Abstract
Although hormone replacement therapy is an option for the loss of ovarian function, hormone delivery through pharmacological means results in various clinical complications. The present study was designed to deliver sex steroids by a functional construct fabricated using encapsulation techniques. Theca and granulosa cells isolated from ovaries of 21-day old rats were encapsulated in multilayer alginate microcapsules to recapitulate the native follicular structure. Cells encapsulated in two other schemes were used as controls to assess the importance of the multilayer structure. The endocrine functions of the encapsulated cells were assessed in vitro for a period of 30 days. Encapsulated cells showed sustained viability during long-term in vitro culture with those encapsulated in multilayer capsules secreting significantly higher and sustained concentrations of 17 β-estradiol (E2) than the two other encapsulation schemes (p<0.05, n=6) in response to follicle-stimulating hormone (FSH) and luteinizing hormone (LH). In addition, cells in the multilayer microcapsules also secreted activin and inhibin in vitro. In contrast, when granulosa and theca cells were cultured in 2-D culture, progesterone (P4) secretion increased while E2 secretion decreased over a 30-day period. In summary, we have designed a multilayer engineered ovarian tissue that secretes sex steroids and peptide hormones and responds to gonadotropins, thus demonstrating the ability to recapitulate native ovarian structure ex vivo.
Keywords: Cell-based hormone replacement therapy, ovarian cells, follicles, encapsulation, alginate, multilayer microcapsules
2. Introduction
Ovaries serve as gonads as well as endocrine glands, and are the primary physiological source for sex steroids. Ovarian follicles are the fundamental units of ovaries and each produces a single oocyte (germ cell), steroids, and protein hormones to regulate the reproductive cycles in females [1;2]. The ovarian hormones produced by the follicles play a crucial role in maintaining the ovarian cycle, determining secondary sexual characteristics and preparing the endometrium for implantation. The follicles become corpora lutea after ovulation and produce hormones in order to maintain the pregnancy if the oocyte is successfully fertilized [3;4]. During the follicular phase (pre-ovulatory phase) estrogens are the predominant steroids produced by the functional follicle and in the luteal phase (post-ovulatory phase) progesterone is the major steroid produced by the corpus luteum. As far as estrogen biosynthesis is concerned there exists a two-cell two-gonadotropin concept [5–9], which involves theca cells, granulosa cells, luteinizing hormone (LH) and follicle stimulating hormone (FSH). Theca cells in the periphery of the follicle possess the key enzyme CYP17A1 (17, 20 lyase) to synthesize the aromatizable androgens (androstenedione and testosterone) under the influence of LH. These aromatizable androgens are then converted by CYP19 (aromatase) in the granulosa cells into estrogens, a process regulated by FSH [10;11]. This cyclic pattern of sex steroid synthesis and secretion occurs during every ovarian cycle until all the embryonically formed primordial follicles for the production of functional follicles are exhausted.
Loss of ovarian function caused by surgical resection, ablative therapy, or menopause not only affects the reproductive ability but also leads to cessation of sex steroid production by the ovary leading to various physiological consequences in women [12;13]. Ovarian hormone-deprived conditions results in various pathological conditions ranging from urogenital complications to osteoporosis [13;14]. Although hormone replacement therapy (HRT) is able to compensate for the loss of ovarian hormone production, hormone delivery through pharmacological means results in consistently higher serum concentrations and clinical complications including increased incidence of heart disease and cancer depending on the HRT regimen used [15–19]. Therefore, cell/tissue-based hormone therapy that provides more physiological serum levels of hormones is an appealing alternative for treatment of ovarian hormone-deprived conditions. We hypothesize that the endocrine cells would respond to the endogenous levels of gonadotropins and secrete sex steroids and, in turn, the gonadotropins would be regulated by sex steroids through the feed back mechanism. In the present study, we have used encapsulation techniques in designing a tissue-engineered endocrine ovary for a cell/tissue-based HRT.
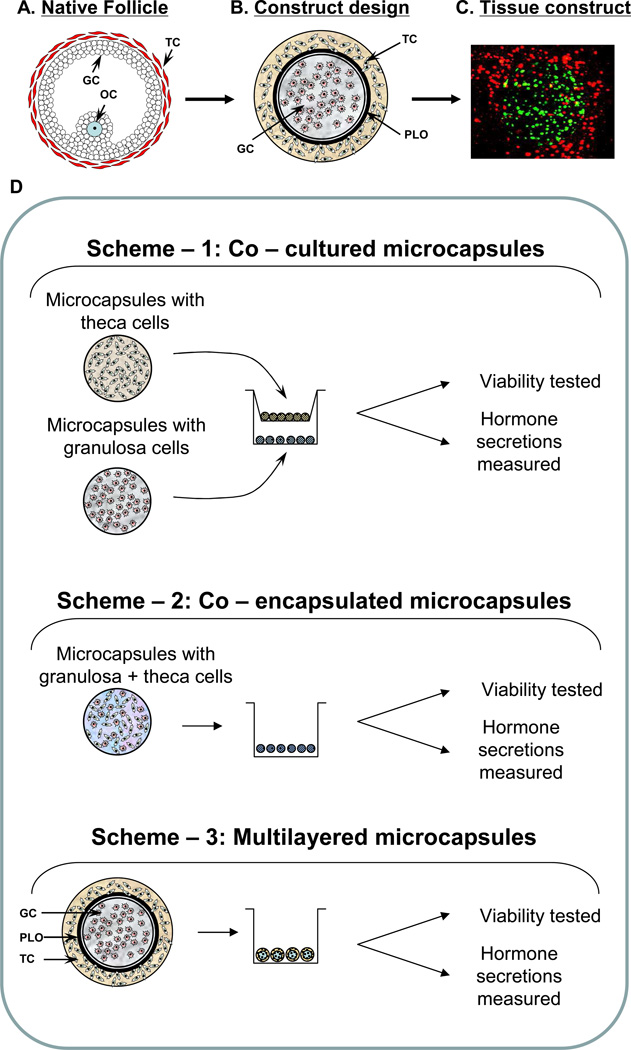
Cell encapsulation techniques are under investigation by an increasing number of research groups for a wide variety of cell/tissue-based therapies and the delivery of therapeutic agents [20–24]. However, despite the need for sustained delivery of sex hormone secretion, an optimum encapsulation technique for ovarian endocrine cells is currently not available. Since sex hormone production involves inter-cellular communication between two primary types of endocrine cells of the ovary (granulosa and theca), we hypothesize that suitable spatial arrangement of these cells in hydrogel microcapsules would be important for their optimum long-term function. Therefore, we have explored three approaches in encapsulating these endocrine cells for the assessment of their functions as ex vivo endocrine tissue constructs for HRT (see Fig. 1). In scheme 1, each cell type was encapsulated separately and co-cultured using a transwell system in the presence of FSH and LH. In scheme 2, both cell types were combined at equal proportion, co-encapsulated in the same microcapsule and then cultured with gonadotropins. In scheme 3, the granulosa and theca cells were encapsulated in different layers of multilayer microcapsules resembling the follicular architecture of ovary and cultured in similar conditions to that of the other two schemes.
