res. 2010;16:5447–5457. doi: 10.1158/1078-0432.CCR-09-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Klamt F. Shacter E. Taurine chloramine, an oxidant derived from neutrophils, induces apoptosis in human B lymphoma cells through mitochondrial damage. J Biol Chem. 2005;280:21346–21352. doi: 10.1074/jbc.M501170200. [DOI] [PubMed] [Google Scholar]
- 122.Klayman DL. Bartosevich JF. Griffin TS. Mason CJ. Scovill JP. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J Med Chem. 1979;22:855–862. doi: 10.1021/jm00193a020. [DOI] [PubMed] [Google Scholar]
- 123.Klayman DL. Scovill JP. Mason CJ. Bartosevich JF. Bruce J. Lin AJ. 2-Acetylpyridine thiosemicarbazones. 6.2-Acetylpyridine and 2-butyrylpyridine thiosemicarbazones as antileukemic agents. Arzneimittelforschung. 1983;33:909–912. doi: 10.1002/chin.198344210. [DOI] [PubMed] [Google Scholar]
- 124.Klionsky DJ. Abeliovich H. Agostinis P. Agrawal DK. Aliev G. Askew DS. Baba M. Baehrecke EH. Bahr BA. Ballabio A. Bamber BA. Bassham DC. Bergamini E. Bi X. Biard-Piechaczyk M. Blum JS. Bredesen DE. Brodsky JL. Brumell JH. Brunk UT. Bursch W. Camougrand N. Cebollero E. Cecconi F. Chen Y. Chin LS. Choi A. Chu CT. Chung J. Clarke PG. Clark RS. Clarke SG. Clave C. Cleveland JL. Codogno P. Colombo MI. Coto-Montes A. Cregg JM. Cuervo AM. Debnath J. Demarchi F. Dennis PB. Dennis PA. Deretic V. Devenish RJ. Di Sano F. Dice JF. Difiglia M. Dinesh-Kumar S. Distelhorst CW. Djavaheri-Mergny M. Dorsey FC. Droge W. Dron M. Dunn WA., Jr. Duszenko M. Eissa NT. Elazar Z. Esclatine A. Eskelinen EL. Fesus L. Finley KD. Fuentes JM. Fueyo J. Fujisaki K. Galliot B. Gao FB. Gewirtz DA. Gibson SB. Gohla A. Goldberg AL. Gonzalez R. Gonzalez-Estevez C. Gorski S. Gottlieb RA. Haussinger D. He YW. Heidenreich K. Hill JA. Hoyer-Hansen M. Hu X. Huang WP. Iwasaki A. Jaattela M. Jackson WT. Jiang X. Jin S. Johansen T. Jung JU. Kadowaki M. Kang C. Kelekar A. Kessel DH. Kiel JA. Kim HP. Kimchi A. Kinsella TJ. Kiselyov K. Kitamoto K. Knecht E. Komatsu M. Kominami E. Kondo S. Kovacs AL. Kroemer G. Kuan CY. Kumar R. Kundu M. Landry J. Laporte M. Le W. Lei HY. Lenardo MJ. Levine B. Lieberman A. Lim KL. Lin FC. Liou W. Liu LF. Lopez-Berestein G. Lopez-Otin C. Lu B. Macleod KF. Malorni W. Martinet W. Matsuoka K. Mautner J. Meijer AJ. Melendez A. Michels P. Miotto G. Mistiaen WP. Mizushima N. Mograbi B. Monastyrska I. Moore MN. Moreira PI. Moriyasu Y. Motyl T. Munz C. Murphy LO. Naqvi NI. Neufeld TP. Nishino I. Nixon RA. Noda T. Nurnberg B. Ogawa M. Oleinick NL. Olsen LJ. Ozpolat B. Paglin S. Palmer GE. Papassideri I. Parkes M. Perlmutter DH. Perry G. Piacentini M. Pinkas-Kramarski R. Prescott M. Proikas-Cezanne T. Raben N. Rami A. Reggiori F. Rohrer B. Rubinsztein DC. Ryan KM. Sadoshima J. Sakagami H. Sakai Y. Sandri M. Sasakawa C. Sass M. Schneider C. Seglen PO. Seleverstov O. Settleman J. Shacka JJ. Shapiro IM. Sibirny A. Silva-Zacarin EC. Simon HU. Simone C. Simonsen A. Smith MA. Spanel-Borowski K. Srinivas V. Steeves M. Stenmark H. Stromhaug PE. Subauste CS. Sugimoto S. Sulzer D. Suzuki T. Swanson MS. Tabas I. Takeshita F. Talbot NJ. Talloczy Z. Tanaka K. Tanaka K. Tanida I. Taylor GS. Taylor JP. Terman A. Tettamanti G. Thompson CB. Thumm M. Tolkovsky AM. Tooze SA. Truant R. Tumanovska LV. Uchiyama Y. Ueno T. Uzcategui NL. van der Klei I. Vaquero EC. Vellai T. Vogel MW. Wang HG. Webster P. Wiley JW. Xi Z. Xiao G. Yahalom J. Yang JM. Yap G. Yin XM. Yoshimori T. Yu L. Yue Z. Yuzaki M. Zabirnyk O. Zheng X. Zhu X. Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kobayashi S. Volden P. Timm D. Mao K. Xu X. Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem. 2010;285:793–804. doi: 10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kobayashi T. Guo LL. Nishida Y. Mechanism of double-strand DNA cleavage effected by iron-bleomycin. Z Naturforsch C. 1998;53:867–870. doi: 10.1515/znc-1998-9-1014. [DOI] [PubMed] [Google Scholar]
- 127.Kohn KW. Grimek-Ewig RA. Alkaline elution analysis, a new approach to the study of DNA single-strand interruptions in cells. Cancer Res. 1973;33:1849–1853. [PubMed] [Google Scholar]
- 128.Kohn KW. Pommier Y. Molecular and biological determinants of the cytotoxic actions of camptothecins. Perspective for the development of new topoisomerase I inhibitors. Ann N Y Acad Sci. 2000;922:11–26. doi: 10.1111/j.1749-6632.2000.tb07021.x. [DOI] [PubMed] [Google Scholar]
- 129.Kohn KW. Shao RG. Pommier Y. How do drug-induced topoisomerase I-DNA lesions signal to the molecular interaction network that regulates cell cycle checkpoints, DNA replication, and DNA repair? Cell Biochem Biophys. 2000;33:175–180. doi: 10.1385/CBB:33:2:175. [DOI] [PubMed] [Google Scholar]
- 130.Kondapi AK. Satyanarayana N. Saikrishna AD. A study of the topoisomerase II activity in HIV-1 replication using the ferrocene derivatives as probes. Arch Biochem Biophys. 2006;450:123–132. doi: 10.1016/j.abb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 131.Kovacevic Z. Kalinowski DS. Lovejoy DB. Quach P. Wong J. Richardson DR. Iron chelators: development of novel compounds with high and selective anti-tumour activity. Curr Drug Deliv. 2010;7:194–207. doi: 10.2174/156720110791560991. [DOI] [PubMed] [Google Scholar]
- 132.Kovacevic Z. Kalinowski DS. Lovejoy DB. Yu Y. Suryo Rahmanto Y. Sharpe PC. Bernhardt PV. Richardson DR. The medicinal chemistry of novel iron chelators for the treatment of cancer. Curr Top Med Chem. 2011;11:483–499. doi: 10.2174/156802611794785190. [DOI] [PubMed] [Google Scholar]
- 133.Kovacs GT. Erlaky H. Toth K. Horvath E. Szabolcs J. Csoka M. Jokuti L. Erdelyi D. Muller J. Subacute cardiotoxicity caused by anthracycline therapy in children: can dexrazoxane prevent this effect? Eur J Pediatr. 2007;166:1187–1188. doi: 10.1007/s00431-006-0370-2. [DOI] [PubMed] [Google Scholar]
