Abstract
Oxidative stress promotes endothelial cell senescence and endothelial dysfunction, important early steps in atherogenesis. To investigate potential antioxidant effects of IGF-1 we treated human aortic endothelial cells (hAECs) with 0–100 ng/mL IGF-1 prior to exposure to native or oxidized low-density lipoprotein (oxLDL). IGF-1 dose- and time- dependently reduced basal- and oxLDL-induced ROS generation. IGF-1 did not alter superoxide dismutase or catalase activity but markedly increased activity of glutathione peroxidase (GPX), a crucial antioxidant enzyme, via a phosphoinositide-3 kinase dependent pathway. IGF-1 did not increase GPX1 mRNA levels but increased GPX1 protein levels by 2.6-fold at 24 hr, and altered selenocysteine-incorporation complex formation on GPX1 mRNA. Furthermore, IGF-1 blocked hydrogen peroxide induced premature cell senescence in hAECs. In conclusion, IGF-1 upregulates GPX1 expression in hAECs via a translational mechanism, which may play an important role in the ability of IGF-1 to reduce endothelial cell oxidative stress and premature senescence. Our findings have major implications for understanding vasculoprotective effects of IGF-1.
Keywords: Oxidative stress, Senescence, Endothelial dysfunction, Atherosclerosis
1. Introduction
Cardiovascular disease is the leading cause of death in the developed world, accounting for more than one third of all deaths in the United States[1]. The underlying etiology responsible for most cardiovascular disease is atherosclerosis, which has a complicated pathogenesis in which increased inflammatory responses and oxidative stress play a major role[2, 3]. An initial early event in atherogenesis is the development of endothelial senescence and endothelial dysfunction[4, 5], both of which have been linked to increased oxidative stress. We have previously shown that a systemic elevation of insulin-like growth factor-1 (IGF-1) by continuous infusion suppressed macrophage infiltration and oxidative stress in the vascular wall, thereby attenuating atherosclerosis in apolipoprotein E deficient (Apoe−/−) mice[6]. However, smooth muscle specific overexpression of IGF-1 did not alter atherosclerotic burden or oxidative stress levels both in the normal vessel wall and in atherosclerotic lesions[7], suggesting that the endothelium was the major site of IGF-1’s antioxidant and anti-atherogenic action. Here we sought to determine potential antioxidant effects of IGF-1 in cultured human aortic endothelial cells. We found that IGF-1 enhances endothelial antioxidant activity, primarily via upregulation of glutathione peroxidase -1 (GPX1) expression and activity. We further characterized mechanisms for IGF-1 upregulation of GPX1, and demonstrated that IGF-1 prevents oxidant stress induced endothelial cell senescence. These findings provide novel insights into mechanisms whereby IGF-1 reduces oxidant-stress induced vascular complications.
2. Materials and Methods
2.1 Materials
Reagents and antibodies were obtained as follows; LY294002, SB202190, and PD98059 from EMD Millipore Chemicals (Billerica, MA); rabbit anti-SBP2 antibody used for Western blot is a generous gift from Dr. Khanna[8]. Mouse monoclonal anti-SBP2 antibody used for immunoprecipitation and mouse monoclonal anti-human GPX1 antibody are from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit anti-human GPX4 antibody from Cayman Chemical (Ann Arbor, MI); 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA) and dihydroethidium from Invitrogen (Grand Island, NY); mouse monoclonal anti-β-actin antibody from Sigma-Aldrich (St. Louis, MO); anti-acetylated lysine, anti-phospho-Tyrosine, and anti-phospho-Ser/Thr (Akt, ATM, and ATR substrates) antibodies from Cell Signaling Technology (Danvers, MA).