Fig 1.
(A) Schematic diagram of an ovarian follicle. (B) Approach of using multilayered alginate microcapsule to mimic native follicular structure. (C) 3D - confocal image of microcapsules demonstrating compartmentalization of different cells - distribution of CellTracker green-labeled cells (granulosa) in the inner layer and CellTracker orange-labeled cells (theca) in the outer layer. (D) Outline of the study demonstrating the co-cultured/co-encapsulated/multilayer microcapsules as the three approaches used. GC – granulosa cells; OC – oocyte; TC – theca cells; PLO – poly-L-ornithine.
3. Methods
3.1 Materials
Medium 199 and McCoy’s 5A media were purchased from Gibco-BRL (Life Technologies/Gibco-BRL, Grand Island, NY). Percoll, oFSH, oLH, 17 β-estradiol, sesame oil, insulin-transferrin-selenium mix (ITS), deoxyribonuclease I (DNase 1), sodium azide, poly-L-ornithine (PLO molecular weight 15 – 30KDa) were purchased from Sigma-Aldrich (St. Louis, MO). Low viscosity (20–200 mPa·s) ultra-pure sodium alginate with high mannuronic acid (LVM) content was purchased from Nova-Matrix (Sandvika, Norway). LVM alginate was reported by the manufacturer to have molecular weights 75–200kDa and guluronic acid to mannuronic acid (G/M) ratios of ≤1. Collagenase type 1 was from Worthington (Lakewood, NJ) and insulin-like growth factor-I (IGF-I) from Peprotech (Rocky Hill, NJ). Solutions for alginate microcapsule synthesis were made using the following chemicals: HEPES, sodium chloride and calcium chloride (Fisher Scientific, Pittsburgh, PA). The vendors for other chemicals, reagents and antibodies used have been indicated in the relevant areas of this method section.
3.2. Animals
All animal studies were conducted with the approval of the Wake Forest University Health Sciences Animal Care and Use Committee. Immature female rats (21 day old Fisher 344 rats) purchased from Harlan Sprague-Dawley Inc. (Indianapolis, IN) were used as donors for the isolation of granulosa and theca cells. The immature rats were injected with 1.5 mg/0.2 ml of E2 dissolved in sesame oil, subcutaneously for three consecutive days to improve the yield of cells (E2-primed rats). The rats were euthanized 24 h after the last injection, ovaries were excised and endocrine cells were isolated from the ovaries.
3.3. Cells isolation and purification
The endocrine cells were isolated from ovaries of E2-primed immature rats according to the procedure described by Li and Hearn [25]. Ovaries were collected in ice cold medium 199 (M199) containing HEPES (25 mM), 1 mg/ml bovine serum albumin (BSA), L-glutamine (2 mM), penicillin (10 000 IU/ml), streptomycin (10 000 µg/ml), and amphotericin B (25 µg/ml). After cleaning the extraneous tissues, the ovaries were washed twice with ice cold M199 and then punctured gently with 27G syringe needles in order to release the loosely packed granulosa from the follicles; cells thus collected were kept on ice. The remaining ovaries were chopped into fine pieces of ~ 0.25 mm2 and the cells released during this process were also collected and kept on ice separately. The pieces of ovaries were then incubated with collagenase (2 mg/ml) and DNase (10 µg/ml) in M199 for 90 min with occasional mixing. The enzyme-digested pieces were dispersed using a Pasteur pipette to obtain a single cell suspension stored on ice as separate fractions. Cells from different fractions collected in the above mentioned steps were purified as described by Magoffin and Erickson [26] using a discontinuous Percoll gradient. To purify granulosa and theca cells, the density of Percoll was adjusted to 1.055 and added in between 44% and 20% Percoll as shown in Fig. 2B. The cells were then loaded on top of the discontinuous Percoll gradient and centrifuged at 400 × g for 20 min at 4°C. Cells from the first interphase (bet ween 20% and d = 1.055 layers) were recovered as granulosa cells and those from the second interphase (between d = 1.055 and 44% layers) were collected as theca cells. The viability of the cells checked using the Trypan blue method was found to be between 85–95%, with some experiment-to-experiment variability. The purity of each cell type was assessed by flow cytometric analysis using cell-specific markers.
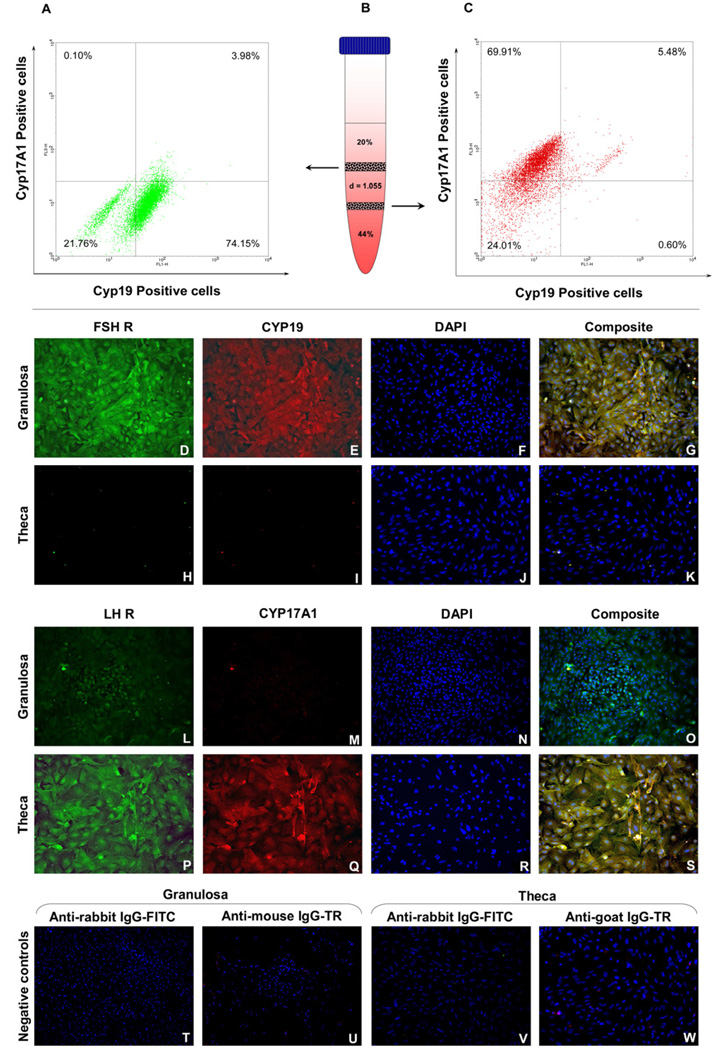
Fig 2.