- 134.Kurz T. Brunk UT. Autophagy of HSP70 and chelation of lysosomal iron in a non-redox-active form. Autophagy. 2009;5:93–95. doi: 10.4161/auto.5.1.7248. [DOI] [PubMed] [Google Scholar]
- 135.Kushner BH. Kramer K. Cheung NK. Oral etoposide for refractory and relapsed neuroblastoma. J Clin Oncol. 1999;17:3221–3225. doi: 10.1200/JCO.1999.17.10.3221. [DOI] [PubMed] [Google Scholar]
- 136.Kwan KY. Wang JC. Mice lacking DNA topoisomerase IIIbeta develop to maturity but show a reduced mean lifespan. Proc Natl Acad Sci U S A. 2001;98:5717–5721. doi: 10.1073/pnas.101132498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lai HW. Chien SY. Kuo SJ. Tseng LM. Lin HY. Chi CW. Chen DR. The potential utility of curcumin in the treatment of HER-2-overexpressed breast cancer: an in vitro and in vivo comparison study with herceptin. Evid Based Complement Alternat Med. 2012:486568. doi: 10.1155/2012/486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lang AJ. Mirski SE. Cummings HJ. Yu Q. Gerlach JH. Cole SP. Structural organization of the human TOP2A and TOP2B genes. Gene. 1998;221:255–266. doi: 10.1016/s0378-1119(98)00468-5. [DOI] [PubMed] [Google Scholar]
- 139.Larsen AK. Escargueil AE. Skladanowski A. Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol Ther. 2003;99:167–181. doi: 10.1016/s0163-7258(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 140.Larsen AK. Skladanowski A. Bojanowski K. The roles of DNA topoisomerase II during the cell cycle. Prog Cell Cycle Res. 1996;2:229–239. doi: 10.1007/978-1-4615-5873-6_22. [DOI] [PubMed] [Google Scholar]
- 141.Lebrecht D. Setzer B. Ketelsen UP. Haberstroh J. Walker UA. Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation. 2003;108:2423–2429. doi: 10.1161/01.CIR.0000093196.59829.DF. [DOI] [PubMed] [Google Scholar]
- 142.Lebrecht D. Walker UA. Role of mtDNA lesions in anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7:108–113. doi: 10.1007/s12012-007-0009-1. [DOI] [PubMed] [Google Scholar]
- 143.Lee S. Jung SR. Heo K. Byl JA. Deweese JE. Osheroff N. Hohng S. DNA cleavage and opening reactions of human topoisomerase IIalpha are regulated via Mg2+-mediated dynamic bending of gate-DNA. Proc Natl Acad Sci U S A. 2012;109:2925–2930. doi: 10.1073/pnas.1115704109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lefrak EA. Pitha J. Rosenheim S. Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 145.Lemasters JJ, editor; Nieminen AL, editor. Mitochondria in Pathogenesis. New York, NY: Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- 146.Lesko SA. Lorentzen RJ. Ts'o PO. Role of superoxide in deoxyribonucleic acid strand scission. Biochemistry. 1980;19:3023–3028. doi: 10.1021/bi00554a029. [DOI] [PubMed] [Google Scholar]
- 147.Levine RL. Wehr N. Williams JA. Stadtman ER. Shacter E. Determination of carbonyl groups in oxidized proteins. Methods Mol Biol. 2000;99:15–24. doi: 10.1385/1-59259-054-3:15. [DOI] [PubMed] [Google Scholar]
- 148.Li TK. Chen AY. Yu C. Mao Y. Wang H. Liu LF. Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidative stress. Genes Dev. 1999;13:1553–1560. doi: 10.1101/gad.13.12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li W. Wang JC. Mammalian DNA topoisomerase IIIalpha is essential in early embryogenesis. Proc Natl Acad Sci U S A. 1998;95:1010–1013. doi: 10.1073/pnas.95.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lipshultz SE. Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28:1276–1281. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 151.Lipshultz SE. Cohen H. Colan SD. Herman EH. The relevance of information generated by in vitro experimental models to clinical doxorubicin cardiotoxicity. Leuk Lymphoma. 2006;47:1454–1458. doi: 10.1080/10428190600800231. [DOI] [PubMed] [Google Scholar]
- 152.Lipshultz SE. Rifai N. Dalton VM. Levy DE. Silverman LB. Lipsitz SR. Colan SD. Asselin BL. Barr RD. Clavell LA. Hurwitz CA. Moghrabi A. Samson Y. Schorin MA. Gelber RD. Sallan SE. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 153.Lipshultz SE. Scully RE. Lipsitz SR. Sallan SE. Silverman LB. Miller TL. Barry EV. Asselin BL. Athale U. Clavell LA. Larsen E. Moghrabi A. Samson Y. Michon B. Schorin MA. Cohen HJ. Neuberg DS. Orav EJ. Colan SD. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol. 2010;11:950–961. doi: 10.1016/S1470-2045(10)70204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu IF. Annamalai T. Sutherland JH. Tse-Dinh YC. Hydroxyl radicals are involved in cell killing by the bacterial topoisomerase I cleavage complex. J Bacteriol. 2009;191:5315–5319. doi: 10.1128/JB.00559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Long BH. Wang L. Lorico A. Wang RC. Brattain MG. Casazza AM. Mechanisms of resistance to etoposide and teniposide in acquired resistant human colon and lung carcinoma cell lines. Cancer Res. 1991;51:5275–5283. [PubMed] [Google Scholar]
- 156.Lovejoy DB. Jansson PJ. Brunk UT. Wong J. Ponka P. Richardson DR. Antitumor activity of metal-chelating compound Dp44mT is mediated by formation of a redox-active copper complex that accumulates in lysosomes. Cancer Res. 2011;71:5871–5880. doi: 10.1158/0008-5472.CAN-11-1218. [DOI] [PubMed] [Google Scholar]
- 157.Lovett BD. Lo Nigro L. Rappaport EF. Blair IA. Osheroff N. Zheng N. Megonigal MD. Williams WR. Nowell PC. Felix CA. Near-precise interchromosomal recombination and functional DNA topoisomerase II cleavage sites at MLL and AF-4 genomic breakpoints in treatment-related acute lymphoblastic leukemia with t(4;11) translocation. Proc Natl Acad Sci U S A. 2001;98:9802–9807. doi: 10.1073/pnas.171309898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lovett BD. Strumberg D. Blair IA. Pang S. Burden DA. Megonigal MD. Rappaport EF. Rebbeck TR. Osheroff N. Pommier YG. Felix CA. Etoposide metabolites enhance DNA topoisomerase II cleavage near leukemia-associated MLL translocation breakpoints. Biochemistry. 2001;40:1159–1170. doi: 10.1021/bi002361x. [DOI] [PubMed] [Google Scholar]
