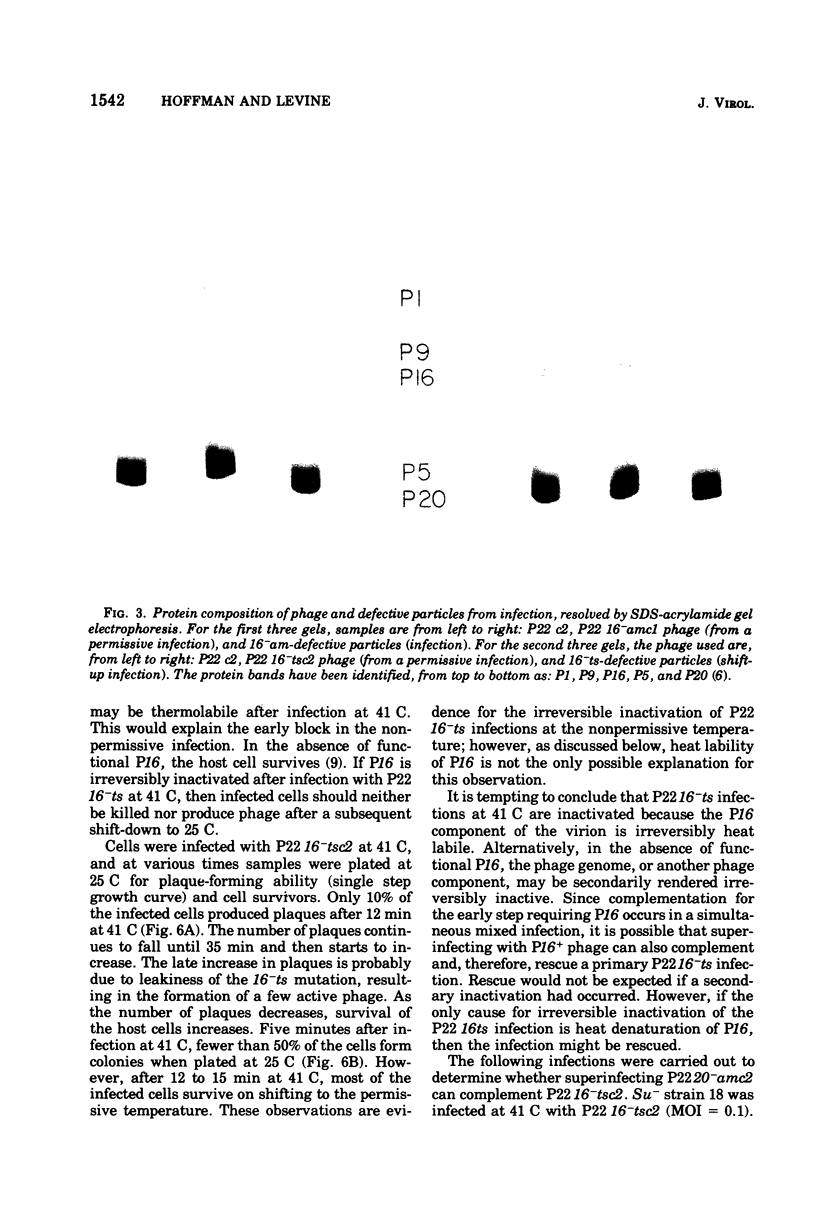
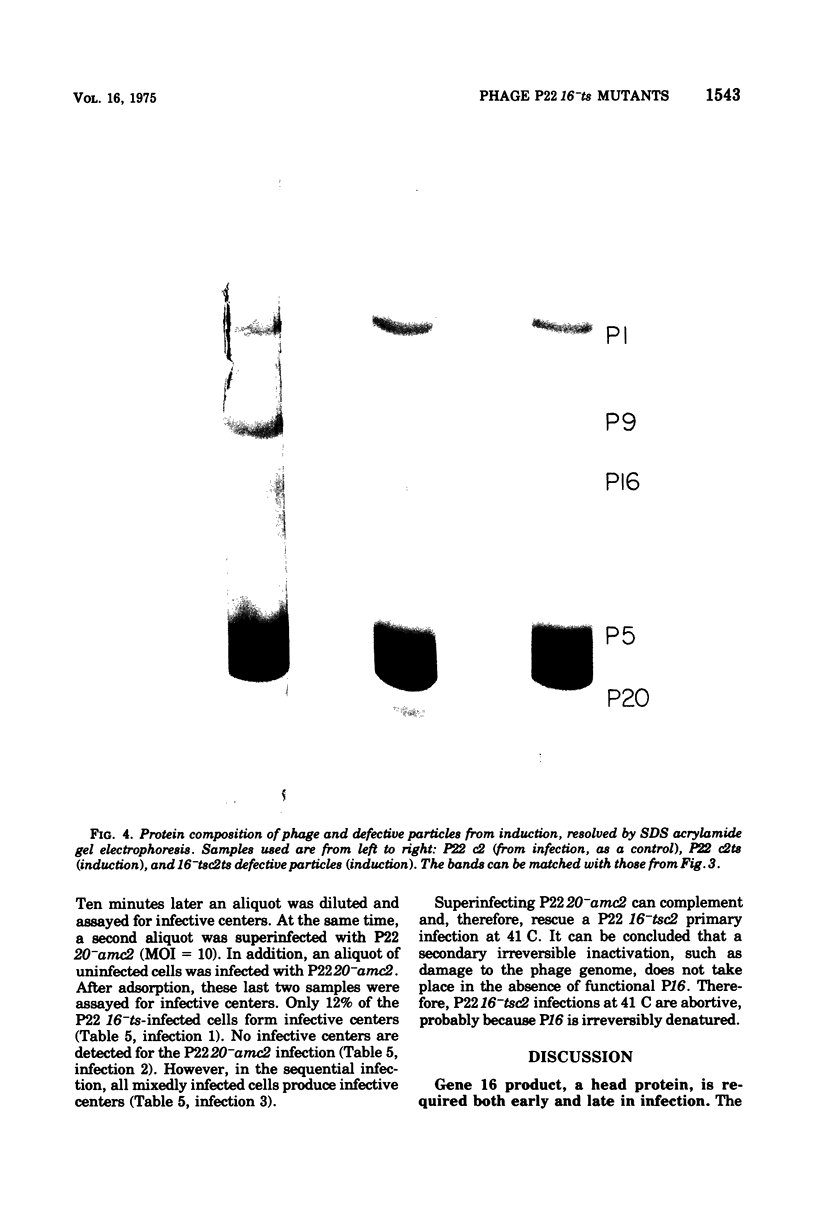
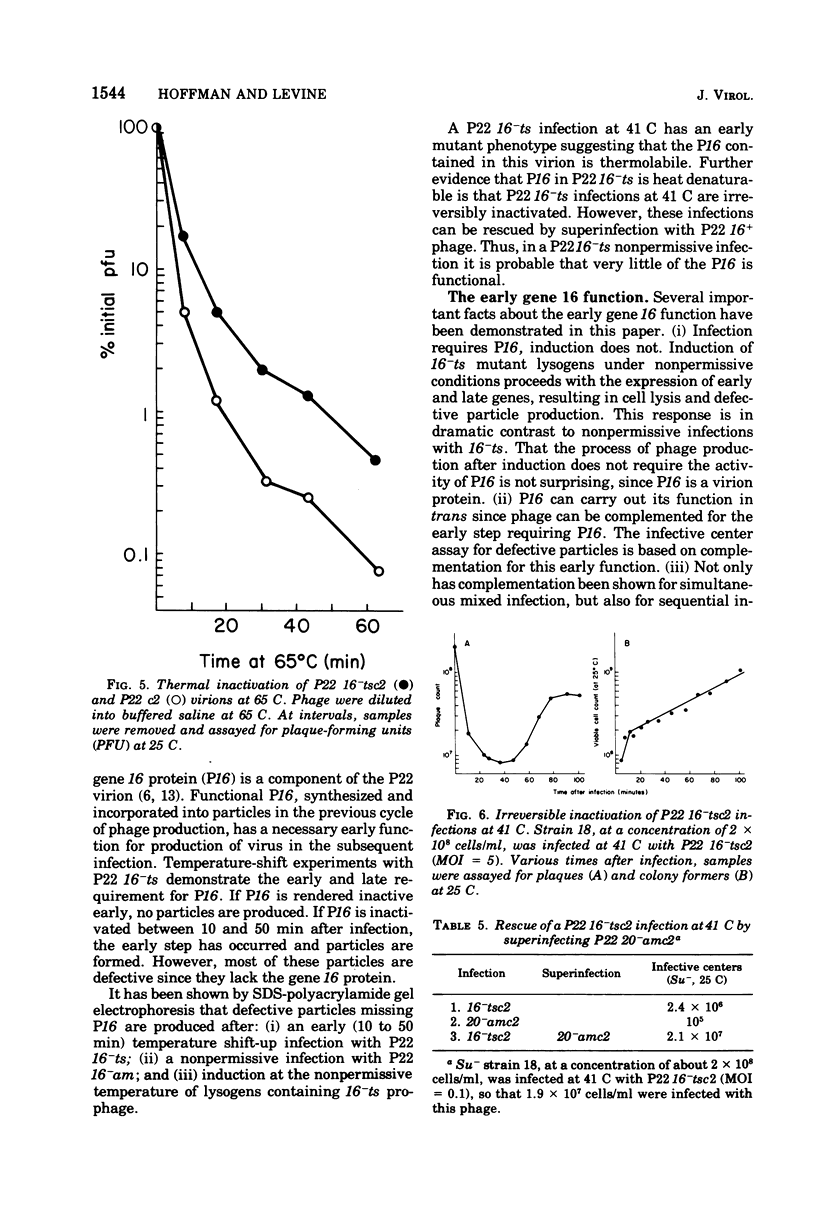
Abstract
The product of gene 16 of phage P22, P16, is a head protein. P16 does not play an essential role in phage assembly since particles formed without this protein appear normal by electron microscopy examination (Botstein et al., 1973). P16 is essential when the particle infects a cell in the following cycle of infection (Botstein et al., 1973; King et al., 1973). We have characterized a mutant of P22 carrying a temperature-sensitive allele of gene 16. This mutant has previously been referred to as P22 25-ts (Levine et al., 1970, 1972) and P22 X-ts (Bezdek and Soska, 1970, 1973). P22 16-ts behaves as an early mutant at the nonpermissive temperature. Temperature shift experiments show that P16 of the infecting virion acts within the first 10 min at 25 C and that gene 16 product is required late in the latent period for incorporation into infectious phage. Induction does not require P16 for the production of particles. Particles produced either in a P22 16-ts thermal shift-up infection or after induction of 16-ts lysogens at 41 C are missing P16 and are, therefore, defective. P16 in P22 16-ts virions formed at the permissive temperature appears to be heat labile; it is inactivated after infection at 41 C. A simple assay for defective particles based on a complementation test is described.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezdek M., Soska J. Evidence for an early regulatory function in Phage P22. Mol Gen Genet. 1970;108(3):243–248. doi: 10.1007/BF00283354. [DOI] [PubMed] [Google Scholar]
