Abstract
Background
It has long been known that red blood cells comprise various subpopulations, which can be separated through Percoll density gradients.
Materials and methods
In this study, we performed integrated flow cytometry, proteomic and metabolomic analyses on five distinct red blood cell subpopulations obtained by Percoll density gradient separation of freshly drawn leucocyte-depleted erythrocyte concentrates. The relation of density gradient fractions to cell age was confirmed through band 4.1a/4.1b assays.
Results
We observed a decrease in size and increase in cell rugosity in older (denser) populations. Metabolomic analysis of fraction 5 (the oldest population) showed a decrease of glycolytic metabolism and of anti-oxidant defence-related mechanisms, resulting in decreased activation of the pentose phosphate pathway, less accumulation of NADPH and reduced glutathione and increased levels of oxidized glutathione. These observations strengthen conclusions about the role of oxidative stress in erythrocyte ageing in vivo, in analogy with results of recent in vitro studies. On the other hand, no substantial proteomic differences were observed among fractions. This result was partly explained by intrinsic technical limitations of the two-dimensional gel electrophoresis approach and the probable clearance from the bloodstream of erythrocytes with membrane protein alterations. Since this clearance effect is not present in vitro (in blood bank conditions), proteomic studies have shown substantial membrane lesions in ageing red blood cells in vitro.
Conclusion
This analysis shows that the three main red blood cell subpopulations, accounting for over 92% of the total RBC, are rather homogeneous soon after withdrawal. Major age-related alterations in vivo probably affect enzyme activities through post-translational mechanisms rather than through changes in the overall proteomic profile of RBC.
Keywords: red blood cell, population, density gradient, proteomics, metabolomics
Introduction
Human red blood cells (RBC) survive in the peripheral circulation for approximately 120 days, while the shelf-life of RBC concentrates stored under refrigeration is currently limited to 42 days1. The clearance of RBC in vivo is the result of a series of progressive events which affect cell viability and lead to an “aberrant” senescent phenotype, resulting in rapid removal from the bloodstream via phagocytosis (for a detailed review about the hypothesised models of erythrocyte clearance through phagocytosis the interested reader is referred to Bratosin et al.)2. Briefly, phagocytosis is mainly triggered and mediated by membrane exposure of phosphatidylserine or, rather, by formation of hemichrome-induced band 3 clusters that are recognised by naturally occurring antibodies2–4.
RBC senescence has so far been investigated through the isolation of RBC populations of different mean cell ages. Most of the investigations have been performed on erythrocytes separated on the basis of differences in cell density or volume/size5,6. Of the various techniques used, only a handful have found extensive application in basic science studies: plain centrifugation, angle-head centrifugation, and the use of several discontinuous gradients, including albumin and stractan, which result in variably efficient separation6. The use of a Percoll gradient has proven to be an easy and efficient way of separating RBC5,6. Nevertheless, it has been suggested that density is not a good criterion to determine RBC age and it has been proposed that separation exploiting differences in RBC volumes through counterflow centrifugation might yield better results. However, a direct comparative study concluded that each of the separation approaches holds specific advantages over the other and both are characterised by one major drawback, that is, the poor yield (low RBC numbers) in every fraction. This issue has so far hampered untargeted strategies which, on the other hand, have now been enabled by the increased sensitivity and specificity of mass spectrometry analytical approaches for proteomic and metabolomic analyses7,8.
Studies have been conducted over the years addressing the peculiar characteristics of RBC sub-populations, from younger to older fractions. RBC ageing has been reported to correlate with decreased cell volume, size and mean corpuscular volume6,9–11, increased mean corpuscular haemoglobin concentration6 and glycated haemoglobin (Hb1Ac),12 reduced 2,3-diphosphoglycerate/haemoglobin ratios13 and cell deformability14,15 and increased osmotic fragility16 consequent to the loss of electrolytes and microvesciculation17,18. Other than phosphatidylserine membrane exposure2 and increased Hb1Ac levels12, older RBC also have higher creatine levels19.
Membrane-related alterations include phosphatidylserine exposure and decreased surface charge density 2, alteration of the membrane lipid content due to loss of sialic acid residues20, susceptibility to phospholipase A221 and microvesiculation22. An age-dependent change in lipid asymmetry correlates with the cells’ propensity to be cleared from the peripheral circulation and bind to autologous mononuclear cells in vitro. Indeed, it has been observed that membrane alterations result in increased adhesiveness to endothelial and reticuloendothelial cells23, changes in membrane cation transport24 and decreased enzymatic activities25, along with the accumulation of lipid peroxidation products26. Most of these phenomena closely resemble apoptosis and have led to the formulation of the concept of eryptosis, an erythrocyte-specific apoptotic phenomenon27.
While alterations of membrane shape and lipid parameters have been widely investigated in RBC populations, other biologically relevant molecules, such as proteins and metabolites, are still poorly investigated in the frame of RBC ageing. Proteomics and metabolomics are two increasingly widespread “omics” strategies which exploit recent advances in the fields of two-dimensional gel-electrophoresis (2D-GE), high performance liquid chromatography (HPLC), mass spectrometry and bioinformatics in order to assay qualitatively and quantitatively all the proteins and metabolite complement to the genome in a given cell type at the exact moment at which the analysis is performed.
Although greater understanding is needed as of whether RBC ageing in vivo and in vitro (blood bank conditions) can actually be compared in order to translate results that have been obtained from application of “-omics” strategies to transfusion medicine issues28, we wanted to determine whether a correlation exists between the proteomic and metabolomic changes that have been observed in the frame of RBC ageing (in vitro)28 and the distribution of these alterations in age-differentiated subsets of erythrocytes. We, therefore, performed integrated flow cytometry and proteomic and metabolomic analyses of five distinct RBC subpopulations obtained by Percoll density gradient separation of freshly drawn leucocyte-depleted erythrocyte concentrates. We observed a decrease in size and increase in cell rugosity in older (denser) populations, which was not accompanied by substantial proteomic changes. Metabolomic analysis of fraction 5 (the oldest population) showed decreased efficiency of anti-oxidant defence-related mechanisms, through reduced activation of the pentose phosphate pathway (PPP) and less accumulation of NADPH and reduced glutathione.
This analysis showed that most RBC populations are rather homogeneous soon after withdrawal. As far as this preliminary study is concerned, there is, therefore, no evident necessity to perform studies to assess storage lesion on separated fractions, since a consistent percentage of all RBC belong to homogenous fractions as far as the proteome and metabolome are concerned.
Materials and methods
Blood sampling
Whole blood (450 mL±10%) was collected from healthy volunteer donors into CPD anticoagulant (63 mL). After separation of the plasma and buffy coat by centrifugation, leucocyte-filtered RBC were suspended in 100 mL of SAGM solution. Samples were collected from four RBC units withdrawn from four different donors (two males, two females, mean±SD age 48±11.5 years).