- 159.Lu DY. Huang M. Xu CH. Zhu H. Xu B. Ding J. Medicinal chemistry of probimane and MST-16: comparison of anticancer effects between bisdioxopiperazines. Med Chem. 2006;2:369–375. doi: 10.2174/157340606777724095. [DOI] [PubMed] [Google Scholar]
- 160.Lu DY. Xu B. Ding J. Antitumor effects of two bisdioxopiperazines against two experimental lung cancer models in vivo. BMC Pharmacol. 2004;4:32. doi: 10.1186/1471-2210-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lu J. Wang W. Tan G. Landry AP. Yi P. Si F. Ren Y. Ding H. Escherichia coli topoisomerase I is an iron and zinc binding protein. Biometals. 2011;24:729–736. doi: 10.1007/s10534-011-9425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lukianova OA. David SS. A role for iron-sulfur clusters in DNA repair. Curr Opin Chem Biol. 2005;9:145–151. doi: 10.1016/j.cbpa.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 163.Lyu YL. Kerrigan JE. Lin CP. Azarova AM. Tsai YC. Ban Y. Liu LF. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67:8839–8846. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 164.Lyu YL. Lin CP. Azarova AM. Cai L. Wang JC. Liu LF. Role of topoisomerase IIbeta in the expression of developmentally regulated genes. Mol Cell Biol. 2006;26:7929–7941. doi: 10.1128/MCB.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lyu YL. Wang JC. Aberrant lamination in the cerebral cortex of mouse embryos lacking DNA topoisomerase IIbeta. Proc Natl Acad Sci U S A. 2003;100:7123–7128. doi: 10.1073/pnas.1232376100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Malik M. Nitiss JL. DNA repair functions that control sensitivity to topoisomerase-targeting drugs. Eukaryot Cell. 2004;3:82–90. doi: 10.1128/EC.3.1.82-90.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mao Y. Desai SD. Ting CY. Hwang J. Liu LF. 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J Biol Chem. 2001;276:40652–40658. doi: 10.1074/jbc.M104009200. [DOI] [PubMed] [Google Scholar]
- 168.Martell AE, editor; Smith RM, editor. Critical Stability Constants. New York: Plenum Press; 1989. [Google Scholar]
- 169.Meier B. Scherk C. Schmidt M. Parak F. pH-dependent inhibition by azide and fluoride of the iron superoxide dismutase from Propionibacterium shermanii. Biochem J. 1998;331(Pt 2):403–407. doi: 10.1042/bj3310403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Miller LD. Coffman LG. Chou JW. Black MA. Bergh J. D'Agostino R., Jr. Torti SV. Torti FM. An iron regulatory gene signature predicts outcome in breast cancer. Cancer Res. 2011;71:6728–6737. doi: 10.1158/0008-5472.CAN-11-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Miller MC., 3rd Stineman CN. Vance JR. West DX. Hall IH. The cytotoxicity of copper(II) complexes of 2-acetyl-pyridyl-4N-substituted thiosemicarbazones. Anticancer Res. 1998;18:4131–4139. [PubMed] [Google Scholar]
- 172.Mizushima N. Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 173.Mordente A. Martorana GE. Meucci E. Santini SA. Littarru GP. Enzyme inactivation by metal-catalyzed oxidation of coenzyme Q1. Biochim Biophys Acta. 1992;1100:235–241. doi: 10.1016/0167-4838(92)90477-u. [DOI] [PubMed] [Google Scholar]
- 174.Mordente A. Miggiano GA. Martorana GE. Meucci E. Santini SA. Castelli A. Alkaline phosphatase inactivation by mixed function oxidation systems. Arch Biochem Biophys. 1987;258:176–185. doi: 10.1016/0003-9861(87)90334-1. [DOI] [PubMed] [Google Scholar]
- 175.Morgan SE. Cadena RS. Raimondi SC. Beck WT. Selection of human leukemic CEM cells for resistance to the DNA topoisomerase II catalytic inhibitor ICRF-187 results in increased levels of topoisomerase IIalpha and altered G(2)/M checkpoint and apoptotic responses. Mol Pharmacol. 2000;57:296–307. [PubMed] [Google Scholar]
- 176.Morham SG. Kluckman KD. Voulomanos N. Smithies O. Targeted disruption of the mouse topoisomerase I gene by camptothecin selection. Mol Cell Biol. 1996;16:6804–6809. doi: 10.1128/mcb.16.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Muller I. Niethammer D. Bruchelt G. Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity (Review) Int J Mol Med. 1998;1:491–494. doi: 10.3892/ijmm.1.2.491. [DOI] [PubMed] [Google Scholar]
- 178.Munoz IG. Moran JF. Becana M. Montoya G. The crystal structure of an eukaryotic iron superoxide dismutase suggests intersubunit cooperation during catalysis. Protein Sci. 2005;14:387–394. doi: 10.1110/ps.04979505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Murren J. Modiano M. Clairmont C. Lambert P. Savaraj N. Doyle T. Sznol M. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clin Cancer Res. 2003;9:4092–4100. [PubMed] [Google Scholar]
- 180.Myers CE. Gianni L. Simone CB. Klecker R. Greene R. Oxidative destruction of erythrocyte ghost membranes catalyzed by the doxorubicin-iron complex. Biochemistry. 1982;21:1707–1712. doi: 10.1021/bi00537a001. [DOI] [PubMed] [Google Scholar]
- 181.Myers CE. McGuire W. Young R. Adriamycin: amelioration of toxicity by alpha-tocopherol. Cancer Treat Rep. 1976;60:961–962. [PubMed] [Google Scholar]
- 182.Myers CE. McGuire WP. Liss RH. Ifrim I. Grotzinger K. Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977;197:165–167. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- 183.Nikolenyi A. Sukosd F. Kaizer L. Csorgo E. Voros A. Uhercsak G. Ormandi K. Lazar G. Thurzo L. Brodowicz T. Kahan Z. Tumor topoisomerase II alpha status and response to anthracycline-based neoadjuvant chemotherapy in breast cancer. Oncology. 2011;80:269–277. doi: 10.1159/000329038. [DOI] [PubMed] [Google Scholar]
- 184.Nitiss JL. Investigating the biological functions of DNA topoisomerases in eukaryotic cells. Biochim Biophys Acta. 1998;1400:63–81. doi: 10.1016/s0167-4781(98)00128-6. [DOI] [PubMed] [Google Scholar]
