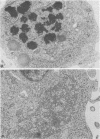
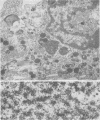
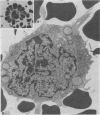
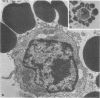
Abstract
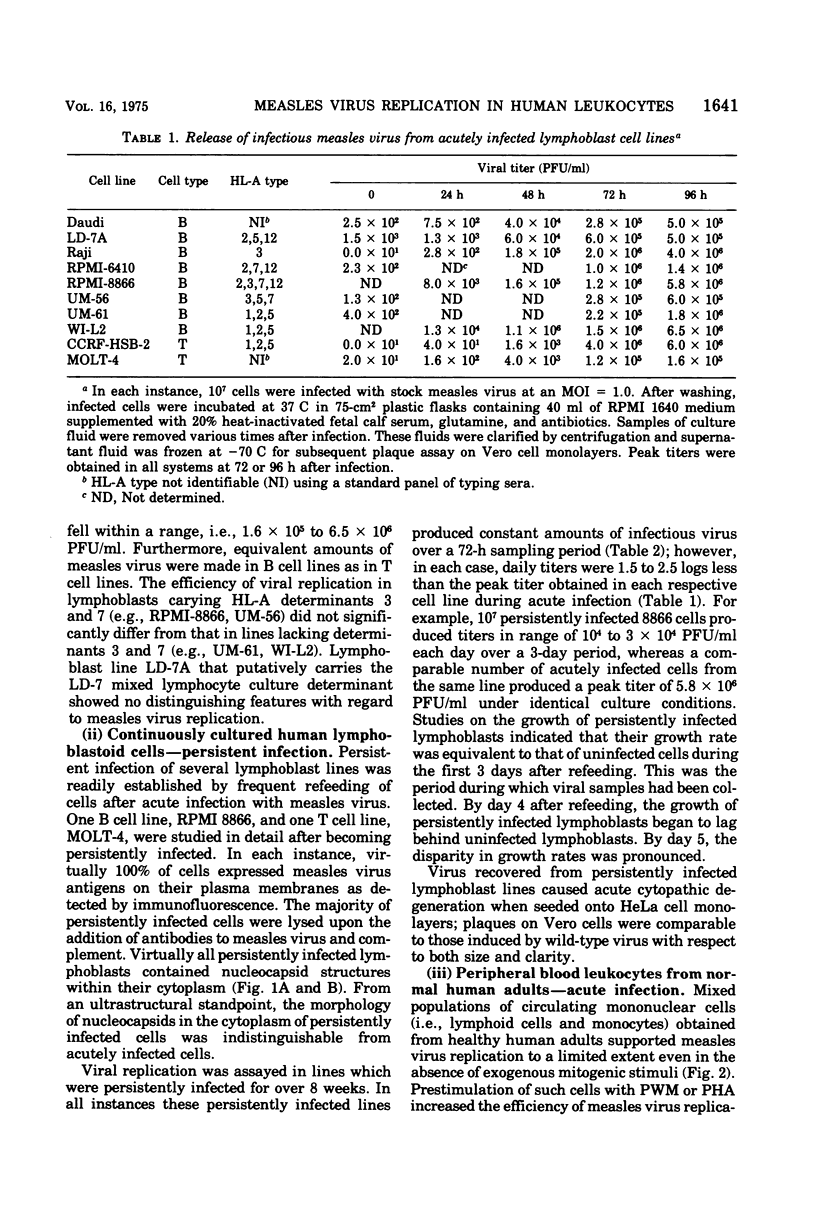
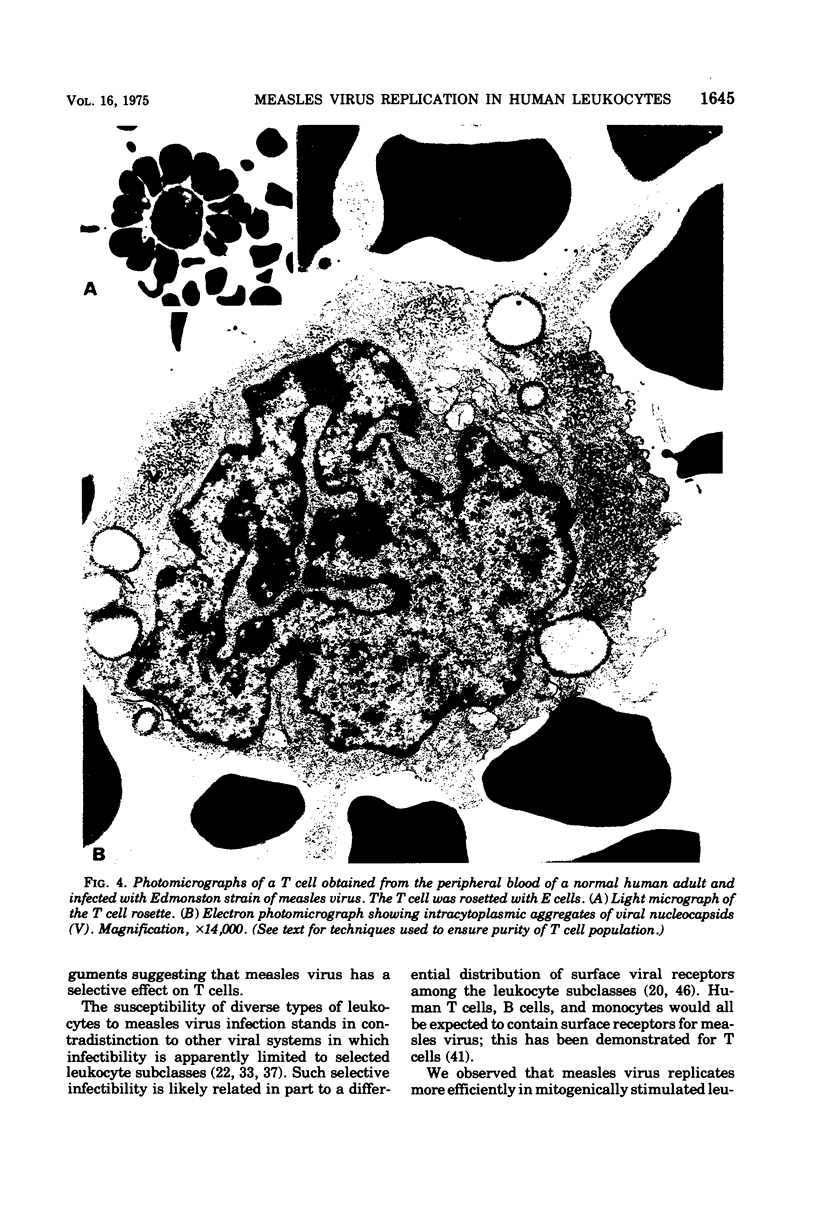
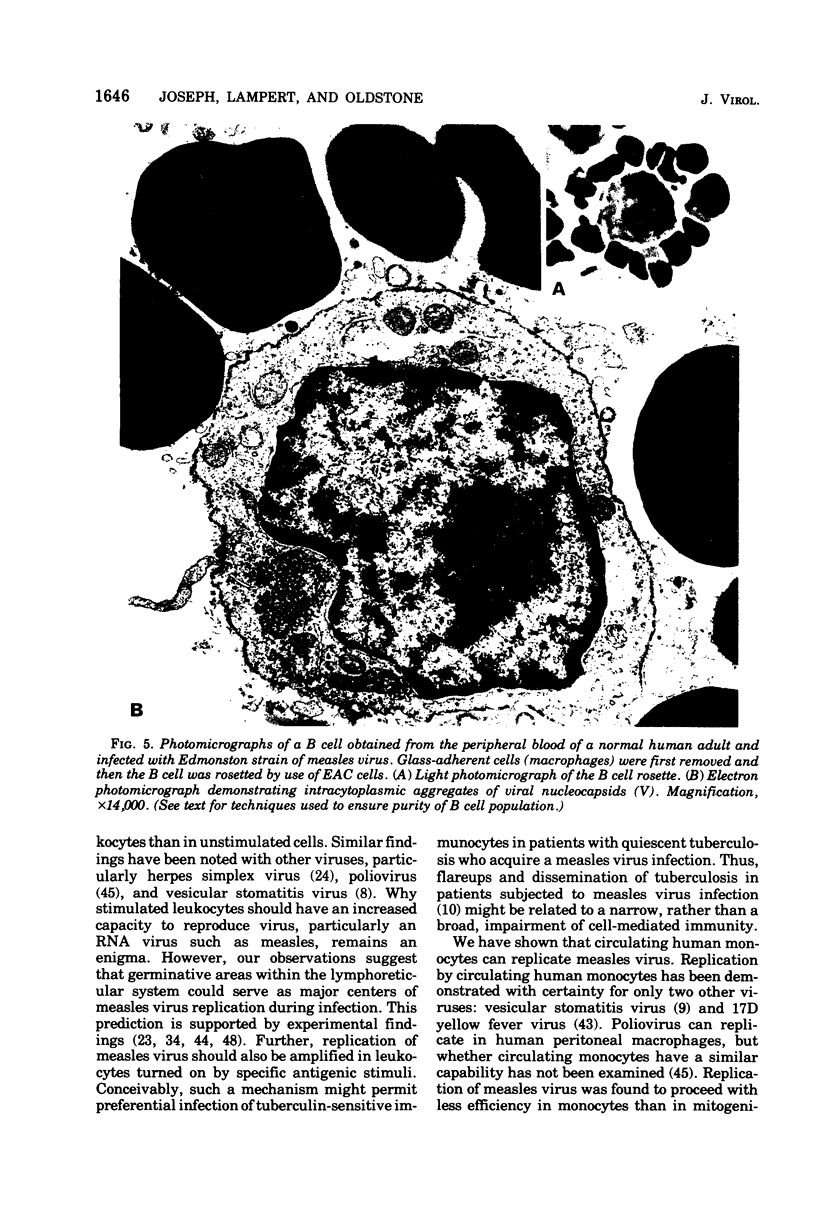
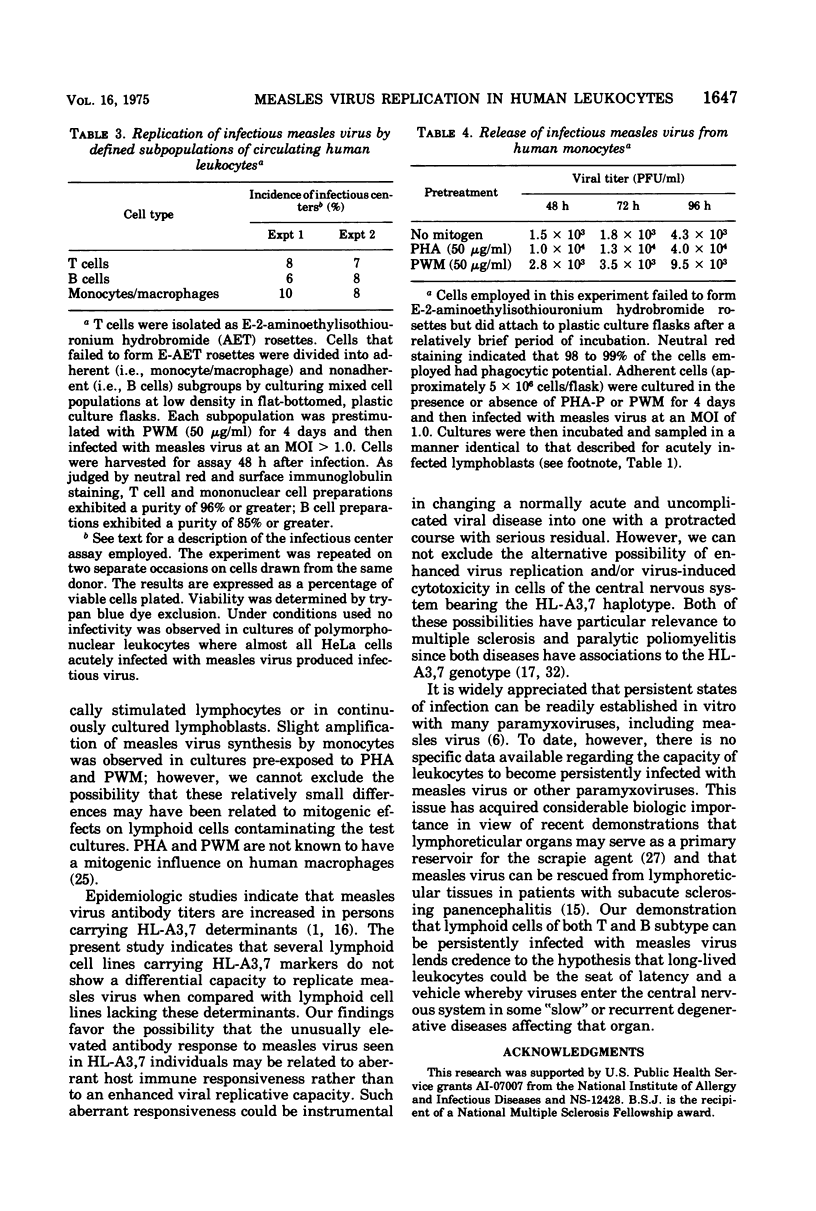
Replication of Edmonston strain measles virus was studied in several human lymphoblast lines, as well as in defined subpopulations of circulating human leukocytes. It was found that measles virus can productively infect T cells, B cells, and monocytes from human blood. These conclusions were derived from infectious center studies on segregated cell populations, as well as from ultrastructural analyses on cells labeled with specific markers. In contrast, mature polymorphonuclear cells failed to synthesize measles virus nucleocapsids even after infection at a relatively high multiplicity of infection. Measles virus replicated more efficiently in lymphocytes stimulated with mitogens than in unstimulated cells. However, both phytohemagglutinin and pokeweed mitogen had a negligible stimulatory effect on viral synthesis in purified populations of monocytes. In all instances the efficiency of measles virus replication by monocytes was appreciably less than that of mitogenically stimulated lymphocytes or of continuously culture lymphoblasts. Under standard conditions of infection, all of the surveyed lymphoblast lines produced equivalent amounts of measles virus regardless of the major histocompatibility (HL-A) haplotype. Hence, no evidence was found that the HL-A3,7 haplotype conferred either an advantage or disadvantage with respect to measles virus synthesis in an immunologically neutral environment. A persistent infection with measles virus could be established in both T and B lymphoblasts. The release of infectious virus from such persistently