Flow cytometric analysis of purity of isolated granulosa cells (A) retrieved from the first interphase of the discontinuous Percoll gradient (B) and theca cells (C) from the second interphase. Immuno-fluorescent staining for FSHR and CYP19 in granulosa (D–G) and theca cells (H–K); LHR and CYP17A1 in granulosa (L–O) and theca cells (P–S); Negative controls with secondary antibodies only on each cell type (T–W). Images acquired at 100 × magnification. FSHR – follicle-stimulating hormone receptor, LHR – luteinizing hormone receptor, CYP19 – aromatase, CYP17A1 – 17, 20 lyase, IgG – immunoglobulin G, FITC – Fluorescein isothiocyanate, TR – Texas Red.
3.4. Flow Cytometry
A fraction of each cell type (5 × 106 cells/ cell type) purified using the discontinuous Percoll gradient was fixed in 3.7% formaldehyde for 15 min. Cells from different interphases were incubated with primary antibodies for CYP19 (mouse anti-CYP19; Abbiotech, San Diego, CA) and CYP17A1 (goat anti-CYP17A1; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. Unbound primary antibodies were then washed off before further incubation for 1 h with FITC-conjugated donkey anti-mouse IgG to detect aromatase positive cells (granulosa) and PerCP-Cy5.5-conjugated donkey anti-goat IgG to detect 17, 20 lyase-positive cells (theca). After washing off the unbound secondary antibodies, cells were then analyzed using flow cytometry. Cells incubated with secondary antibodies only were used as control.
3.5. Culture of granulosa and theca cells
Purified granulosa and theca cells were incubated at 37°C under an atmosphere of 5% CO2 in humidified air until they reached 80% – 90% confluency. The granulosa cells were cultured in granulosa growth medium [McCoy’s 5A with 2 mM L-glutamine, 1 mg/ml BSA, 10 000 IU/ml penicillin, 10 000 µg/ml streptomycin, 25 µg/ml amphotericin B, ITS (10 µg/ml insulin; 5.5 µg/ml transferrin; 5 ng/ml selenium; 0.5 mg/ml BSA; 4.7 µg/ml linoleic acid), 200 ng/ml FSH, 100 nM E2 and 10 nM IGF-I]. Similarly the theca cells were grown in theca growth medium [McCoy’s 5A with 2 mM L-glutamine, 1 mg/ml BSA, 10 000 IU/ml penicillin, 10 000 µg/ml streptomycin, 25 µg/ml amphotericin B, ITS (10 µg/ml insulin; 5.5 µg/ml transferrin; 5 ng/ml selenium; 0.5 mg/ml BSA; 4.7 µg/ml linoleic acid), 100 ng/ml LH, and 10 nM IGF-I]. Upon reaching 80% – 90% confluent stage (~ 96 h), the granulosa and theca cells were lifted from the culture flask with a cell scrapper and used for further studies.
3.6. Immuno-fluorescence staining
Each cell type was cultured on chamber slides in respective growth medium and was screened for the expression of essential cellular components for steroidogenesis. After fixing the cells in 3.7% formaldehyde for 15 min, they were washed with PBS and blocked with PBS containing BSA (1% w/v). The monolayer was then incubated overnight with primary antibodies at 4°C. Granulosa cells were incubated with rabbit anti-FSHR (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-CYP19 (Abbiotech, San Diego, CA). Similarly theca cells were incubated with rabbit anti-LHR (Santa Cruz Biotechnology, Santa Cruz, CA) and goat anti-CYP17A1 (Santa Cruz Biotechnology, Santa Cruz, CA). After overnight incubation with primary antibodies the slides were washed with PBS and incubated with secondary antibodies for 2 h at 4°C. The unbound secondary antibodies were washed away and the nucleus was counterstained with DAPI and cover slips were mounted. The images were acquired using fluorescence microscope (Zeiss Axiovert 200M) and composite images were made with the help of Image-Pro plus software (version 6.3.1.542).
3.7 Evaluation of the steroidogenic potential of granulosa and theca cells in monolayer (2D) culture system
To assess the steroidogenic potential of granulosa and theca cells in response to FSH and LH during long-term monolayer culture, granulosa and theca cells (105 cells/cell type/well) were co-cultured using a transwell system (EMD Millipore, Billerica, MA). Granulosa and/or theca cells were seeded at the same density separately and cultured as single-cell controls. Cells from all the groups (both in co-culture and the single-cell culture groups) were cultured in the absence and the presence of 100 ng/ml FSH and 100 ng/ml LH for a period of 30 days and the culture media were collected every alternate day to assess the secretion of sex steroids. The removed incubation media were replaced with fresh media containing the above-mentioned concentrations of gonadotropins.
3.8. Encapsulation
The granulosa and theca cells were encapsulated in alginate hydrogel using a micro-fluidic device recently described by our group [27] and cultured using three different schemes (Fig. 1D). In first scheme, each cell type was encapsulated in 1.5% (w/v) ultrapure LVM alginate as separate microcapsules and then co-cultured using a transwell (EMD Millipore, Billerica, MA) system (referred as co-culture microcapsules). In the second scheme, both cell types were mixed together in equal proportion prior to encapsulation in 1.5% (w/v) LVM, so that each microcapsule contained both cells (referred to as co-encapsulated microcapsules). In the third scheme, using a multilayer microcapsule design, which we have previously described [21;23], first the granulosa cells were encapsulated in 1.5% (w/v) LVM and coated with PLO (0.1% w/v) for 20 min. The PLO-coated microcapsules were then mixed with theca cells suspended in 1.5% (w/v) LVM and encapsulated again using the micro-fluidic device in order to obtain multilayer microcapsules whose structural architecture resembles that of native follicles as illustrated in Fig. 1 (referred to as multilayer microcapsules). To demonstrate the differential compartmentalization of the different cell types in the multilayer microcapsules, the granulosa cells were pre-stained with Cell Tracker green (Life Technologies/Invitrogen, Grand Island, NY) and the theca cells were pre-stained with Cell-tracker Orange (Life Technologies/Invitrogen, Grand Island, NY), prior to the synthesis of the multilayered microcapsules. The multilayer microcapsules were then imaged to visualize the cell types using confocal microscope (Zeiss LSM510). In addition, to determine the importance of the cell-cell interactions between the theca cells (TC) and granulosa cells (GC) each cell type was encapsulated individually as mentioned in scheme 1 and cultured separately in the presence of gonadotropins as “single-cell” controls for all the experiments. In each group, the number of microcapsules was adjusted to accommodate 105 cells of each cell type/ well.