- 185.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Nohl H. Demonstration of the existence of an organo-specific NADH dehydrogenase in heart mitochondria. Eur J Biochem. 1987;169:585–591. doi: 10.1111/j.1432-1033.1987.tb13649.x. [DOI] [PubMed] [Google Scholar]
- 187.Nohl H. Identification of the site of adriamycin-activation in the heart cell. Biochem Pharmacol. 1988;37:2633–2637. doi: 10.1016/0006-2952(88)90257-2. [DOI] [PubMed] [Google Scholar]
- 188.Noulsri E. Richardson DR. Lerdwana S. Fucharoen S. Yamagishi T. Kalinowski DS. Pattanapanyasat K. Antitumor activity and mechanism of action of the iron chelator, Dp44mT, against leukemic cells. Am J Hematol. 2009;84:170–176. doi: 10.1002/ajh.21350. [DOI] [PubMed] [Google Scholar]
- 189.Olive PL. The comet assay. An overview of techniques. Methods Mol Biol. 2002;203:179–194. doi: 10.1385/1-59259-179-5:179. [DOI] [PubMed] [Google Scholar]
- 190.Olson RD. Boerth RC. Gerber JG. Nies AS. Mechanism of adriamycin cardiotoxicity: evidence for oxidative stress. Life Sci. 1981;29:1393–1401. doi: 10.1016/0024-3205(81)90001-1. [DOI] [PubMed] [Google Scholar]
- 191.Omichinski JG. Trainor C. Evans T. Gronenborn AM. Clore GM. Felsenfeld G. A small single-“finger” peptide from the erythroid transcription factor GATA-1 binds specifically to DNA as a zinc or iron complex. Proc Natl Acad Sci U S A. 1993;90:1676–1680. doi: 10.1073/pnas.90.5.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ott R. Krämer R. DNA hydrolysis by inorganic catalysts. Appl Microbiol Biotechnol. 1999;52:761–767. [Google Scholar]
- 193.Pedersen-Bjergaard J. Andersen MK. Christiansen DH. Nerlov C. Genetic pathways in therapy-related myelodysplasia and acute myeloid leukemia. Blood. 2002;99:1909–1912. doi: 10.1182/blood.v99.6.1909. [DOI] [PubMed] [Google Scholar]
- 194.Petering DH. Byrnes RW. Antholine WE. The role of redox-active metals in the mechanism of action of bleomycin. Chem Biol Interact. 1990;73:133–182. doi: 10.1016/0009-2797(90)90001-4. [DOI] [PubMed] [Google Scholar]
- 195.Petering DH. Mao Q. Li W. DeRose E. Antholine WE. Metallobleomycin-DNA interactions: structures and reactions related to bleomycin-induced DNA damage. Met Ions Biol Syst. 1996;33:619–648. [PubMed] [Google Scholar]
- 196.Pfister TD. Reinhold WC. Agama K. Gupta S. Khin SA. Kinders RJ. Parchment RE. Tomaszewski JE. Doroshow JH. Pommier Y. Topoisomerase I levels in the NCI-60 cancer cell line panel determined by validated ELISA and microarray analysis and correlation with indenoisoquinoline sensitivity. Mol Cancer Ther. 2009;8:1878–1884. doi: 10.1158/1535-7163.MCT-09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pilch DR. Sedelnikova OA. Redon C. Celeste A. Nussenzweig A. Bonner WM. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol. 2003;81:123–129. doi: 10.1139/o03-042. [DOI] [PubMed] [Google Scholar]
- 198.Pommier Y. Camptothecins and topoisomerase I: a foot in the door. Targeting the genome beyond topoisomerase I with camptothecins and novel anticancer drugs: importance of DNA replication, repair and cell cycle checkpoints. Curr Med Chem Anticancer Agents. 2004;4:429–434. doi: 10.2174/1568011043352777. [DOI] [PubMed] [Google Scholar]
- 199.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 200.Pommier Y. Barcelo JM. Rao VA. Sordet O. Jobson AG. Thibaut L. Miao ZH. Seiler JA. Zhang H. Marchand C. Agama K. Nitiss JL. Redon C. Repair of topoisomerase I-mediated DNA damage. Prog Nucleic Acid Res Mol Biol. 2006;81:179–229. doi: 10.1016/S0079-6603(06)81005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pommier Y. Leo E. Zhang H. Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pommier Y. Tanizawa A. Kohn KW. Mechanisms of topoisomerase I inhibition by anticancer drugs. Adv Pharmacol. 1994;29B:73–92. doi: 10.1016/s1054-3589(08)61132-1. [DOI] [PubMed] [Google Scholar]
- 203.Pouillart P. Evaluating the role of dexrazoxane as a cardioprotectant in cancer patients receiving anthracyclines. Cancer Treat Rev. 2004;30:643–650. doi: 10.1016/j.ctrv.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 204.Pourquier P. Ueng LM. Fertala J. Wang D. Park HJ. Essigmann JM. Bjornsti MA. Pommier Y. Induction of reversible complexes between eukaryotic DNA topoisomerase I and DNA-containing oxidative base damages. 7, 8-dihydro-8-oxoguanine and 5-hydroxycytosine. J Biol Chem. 1999;274:8516–8523. doi: 10.1074/jbc.274.13.8516. [DOI] [PubMed] [Google Scholar]
- 205.Qian H. Yang Y. Alterations of cellular organelles in human liver-derived hepatoma G2 cells induced by adriamycin. Anticancer Drugs. 2009;20:779–786. doi: 10.1097/CAD.0b013e32832f4e6f. [DOI] [PubMed] [Google Scholar]
- 206.Quiles JL. Huertas JR. Battino M. Mataix J. Ramirez-Tortosa MC. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002;180:79–95. doi: 10.1016/s0300-483x(02)00383-9. [DOI] [PubMed] [Google Scholar]
- 207.Rao VA. Agama K. Holbeck S. Pommier Y. Batracylin (NSC 320846), a dual inhibitor of DNA topoisomerases I and II induces histone gamma-H2AX as a biomarker of DNA damage. Cancer Res. 2007;67:9971–9979. doi: 10.1158/0008-5472.CAN-07-0804. [DOI] [PubMed] [Google Scholar]