infected cells was stable over a period of several weeks and was approximately 100-fold less than peak viral titers obtained in each respective line after acute infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnason B. G., Fuller T. C., Lehrich J. R., Wray S. H. Histocompatibility types and measles antibodies in multiple sclerosis and optic neuritis. J Neurol Sci. 1974 Aug;22(4):419–428. doi: 10.1016/0022-510x(74)90078-1. [DOI] [PubMed] [Google Scholar]
- Bloom A. D., Wong A., Tsuchimoto T. Bursa-dependent lymphocyte function in established cell lines: an in vitro model for the study of immunoglobulin and specific antibody synthesis. Birth Defects Orig Artic Ser. 1973 Jan;9(1):62–72. [PubMed] [Google Scholar]
- Burnet F. M. Measles as an index of immunological function. Lancet. 1968 Sep 14;2(7568):610–613. doi: 10.1016/s0140-6736(68)90701-0. [DOI] [PubMed] [Google Scholar]
- Choppin P. W., Scheid A., Mountcastle W. E. Proceedings: Paramyxoviruses, membranes, and persistent infections. Neurology. 1975 May;25(5):494–494. [PubMed] [Google Scholar]
- Dierich M. P., Pellegrino M. A., Ferrone S., Reisfeld R. A. Evaluation of C3 receptors on lymphoid cells with different complement sources. J Immunol. 1974 May;112(5):1766–1773. [PubMed] [Google Scholar]
- Edelman R., Wheelock E. F. Specific role of each human leukocyte type in viral infections. I. Monocyte as host cell for vesicular stomatitis virus replication in vitro. J Virol. 1967 Dec;1(6):1139–1149. doi: 10.1128/jvi.1.6.1139-1149.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R., Wheelock E. F. Vesicular stomatitis virus replication in human leukocyte cultures: enhancement by phytohemagglutinin. Science. 1966 Nov 25;154(3752):1053–1055. doi: 10.1126/science.154.3752.1053. [DOI] [PubMed] [Google Scholar]