2.2 Cell culture
Human aortic endothelial cells (hAECs) were purchased from Lonza (Basel, Switzerland) and maintained in Endothelial Growth Medium 2 with 2 % fetal bovine serum and supplements provided by the manufacturer (Lonza). Actively dividing cells (passage 4 to 8) were used for experiments. All the experiments were conducted using fully confluent culture in serum-free/ phenol red-free Endothelial cell Basal Medium (Lonza; Basel, Switzerland) supplemented with 0.5 % bovine serum albumin (fraction V; Sigma-Aldrich, St. Louis, MO).
2.3 Enzyme activity assay
Glutathione peroxidase activity, superoxide dismutase activity, and catalase activity were determined in hAECs using commercially available kits as follows; Glutathione Peroxidase Assay kit and Catalase Assay kit from Cayman Chemical (Ann Arbor, MI); and Superoxide Dismutase Assay kit from R&D Systems (Minneapolis, MN). Cellular glutathione levels were determined using Glutathione Assay kit obtained from Cayman Chemical (Ann Arbor, MI). All the assays were performed accordingly to the instructions provided by the manufacturers.
2.4 Western blot analysis
Western blot analysis was performed as described previously [9]. In brief, cells were washed with PBS and lysed in RIPA buffer, containing 150 mM NaCl, 20 mM Tris-Cl, pH 7.2, 1 mM EDTA, 1% Nonidet P-40, 0.5% deoxycholate, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 0.1 M okadaic acid, 0.1 µM aprotinin, 10µg/ml leupeptin, and 10 mM NaF. Lysates were subjected to 10% SDS-PAGE and western blotting analysis. Immunopositive bands were visualized by enhanced chemiluminescence (ECL, Amersham). Blots were stripped and reprobed with monoclonal anti-β-actin antibody as a control for equal loading.
2.5 Lipoprotein preparation
Human plasma derived native LDL (nLDL) was purchased from Kalen Biomedical (Montgomery Village, MD). OxLDL was prepared as previously described. Briefly, an aliquot of the nLDL fraction was passed through a 10DG desalting column (Bio-Rad) to remove EDTA, then the nLDL fraction (0.2 mg/ml, diluted in PBS) was incubated with 5 µM CuSO4 at 37 °C for 3h. The reaction was stopped by adding EDTA (final concentration 0.25 mM). The oxLDL prepared under these conditions showed an increase in relative mobility on agarose gel electrophoresis, and the value for thiobarbituric acid-reactive substances (TBARS) in oxLDL was 37.2 ± 1.2 nmol malondialdehyde per milligram protein. TBARS were not detectable in nLDL.
2.6 Realtime PCR analysis of gene expression
After exposure to testing agents for 6–24 hr, cells were lysed in Tripure reagent (Roche). Total RNA was extracted and precipitated by isopropanol, and was further purified using RNeasy kit (Qiagen). The total RNA was subjected to a reverse-transcriptase reaction using RT2 First Strand Kit (Qiagen), followed by the realtime PCR analysis in RT2 SYBR Green Master Mix (Qiagen) using iCycler iQ Realtime PCR Detection System (Bio-Rad). Specific primer sets for following genes were purchased from Qiagen; GPX1 (catalog number, PPH00154F), GPX2 (PPH01710B), GPX3 (PPH05746F), GPX4 (PPH05586B), GPX5 (PPH00454A), GPX6 (PPH06081A), GPX7 (PPH09224F), and β-actin (used as an internal control, PPH00073G).