Percoll gradient
Density-fractionated RBC were prepared using Percoll (Sigma-Aldrich, St. Louis, MO, USA) discontinuous gradients, as previously described6,29. Briefly, the gradient was built up in five layers of 2 mL containing 80% (1.096 g/mL), 71% (1.087 g/mL), 67% (1.083 g/mL), 64% (1.080 g/mL) and 40% (1.060g/mL) Percoll, buffered with buffer A (26.3 g/L bovine serum albumin, 132 mmol/L NaCl, 4.6 mmol/L KCl, and 10 mmol/L HEPES pH 7.1). The RBC were then washed with buffer B (9 mmol/L Na2HPO4, 1.3 mmol/L NaH2PO4, 140 mmol/L NaCl, 5.5 mmol/L glucose, and 0.8 g/L bovine serum albumin) and diluted with one volume of buffer A. Of this suspension 0.5 mL were layered on the Percoll gradient and separated by centrifugation at 3,000 rpm for 15 minutes at room temperature. Fractions were collected by careful pipetting and extensively rinsed with buffer B to remove residual Percoll.
Flow cytometry assay
The five different erythrocyte populations were washed twice in 5 mmol/L phosphate buffer, pH 8.0, containing 0.9% (w/v) NaCl to remove Percoll and isolated by centrifuging twice at 1000 × g for 10 minutes at 4 °C. Subsequently they were analysed by flow cytometry with a sample of whole erythrocytes as a control. The morphology of the cells was assessed by a FACScalibur (Becton-Dickinson, USA). Analyses were conducted using the Cellquest program on 10,000 events acquired without gating. Events were analysed by side scatter and forward scatter.
Preparation of red blood cell membrane
Human RBC membrane proteins were extracted using the conventional method described by Olivieri and colleagues30 with some modifications. The five RBC populations were washed twice in 5 mmol/L phosphate buffer, pH 8.0, containing 0.9% (w/v) NaCl to remove Percoll and isolated by centrifuging twice at 1,000 × g for 10 minutes at 4 °C. The RBC were lysed with 9 vol of cold 5 mmol/L phosphate buffer, pH 8.0, containing 1 mmol/L EDTA and 1 mmol/L phenylmethanesulfonyl fluoride. Membranes were collected by centrifugation at 17,000 × g for 20 minutes at 4 °C and further washed until free of haemoglobin. To remove non-specifically membrane-bound cytosolic proteins, RBC membranes were further washed three times with 0.9% NaCl and collected by centrifugation at 17,000 × g for 20 minutes at 4 °C. The protein content was estimated by the bicinchoninic acid method31. The resulting membrane protein extracts were used for the subsequent analytical steps.
Determination of the band 4.1a/4.1b ratio
Membrane proteins were electrophoresed on a sodium dodecyl (SDS) polyacrylamide gel as described elseswhere32. Using Coomassie blue staining, bands 4.1a and 4.1b were quantified with a GS-800 calibrated densitometer (Bio-Rad Laboratories, Hercules, CA, USA), and the 4.1a/4.1b ratio was calculated.
Two-dimensional isoelectric focusing sodium dodecylsulphate polyacrylamide gel electrophoresis
To remove lipids, proteins were precipitated from a desired volume (containing 400 μg of proteins) of each sample with cold (4 °C) acetone (80% v/v) over-night, then centrifuged at 18,000 g for 20 minutes. The supernatant was removed and the pellet was air-dried and then dissolved in the focusing solution of 8 M urea, 2% (w/v) ASB-14, 0.5% (w/v) pH 3–10 carrier ampholyte (Bio-lyte; Bio-Rad) and 40 mM Tris base with continuous stirring. Proteins were subsequently reduced (by 10 mM tributylphosphine for 1 hour) and alkylated (by 40 mM iodoacetamide for 1 hour). To prevent over-alkylation, an excess of iodoacetamide was eliminated by adding 10 mM dithioerythritol. Isoelectric focusing (IEF) was performed usinga Biorad Multiphore II and Dry Strip Kit (Bio-Rad-Protean-IEF-Cell-System). Seventeen-centimetre immobilised pH gradient (IPG) strips (Bio-Rad) pH 3–10 were rehydrated overnight with 345 μL of rehydration solution containing 8 M urea, 2% (w/v) ASB, 0.5% (w/v) pH 3–10 carrier ampholyte (Bio-lyte; Bio-Rad), 10 mM dithioerythritol and 100 μL of sample were loaded using the cup-loading method. The total product time × voltage applied was 80,000 V h for each strip at 20 °C. For the second dimension, IPG strips were incubated in the equilibration solution [6 M urea, 50 mM Tris-HCl (pH 6.8), 30% (v/v) glycerol, 3% (w/v) SDS, 0.002% (w/v) bromophenol blue] for 30 minutes with gentle agitation. Equilibrated strips were then placed on SDS-polyacrylamide gels, 16×20 cm, 11% acrylamide, and sealed with 0.5% (w/v) agarose. SDS-polylacrylamide gel electrophoresis (PAGE) was performed using the Protean II xi Cell, large gel format (Bio-Rad) at a constant current (35 mA per gel) at 7 °C until the bromophenol blue tracking dye was approximately 2–3 mm from the bottom of the gel. Protein spots were stained with Coomassie brilliant blue G-250 stain33.
Image analysis
Twenty stained gels (1 technical replicate × 4 biological replicates × 5 RBC fractions) were digitalised using an ImageScanner and LabScan software 3.01 (Bio-Rad). It was not possible to perform more than one replicate per fraction per individual, as cell recovery and membrane extraction steps reduced the biological material available for 2D-GE analyses. The 2D-GE image analysis was carried out and spots were detected and quantified using Progenesis SameSpots software v.2.0.2733.19819 (Nonlinear Dynamics, Newcastle, UK). Each gel was analysed for spot detection and background subtraction. Among-fraction comparisons were performed by analysis of variance (ANOVA) in order to classify sets of proteins for which statistically significant differences with a confidence level of 0.05 were found. All statistical analyses were performed with the Progenesis SameSpots software v.2.0.2733.19819. After the background subtraction, spot detection and match, one standard gel was obtained for each group (RBC fractions) through normalisation of the biological replicates. These standard gels were then matched to yield information about the spots of differentially modulated proteins. Differentially modulated protein spots were considered significant at P values <0.05 and the change in the photodensity of protein spots among fractions had to be more than 2-fold. Given that it was impossible to perform technical replicates for each fraction because of the small amounts of membrane protein material, we performed Bonferroni’s post-tests to exclude false positive results.
Metabolomics
Samples containing 5×105 cells from each separated fraction were extracted using the protocol described by D’Alessandro et al.34. Briefly, for each sample, 0.5 mL of the pooled erythrocyte stock were transferred into a microcentrifuge tube (Eppendorf® Germany). Erythrocyte samples were then centrifuged at 1,000 g for 2 minutes at 4 °C. The centrifuged tubes were next placed on ice while the supernatants were carefully aspirated, paying attention not to remove any erythrocytes at the interface. The erythrocytes were resuspended in 0.15 mL of ice cold ultra-pure water (18 MΩ) to lyse cells, then the tubes were plunged into a water bath at 37 °C for 0.5 min. Samples were mixed with 0.6 mL of −20 °C methanol and then with 0.45 mL chloroform. Subsequently, 0.15 mL of ice cold ultra-pure water were added to each tube and the tubes were transferred to a freezer and kept at −20°C for 2–8 hours. An equivalent volume of acetonitrile was added to the tubes which were transferred to a refrigerator and stored at 4 °C for 20 minutes. Samples with precipitated proteins were then centrifuged at 10,000 × g for 10 minutes at 4 °C. Finally, the samples were dried in a rotational vacuum concentrator (RVC 2–18 - Christ Gmbh; Osterode am Harz, Germany) and re-suspended in 200 μL of water, 5% formic acid and transferred to glass auto-sampler vials for liquid chromatography/mass spectrometry (LC/MS) analysis.