- Finkel A., Dent P. B. Abnormalities in lymphocyte proliferation in classical and atypical measles infection. Cell Immunol. 1973 Jan;6(1):41–48. doi: 10.1016/0008-8749(73)90004-x. [DOI] [PubMed] [Google Scholar]
- Fireman P., Friday G., Kumate J. Effect of measles vaccine on immunologic responsiveness. Pediatrics. 1969 Feb;43(2):264–272. [PubMed] [Google Scholar]
- GRESSER I., CHANY C. Isolation of measles virus from the washed leucocytic fraction of blood. Proc Soc Exp Biol Med. 1963 Jul;113:695–698. doi: 10.3181/00379727-113-28465. [DOI] [PubMed] [Google Scholar]
- Gresser I., Lang D. J. Relationships between viruses and leucocytes. Prog Med Virol. 1966;8:62–130. [PubMed] [Google Scholar]
- Horta-Barbosa L., Hamilton R., Wittig B., Fuccillo D. A., Sever J. L., Vernon M. L. Subacute sclerosing panencephalitis: isolation of suppressed measles virus from lymph node biopsies. Science. 1971 Aug 27;173(3999):840–841. doi: 10.1126/science.173.3999.840. [DOI] [PubMed] [Google Scholar]
- Jersild C., Ammitzboll T., Clausen J., Fog T. Association between HL-A antigens and measles antibody in multiple sclerosis. Lancet. 1973 Jan 20;1(7795):151–152. doi: 10.1016/s0140-6736(73)90224-9. [DOI] [PubMed] [Google Scholar]
- Jersild C., Dupont B., Svejgaard A., Platz P. J., Ciongoli K. A., Fog T. Proceedings: Genetic factors in multiple sclerosis: the major histocompatibility system (HL-A) and immunity. Neurology. 1975 May;25(5):488–489. doi: 10.1212/wnl.25.5.488. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M. Surface markers on human B and T lymphocytes. V. Characterization of the lymphoproliferative response to three different lectins and allogeneic lymphocytes by surface markers. Scand J Immunol. 1974;3(6):749–755. doi: 10.1111/j.1365-3083.1974.