3.9. Live/dead analysis of cells in micro-capsules
The viability of the encapsulated cells was assessed using live/dead assay as follows: Once in every three days some of capsules from each scheme (cultured separately in the similar conditions) were transferred to a 24-well plate and incubated with 25 µM CFDA SE (carboxyfluorescein diacetate, succinimidyl ester) (Life Technologies/Invitrogen, Grand Island, NY) in serum-free medium for 15 min at 37°C under an atmosphere of 5% CO2 in humidified air. Then the CFDA SE containing medium was replaced with medium containing 10% FBS and incubated again under the above-mentioned conditions for another 30 min. The serum-containing medium was then replaced with 50 µg/ml of propidium iodide (PI) (Life Technologies/Invitrogen, Grand Island, NY) and incubated at room temperature for 2 min and the microcapsules were washed to remove excess PI. The microcapsules were then observed under inverted fluorescence microscope and imaged. The number of live and dead cells was determined qualitatively from the composite image acquired using Image-Pro plus software (version 6.3.1.542).
3.10. Measurement of 17 β-estradiol, progesterone, activin and inhibin
To evaluate the endocrine functions of un-encapsulated and encapsulated ovarian cells, the levels of E2, P4, activin and inhibin were measured in the culture media. Encapsulated granulosa and theca cells of all three schemes were cultured in the absence and the presence of FSH (100 ng/ml) and LH (100 ng/ml) for a period of 30 days and the culture media were collected every alternate day to assess the secretion of sex steroids, activin and inhibin. The levels of sex steroids (E2, P4) (Enzo, Life sciences, Plymouth Meeting, PA), activin (R&D systems Inc., Minneapolis, MN) and inhibin (Life Sciences Advance Technologies Inc., St. Petersburg, FL) in the culture media were measured using ELISA kits following the manufacturers’ instructions. While E2 and P4 were measured using competitive immunoassay kits, activin and inhibin were analyzed using sandwich immunoassay kits. According to manufacturer’s instructions, the inhibin ELISA kit detects both inhibin–A and –B levels (total inhibin levels), whereas activin ELISA kit measures the levels of activin-A.
3.11. Statistical Analysis
Statistical analyses were performed using SPSS software (version 10.0.1). Results are presented as the mean ± S.E.M unless stated otherwise. Comparisons between the means of hormone levels in different groups were performed using analysis of variance (ANOVA) followed by post-hoc testing using Bonferroni correction when appropriate. Differences were considered to be statistically significant when p<0.05.
4. Results
4.1. Expression of gonadotropin receptors and steroidogenic enzymes
The purity of granulosa and theca cells separated on the discontinuous Percoll gradient was assessed by flow cytometry using cell-specific markers. While antibody for CYP19 and FITC-conjugated secondary antibody was used to detect the granulosa cells, antibody for CYP17A1 and PerCP Cy5.5-conjugated secondary antibody was used to detect the theca cells. The flow cytometric analysis revealed that 74.15% of the cells recovered from the first interphase in the Percoll gradient stained positive for CYP19 (Fig. 2A) and 69.91% of the cells obtained from the second interphase were stained for CYP17A1 (Fig. 2C). To ensure that both endocrine cells possess other essential cellular components for steroidogenesis, the granulosa and theca cells were screened for the expression of cell-specific gonadotropin receptor and steroidogenic enzyme by immunofluorescence staining. While granulosa cells stained positive for FSH receptor (FSHR) and CYP19 (Fig. 2D–G), theca cells stained negative for both FSHR and CYP19 (Fig. 2H–K). On the other hand theca cells and some of the granulosa cells stained positive for LH receptor (LHR), but CYP17A1 was detectable only in theca cells (Fig. 2L–S).
4.2. Endocrine functions of granulosa and theca cells in vitro 2D culture
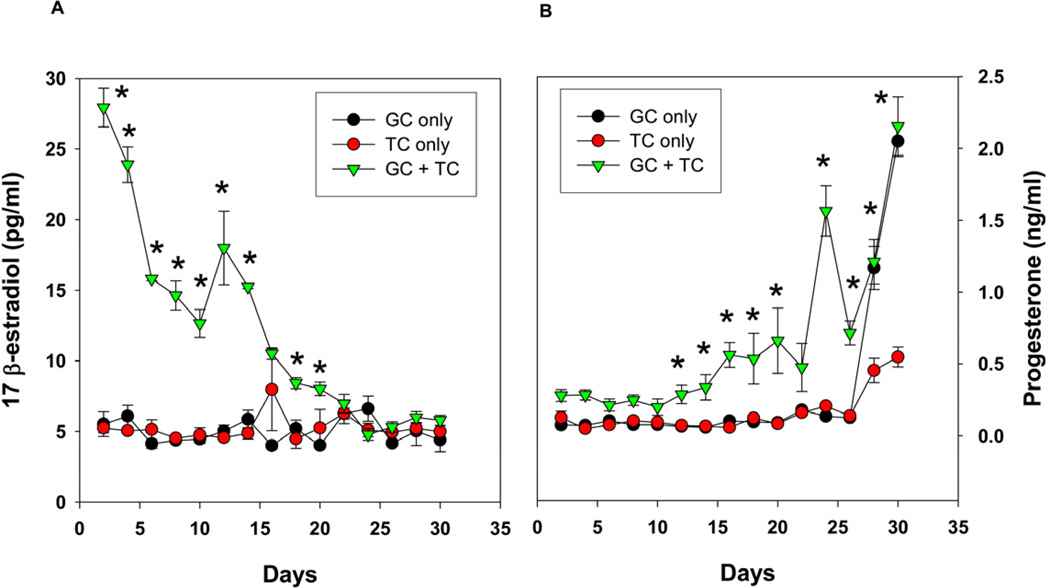
In order to determine the baseline steroidogenic potential of these cells cultured in 2-D for comparison to our 3-D encapsulation system, the granulosa and theca cells were co-cultured in the absence and the presence of FSH and LH in a two-dimensional culture system using culture inserts and their secretion of E2 and P4 were measured in the culture media collected every alternate day. When compared to either granulosa or theca cells alone, the co-cultured cells secreted significantly higher levels of E2 after 48 h of treatment with FSH and LH, but the levels of E2 declined with time and reached their basal detectable levels after 3 weeks (Fig. 3A). Similarly, progesterone secretion in co-culture was also significantly higher than that of individual cell culture. In contrast to E2, progesterone secretion in the culture media was at the basal detection limit initially, but increased after two weeks and reached maximum by the end of the long-term culture (Fig. 3B).
Fig 3.