Rapid resolution reversed-phase high performance liquid chromatography
An Ultimate 3000 Rapid Resolution HPLC system (LC Packings, DIONEX, Sunnyvale, USA) was used to separate metabolites. The system featured a binary pump and vacuum degasser, well-plate autosampler with a six-port micro-switching valve and a thermostated column compartment. A Dionex Acclaim RSLC 120 C18 column 2.1×150 mm, 2.2 μm was used to separate the extracted metabolites. Acetonitrile, formic acid and HPLC-grade water were purchased from Sigma Aldrich (Milan, Italy). The LC parameters were: injection volume, 20 μL; column temperature, 30 °C; and flow rate of 0.2 mL/min. The LC solvent gradient and timetable were identical during the whole period of the analyses. A 0–95% linear gradient of solvent A (0.1% formic acid in water) to B (0.1% formic acid in acetonitrile) was employed over 15 minutes followed by a solvent B hold of 2 minutes, returning to 100% A in 2 minutes and a 6-minute post-time solvent A hold.
Electrospray ionisation mass spectrometry
Metabolites were directly eluted into a High Capacity ion Trap HCTplus (Bruker-Daltonik, Bremen, Germany). Mass spectra for metabolite-extracted samples were acquired in positive and negative ion modes, as previously described34. The electrospray ionisation (ESI) capillary voltage was set at 3,000 V in (+) ion mode. The liquid nebuliser was set at 30 psig and the flow rate of the nitrogen drying gas was set at 9 L/min. The dry gas temperature was maintained at 300 °C. Internal reference ions were used to maintain mass accuracy continuously. Data were acquired at a rate of 5 spectra/sec with a stored mass range of m/z 50–1500. Data were collected using Bruker Esquire Control (v. 5.3 - build 11) data acquisition software. In the multiple reaction monitoring (MRM) analysis, the m/z of interest were isolated, fragmented and monitored (both the parental and fragment ions) throughout the whole RT range. HPLC on-line MS-eluted metabolites were validated by comparing transition fingerprints, upon fragmentation and matching against the standard metabolites through direct infusion with a syringe pump (infusion rate 4 μL/min). Standard curve calibrations were performed on precursor and fragment ion signals. Only the former were adopted for quantitation, as precursor ion signals guaranteed higher intensity and thus improved limit of detection (LOD) and quantitation of metabolites of interest34. However, transitions were monitored in independent runs to validate each detected metabolite.
Metabolite analysis and data elaboration
Quantitative analyses of standard compounds were performed on MRM data compared to standard metabolite runs. Each standard compound was weighed and dissolved in nanopure water (18 mΩ). Calibration curves were calculated as previously reported34. In brief, each standard metabolite was run in triplicate, at incremental dilutions until the LOD was reached. The LOD for each compound was calculated as the minimum amount injected which gave a detector signal response higher than three times the noise (S/N >3).
Standards (≥98% chemical purity) D-fructose 6-phosphate (F6P), D-glucose 6-phosphate (G6P), D-fructose 1,6 diphosphate (FDP), glyceraldehyde phosphate (G3P), 1,3 and 2,3 diphosphoglycerate (DPG), phosphoenolpyruvic acid (PEP), L-lactic acid (LA), NADPH, 6-phosphogluconic acid (6PG), ATP, NADH, glutathione (GSH), oxidized glutathione (GSSG), glutamine (GLTM) and glutamate (GLUT) were purchased from Sigma Aldrich (Milan, Italy).
Standards were stored at −25 °C, 4 °C or room temperature, following the manufacturer’s instructions.
LC/MS data files were processed by Bruker DataAnalysis 4.0 (build 234) software. Results were plotted with GraphPad Prism 5.0 (GraphPad Software Inc.) as fold-change variations upon normalisation of the results obtained among the five fractions for each independent metabolite, as described by D’Alessandro et al.28 and Nishino et al.35.
Results and discussion
In vivo RBC ageing is an extensively investigated topic in biological research, as erythrocytes are widely available and substantially less complex than most other cellular biological matrices36. As thoroughly reviewed by Shinozuka36, researchers first addressed the main alterations affecting RBC as they age in blood vessels, including modified membrane sialiation, appearance of band-3 dimer neo-epitopes at the membrane and shape alterations (decreased size and surface/volume ratios). Biochemical studies have been performed over the last decades in order to shed light on the observed increases in mean corpuscular haemoglobin concentration and mean corpuscular haemoglobin in older cells, as well as slightly increased oxygen affinity and altered enzymatic activities36. However, most of the information collected up to now has been related to alterations to single parameters, while to the best of the authors’ knowledge no untargeted “omics” study has been reported so far.
In the present study, we performed flow cytometric, proteomic and metabolomic investigations on Percoll density gradient-fractionated RBC. Percoll density gradients allowed us to separate five distinct subpopulations (Figure 1) from the erythrocyte fraction, obtained by centrifugation and leucofiltration, of freshly drawn blood from healthy donor volunteers. It has already been reported that it is possible to obtain from four to nine distinct populations, depending on the density gradient ladder5,6,37,38.
Figure 1.
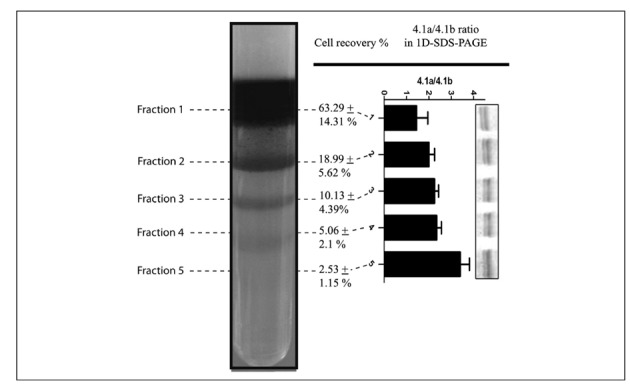
Percoll density gradient of freshly drawn, leucocyte-filtered, RBC concentrates. Five distinct subpopulations are visible, which are numbered from top to bottom. The gradient was prepared by stacking layers of different densities, in agreement with Bosch et al.28: 1.096 g/mL, 1.087 g/mL, 1.083 g/mL, 1.080 g/mL and 1.060 g/mL. Percentages of cell recovery are reported for each fractions as means+SD (total=100%). In the right panel, the graph reports densitometric analysis for the band 4.1a/4.1b ratio from the 1D-GE runs for each distinct subpopulation.