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Cooper N. R., Oldstone M. B. Immunologic injury of cultured cells infected with measles virus. I. role of IfG antibody and the alternative complement pathway. J Exp Med. 1975 Apr 1;141(4):761–774. [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Shope T. C., Peterson W. D., Jr Epstein-barr virus-negative human malignant T-cell lines. J Exp Med. 1974 May 1;139(5):1070–1076. doi: 10.1084/jem.139.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman L. F., Kibrick S., Ennis F., Polgar P. Herpes simplex virus replication in human lymphocyte cultures stimulated with phytomitogens and anti-lymphocyte globulin. Proc Soc Exp Biol Med. 1972 Dec;141(3):1095–1099. doi: 10.3181/00379727-141-36941. [DOI] [PubMed] [Google Scholar]
- Koller C. A., King G. W., Hurtubise P. E., Sagone A. L., LoBuglio A. F. Characterization of glass adherent human mononuclear cells. J Immunol. 1973 Nov;111(5):1610–1612. [PubMed] [Google Scholar]
- Lampert P. W., Joseph B. S., Oldstone M. B. Antibody-induced capping of measles virus antigens on plasma membrane studied by electron microscopy. J Virol. 1975 May;15(5):1248–1255. doi: 10.1128/jvi.15.5.1248-1255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle G. C., Sturman L., Hadlow W. J. Isolation from mouse spleen of cell populations with high specific infectivity for scrapie virus. Infect Immun. 1972 Mar;5(3):319–323. doi: 10.1128/iai.5.3.319-323.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland H. F. The effect of measles virus infection on T and B lymphocytes in the mouse. I. Suppression of helper cell activity. J Immunol. 1974 Dec;113(6):1978–1983. [PubMed] [Google Scholar]
- Mellstedt H. In vitro activation of human T and B lymphocytes by pokeweed mitogen. Clin Exp Immunol. 1975 Jan;19(1):75–82. [PMC free article] [PubMed] [Google Scholar]