(A) 17 β-estradiol production as a function of time by co-cultured granulosa cells with theca cells in the presence of FSH and LH in a 2D co-culture system. (B) Progesterone production by co-cultured granulosa cells with theca cells in response to FSH and LH in 2D system. The results indicate the loss of estrogen production with time and the increase in progesterone, indicating luteal differentiation. Each data point represents mean ± SEM of 6 values (3 wells/group assayed in duplicates). * denotes statistical significance at P <0.05 compared to granulosa and/or theca cells alone. The figures represent data from one of two separate experiments.
4.3. Serial encapsulation facilitates the synthesis of multi-layered microcapsules
In an effort to fabricate a more physiologically-relevant construct for the tissue-engineered ovarian tissue, the architectural design of native follicle was adopted wherein the granulosa cells were placed in a central alginate core enveloped by a semi-permeable membrane made with PLO, which was then surrounded by an outer layer of alginate with theca cells (Fig. 1A – B). To demonstrate the differential compartmentalization of cells in the multilayer microcapsules, the granulosa and theca cells were stained with different Cell-Tracker flurophores as mentioned earlier. Confocal imaging showed the distribution of granulosa cells in the inner alginate core and the theca cells in the outer alginate layer (Fig. 1C).
4.4. Viability of encapsulated ovarian endocrine cells
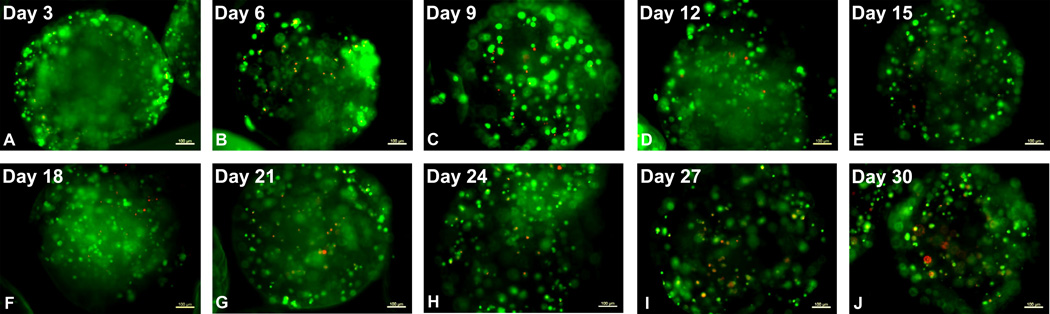
The viability of encapsulated ovarian endocrine cells was determined by live/dead staining using CFDA and PI respectively. The images presented in Fig. 4 are representative of several microcapsules stained at each time point. As shown in Fig. 4, cell viability was maintained over the course of 30 days in culture (indicated by green pseudocolor fluorescence for CFDA). While the presence of dead cells increased over time (as indicated by red pseudocolor fluorescence for PI), there were relatively few dead cells observed even at 30 days in culture. The images shown are for the multilayer scheme, but similar results were obtained with all schemes (data not shown).
Fig 4.
Live/Dead staining of cells in the multilayer microcapsules at specified time points. The image presented for each time point is a single construct and representative of several constructs stained at each time point. The number of live and dead cells was determined qualitatively from the composite image. Green represents live cells and red represents the dead cells and the results indicate that cell viability was maintained over the course of 30 days in culture. Scale bar on the images depict 100 µm.
4.5. Sex steroid secretion by the encapsulated ovarian cells in vitro
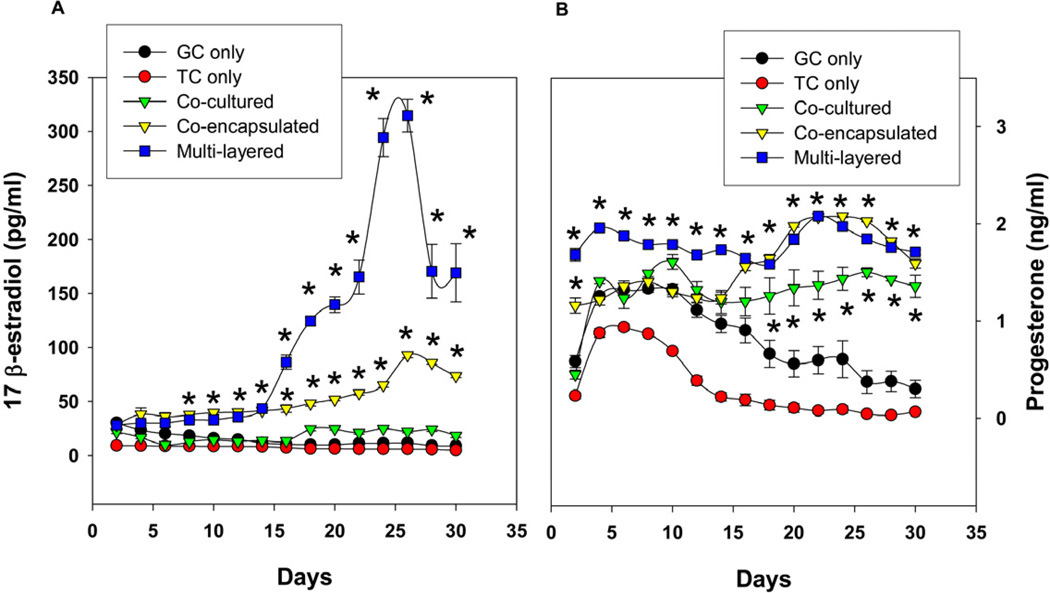
To assess the endocrine function of encapsulated ovarian cells, the two cell types were encapsulated in alginate hydrogel and cultured using three different schemes as mentioned earlier (Fig. 1D). In the first scheme E2 secretion by encapsulated cells in response to FSH and LH was detected only after 2 weeks where their levels were twice that of the single-cell controls (p<0.05, n = 6; Fig. 5A). Unlike scheme 1, cells encapsulated in the second scheme responded to gonadotropins from day 2 onwards and the levels constantly increased with time and reached four fold around the fourth week in comparison with their initial levels (Fig. 5B). Similarly, the cells encapsulated in multilayer microcapsules also exhibited an early response to FSH and LH in E2 secretion. However importantly, there was a ten-fold increase in the E2 secretion observed in the third scheme (multilayer) compared to their initial levels after two weeks, which was significantly higher than the other two schemes (p<0.05, n=6; Fig. 5A). Progesterone (P4) secretions by cells from all three encapsulation schemes were significantly higher with FSH and LH stimulation at all the time points when compared to the basal levels and single-cell controls (Fig. 5B). The secretion of E2 and P4 in response to FSH and LH were significantly higher than the basal conditions in all three schemes at most of the time points (Supplement to figure 5).
Fig 5.