- 208.Rao VA. Fan AM. Meng L. Doe CF. North PS. Hickson ID. Pommier Y. Phosphorylation of BLM, dissociation from topoisomerase IIIalpha, and colocalization with gamma-H2AX after topoisomerase I-induced replication damage. Mol Cell Biol. 2005;25:8925–8937. doi: 10.1128/MCB.25.20.8925-8937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rao VA. Klein SR. Agama KK. Toyoda E. Adachi N. Pommier Y. Shacter EB. The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIalpha in breast cancer cells. Cancer Res. 2009;69:948–957. doi: 10.1158/0008-5472.CAN-08-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rao VA. Klein SR. Bonar SJ. Zielonka J. Mizuno N. Dickey JS. Keller PW. Joseph J. Kalyanaraman B. Shacter E. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J Biol Chem. 2010;285:34447–34459. doi: 10.1074/jbc.M110.133579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rao VA. Zhang J. Klein SR. Espandiari P. Knapton A. Dickey JS. Herman E. Shacter EB. The iron chelator Dp44mT inhibits the proliferation of cancer cells but fails to protect from doxorubicin-induced cardiotoxicity in spontaneously hypertensive rats. Cancer Chemother Pharmacol. 2011;68:1125–1134. doi: 10.1007/s00280-011-1587-y. [DOI] [PubMed] [Google Scholar]
- 212.Ren B. Duan X. Ding H. Redox control of the DNA damage-inducible protein DinG helicase activity via its iron-sulfur cluster. J Biol Chem. 2009;284:4829–4835. doi: 10.1074/jbc.M807943200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Requena JR. Fu MX. Ahmed MU. Jenkins AJ. Lyons TJ. Baynes JW. Thorpe SR. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem J. 1997;322(Pt 1):317–325. doi: 10.1042/bj3220317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Richardson DR. Molecular mechanisms of iron uptake by cells and the use of iron chelators for the treatment of cancer. Curr Med Chem. 2005;12:2711–2729. doi: 10.2174/092986705774462996. [DOI] [PubMed] [Google Scholar]
- 215.Richardson DR. Kalinowski DS. Lau S. Jansson PJ. Lovejoy DB. Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim Biophys Acta. 2009;1790:702–717. doi: 10.1016/j.bbagen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 216.Richardson DR. Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta. 1997;1331:1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- 217.Richardson J. Thomas KA. Rubin BH. Richardson DC. Crystal structure of bovine Cu, Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975;72:1349–1353. doi: 10.1073/pnas.72.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Robert J. Preclinical assessment of anthracycline cardiotoxicity in laboratory animals: predictiveness and pitfalls. Cell Biol Toxicol. 2007;23:27–37. doi: 10.1007/s10565-006-0142-9. [DOI] [PubMed] [Google Scholar]
- 219.Robinson KM. Janes MS. Pehar M. Monette JS. Ross MF. Hagen TM. Murphy MP. Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Roca J. Ishida R. Berger JM. Andoh T. Wang JC. Antitumor bisdioxopiperazines inhibit yeast DNA topoisomerase II by trapping the enzyme in the form of a closed protein clamp. Proc Natl Acad Sci U S A. 1994;91:1781–1785. doi: 10.1073/pnas.91.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 222.Rudolf J. Makrantoni V. Ingledew WJ. Stark MJ. White MF. The DNA repair helicases XPD and FancJ have essential iron-sulfur domains. Mol Cell. 2006;23:801–808. doi: 10.1016/j.molcel.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 223.Sabourin M. Osheroff N. Sensitivity of human type II topoisomerases to DNA damage: stimulation of enzyme-mediated DNA cleavage by abasic, oxidized and alkylated lesions. Nucleic Acids Res. 2000;28:1947–1954. doi: 10.1093/nar/28.9.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Saletta F. Rahmanto YS. Siafakas AR. Richardson DR. Cellular iron depletion and the mechanisms involved in the iron-dependent regulation of the growth arrest and DNA damage family of genes. J Biol Chem. 2011;286:35396–35406. doi: 10.1074/jbc.M111.273060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sawyer DB. Peng X. Chen B. Pentassuglia L. Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53:105–113. doi: 10.1016/j.pcad.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Schindlbeck C. Mayr D. Olivier C. Rack B. Engelstaedter V. Jueckstock J. Jenderek C. Andergassen U. Jeschke U. Friese K. Topoisomerase IIalpha expression rather than gene amplification predicts responsiveness of adjuvant anthracycline-based chemotherapy in women with primary breast cancer. J Cancer Res Clin Oncol. 2010;136:1029–1037. doi: 10.1007/s00432-009-0748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schmidt BH. Burgin AB. Deweese JE. Osheroff N. Berger JM. A novel and unified two-metal mechanism for DNA cleavage by type II and IA topoisomerases. Nature. 2010;465:641–644. doi: 10.1038/nature08974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schneider E. Horton JK. Yang CH. Nakagawa M. Cowan KH. Multidrug resistance-associated protein gene overexpression and reduced drug sensitivity of topoisomerase II in a human breast carcinoma MCF7 cell line selected for etoposide resistance. Cancer Res. 1994;54:152–158. [PubMed] [Google Scholar]
- 229.Schneider YJ. Baurain R. Zenebergh A. Trouet A. DNA-binding parameters of daunorubicin and doxorubicin in the conditions used for studying the interaction of anthracycline-DNA complexes with cells in vitro. Cancer Chemother Pharmacol. 1979;2:7–10. doi: 10.1007/BF00253097. [DOI] [PubMed] [Google Scholar]
- 230.Seifried HE. McDonald SS. Anderson DE. Greenwald P. Milner JA. The antioxidant conundrum in cancer. Cancer Res. 2003;63:4295–4298. [PubMed] [Google Scholar]
- 231.Shacter E. Williams JA. Lim M. Levine RL. Differential susceptibility of plasma proteins to oxidative modification: examination by western blot immunoassay. Free Radic Biol Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 232.Shires TK. Iron-induced DNA damage and synthesis in isolated rat liver nuclei. Biochem J. 1982;205:321–329. doi: 10.1042/bj2050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shterman N. Kupfer B. Moroz C. Comparison of transferrin receptors, iron content and isoferritin profile in normal and malignant human breast cell lines. Pathobiology. 1991;59:19–25. doi: 10.1159/000163611. [DOI] [PubMed] [Google Scholar]