2.7 Immunoprecipitation of SBP2- mRNA complex and mRNA quantification
Messenger RNA – protein complexes immunoprecipitation has been performed as described[10, 11]. Briefly, mRNA-protein complexes were extracted from the cells using Polysome lysis buffer (100 mM KCl, 5 mM MgCl210 mM HEPES pH 7.0, 0.5% IGEPAL CA-630 (Sigma-Aldrich, St. Louis, MO), 1 mM dithiothreitol, 100 units/mL RNase OUT (Invitrogen, Grand Island, NY), and 0.2 % Ribonucleoside Vanadyl Complex, protease inhibitor cocktail (Halt Protease Inhibitor Cocktails, Thermo Scientific, Rockford, IL)). Protein contents in the extract were determined using RC DC Protein Assay kit (Bio-Rad, Hercules, CA), and the equal amount of protein for each sample was subjected to an immunoprecipitation. Immunoprecipitation reaction was achieved by mixing the extract with anti-SBP2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in 50 mM Tris pH 7.4, 150 mM NaCl, 1 mM MgCl20.05 % IGEPAL CA-630, 15 mM EDTA, 1 mM dithiothreitol, 100 units/mL RNase OUT (Invitrogen, Grand Island, NY), and 0.2 % Ribonucleoside Vanadyl Complex containing protease inhibitor cocktail for 18 hr< at 4 °C. The antibody-SBP2-mRNA complexes were collected by Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) and extensively washed using a buffer solution with the same composition of the immunoprecipitation mix. The resulted immunoprecipitates were extracted for RNAs by using Tripure reagent (Roche) and further purified using RNeasy kit (Qiagen). GPX1 and GPX4 mRNA levels were determined as described above by quantitative realtime-PCR. Mouse non-immune IgG was used replacing the anti-SBP2 antibody in the immunoprecipitation procedure to confirm specific precipitation of SBP2-mRNA complexes, and produced no detection of GPX1 or GPX4 mRNA (data not shown).
2.8 Senescence associated β-galactosidase expression
Semi-confluent hAEC culture was exposed to IGF-1 for 24 hr, followed by exposure to 100 µM hydrogen peroxide for 1 hr. Subsequently, cells were trypsinized and counted to re-plate in an equal cell number on a new culture dish. Cells were then maintained for a week in the regular Endothelial Growth Medium 2 containing 2 % fetal bovine serum and supplements, and then stained for senescence associated β-galactosidase expression using Senescence β-Galactosidase Staining Kit (Cell Signaling Technology, Danvers, MA). Positively stained cells were captured in images using a DP70 digital camera connected to a microscope (Olympus) and counted using ImageJ software. Data are expressed as a % of positively stained cells in a total cell count.