Sustained E2 (A) and P4 (B) secretion by granulosa and theca cells encapsulated using three different schemes. In contrast to 2-D cultures, E2 levels are maintained or increased with time and the presence of both GC and TC (either through Schemes 1, 2, or 3) led to increased levels of E2 production compared to GC or TC alone. Multi-layer cultures led to significantly increased levels of E2 production. Each data point represents mean ± SEM of 6 values (3 wells/group assayed in duplicates). * denotes statistical significance at P <0.05 compared to granulosa and/or theca cells alone. The figures represent data from one of two separate experiments.
4.6. Ovarian peptide secretion by the encapsulated ovarian cells in vitro
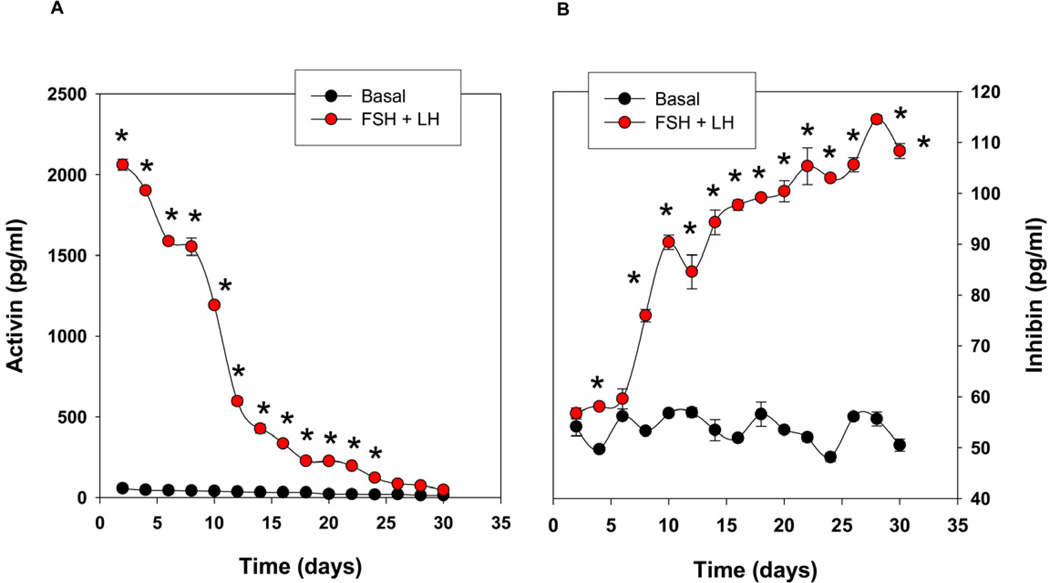
In addition to secreting sex steroids, the ovary is also responsible for the secretion of activin and inhibin that establish the feedback loop with the hypothalamus and pituitary. Owing to the profound response of cells in the multilayer capsules to the gonadotropins, we further explored the production of these two key polypeptide hormones, activin and inhibin secreted by the follicular cells, in scheme 3. Compared with the basal condition (without LH/FSH), cells encapsulated in the multilayer microcapsules secreted significantly higher levels of activin in response to FSH and LH after 48 h, but its levels gradually declined with time during the long-term culture (Fig. 6A). In contrast to activin, the levels of inhibin production remained at baseline up to day 6 and increased ~ 2 fold basal after two weeks of the culture (Fig. 6B).
Fig 6.
Secretion of activin (A) and inhibin (B) in vitro by the encapsulated cells in the multilayered microcapsules suggesting that the construct is also capable of secreting peptide hormones in addition to the sex steroids. Each data point represents mean ± SEM of 6 values (3 wells/group assayed in duplicates). * denotes statistical significance at P <0.05 compared to the basal level (without FSH/LH). The figures represent data from one of two separate experiments.
5. Discussion
Even though HRT has been reported to rectify the imbalance in the hormonal levels after the loss of ovarian function [17;28], the risks with such therapies may outweigh the benefits [15;18;19]. The major drawback in using the current pharmacological means is that the hormone delivery is not controlled by the innate hypothalamo-pituitary-gonadal (HPG) axis. To address this problem, we have designed an ovarian endocrine cell/tissue construct for cell-based HRT as an alternative to the pharmacological agents. The construct would permit endocrine tissue interaction with the HPG axis to release sex steroids accordingly. The greatest challenge for cell/tissue-based HRT has been the lack of an appropriate means of delivering these cells into the body. Cell encapsulation techniques with biomaterials such as alginate have the potential to provide an elegant solution to this problem.
Alginate has been predominantly used in encapsulation because of its several appealing properties [29]. The anchorage-independent culture helps the cells to attain a more natural, physical configuration resembling in vivo three-dimensionality. In addition, this configuration provides much better exposure of the cell membrane which augments the exchange of nutrients and gases from multiple directions unlike the 2D system. The suitable pore size formed by the hydrogel facilitates the exchange of bio-molecules in and out of the microcapsules quickly [30;31]. The soft nature of the hydrogel has been shown to allow the expansion of the growing cells in this 3D scaffold structure [32;33]. The controllable gelation property adds advantage to this material in attaining desired shape and size [34]. The anionic nature of the alginate allows the interaction with polycationic molecules like poly-L-Lysine (PLL), poly-D-Lysine (PDL) and poly-L-Ornithine (PLO) and helps not only in the immunoisolation of the cells but also in making layer-by-layer architecture for the synthesis of follicle-like microcapsules [35–37].
Since encapsulation with alginate has been successfully employed for delivering insulin in type-1 diabetes [23;35], we reasoned that the use of an alginate-based system would be suitable for the delivery of sex hormones. In addition, alginate or alginate-composite materials have been successfully employed for in vitro maturation of ovarian follicles [31;38;39], suggesting that endocrine cells of the ovary may possess sustained viability ex vivo. Lastly, the encapsulation techniques employed for alginate-based materials and the resulting microcapsules serendipitously mimic the native shape of ovary follicles and techniques to achieve multiple alginate layers have previously been reported [23] suggesting that similar techniques could be employed to further recapitulate the native architecture of the endocrine ovary as a means to create a cell/tissue-based HRT.
Since the ovary involves two cell types for the biosynthesis and secretion of estrogens, both granulosa and theca cells were isolated from immature rats and cultured in the presence of FSH and LH, respectively in order to maintain their phenotypes. The immuno-fluorescence staining confirmed the presence of essential receptor and enzymes in each cell type for the successful steroidogenesis of estrogens. Still these cells were not able to produce sustained levels of estrogens during the long-term co-culture in a 2D system, instead, the levels of progesterone increased with time in the culture media. Ovarian cells cultured on 2D surfaces have been previously reported to differentiate into luteal cells due to the limitation of space for the growing cells in the 2D system and the morphological phenotype acquired on such surfaces [40;41]. Moreover, the two dimensional culture does not replicate the conditions of in vivo system. It has been shown in several studies that cells grown in a hydrogel system exhibited improved function that resembles that of in vivo conditions [42–45].