It is has long been known that denser populations correspond to older RBC6. The causes of the altered hydrodynamic density of older RBC have been postulated to depend on membrane lipid scrambling resulting in shape alterations14,15 and/or altered haemoglobin/water ratios due to unbalanced loss of the latter during the life of erythrocytes39. The relation of fraction density to cell age was further confirmed by monitoring the band 4.1a/4.1b ratio via one-dimensional SDS-PAGE (1D-GE) (Figure 1). The ratio between the amounts of the band 4.1 and 4.1b proteins is known to increase proportionally to age40. This phenomenon has been reported to occur in several mammals and has been related to deamidation of Asn 478 and 502 of the band 4.1b protein which results in altered electrophoretic mobility and thus different apparent molecular weight in SDS-PAGE runs41.
Upon Percoll gradient separation, the distribution of RBC populations was strongly biased towards the youngest subpopulation (least dense, fraction 1 in Figure 1), which was significantly more abundant that the other subpopulations (cell recovery for this fraction was 63.29±14.31% of the total - Figure 1). Taken together, the three upper (least dense) bands accounted for >92% of the total RBC, while the denser/older populations represented a minority of the cells, especially as far as the densest/oldest and barely visible fraction 5 was concerned (approximately 2% of the total).
While it has been reported in the literature that Percoll separation might have some limitations and does not necessarily yield RBC which are also separated by size6, in the present study we confirmed through flow cytometry that there were differences in volumes (forward scattering - FS) and membrane rugosity (side scattering - SS) among the five different fractions (Figure 2). In particular, older cell fractions displayed higher rugosity (SS distributions moved upwards from fraction 1 to 5 - Figure 2) and lower cell volume (FS distributions moved leftwards to the vertical axis from fraction 1 to 5 - Figure 2), as we would have expected9–11,14,15. A decrease in cell size and increase in cell rugosity have, so far, been related to progressive dehydration39, alterations to the membrane shape deriving from membrane shedding through vesiculation17,18,22, membrane lipid scrambling4,14,15 and a subsequent increase in osmotic fragility as a result of the decreased surface/volume ratio16 that develops upon acquisition of a spheroechinocyte/spherocyte shape29,42.
Figure 2.
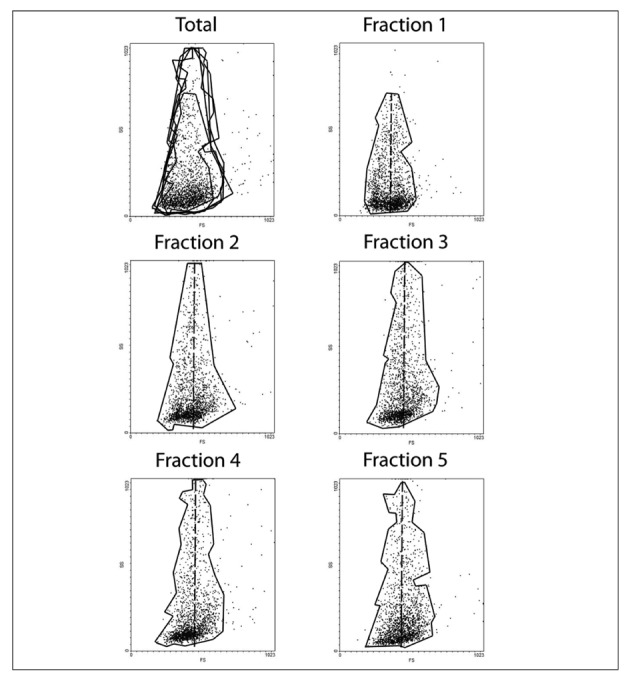
Flow cytometry analysis representing forward scattering (FS) and side scattering (SS) on the x and y axis, respectively, for the total red blood cell population (upper left frame) and for each one of the five fractions, as labelled. Each subpopulation has been delimited into a shape enclosing >95% of the counted events, and then superimposed in the frame labelled Total (upper left corner). Fractions 2 to 5 showed greater SS in comparison to fraction 1. The core of events is counted with a homogeneous distribution for fraction 1 as far as FS is concerned. For the other fractions, FS events are mainly shifted leftwards from the main axis (dotted line).
The trends for FS to decrease and SS to increase (Figure 2) were particularly evident despite the limited number of events (10,000) recorded through flow cytometry. Indeed, this minor technical limitation, which did not hamper us from drawing conclusions in line with those in the literature, was mainly due to the poor recovery rate of cells from Percoll fractions. Since flow cytometry assays were planned only to confirm the quality of our separation, in agreement with published literature, we decided to limit the extent of this part of the experimental workflow while looking for a compromise which could guarantee the most meaningful information. On the other hand, the main goal of the present study was to exploit exactly the same samples in order to carry out multiple “omics” investigations, such as proteomics and metabolomics, the former being extremely demanding in terms of samples needed to perform the analyses.
No proteome targeting study has been reported so far in the frame of RBC aging in vivo, except for 1D-GE-based investigations43–45, while recent literature has provided a consistent body of data on protein-targeting storage lesions in in vitro refrigerated models (i.e. (blood-bank conditions)28,46–48. The question is whether it is possible to approximate the 120-day of life-span of RBC in vivo with the 42-day shelf-life in vitro, as discussed in recent years49.
In the present study, we did not observe any significant (P < 0.05 ANOVA; fold-change variation > 2) differences among spots (number of spots and spot intensities) from 2D-GE electrophoresis of membrane proteins of RBC from the five fractions (Figure 3). However, the overall number of spots detected through Coomassie staining in the total population (136±16 spots) was always higher than in each subfraction (fraction 1=118±10; fraction 2=109±26; fraction 3=116±12; fraction 4=111±14; fraction 5=125±14). Nevertheless, due to the poor technical reproducibility and the intrinsic limitations of the 2D-GE approach, we were not able to identify spots whose apparent amounts were modulated in a statistically significant fashion. While it was to be expected that only a few proteins (band 4.1a/4.1b; glycated haemoglobin) would vary significantly in the frame of RBC subpopulations, as emerged from previous 1D-GE approaches43–45, it appears technically difficult to unravel these finely tuned alterations in RBC proteins through 2D-GE approaches. One major technical limitation is the poor membrane protein recovery, which is also a function of cell fraction recovery, and hampers the possibility of performing further technical replicates, thus affecting statistical analyses and forcing us to run stringent post-test analyses in order to exclude false positive results. Since our inability to identify statistically significant results might be attributed to either biological or technical variability, affecting statistical outcomes, further studies are essential to determine whether differences are truly minimal or whether they are present but difficult to demonstrate. Taken together, these considerations further support the recent conclusion that 1D-GE still represents a reliable analytical approach despite the introduction of a large number of gel-based techniques over the last 40 years50.
Figure 3.