2.9 Statistical analysis
Data are presented as means ± SEM. Statistical analysis was performed using one-way ANOVA or Student’s t-test, with P<0.05 considered significant. All experiments were performed a minimum of three times.
3. Results
3.1 IGF-1 reduces reactive oxygen species levels in cultured human aortic endothelial cells
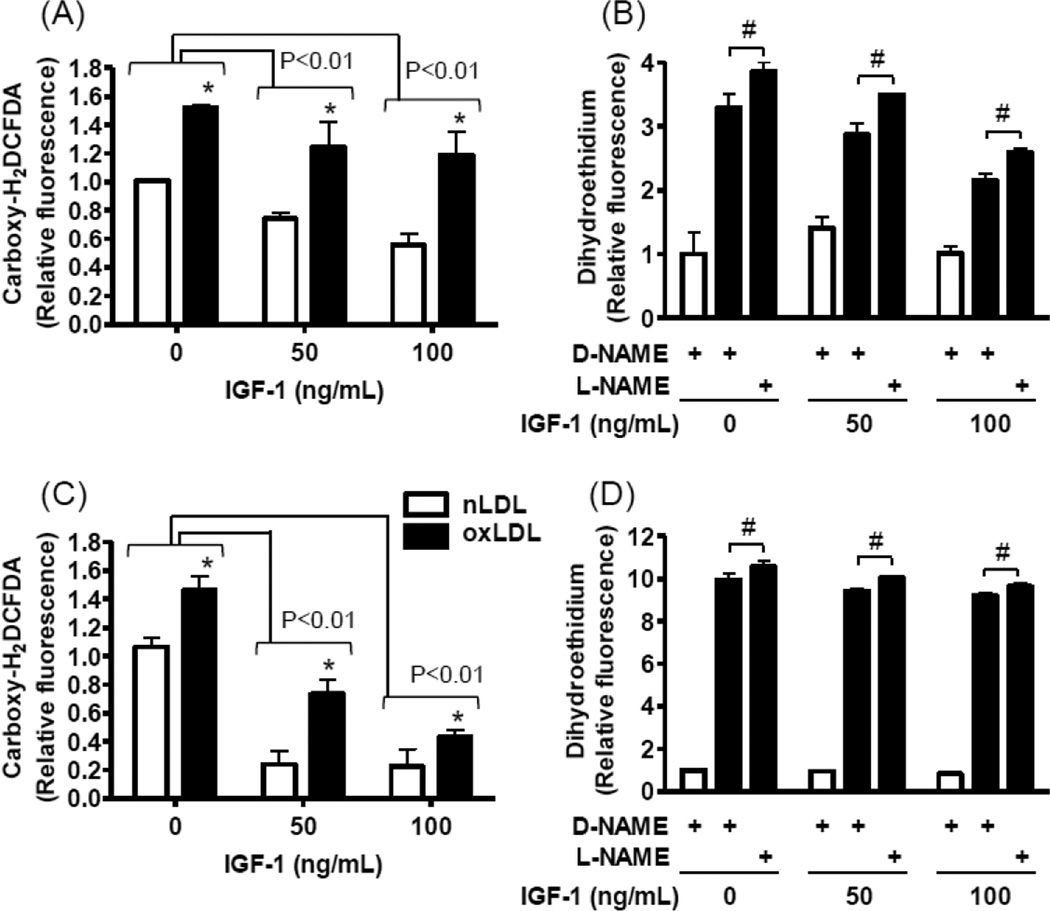
To assess potential IGF-1 effects on reactive oxygen species (ROS) levels in endothelial cells, cultured human aortic endothelial cells (hAECs) were exposed to native or oxidized LDL and intracellular ROS levels were probed using 6-carboxy-2',7'-dichlorodihydrofluorescein diacetate (Carboxy-H2DCFDA; Fig 1A and 1C). Consistent with previous reports[12, 13] oxidized LDL enhanced ROS generation (Fig 1A and 1C); and pre-exposure to IGF-1 dose-dependently suppressed ROS levels in both basal and oxidized LDL-stimulated cells (Fig 1A, 1C). IGF-1’s antioxidant effect was also time-dependent, since the ROS suppression by IGF-1 was more pronounced after 24 hours exposure (Fig 1C) than at 1 hour (Fig 1A). Dihydroethidium preferably detects superoxide, whereas carboxy-H2DCFDA detects a wider spectrum of different oxidant molecules[14]. IGF-1 also decreased dihydroethidium-detectable ROS levels in a dose-dependent manner, but the effect was more modest and only detectable at 1 hr (Fig 1B), contrasting to the carboxy-H2DCFDA results, a longer exposure time caused loss of the IGF-1 effect (Fig 1B and D). Of note, a nitric oxide (NO) synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME) did not significantly inhibit IGF-1’s antioxidant effect (Fig 1B and D, the control was D-NAME, an inactive enantiomer of L-NAME). These results suggest that IGF-1 has NO synthase independent antioxidant effects[15], which suppress both basal ROS (associated with general cellular activities such as mitochondrial respiratory chain reactions[16]) and oxidized LDL induced ROS (generated via specific enzymatic sources such as NADPH-oxidase[17, 18]). These findings prompted us to investigate potential IGF-1 effects on enzymatic antioxidant systems.
Figure 1. IGF-1 reduced reactive oxygen species levels in hAECs.