- Bezdek M., Soska J. The kinetics of the requirement for X gene product in bacteriophage P 22. Mol Gen Genet. 1973 Sep 5;125(2):189–196. doi: 10.1007/BF00268871. [DOI] [PubMed] [Google Scholar]
- Botstein D., Chan R. K., Waddell C. H. Genetics of bacteriophage P22. II. Gene order and gene function. Virology. 1972 Jul;49(1):268–282. doi: 10.1016/s0042-6822(72)80028-x. [DOI] [PubMed] [Google Scholar]
- Botstein D., Levine M. Synthesis and maturation of phage P22 DNA. II. Properties of temperature-sensitive phage mutants defective in DNA metabolism. J Mol Biol. 1968 Jun 28;34(3):643–654. doi: 10.1016/0022-2836(68)90186-1. [DOI] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Bronson M. J., Levine M. Virulent mutants of bacteriophage p22.I. Isolation and genetic analysis. J Virol. 1971 May;7(5):559–568. doi: 10.1128/jvi.7.5.559-568.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B., Levine M. Bacteriophage P22 virion protein which performs an essential early function. II. Characterization of the gene 16 function. J Virol. 1975 Dec;16(6):1547–1559. doi: 10.1128/jvi.16.6.1547-1559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V., Rosen H., Levine M. Binding of bacteriophage P22 tail parts to cells. J Virol. 1972 Dec;10(6):1152–1158. doi: 10.1128/jvi.10.6.1152-1158.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V. The production of inactive phage P22 particles following induction. Virology. 1967 Oct;33(2):317–322. doi: 10.1016/0042-6822(67)90150-x. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- LEVINE M., CURTISS R. Genetic fine structure of the C region and the linkage map of phage P22. Genetics. 1961 Dec;46:1573–1580. doi: 10.1093/genetics/46.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE M. Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology. 1957 Feb;3(1):22–41. doi: 10.1016/0042-6822(57)90021-1. [DOI] [PubMed] [Google Scholar]
- Levine M., Chakravorty M., Bronson M. J. Control of the replication complex of bacteriophage P22. J Virol. 1970 Oct;6(4):400–405. doi: 10.1128/jvi.6.4.400-405.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. Replication and lysogeny with phage P22 in Salmonella typhimurium. Curr Top Microbiol Immunol. 1972;58:135–156. doi: 10.1007/978-3-642-65357-5_4. [DOI] [PubMed] [Google Scholar]
- Levine M., Schott C. Mutations of phage P22 affecting phage DNA synthesis and lysogenization. J Mol Biol. 1971 Nov 28;62(1):53–64. doi: 10.1016/0022-2836(71)90130-6. [DOI] [PubMed] [Google Scholar]
- SMITH H. O., LEVINE M. TWO SEQUENTIAL REPRESSIONS OF DNA SYNTHESIS IN THE ESTABLISHMENT OF LYSOGENY BY PHAGE P22 AND ITS MUTANTS. Proc Natl Acad Sci U S A. 1964 Aug;52:356–363. doi: 10.1073/pnas.52.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Defective phage formation by lysogens of integration deficient phage P22 mutants. Virology. 1968 Feb;34(2):203–223. doi: 10.1016/0042-6822(68)90231-6. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Lysogenization and superinfection immunity in Salmonella. Virology. 1958 Apr;5(2):291–326. doi: 10.1016/0042-6822(58)90025-4. [DOI] [PubMed] [Google Scholar]