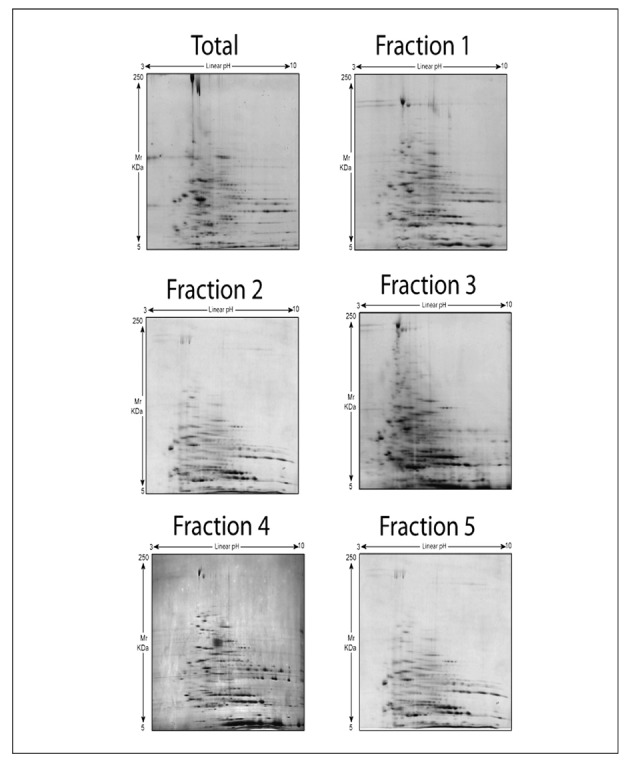
Two-dimensional gel electrophoresis of freshly drawn RBC after separation into five distinct subpopulations through a Percoll density gradient. First dimension isoelectric focusing pI values linearly span between 3 and 10, while molecular weights are indicated on the left.
On the other hand, RBC membrane alterations have been reported to be irreversible in long-term SAGM-stored erythrocytes under blood-bank conditions28. It is likely that these RBC membrane protein lesions also arise in older RBC populations in vivo, although at this very stage RBC might be promptly cleared from the bloodstream and, therefore, no longer be present, or be present in traces, in freshly drawn blood. In other terms, a closed system such as a stored RBC unit allows a model to be pushed to its limits, while in vivo ageing in healthy subjects results in a continuous turn-over hampering the observation of extreme phenotypes at the proteome level.
While RBC membrane proteome-targeting lesions are known to occur on average from day 21 onwards in vitro (blood bank conditions)28, RBC stored under refrigeration are known to suffer from early age-related symptoms of reduced cell integrity which affect RBC metabolism28,35,51.
The rationale behind our simultaneous investigation of the RBC membrane proteome and metabolism stems from previous observations about a strong intertwining between glycolytic rate and the oxygen-dependent binding of glycolytic enzymes to the cytosolic domain of band 3, the most abundant integral membrane protein in RBC52,53. While we did not observe significant proteomic differences among subpopulations at the membrane level, a limited, albeit biologically meaningful, number of changes are known to occur in senescent erythrocytes45.
In the frame of in vivo ageing, RBC metabolism has been studied only by addressing enzyme activities, phosphate intermediates (ATP, 2,3-DPG) or creatine29,54–62. Unlike proteome-targeting studies, little - albeit relevant - information is available on RBC metabolic fluxes as cells age in in vivo conditions. It has been reported that the activities of the main rate-limiting enzymes of glycolysis, including hexokinase, glucose 6-phosphate dehydrogenase and pyruvate kinase, decrease in Percoll density gradient-separated older RBC populations51,57. This is consistent with the increased alkalosis (in older RBC pH is higher by 0.2 units on average) and decreased content of organic phosphate compounds, both of which positively influence haemoglobin affinity for oxygen and thus result in a theoretically reduced capacity of older RBC to oxygenate peripheral tissues59. With regards to ATP and 2,3-DPG, it has been reported that older cells contain approximately 76% to 79% of the amounts detected in younger populations29,60. In the present investigation, we were able to confirm the same trend for ATP, as the level detected in fraction 5 corresponded to 78.1% of that in the normalised group (values for ATP and other metabolites are reported as means ± SD of fold-change variation against inter-fraction normalised values for each tested individual - Figure 4). Interestingly, through direct assays of a handful of glycolytic metabolic intermediates such as G6P/F6P, FBP, G3P, PEP and LH, we found a general trend to a gradual decrease of the amounts of these metabolites in older cell subpopulations (especially in fractions 4 and 5) in comparison to fraction 1 and to fractions 2 to 3 (Figure 4 - upper panel). The most significant of these alterations was in G6P/F6P, whose levels in fraction 5 were half those in fraction 1, in agreement with reports of decreased hexokinase activity in older RBC populations54.
Figure 4.
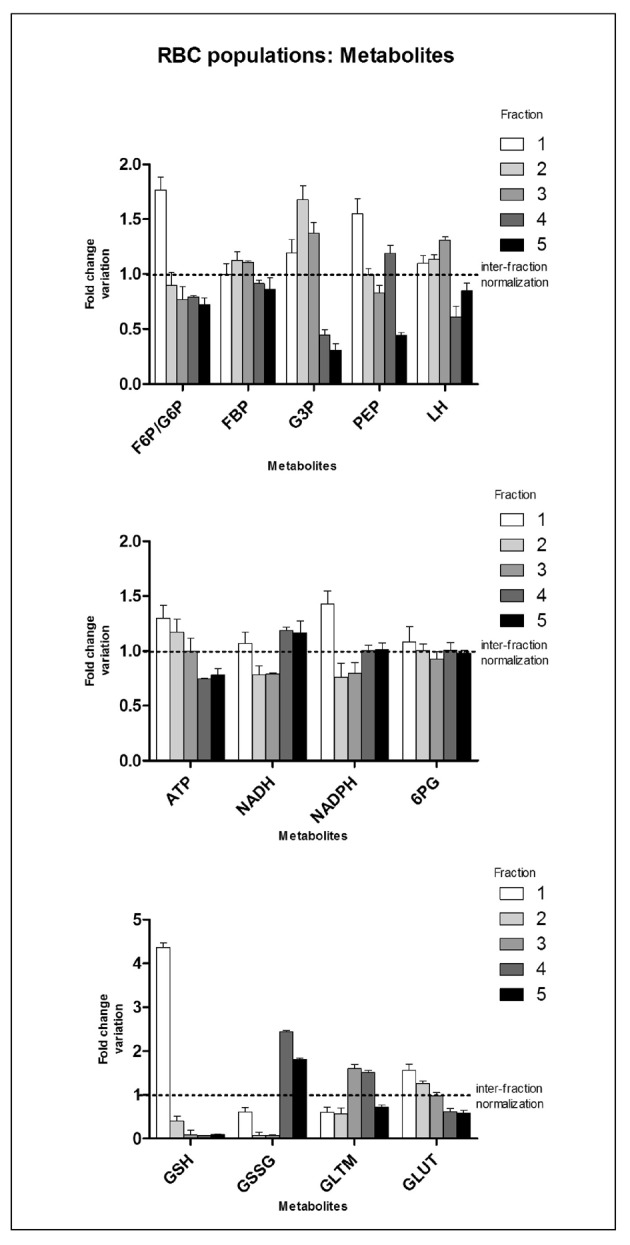
Time-course metabolomic analyses of leucocyte-filtered RBC subpopulations after separation through a Percoll density gradient. Internal normalisation was performed against the average values for each metabolite among the five distinct subpopulations for all the tested individuals (results are plotted as means+SD). Abbreviations: F6P/G6P=fructose/glucose 6-phosphate; FBP=fructose 1,6 biphosphate; G3P=glyceraldehyde 3-phosphate; PEP=phosphoenolpyruvate; LH=lactate; ATP=adenosine triphosphate; NADH=reduced nicotinamide adenine dinucleotide; NADPH=nicotinamide adenine dinucleotide phosphate; PG=6-phosphogluconate; GSH=reduced glutathione; GSSG=oxidized glutathione; GLTM=glutamine; GLUT=glutamate.