Human AECs were pre-exposed to 0, 50, and 100 ng/mL IGF-1 for 1 hr (A and B) or 24 hr (C and D), and then exposed to 60 µg/mL oxidized LDL (closed bars) for 6 hr. As a control, native LDL (60 µg/mL) was used instead of oxidized LDL (open bars). (A and C) reactive oxygen species levels were determined by using carboxy-H2DCFDA as an indicator and expressed relative to the detection in native LDL/ 0 ng/mL IGF-1 treated cells. Significant differences are indicated; Brackets represent IGF-1’s effect, P<0.01, n=4, and the symbol (*) represents oxLDL’s effect, P<0.01, n=4. (B and D) Dihydroethidium was used to selectively determine superoxide levels and results are expressed relative to the detection in native LDL/ D-NAME/ 0 ng/mL IGF-1 treated cells. L-NAME was used to inhibit nitric oxide synthase activity, and an inactive enantiomer, D-NAME, was used as a control. #P<0.01, L-NAME vs. D-NAME, n=4.
3.2 IGF-1 upregulates GPX activity and GPX1 expression in hAEC
To determine mechanisms whereby IGF-1 exerted its antioxidant effects, we assessed activities of major antioxidant systems in hAECs after exposure to IGF-1 (Fig 2). An exposure to IGF-1 for 24 hr caused a modest and non-significant trend of an increase in superoxide dismutase activity and did not regulate catalase activity (Fig 2A and 2B). Conversely, glutathione peroxidase (GPX) activity was upregulated in a dose-dependent and time-dependent manner (~4.8-fold increase at 24 h with 100 ng/mL IGF-1, P<0.01, n=4; Fig 2C). Glutathione is an essential substrate for GPX to express antioxidant activity, and it has been reported that growth hormone and IGF-1 potentially regulate glutathione levels in tissues such as kidney, brain and aorta[19, 20]. However, IGF-1 did not increase intracellular glutathione levels (Fig 2D). In order to further characterize IGF-1’s effect on GPX activity we profiled gene expression of seven GPX isozymes (GPX-1 to -7) that the assay used in Fig 2 does not discriminate. Reverse-transcription and realtime-PCR analysis revealed that hAECs significantly express GPX1 and GPX4 (data not shown), thus we further assessed GPX1 and GPX4 expressions by Western blot analysis (Fig 3A). IGF-1 upregulated GPX1 (2.6-fold increase, 100 ng/mL IGF-1 vs 0 ng/mL IGF-1, n=4, p<0.01), whereas it did not change GPX4 expression levels, suggesting that IGF-1 specifically regulates GPX1 expression. Moreover, IGF-1 did not alter GPX1 protein levels in cultured human aortic smooth muscle cells (Fig 3B), thus the IGF-1 effect is likely cell-type specific.
Figure 2. IGF-1 upregulates glutathione peroxidase activity in a dose-dependent and time-dependent manner.
In hAECs (A) Superoxide dismutase and (B) catalase activity were determined after 24 hr exposure to IGF-1. There was no statistically significant alteration in enzyme activities. (C) Glutathione peroxidase activity was determined after 2 hr, 6hr, and 24 hr of exposure to IGF-1 (0 ng/mL; closed square, 50 ng/mL; open circle, 100 ng/mL; closed circle). *P<0.01 vs. 0 ng/mL IGF-1, n=4. #P<0.01 vs. 50 ng/mL IGF-1, n=4. (D) Total glutathione (GSH) levels were determined after 24 hr exposure to IGF-1.
Figure 3. IGF-1 upregulated GPX1, but not GPX4, expression in hAECs.
(A) hAECs were exposed to IGF-1 for 24 hr, and GPX1 and GPX4 protein levels were determined by Western blot analysis. *P<0.01 vs 0 ng/mL IGF-1, n=4. (B) Human aortic smooth muscle cells were exposed to IGF-1 for 24 hr, and then GPX1 protein levels were assessed.