- 234.Shukla S. Srivastava RS. Shrivastava SK. Sodhi A. Kumar P. Synthesis, characterization and antiproliferative activity of 1,2-naphthoquinone and its derivatives. Appl Biochem Biotechnol. 2012;167:1430–1435. doi: 10.1007/s12010-012-9551-9. [DOI] [PubMed] [Google Scholar]
- 235.Simonart T. Boelaert JR. Andrei G. Clercq ED. Snoeck R. Iron withdrawal strategies fail to prevent the growth of SiHa-induced tumors in mice. Gynecol Oncol. 2003;90:91–95. doi: 10.1016/s0090-8258(03)00226-9. [DOI] [PubMed] [Google Scholar]
- 236.Simonart T. Boelaert JR. Andrei G. van den Oord JJ. Degraef C. Hermans P. Noel JC. Van Vooren JP. Heenen M. De Clercq E. Snoeck R. Desferrioxamine enhances AIDS-associated Kaposi's sarcoma tumor development in a xenograft model. Int J Cancer. 2002;100:140–143. doi: 10.1002/ijc.10475. [DOI] [PubMed] [Google Scholar]
- 237.Simunek T. Sterba M. Popelova O. Adamcova M. Hrdina R. Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 238.Sinha BK. Mimnaugh EG. Rajagopalan S. Myers CE. Adriamycin activation and oxygen free radical formation in human breast tumor cells: protective role of glutathione peroxidase in adriamycin resistance. Cancer Res. 1989;49:3844–3848. [PubMed] [Google Scholar]
- 239.Sissi C. Palumbo M. Effects of magnesium and related divalent metal ions in topoisomerase structure and function. Nucleic Acids Res. 2009;37:702–711. doi: 10.1093/nar/gkp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Smith PJ. Ferrous iron-mediated enhancement of DNA damage and recovery potential in bleomycin-treated human cells. Biochem Pharmacol. 1987;36:475–480. doi: 10.1016/0006-2952(87)90354-6. [DOI] [PubMed] [Google Scholar]
- 241.Sng JH. Heaton VJ. Bell M. Maini P. Austin CA. Fisher LM. Molecular cloning and characterization of the human topoisomerase IIalpha and IIbeta genes: evidence for isoform evolution through gene duplication. Biochim Biophys Acta. 1999;1444:395–406. doi: 10.1016/s0167-4781(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 242.Sordet O. Khan QA. Kohn KW. Pommier Y. Apoptosis induced by topoisomerase inhibitors. Curr Med Chem Anticancer Agents. 2003;3:271–290. doi: 10.2174/1568011033482378. [DOI] [PubMed] [Google Scholar]
- 243.Sordet O. Khan QA. Plo I. Pourquier P. Urasaki Y. Yoshida A. Antony S. Kohlhagen G. Solary E. Saparbaev M. Laval J. Pommier Y. Apoptotic topoisomerase I-DNA complexes induced by staurosporine-mediated oxygen radicals. J Biol Chem. 2004;279:50499–50504. doi: 10.1074/jbc.M410277200. [DOI] [PubMed] [Google Scholar]
- 244.Spagnuolo RD. Recalcati S. Tacchini L. Cairo G. Role of hypoxia-inducible factors in the dexrazoxane-mediated protection of cardiomyocytes from doxorubicin-induced toxicity. Br J Pharmacol. 2011;163:299–312. doi: 10.1111/j.1476-5381.2011.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 245.Spickett CM. Wiswedel I. Siems W. Zarkovic K. Zarkovic N. Advances in methods for the determination of biologically relevant lipid peroxidation products. Free Radic Res. 2010;44:1172–1202. doi: 10.3109/10715762.2010.498476. [DOI] [PubMed] [Google Scholar]
- 246.Stadtman ER. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med. 1990;9:315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- 247.Subramanian D. Furbee CS. Muller MT. ICE bioassay. Isolating in vivo complexes of enzyme to DNA. Methods Mol Biol. 2001;95:137–147. doi: 10.1385/1-59259-057-8:137. [DOI] [PubMed] [Google Scholar]
- 248.Swain SM. Whaley FS. Gerber MC. Weisberg S. York M. Spicer D. Jones SE. Wadler S. Desai A. Vogel C. Speyer J. Mittelman A. Reddy S. Pendergrass K. Velez-Garcia E. Ewer MS. Bianchine JR. Gams RA. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15:1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 249.Synold TW. Tetef ML. Doroshow JH. Antineoplastic activity of continuous exposure to dexrazoxane: potential new role as a novel topoisomerase II inhibitor. Semin Oncol. 1998;25:93–99. [PubMed] [Google Scholar]
- 250.Tan C. Tasaka H. Yu KP. Murphy ML. Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer. 1967;20:333–353. doi: 10.1002/1097-0142(1967)20:3<333::aid-cncr2820200302>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 251.Tanabe K. Ikegami Y. Ishida R. Andoh T. Inhibition of topoisomerase II by antitumor agents bis(2,6-dioxopiperazine) derivatives. Cancer Res. 1991;51:4903–4908. [PubMed] [Google Scholar]
- 252.Tanida I. Minematsu-Ikeguchi N. Ueno T. Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 253.Tanigawa N. Katoh H. Kan N. Mizuno Y. Tanimura H. Satomura K. Hikasa Y. Effect of vitamin E on toxicity and antitumor activity of adriamycin in mice. Jpn J Cancer Res. 1986;77:1249–1255. [PubMed] [Google Scholar]
- 254.Tennyson RB. Lindsley JE. Type II DNA topoisomerase from Saccharomyces cerevisiae is a stable dimer. Biochemistry. 1997;36:6107–6114. doi: 10.1021/bi970152f. [DOI] [PubMed] [Google Scholar]
- 255.Tetef ML. Synold TW. Chow W. Leong L. Margolin K. Morgan R. Raschko J. Shibata S. Somlo G. Yen Y. Groshen S. Johnson K. Lenz HJ. Gandara D. Doroshow JH. Phase I trial of 96-hour continuous infusion of dexrazoxane in patients with advanced malignancies. Clin Cancer Res. 2001;7:1569–1576. [PubMed] [Google Scholar]
- 256.Thelander L. Graslund A. Mechanism of inhibition of mammalian ribonucleotide reductase by the iron chelate of 1-formylisoquinoline thiosemicarbazone. Destruction of the tyrosine free radical of the enzyme in an oxygen-requiring reaction. J Biol Chem. 1983;258:4063–4066. [PubMed] [Google Scholar]
- 257.Thongphasuk P. Stremmel W. Chamulitrat W. 2,3-Dehydrosilybin is a better DNA topoisomerase I inhibitor than its parental silybin. Chemotherapy. 2009;55:42–48. doi: 10.1159/000175466. [DOI] [PubMed] [Google Scholar]