- Minagawa T., Nakaya C., Iida H. Host DNA synthesis-suppressing factor in culture fluid of tissue cultures infected with measles virus. J Virol. 1974 May;13(5):1118–1125. doi: 10.1128/jvi.13.5.1118-1125.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Morris P. J., Pietsch M. C. Letter: A possible association between paralytic poliomyelitis and multiple sclerosis. Lancet. 1973 Oct 13;2(7833):847–848. doi: 10.1016/s0140-6736(73)90891-x. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Jensen F. C., Oldstone M. B. Pathogenesis of of cytomegalovirus infection. I. Activation of virus from bone marrow-derived lymphocytes by in vitro allogenic reaction. J Exp Med. 1975 Mar 1;141(3):561–572. doi: 10.1084/jem.141.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Welsh R. M., Joseph B. S. Pathogenic mechanisms of tissue injury in persistent viral infections. Ann N Y Acad Sci. 1975 Jun 13;256:65–72. doi: 10.1111/j.1749-6632.1975.tb36035.x. [DOI] [PubMed] [Google Scholar]
- Ono K., Iwa N., Kato S., Konobe T. Demonstration of viral antigen in giant cells formed in monkeys experimentally infected with measles virus. Biken J. 1970 Dec;13(4):329–337. [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Phillips S. M., Hirsch M. S., André-Schwartz J., Solnik C., Black P., Schwartz R. S., Merrill J. P., Carpenter C. B. Cellular immunity in the mouse. V. Further studies on leukemia virus activation in allogeneic reactions of mice: stimulatory parameters. Cell Immunol. 1975 Jan;15(1):169–179. doi: 10.1016/0008-8749(75)90173-2. [DOI] [PubMed] [Google Scholar]
- Smithwick E. M., Berkovich S. In vitro suppression of the lymphocyte response to tuberculin by live measles virus. Proc Soc Exp Biol Med. 1966 Oct;123(1):276–278. doi: 10.3181/00379727-123-31465. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Albrecht P., Lucas S. J. Inhibition of lymphocyte stimulation by measles virus. J Immunol. 1975 May;114(5):1458–1461. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J., Bokisch V. A. Binding of soluble immune complexes to human lymphoblastoid cells. I. Characterization of receptors for IgG Fc and complement and description of the binding mechanism. J Exp Med. 1974 Oct 1;140(4):877–894. doi: 10.1084/jem.140.