3.3 IGF-1 potentially regulates selenocysteine-decoding complex on GPX1 mRNA, thereby regulating GPX1 translation
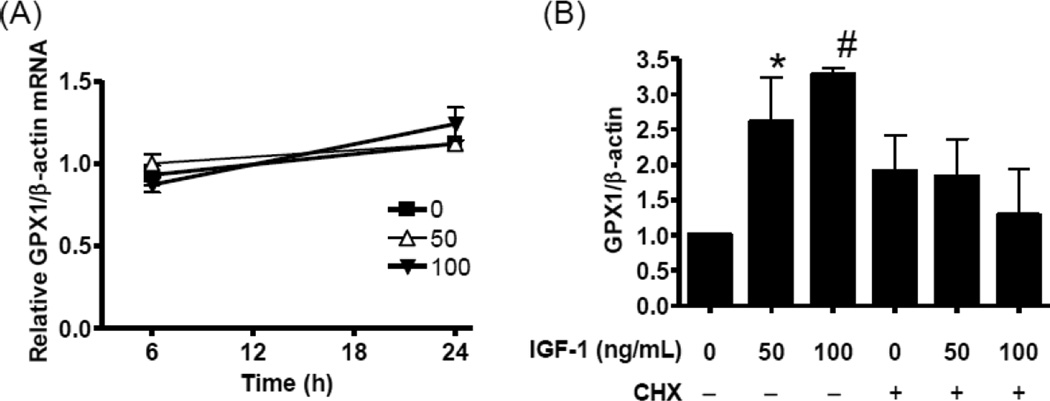
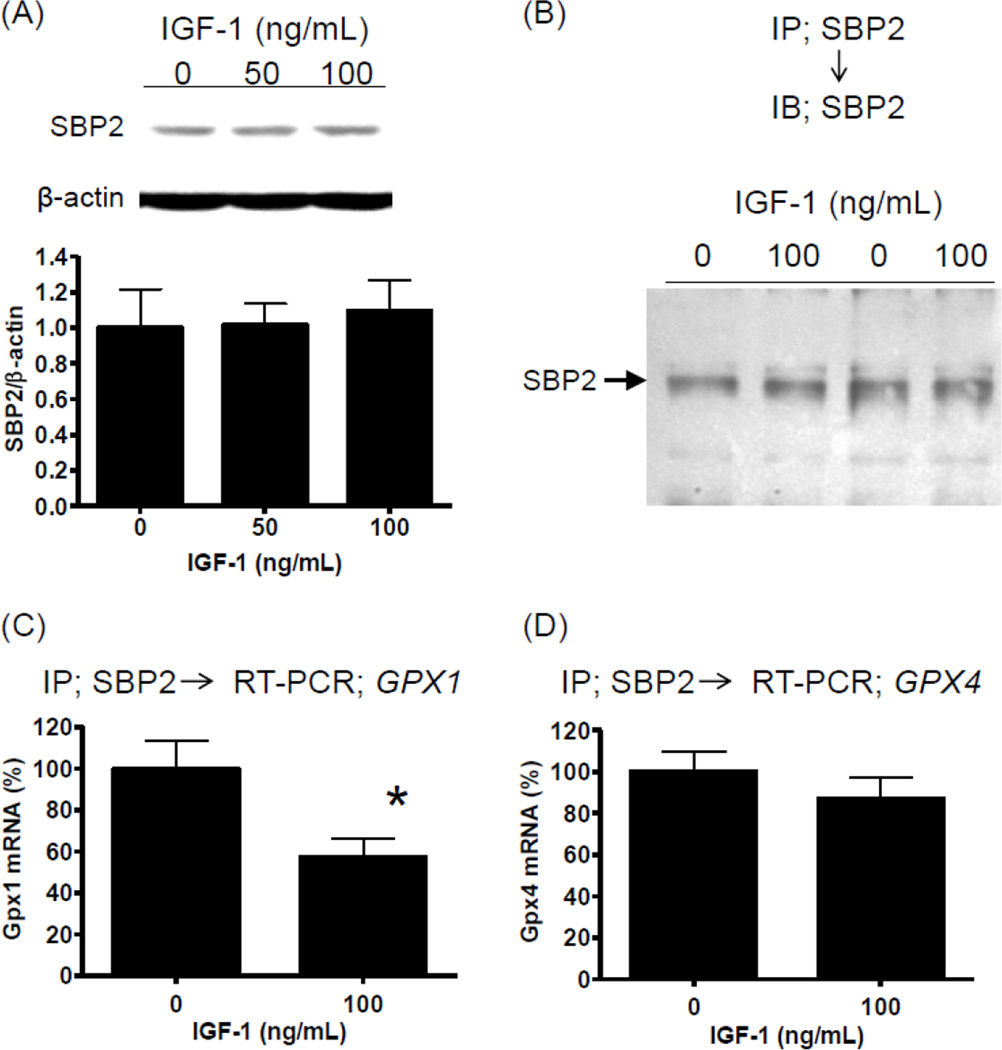
To determine if IGF-1 upregulation of GPX1 is transcriptionally mediated, we assessed GPX1 mRNA levels after 6 hr and 24 hr of IGF-1 exposure. IGF-1 did not alter GPX1 mRNA levels (Fig 4A). However, cycloheximide blocked the upregulation of GPX1 by IGF-1 (Fig 4B), suggesting that the IGF-1 effect is translational. It is of note CHX upregulated basal GPX1 levels (Fig 4B). IGF-1 has been shown to upregulate translation activity in general by promoting 5’-cap dependent translation[21]. In fact, in our experimental setting, IGF-1 induced eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4EBP1) phosphorylation[22], but not eIF4E phosphorylation[23, 24], (Supplemental Figure 1) suggesting general upregulation of translation activity. Our results (Fig 3 and 4) suggest that IGF-1 regulates specifically GPX1 but not GPX4. GPX1 is one of several proteins referred to as a selenoprotein. Selenoproteins are characterized by a selenocysteine residue(s) in their primary structure and thus require unique processes to incorporate selenocysteine upon translation [25–27]. Since it has been suggested that the selenocysteine incorporation step serves as a major regulatory point for altering GPX1 expression levels [25–27], we assessed selenocysteine insertion sequence (SECIS)-binding protein 2 (SBP2) binding to GPX1 mRNA. SBP2 has been shown to be essential for selenocysteine