- 258.Tian J. Peehl DM. Zheng W. Knox SJ. Anti-tumor and radiosensitization activities of the iron chelator HDp44mT are mediated by effects on intracellular redox status. Cancer Lett. 2010;298:231–237. doi: 10.1016/j.canlet.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 259.Toyokuni S. Sagripanti JL. Iron-mediated DNA damage: sensitive detection of DNA strand breakage catalyzed by iron. J Inorg Biochem. 1992;47:241–248. doi: 10.1016/0162-0134(92)84069-y. [DOI] [PubMed] [Google Scholar]
- 260.Traore HN. Meyer D. Necrosis of host cells and survival of pathogens following iron overload in an in vitro model of co-infection with human immunodeficiency virus (HIV) and Mycobacterium tuberculosis. Int J Antimicrob Agents. 2007;29:465–470. doi: 10.1016/j.ijantimicag.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 261.Tukenova M. Guibout C. Oberlin O. Doyon F. Mousannif A. Haddy N. Guerin S. Pacquement H. Aouba A. Hawkins M. Winter D. Bourhis J. Lefkopoulos D. Diallo I. de Vathaire F. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 262.Tutem E. Apak R. Sozgen K. The interaction of antitumor-active anthraquinones with biologically important redox couples: I. Spectrophotometric investigation of the interaction of carminic acid and mitoxantrone with the iron (II, III) and copper (I, II) redox couples. J Inorg Biochem. 1996;61:79–96. doi: 10.1016/0162-0134(95)00023-2. [DOI] [PubMed] [Google Scholar]
- 263.Valko M. Morris H. Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 264.Valko M. Rhodes CJ. Moncol J. Izakovic M. Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 265.van Hille B. Etievant C. Barret JM. Kruczynski A. Hill BT. Characterization of the biological and biochemical activities of F 11782 and the bisdioxopiperazines, ICRF-187 and ICRF-193, two types of topoisomerase II catalytic inhibitors with distinctive mechanisms of action. Anticancer Drugs. 2000;11:829–841. doi: 10.1097/00001813-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 266.Vassal G. Pondarre C. Cappelli C. Terrier-Lacombe MJ. Boland I. Morizet J. Benard J. Venuat AM. Ardouin P. Hartmann O. Gouyette A. DNA-topoisomerase I, a new target for the treatment of neuroblastoma. Eur J Cancer. 1997;33:2011–2015. doi: 10.1016/s0959-8049(97)00296-7. [DOI] [PubMed] [Google Scholar]
- 267.Velez JM. Miriyala S. Nithipongvanitch R. Noel T. Plabplueng CD. Oberley T. Jungsuwadee P. Van Remmen H. Vore M. St. Clair DK. p53 regulates oxidative stress-mediated retrograde signaling: a novel mechanism for chemotherapy-induced cardiac injury. PLoS One. 2011;6:e18005. doi: 10.1371/journal.pone.0018005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Venable RO. Saba CF. Endicott MM. Northrup NC. Dexrazoxane treatment of doxorubicin extravasation injury in four dogs. J Am Vet Med Assoc. 2012;240:304–307. doi: 10.2460/javma.240.3.304. [DOI] [PubMed] [Google Scholar]
- 269.Voest EE. van Acker SA. van der Vijgh WJ. van Asbeck BS. Bast A. Comparison of different iron chelators as protective agents against acute doxorubicin-induced cardiotoxicity. J Mol Cell Cardiol. 1994;26:1179–1185. doi: 10.1006/jmcc.1994.1136. [DOI] [PubMed] [Google Scholar]
- 270.Von Hoff DD. Phase I trials of dexrazoxane and other potential applications for the agent. Semin Oncol. 1998;25:31–36. [PubMed] [Google Scholar]
- 271.Von Hoff DD. Layard M. Risk factors for development of daunorubicin cardiotoxicity. Cancer Treat Rep. 1981;65(Suppl 4):19–23. [PubMed] [Google Scholar]
- 272.Von Hoff DD. Layard M. Rozencweig M. Muggia FM. Time relationship between last dose of daunorubicin and congestive heart failure. Cancer Treat Rep. 1977;61:1411–1413. [PubMed] [Google Scholar]
- 273.Von Hoff DD. Layard MW. Basa P. Davis HL., Jr. Von Hoff AL. Rozencweig M. Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 274.Von Hoff DD. Rozencweig M. Layard M. Slavik M. Muggia FM. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med. 1977;62:200–208. doi: 10.1016/0002-9343(77)90315-1. [DOI] [PubMed] [Google Scholar]
- 275.Wadler S. Green MD. Basch R. Muggia FM. Lethal and sublethal effects of the combination of doxorubicin and the bisdioxopiperazine, (+)-1,2,-bis (3–5-dioxopiperazinyl-1-yl) propane (ICRF 187), on murine sarcoma S180 in vitro. Biochem Pharmacol. 1987;36:1495–1501. doi: 10.1016/0006-2952(87)90116-x. [DOI] [PubMed] [Google Scholar]
- 276.Wadler S. Green MD. Muggia FM. Synergistic activity of doxorubicin and the bisdioxopiperazine (+)-1,2-bis(3,5-dioxopiperazinyl-1-yl)propane (ICRF 187) against the murine sarcoma S180 cell line. Cancer Res. 1986;46:1176–1181. [PubMed] [Google Scholar]
- 277.Wang JC. DNA topoisomerases: from a laboratory curiosity to a subject in cancer chemotherapy. NCI Monogr. 1987:3–6. [PubMed] [Google Scholar]
- 278.Wang JC. DNA topoisomerases as targets of therapeutics: an overview. Adv Pharmacol. 1994;29A:1–19. doi: 10.1016/s1054-3589(08)60537-2. [DOI] [PubMed] [Google Scholar]
- 279.Wang JC. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 280.Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 281.Wang L. Eastmond DA. Catalytic inhibitors of topoisomerase II are DNA-damaging agents: induction of chromosomal damage by merbarone and ICRF-187. Environ Mol Mutagen. 2002;39:348–356. doi: 10.1002/em.10072. [DOI] [PubMed] [Google Scholar]
- 282.Wattanapitayakul SK. Chularojmontri L. Herunsalee A. Charuchongkolwongse S. Niumsakul S. Bauer JA. Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clin Pharmacol Toxicol. 2005;96:80–87. doi: 10.1111/j.1742-7843.2005.pto960112.x. [DOI] [PubMed] [Google Scholar]
- 283.Webb MR. Ebeler SE. Comparative analysis of topoisomerase IB inhibition and DNA intercalation by flavonoids and similar compounds: structural determinates of activity. Biochem J. 2004;384:527–541. doi: 10.1042/BJ20040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wei L. Easmon J. Nagi RK. Muegge BD. Meyer LA. Lewis JS. 64Cu-azabicyclo[3.2.2]nonane thiosemicarbazone complexes: radiopharmaceuticals for PET of topoisomerase II expression in tumors. J Nucl Med. 2006;47:2034–2041. [PubMed] [Google Scholar]