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdirmarsson H., Agnarsdottir G., Lachmann P. J. Measles virus receptor on human T lymphocytes. Nature. 1975 Jun 12;255(5509):554–556. doi: 10.1038/255554a0. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F., Edelman R. Specific role of each human leukocyte type in viral infections. 3. 17D yellow fever virus replication and interferon production in homogeneous leukocyte cultures treated with phytohemagglutinin. J Immunol. 1969 Sep;103(3):429–436. [PubMed] [Google Scholar]
- White R. G., Boyd J. F. The effect of measles on the thymus and other lymphoid tissues. Clin Exp Immunol. 1973 Mar;13(3):343–357. [PMC free article] [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Replication of poliovirus in phytohemagglutinin-stimulated human lymphocytes. J Virol. 1969 May;3(5):451–457. doi: 10.1128/jvi.3.5.451-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Lymphocyte receptors for myxoviruses and paramyxoviruses. J Immunol. 1974 Jun;112(6):2176–2183. [PubMed] [Google Scholar]
- Woodruff J. J., Woodruff J. F. Virus-induced alterations of lymphoid tissue. IV. The effect of Newcastle disease virus on the fate of transfused thoracic duct lymphocytes. Cell Immunol. 1974 Jan;10(1):78–85. doi: 10.1016/0008-8749(74)90153-1. [DOI] [PubMed] [Google Scholar]
- Yamanouchi K., Chino F., Kobune F., Kodama H., Tsuruhara T. Growth of measles virus in the lymphoid tissues of monkeys. J Infect Dis. 1973 Dec;128(6):795–799. doi: 10.1093/infdis/128.6.795. [DOI] [PubMed] [Google Scholar]
- Zweiman B., Pappagianis D., Maibach H., Hildreth E. A. Effect of measles immunization on tuberculin hypersensitivity and in vitro lymphocyte reactivity. Int Arch Allergy Appl Immunol. 1971;40(6):834–841. doi: 10.1159/000230466. [DOI] [PubMed] [Google Scholar]