The age-related decline in enzymatic activities has been shown to involve a series of enzymes including GSH-transferase,54 glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase,55 which are related to anti-oxidant stress responses, through the activation of the PPP, production of reducing intermediates such as NADPH, regeneration of GSH levels from GSSG and reduction of oxidized anti-oxidant defence proteins, such as superoxide dismutases63. Only GSH levels have been assayed in younger and older RBC populations so far64, and have shown a trend to decrease in proportion to RBC age.
In the present study, we confirmed this trend (Figure 4 - lower panel), through a substantial decrease in GSH levels from fraction 1 to fraction 2 and from fraction 2 to the other fractions. Furthermore, we found a substantial increase in oxidized glutathione (GSSG) levels in fractions 4 and 5, in comparison to the first three fractions. As far as the PPP is concerned, fraction 1 displayed a significantly greater (P <0.01 ANOVA) fold-change in G6P levels, while 6PG was rather homogeneous in all the tested populations (though still approximately 10% higher in fraction 1) (Figure 4 - central panel). NADPH is a reduced intermediate of the oxidative phase of the PPP which is required for reduction of GSSG to GSH and for restoring the activity of several anti-oxidant enzymes, including glutathione peroxidase. Our analyses showed a net decrease of NADPH from fraction 1 to fraction 2 and, consistently, in all the other fractions (Figure 4 - central panel). Finally, since GSH is a tripeptide of glutamate (GLUT), glycine and cysteine, reduced levels of GSH might be affected by the observed decrease in GLUT levels from fraction 1 to fraction 4 and 5, other than by the already mentioned enzyme changes55.
It is worth stressing that analogous results have been obtained from the analysis of in vitro ageing of RBC under blood bank conditions (refrigerated storage in CPD-SAGM-containing plastic bags at 4 °C), in which early accumulating storage lesions affect metabolic fluxes of RBC through a decrease in glycolytic rates and an increase of the PPP from day 14 onwards, while reaching unsustainable levels of oxidation from day 28 onwards28. Furthermore, it is noteworthy that measurable alterations of normal metabolic fluxes occur prior to any evident alteration of the proteome machinery either in vitro28 or in vivo (present study).
Through the present metabolomic analyses we provide confirmatory evidence of the theory relating RBC ageing, both in vitro28 and in vivo (present study), to an exacerbation of oxidative stress and a decreased capacity of RBC to cope with this stress28,48,65,66. Anti-oxidant defences represent the central core of protein activities in RBC as proteins involved in these phenomena are direct or indirect interactors of the great majority of the residual proteome65.
Conclusion
In the present study, we integrated flow cytometry, proteomics and metabolomics to investigate the differences among RBC subpopulations from leucocyte-filtered erythrocyte concentrates obtained from freshly drawn blood by Percoll density gradient separation.
We confirmed the efficiency of the separation process through flow cytometry, which evidenced a decrease in cell size and increase in rugosity, probably due to the accumulation of membrane shape alterations, as previously reported6,9–11,14,15.
Proteomic analyses did not show any substantial differences among RBC fractions. The main, potential reasons for this are: (i) the probable clearance of those RBC with altered membrane protein profiles from the bloodstream, and/or (ii) the difference between the in vivo and in vitro (blood bank conditions) models of RBC ageing28, in which stresses to RBC tend to accumulate in the latter models because oxidative stress and reactive oxygen radical species are catalytic processes, thus allowing the investigations of extreme conditions.
We compared alterations of RBC metabolic fluxes in different fractions to the metabolic storage lesions which arise early during RBC storage under blood banking conditions28, concluding that oxidative stress seems to be the leading cause of the senescent phenotype of RBC also in vivo28,48,65,66.
On the other hand, the observations that the RBC fractions showing the greatest differences accounted for less than 8% of the total original RBC population prompted us to conclude that the great majority of RBC from freshly drawn blood undergoing treatment for blood banking in the transfusion setting can be considered as homogeneous. This consideration underpins the statement that, when planning studies to assess RBC storage lesions for transfusion purposes, it appears that fractionation of RBC into distinct populations is not essential, as more than 92% of the total population have homogeneous properties. Indeed, previous studies have already reported that only RBC from the oldest (gerocytes) and youngest (neocytes) subpopulations are differentially affected by storage conditions43,44. This prompts two main considerations: (i) alternative mechanisms (e.g. cationic dysregulation43) affect RBC survival in vitro and these are not necessarily the same as those occurring during in vivo ageing; (ii) changes affecting youngest RBC subpopulations are also the ones targeting a substantial percentage (from 65 to 92 %) of the whole unfractionated RBC population, which makes it statistically likely that most of the observations so far reported on unfractionated RBC predominantly reflect molecular lesions to the most abundant fractions.
In the near future, besides delving into the storage issue in greater detail, it would be worth exploring the changes to these very same parameters (flow cytometry, proteomics, metabolomics on Percoll density gradient-separated fractions) in scenarios in which RBC are partially compromised by genetic defects (e.g. glucose 6-phosphate dehydrogenase deficiency, beta thalassemia) or diseases (for example, malaria).
Acknowledgments
Angelo D’Alessandro, Barbara Blasi, Gian Maria D’Amici, Cristina Marocco and Lello Zolla are supported by funds from the Italian National Blood Centre (Centro Nazionale Sangue - CNS - Istituto Superiore Sanità - Rome, Italy).
Footnotes
The Authors declare no conflicts of interest.