- 285.Wessel I. Jensen LH. Jensen PB. Falck J. Rose A. Roerth M. Nitiss JL. Sehested M. Human small cell lung cancer NYH cells selected for resistance to the bisdioxopiperazine topoisomerase II catalytic inhibitor ICRF-187 demonstrate a functional R162Q mutation in the Walker A consensus ATP binding domain of the alpha isoform. Cancer Res. 1999;59:3442–3450. [PubMed] [Google Scholar]
- 286.Whitnall M. Howard J. Ponka P. Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci U S A. 2006;103:14901–14906. doi: 10.1073/pnas.0604979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Whitnall M. Suryo Rahmanto Y. Sutak R. Xu X. Becker EM. Mikhael MR. Ponka P. Richardson DR. The MCK mouse heart model of Friedreich's ataxia: alterations in iron-regulated proteins and cardiac hypertrophy are limited by iron chelation. Proc Natl Acad Sci U S A. 2008;105:9757–9762. doi: 10.1073/pnas.0804261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Xiang P. Deng HY. Li K. Huang GY. Chen Y. Tu L. Ng PC. Pong NH. Zhao H. Zhang L. Sung RY. Dexrazoxane protects against doxorubicin-induced cardiomyopathy: upregulation of Akt and Erk phosphorylation in a rat model. Cancer Chemother Pharmacol. 2009;63:343–349. doi: 10.1007/s00280-008-0744-4. [DOI] [PubMed] [Google Scholar]
- 289.Xu X. Chen K. Kobayashi S. Timm D. Liang Q. Resveratrol attenuates doxorubicin-induced cardiomyocyte death via inhibition of S6k1-mediated autophagy. J Pharmacol Exp Ther. 2012;341:183–195. doi: 10.1124/jpet.111.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Xu X. Persson HL. Richardson DR. Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol. 2005;68:261–271. doi: 10.1124/mol.105.013383. [DOI] [PubMed] [Google Scholar]
- 291.Xu X. Sutak R. Richardson DR. Iron chelation by clinically relevant anthracyclines: alteration in expression of iron-regulated genes and atypical changes in intracellular iron distribution and trafficking. Mol Pharmacol. 2008;73:833–844. doi: 10.1124/mol.107.041335. [DOI] [PubMed] [Google Scholar]
- 292.Yalowich JC. Wu X. Zhang R. Kanagasabai R. Hornbaker M. Hasinoff BB. The anticancer thiosemicarbazones Dp44mT and triapine lack inhibitory effects as catalytic inhibitors or poisons of DNA topoisomerase IIalpha. Biochem Pharmacol. 2012;84:52–58. doi: 10.1016/j.bcp.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yan T. Deng S. Metzger A. Godtel-Armbrust U. Porter AC. Wojnowski L. Topoisomerase II{alpha}-dependent and -independent apoptotic effects of dexrazoxane and doxorubicin. Mol Cancer Ther. 2009;8:1075–1085. doi: 10.1158/1535-7163.MCT-09-0139. [DOI] [PubMed] [Google Scholar]
- 294.Yanauchi O. Odani A. Stability constants of metal complexes of amino acids with charges side chains - Part I: positive charged side chains. Pure Appl Chem. 1996;68:469–496. [Google Scholar]
- 295.Yang DC. Wang F. Elliott RL. Head JF. Expression of transferrin receptor and ferritin H-chain mRNA are associated with clinical and histopathological prognostic indicators in breast cancer. Anticancer Res. 2001;21:541–549. [PubMed] [Google Scholar]
- 296.Yu Y. Kovacevic Z. Richardson DR. Tuning cell cycle regulation with an iron key. Cell Cycle. 2007;6:1982–1994. doi: 10.4161/cc.6.16.4603. [DOI] [PubMed] [Google Scholar]
- 297.Yuan J. Lovejoy DB. Richardson DR. Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity: in vitro and in vivo assessment. Blood. 2004;104:1450–1458. doi: 10.1182/blood-2004-03-0868. [DOI] [PubMed] [Google Scholar]
- 298.Zamocky M. Koller F. Understanding the structure and function of catalases: clues from molecular evolution and in vitro mutagenesis. Prog Biophys Mol Biol. 1999;72:19–66. doi: 10.1016/s0079-6107(98)00058-3. [DOI] [PubMed] [Google Scholar]
- 299.Zeng Z. Cortes-Ledesma F. El Khamisy SF. Caldecott KW. TDP2/TTRAP is the major 5′-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage. J Biol Chem. 2011;286:403–409. doi: 10.1074/jbc.M110.181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhang A. Lyu YL. Lin CP. Zhou N. Azarova AM. Wood LM. Liu LF. A protease pathway for the repair of topoisomerase II-DNA covalent complexes. J Biol Chem. 2006;281:35997–36003. doi: 10.1074/jbc.M604149200. [DOI] [PubMed] [Google Scholar]
- 301.Zhou L. Sung RY. Li K. Pong NH. Xiang P. Shen J. Ng PC. Chen Y. Cardioprotective effect of dexrazoxane in a rat model of myocardial infarction: anti-apoptosis and promoting angiogenesis. Int J Cardiol. 2011;152:196–201. doi: 10.1016/j.ijcard.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 302.Zhou Y. Hileman EO. Plunkett W. Keating MJ. Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
- 303.Zielonka J. Hardy M. Kalyanaraman B. HPLC study of oxidation products of hydroethidine in chemical and biological systems: ramifications in superoxide measurements. Free Radic Biol Med. 2009;46:329–338. doi: 10.1016/j.freeradbiomed.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zielonka J. Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zielonka J. Srinivasan S. Hardy M. Ouari O. Lopez M. Vasquez-Vivar J. Avadhani NG. Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic Biol Med. 2008;44:835–846. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zielonka J. Vasquez-Vivar J. Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 307.Zunino F. Di Marco A. Zaccara A. Gambetta RA. The interaction of daunorubicin and doxorubicin with DNA and chromatin. Biochim Biophys Acta. 1980;607:206–214. doi: 10.1016/0005-2787(80)90073-8. [DOI] [PubMed] [Google Scholar]
- 308.Zuo L. Clanton TL. Detection of reactive oxygen and nitrogen species in tissues using redox-sensitive fluorescent probes. Methods Enzymol. 2002;352:307–325. doi: 10.1016/s0076-6879(02)52028-0. [DOI] [PubMed] [Google Scholar]