In contrast to the 2D culture, we found that 3D culture promoted maintenance of several key physiological aspects of ovary endocrine cells. First, the alginate hydrogel supported the long-term survival of encapsulated cells in our study, probably because of its soft material properties, 3D aspects, and porous nature to allow nutrient diffusion in and waste and hormonal secretions out of the microcapsules. The 3D spatial arrangement of these two endocrine cell types in the native ovarian follicle is known to be very critical, since intercellular communications are required between them for their functions [46–48]. This important property of the in vivo system was the motivation for exploring the multi-layer approach (scheme 3) in the present study. Our results highlight the importance of the structure-function relationship of the granulosa and theca cells as evidenced by the significantly increased levels of E2 secretion in the multi-layer scheme compared to schemes 1 and 2. The multilayer microcapsules also produced a steady level of progesterone suggesting that this design mimics the in vivo set-up of the cells within the pre-ovulatory follicles. In scheme 2 where both cells were mixed and co-encapsulated in same capsule, the levels of progesterone levels increased with time indicating enhanced luteal differentiation of the endocrine cells over time. This may have been due to the over-crowding of cells in the capsules with time or mixing of both cell types, as both conditions are known to induce luteal differentiation, where biosynthesis of estrogen diminishes and progesterone increases [41;49]. The presence of FSH and E2 has been reported not only to prevent ovarian cell atresia but also to maintain the aromatase activity during the long term culture. In addition, the inhibin secreted by these cells has been shown to potentiate steroidogenesis which may have contributed to the improved E2 secretion by these endocrine cells [50;51].
Since cells in the multilayer capsules were observed to function in similar fashion to the native follicle, and it is possible to expand the isolated endocrine cells in culture, large quantities of endocrine tissue constructs could be fabricated from the limited number of available follicles. The inhibin secretion by these constructs suggests that these multilayer microcapsules could function as a potential tissue-engineered endocrine ovary by synchronizing with the innate HPG axis. Addition of another layer of PLO around the theca cells in the multilayer microcapsule would provide protection from host immune system for both the granulosa cells and theca cells, thus making it possible to use the construct as either allografts or xenografts.
5. Conclusion
Our study demonstrates that cell encapsulation techniques applied to ovarian endocrine cells can be used to achieve sustained release of key sex hormones. More importantly, our results indicate that recapitulating the structure-function relationships of the native follicles of ovary in a construct (see Fig. 1) leads to sustained secretion of adequate levels of 17 β-estradiol (E2), progesterone (P4), activin and inhibin. Specifically, the multilayer alginate microcapsule that recapitulates the structural architecture of native follicle demonstrates the importance of spatial arrangement of two cell types in order to maximize the function of the ovarian endocrine cells. We have found that tissue-engineered ovarian endocrine tissue are capable of enhanced secretion of E2, P4, activin, and inhibin in vitro, thus showing that the endocrine unit of ovaries could be recapitulated ex vivo. This study thus underlies the importance of maintaining structure-function relationships within tissue engineered constructs and highlights the role that biomaterials can play in forming these relationships in tissue engineering.
Supplementary Material
Secretion of E2 and P4 in vitro by encapsulated ovarian cells at basal conditions and in response to FSH and/or LH. 48 h secretion of E2 and P4 were measured in the culture media of all the groups at day 10 (A, C) and day 20 (B, D). Different alphabets above the bars denote significance at P < 0.05 between groups.
Acknowledgments
We gratefully acknowledge the financial supported by Jack and Pamela Egan Family Foundation and the National Institutes of Health (grant # R01DK080897).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sivanandane Sittadjody, Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC 27101
Justin M. Saul, Department of Chemical and Paper Engineering, Miami University, Oxford, OH 45056
Sunyoung Joo, Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC 27101
James J. Yoo, Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC 27101
Anthony Atala, Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC 27101.
Emmanuel C. Opara, Email: eopara@wakehealth.edu, Wake Forest Institute for Regenerative Medicine, Wake Forest University School of Medicine, Winston-Salem, NC 27101, Tel: 336-713-1297, Fax: 336-713-7290.
Reference List
- 1.Adashi EY. Endocrinology of the ovary. Hum Reprod. 1994;9(5):815–827. doi: 10.1093/oxfordjournals.humrep.a138602. [DOI] [PubMed] [Google Scholar]
- 2.Drummond AE. The role of steroids in follicular growth. Reprod Biol Endocrinol. 2006;4:16. doi: 10.1186/1477-7827-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wierman ME. Sex steroid effects at target tissues: mechanisms of action. Adv Physiol Educ. 2007;31(1):26–33. doi: 10.1152/advan.00086.2006. [DOI] [PubMed] [Google Scholar]
- 5.Falck B. Site of production of oestrogen in the ovary of the rat. Nature. 1959;184(Suppl 14):1082. doi: 10.1038/1841082a0. [DOI] [PubMed] [Google Scholar]
- 6.Falck B. Site of production of oestrogen in rat ovary as studied in micro-transplants. Acta Physiol Scand Suppl. 1959;47(163):1–101. doi: 10.1111/j.1748-1716.1960.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 7.Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the 'two-cell, two-gonadotrophin' model revisited. Mol Cell Endocrinol. 1994;100(1–2):51–54. doi: 10.1016/0303-7207(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi M, Nakano R, Ooshima A. Immunohistochemical localization of pituitary gonadotrophins and gonadal steroids confirms the 'two-cell, two-gonadotrophin' hypothesis of steroidogenesis in the human ovary. J Endocrinol. 1990;126(3):483–488. doi: 10.1677/joe.0.1260483. [DOI] [PubMed] [Google Scholar]
- 9.Short RV. Ovarian steroid synthesis and secretion in vivo. Recent Prog Horm Res. 1964;20:303–340. [PubMed] [Google Scholar]
- 10.Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev. 1985;6(3):371–399. doi: 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- 11.Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15(6):725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med. 2004;229(10):977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson JC. A woman's journey through the reproductive, transitional and postmenopausal periods of life: impact on cardiovascular and musculo-skeletal risk and the role of estrogen replacement. Maturitas. 2011;70(2):197–205. doi: 10.1016/j.maturitas.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Gambrell RD., Jr The menopause. Invest Radiol. 1986;21(4):369–378. doi: 10.1097/00004424-198604000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Gambacciani M, Monteleone P, Sacco A, Genazzani AR. Hormone replacement therapy and endometrial, ovarian and colorectal cancer. Best Pract Res Clin Endocrinol Metab. 2003;17(1):139–147. doi: 10.1016/s1521-690x(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 16.Gambacciani M, Genazzani AR. Hormone therapy. Menopause. 2003;10(3):266–267. doi: 10.1097/00042192-200310030-00015. [DOI] [PubMed] [Google Scholar]