References
- 1.D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8:82–8. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bratosin D, Mazurier J, Tissier JP, et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173–95. doi: 10.1016/s0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 3.Lutz HU, Fasler S, Stammler P, et al. Naturally occurring anti-band 3 antibodies and complement in phagocytosis of oxidatively-stressed and in clearance of senescent red cells. Blood Cells. 1988;14:175–203. [PubMed] [Google Scholar]
- 4.Schroit AJ, Madsen JW, Tanaka Y. In vivo recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J Biol Chem. 1985;260:5131–8. [PubMed] [Google Scholar]
- 5.Connor J, Pak CC, Schroit AJ. Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J Biol Chem. 1994;269:2399–404. [PubMed] [Google Scholar]
- 6.Bosch FH, Werre JM, Roerdinkholder-Stoelwinder B, et al. Characteristics of red blood cell populations fractionated with a combination of counterflow centrifugation and Percoll separation. Blood. 1992;79:254–60. [PubMed] [Google Scholar]
- 7.D’Alessandro A, Zolla L. Proteomics for quality-control processes in transfusion medicine. Anal Bioanal Chem. 2010;398:111–24. doi: 10.1007/s00216-010-3799-0. [DOI] [PubMed] [Google Scholar]
- 8.D’Alessandro A, Gevi F, Zolla L. Targeted mass spectrometry-based metabolomic profiling through Multiple Reaction Monitoring of Liver and other biological matrices. In Liver Proteomics. In: Josic D, Hixson DC, editors. Methods and Protocols Series: Methods in Molecular Biology, vol. 909. New York, NY, USA: Springer Protocols, Humana Press; 2012. [DOI] [PubMed] [Google Scholar]
- 9.Nash GB, Wyard SJ. Changes in surface area and volume measured by micropipette aspiration for erythrocytes ageing in vivo. Biorheology. 1980;17:479–84. [PubMed] [Google Scholar]
- 10.Linderkamp O, Meiselman HJ. Geometric, osmotic, and membrane mechanical properties of density-separated human red cells. Blood. 1982;59:1121–7. [PubMed] [Google Scholar]
- 11.van Oss CJ. Shape of ageing erythrocytes. Biorheology. 1982;19:725. doi: 10.3233/bir-1982-19606. [DOI] [PubMed] [Google Scholar]
- 12.Bunn HF, Haney DN, Kamin S, et al. The biosynthesis of human hemoglobin Alc. Slow glycosylation of hemoglobin in vivo. J Clin Invest. 1976;57:1652–9. doi: 10.1172/JCI108436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samaja M, Rovida E, Motterlini R, et al. Human red cell age, oxygen affinity and oxygen transport. Respir Physiol. 1990;79:69–79. doi: 10.1016/0034-5687(90)90061-3. [DOI] [PubMed] [Google Scholar]
- 14.Clark MR, Mohandas N, Shohet SB. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood. 1983;61:899–910. [PubMed] [Google Scholar]
- 15.Gifford SC, Derganc J, Shevkoplyas SS, et al. A detailed study of time-dependent changes in human red blood cells: from reticulocyte maturation to erythrocyte senescence. Br J Haematol. 2006;135:395–404. doi: 10.1111/j.1365-2141.2006.06279.x. [DOI] [PubMed] [Google Scholar]
- 16.Rifkind JM, Araki K, Hadley EC. The relationship between the osmotic fragility of human erythrocytes and cell age. Arch Biochem Biophys. 1983;222:582–9. doi: 10.1016/0003-9861(83)90556-8. [DOI] [PubMed] [Google Scholar]
- 17.Dumaswala UJ, Greenwalt TJ. Human erythrocytes shed exocytic vesicles in vivo. Transfusion. 1984;24:490–2. doi: 10.1046/j.1537-2995.1984.24685066807.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenwalt TJ, Dumaswala UJ. Effect of red cell age on vesiculation in vitro. Br J Haematol. 1988;68:465–7. doi: 10.1111/j.1365-2141.1988.tb04237.x. [DOI] [PubMed] [Google Scholar]
- 19.Syllm-Rapoport I, Daniel A, Starck H, et al. Creatine in density-fractionated red cells, a useful indicator of erythropoietic dynamics and of hypoxia past and present. Acta Haematol. 1981;66:86–95. doi: 10.1159/000207103. [DOI] [PubMed] [Google Scholar]
- 20.Bartosz G, Grzelinska E, Bartkowiak A. Aging of the erythrocyte. XIX. Decrease in surface charge density of bovine erythrocytes. Mech Ageing Dev. 1984;24:1–7. doi: 10.1016/0047-6374(84)90172-6. [DOI] [PubMed] [Google Scholar]
- 21.Shukla SD, Hanahan DJ. Membrane alterations in cellular aging: susceptibility of phospholipids in density (age)-separated human erythrocytes to phospholipase A2. Arch Biochem Biophys. 1982;214:335–41. doi: 10.1016/0003-9861(82)90038-8. [DOI] [PubMed] [Google Scholar]
- 22.Bartosz G. Aging of the erythrocyte. IV. Spin-label studies of membrane lipids, proteins and permeability. Biochim Biophys Acta. 1981;644:69–73. doi: 10.1016/0005-2736(81)90059-6. [DOI] [PubMed] [Google Scholar]
- 23.Dhermy D, Simeon J, Wautier MP, et al. Role of membrane sialic acid content in the adhesiveness of aged erythrocytes to human cultured endothelial cells. Biochim Biophys Acta. 1987;904:201–6. doi: 10.1016/0005-2736(87)90369-5. [DOI] [PubMed] [Google Scholar]
- 24.Hentschel WM, Wu LL, Tobin GO, et al. Erythrocyte cation transport activities as a function of cell age. Clin Chim Acta. 1986;157:33–43. doi: 10.1016/0009-8981(86)90315-3. [DOI] [PubMed] [Google Scholar]
- 25.Bartosz G. In: Blood Cell Biochemistry: Erythroid Cells. Harris JR, editor. Vol. 1. Plenum Press; New York: 1990. pp. 45–79. [Google Scholar]
- 26.Jain SK. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biochim Biophys Acta. 1988;937:205–10. doi: 10.1016/0005-2736(88)90242-8. [DOI] [PubMed] [Google Scholar]
- 27.Lang F, Gulbins E, Lerche H, et al. Eryptosis, a window to systemic disease. Cell Physiol Biochem. 2008;22:373–80. doi: 10.1159/000185448. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered erythrocyte concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vettore L, Concetta de matteis M, Zampini P. A new density gradient system for the separation of human red blood cells. Am J Hematol. 1980;8:291. doi: 10.1002/ajh.2830080307. [DOI] [PubMed] [Google Scholar]
- 30.Olivieri E, Herbert B, Righetti PG. The effect of protease inhibitors on the two-dimensional electrophoresis pattern of red blood cell membranes. Electrophoresis. 2001;22:560–5. doi: 10.1002/1522-2683(200102)22:3<560::AID-ELPS560>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 31.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 32.Alaia V, Frey BM, Siderow A, et al. A pair of naturally occurring antibodies may dampen complement-dependent phagocytosis of red cells with a positive antiglobulin test in healthy blood donors. Vox Sang. 2009;97:338–47. doi: 10.1111/j.1423-0410.2009.001214.x. [DOI] [PubMed] [Google Scholar]
- 33.Candiano G, Bruschi M, Musante L, et al. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–33. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- 34.D’Alessandro A, Gevi F, Zolla L. A robust high resolution reversed-phase HPLC strategy to investigate various metabolic species in different biological models. Mol Biosyst. 2011;7:1024–32. doi: 10.1039/c0mb00274g. [DOI] [PubMed] [Google Scholar]