- 17.Maclaran K, Stevenson JC. Primary prevention of cardiovascular disease with HRT. Womens Health. 2012;8(1):63–74. doi: 10.2217/whe.11.87. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson JC, Hodis HN, Pickar JH, Lobo RA. HRT and breast cancer risk: a realistic perspective. Climacteric. 2011;14(6):633–636. doi: 10.3109/13697137.2011.590618. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson JC. Prevention of osteoporosis: one step forward, two steps back. Menopause Int. 2011;17(4):137–141. doi: 10.1258/mi.2011.011112. [DOI] [PubMed] [Google Scholar]
- 20.Darrabie MD, Kendall WF, Jr, Opara EC. Characteristics of Poly-L-Ornithine-coated alginate microcapsules. Biomaterials. 2005;26(34):6846–6852. doi: 10.1016/j.biomaterials.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Khanna O, Moya ML, Opara EC, Brey EM. Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A. 2010;95(2):632–640. doi: 10.1002/jbm.a.32883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 23.Opara EC, Mirmalek-Sani SH, Khanna O, Moya ML, Brey EM. Design of a bioartificial pancreas(+) J Investig Med. 2010;58(7):831–837. doi: 10.231/JIM.0b013e3181ed3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong H, Chang TM. Bioartificial liver: implanted artificial cells microencapsulated living hepatocytes increases survival of liver failure rats. Int J Artif Organs. 1986;9(5):335–336. [PubMed] [Google Scholar]
- 25.Li SK, Hearn MT. Isolation of thecal cells: an assessment of purity and steroidogenic potential. J Biochem Biophys Methods. 2000;45(2):169–181. doi: 10.1016/s0165-022x(00)00107-x. [DOI] [PubMed] [Google Scholar]
- 26.Magoffin DA, Erickson GF. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology. 1988;122(5):2345–2347. doi: 10.1210/endo-122-5-2345. [DOI] [PubMed] [Google Scholar]
- 27.Tendulkar S, Mirmalek-Sani SH, Childers C, Saul J, Opara EC, Ramasubramanian MK. A three-dimensional microfluidic approach to scaling up microencapsulation of cells. Biomed Microdevices. 2012;14(3):461–469. doi: 10.1007/s10544-011-9623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 29.Santos E, Zarate J, Orive G, Hernandez RM, Pedraz JL. Biomaterials in cell microencapsulation. Adv Exp Med Biol. 2010;670:5–21. doi: 10.1007/978-1-4419-5786-3_2. [DOI] [PubMed] [Google Scholar]
- 30.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101(7):1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 31.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28(30):4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rokstad AM, Kulseng B, Strand BL, Skjak-Braek G, Espevik T. Transplantation of alginate microcapsules with proliferating cells in mice: capsular overgrowth and survival of encapsulated cells of mice and human origin. Ann N Y Acad Sci. 2001;944:216–225. doi: 10.1111/j.1749-6632.2001.tb03834.x. [DOI] [PubMed] [Google Scholar]
- 33.Stabler CL, Sambanis A, Constantinidis I. Effects of alginate composition on the growth and overall metabolic activity of betaTC3 cells. Ann N Y Acad Sci. 2002;961:130–133. doi: 10.1111/j.1749-6632.2002.tb03065.x. [DOI] [PubMed] [Google Scholar]
- 34.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 35.de VP, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27(32):5603–5617. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Orive G, Tam SK, Pedraz JL, Halle JP. Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials. 2006;27(20):3691–3700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 37.Ponce S, Orive G, Hernandez R, Gascon AR, Pedraz JL, de Haan BJ, et al. Chemistry and the biological response against immunoisolating alginate-polycation capsules of different composition. Biomaterials. 2006;27(28):4831–4839. doi: 10.1016/j.biomaterials.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 38.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27(5):714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Lawson MS, Yeoman RR, Pau KY, Barrett SL, Zelinski MB, et al. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26(5):1061–1072. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meidan R, Girsh E, Blum O, Aberdam E. In vitro differentiation of bovine theca and granulosa cells into small and large luteal-like cells: morphological and functional characteristics. Biol Reprod. 1990;43(6):913–921. doi: 10.1095/biolreprod43.6.913. [DOI] [PubMed] [Google Scholar]
- 41.Portela VM, Zamberlam G, Price CA. Cell plating density alters the ratio of estrogenic to progestagenic enzyme gene expression in cultured granulosa cells. Fertil Steril. 2010;93(6):2050–2055. doi: 10.1016/j.fertnstert.2009.01.151. [DOI] [PubMed] [Google Scholar]
- 42.Parsons-Wingerter PA, Saltzman WM. Growth versus function in the three-dimensional culture of single and aggregated hepatocytes within collagen gels. Biotechnol Prog. 1993;9(6):600–607. doi: 10.1021/bp00024a006. [DOI] [PubMed] [Google Scholar]
- 43.Saltzman WM, Parkhurst MR, Parsons-Wingerter P, Zhu WH. Three-dimensional cell cultures mimic tissues. Ann N Y Acad Sci. 1992;665:259–273. doi: 10.1111/j.1749-6632.1992.tb42590.x. [DOI] [PubMed] [Google Scholar]
- 44.Sharma R, Greenhough S, Medine CN, Hay DC. Three-dimensional culture of human embryonic stem cell derived hepatic endoderm and its role in bioartificial liver construction. J Biomed Biotechnol. 2010;2010:236147. doi: 10.1155/2010/236147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber LM, Hayda KN, Haskins K, Anseth KS. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28(19):3004–3011. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res. 2009;2(1):9. doi: 10.1186/1757-2215-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13(3):289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 48.Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140(4):489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- 49.Bosc MJ, Nicolle A. Steroid productions by co-cultures of granulosa cells with inner and outer theca cells in preovulatory follicles of gonadotropin stimulated calves. J Steroid Biochem Mol Biol. 1997;62(2–3):213–221. doi: 10.1016/s0960-0760(97)00030-7. [DOI] [PubMed] [Google Scholar]
- 50.Campbell BK, Baird DT. Inhibin A is a follicle stimulating hormone-responsive marker of granulosa cell differentiation, which has both autocrine and paracrine actions in sheep. J Endocrinol. 2001;169(2):333–345. doi: 10.1677/joe.0.1690333. [DOI] [PubMed] [Google Scholar]
- 51.Young JM, McNeilly AS. Inhibin removes the inhibitory effects of activin on steroid enzyme expression and androgen production by normal ovarian thecal cells. J Mol Endocrinol. 2012;48(1):49–60. doi: 10.1530/JME-11-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Secretion of E2 and P4 in vitro by encapsulated ovarian cells at basal conditions and in response to FSH and/or LH. 48 h secretion of E2 and P4 were measured in the culture media of all the groups at day 10 (A, C) and day 20 (B, D). Different alphabets above the bars denote significance at P < 0.05 between groups.