- 35.Nishino T, Yachie-Kinoshita A, Hirayama A, et al. In silico modeling and metabolome analysis of long-stored erythrocytes to improve blood storage methods. J Biotechnol. 2009;144:212–23. doi: 10.1016/j.jbiotec.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Shinozuka T. Changes in human red blood cells during aging in vivo. Keio J Med. 1994;43:155–63. doi: 10.2302/kjm.43.155. [DOI] [PubMed] [Google Scholar]
- 37.Salvo G, Caprari P, Samoggia P, et al. Human erythrocyte separation according to age on a discontinuous “Percoll” density gradient. Clin Chim Acta. 1982;122:293–300. doi: 10.1016/0009-8981(82)90290-x. [DOI] [PubMed] [Google Scholar]
- 38.Mosca A, Paleari R, Modenese A, et al. Clinical utility of fractionating erythrocytes into “Percoll” density gradients. Adv Exp Med Biol. 1991;307:227–38. doi: 10.1007/978-1-4684-5985-2_21. [DOI] [PubMed] [Google Scholar]
- 39.Waugh RE, Narla M, Jackson CW, et al. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood. 1992;79:1351–8. [PubMed] [Google Scholar]
- 40.Inaba M, Maede Y. Correlation between protein 4.1a/4.1b ratio and erythrocyte life span. Biochim Biophys Acta. 1988;944:256–64. doi: 10.1016/0005-2736(88)90439-7. [DOI] [PubMed] [Google Scholar]
- 41.Inaba M, Gupta KC, Kuwabara M, et al. Deamidation of human erythrocyte protein 4.1: possible role in aging. Blood. 1992;79:3355–61. [PubMed] [Google Scholar]
- 42.Shinozuka T, Takei S, Yanagida J, et al. Binding of lectins to “young” and “old” human erythrocytes. Blut. 1988;57:117–23. doi: 10.1007/BF00320150. [DOI] [PubMed] [Google Scholar]
- 43.Minetti G, Ciana A, Profumo A, et al. Cell age-related monovalent cations content and density changes in stored human erythrocytes. Biochim Biophys Acta. 2001;1527:149–55. doi: 10.1016/s0304-4165(01)00159-3. [DOI] [PubMed] [Google Scholar]
- 44.Keegan TE, Heaton A, Holme S, et al. Improved post-transfusion quality of density separated AS-3 red cells after extended storage. Br J Haematol. 1992;82:114–21. doi: 10.1111/j.1365-2141.1992.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki T, Dale GL. Membrane proteins in senescent erythrocytes. Biochem J. 1989;257(1):37–41. doi: 10.1042/bj2570037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bosman GJ, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 47.Lion N, Crettaz D, Rubin O, Tissot JD. Stored red blood cells: a changing universe waiting for its map(s) J Proteomics. 2010;73:374–85. doi: 10.1016/j.jprot.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Antonelou MH, Kriebardis AG, Stamoulis KE, et al. Red blood cell aging markers during storage in citrate-phosphate-dextrose-saline-adenine-glucose-mannitol. Transfusion. 2010;50:376–89. doi: 10.1111/j.1537-2995.2009.02449.x. [DOI] [PubMed] [Google Scholar]
- 49.Bosman GJ, Lasonder E, Groenen-Döpp YA, et al. Comparative proteomics of erythrocyte aging in vivo and in vitro. J Proteomics. 2010;73:396–402. doi: 10.1016/j.jprot.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Cottingham K. 1DE proves its worth... again. J Proteome Res. 2010;9:1636. doi: 10.1021/pr100103x. [DOI] [PubMed] [Google Scholar]
- 51.Bennett-Guerrero E, Stafford-Smith M, Waweru PM, et al. A prospective, double-blind, randomized clinical feasibility trial of controlling the storage age of red blood cells for transfusion in cardiac surgical patients. Transfusion. 2009;49:1375–83. doi: 10.1111/j.1537-2995.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 52.Campanella E, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. PNAS. 2005;102:2402–7. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castagnola M, Messana I, Sanna MT, Giardina B. Oxygen-linked modulation of erythrocyte metabolism: state of the art. Blood Transfus. 2010;8:53–8. doi: 10.2450/2010.009S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jansen G, Koenderman L, Rijksen G, et al. Characteristics of hexokinase, pyruvate kinase, and glucose-6-phosphate dehydrogenase during adult and neonatal reticulocyte maturation. Am J Hematol. 1985;20:203–15. doi: 10.1002/ajh.2830200302. [DOI] [PubMed] [Google Scholar]
- 55.Strange RC, Johnson PH, Lawton A, et al. Studies on the variability of glutathione S-transferase from human erythrocytes. Clin Chim Acta. 1982;120:251–60. doi: 10.1016/0009-8981(82)90162-0. [DOI] [PubMed] [Google Scholar]
- 56.Mosca A, Paleari R, Modenese A, et al. Clinical utility of fractionating erythrocytes into “Percoll” density gradients. Adv Exp Med Biol. 1991;307:227–38. doi: 10.1007/978-1-4684-5985-2_21. [DOI] [PubMed] [Google Scholar]
- 57.Rennie CM, Thompson S, Parker AC, Maddy A. Human erythrocyte fraction in “Percoll” density gradients. Clin Chim Acta. 1979;98:119–25. doi: 10.1016/0009-8981(79)90172-4. [DOI] [PubMed] [Google Scholar]
- 58.Brajovich ML, Rucci A, Acosta IL, et al. Effects of aging on antioxidant response and phagocytosis in senescent erythrocytes. Immunol Invest. 2009;38:551–9. doi: 10.1080/08820130902888383. [DOI] [PubMed] [Google Scholar]
- 59.Romero PJ, Romero EA. Determinant factors for an apparent increase in oxygen affinity of senescent human erythrocytes. Acta Cient Venez. 2004;55:83–5. [PubMed] [Google Scholar]
- 60.Nakao K, Wada T, Kamiyama T, et al. A direct relationship between adenosine triphosphate-level and in vivo viability of erythrocytes. Nature. 1962;194:877–8. doi: 10.1038/194877a0. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki T, Dale GL. Senescent erythrocytes: isolation of in vivo aged cells and their biochemical characteristics. Proc Natl Acad Sci USA. 1988;85:1647–51. doi: 10.1073/pnas.85.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dale GL, Norenberg SL, Suzuki T, Forman L. Altered adenine nucleotide metabolism in senescent erythrocytes from the rabbit. Prog Clin Biol Res. 1989;319:259–69. [PubMed] [Google Scholar]
- 63.D’Alessandro A, Zolla L. The SODyssey: superoxide dismutases from biochemistry, through proteomics, to oxidative stress, aging and nutraceuticals. Expert Rev Proteomics. 2011;8:405–21. doi: 10.1586/epr.11.13. [DOI] [PubMed] [Google Scholar]
- 64.Sass MD, Caruso CJ, O’Connell DJ. Decreased glutathione in aging red cells. Clin Chim Acta. 1965;11:334. doi: 10.1016/0009-8981(65)90223-8. [DOI] [PubMed] [Google Scholar]
- 65.D’Alessandro A, Righetti PG, Zolla L. The red blood cell proteome and interactome: an update. J Proteome Res. 2010;9:144–63. doi: 10.1021/pr900831f. [DOI] [PubMed] [Google Scholar]
- 66.Kanias T, Acker JP. Biopreservation of red blood cells--the struggle with hemoglobin oxidation. FEBS J. 2010;277:343–56. doi: 10.1111/j.1742-4658.2009.07472.x. [DOI] [PubMed] [Google Scholar]