incorporation by binding to the SECIS element, which is a cis-acting element in the 3’-UTR of GPX1 mRNA[8, 28, 29]. IGF-1 did not alter SBP2 protein levels in hAECs (Fig 5A). Immunoprecipitation using anti-SBP2 antibody yielded the same levels of SBP2 from polysome fractions of control and IGF-1 treated hAECs (Fig 5B); however, GPX1 mRNA levels associated with anti-SBP2-immunoprecipitates were lowered by approximately 40 % by IGF-1 (Fig 5C; P<0.01, n=8), whereas GPX4 mRNA levels were not altered (Fig 5D). These data strongly suggest that IGF-1 modifies the SBP2 - GPX1 mRNA complex formation. By performing sequence motif analysis[30, 31] for SBP2, we found numerous sites for potential post-translational modifications, such as acetylation, Ser/Thr-phosphorylation, and Tyr-phosphorylation. We tested these potential modifications by using specific antibodies and found that SBP2 is Tyr-phosphorylated (Supplemental Figure 2) but is not acetylated or Ser/Thr phosphorylated (data not shown). However, IGF-1 did not change the Tyr-phosphorylation status of SBP2 (Supplemental Figure 2).
Figure 4. IGF-1 upregulation of GPX1 is translation dependent.
(A) GPX1 mRNA levels were assessed by quantitative RT-PCR in hAECs exposed to 0–100 ng/mL IGF-1 for 6 hr and 24 hr. No significant difference was detected, n=3. (B) hAECs were exposed to 0–100 ng/mL IGF-1 and cycloheximide (CHX; 10 µg/mL) for 24 hr and GPX1 protein expression was assessed by Western blot analysis. IGF-1 increased GPX1 levels (*P<l0.05 vs. 0 ng/mL IGF-1, #P<0.01 vs. 0 ng/mL IGF-1, n=4), however CHX blocked the IGF-1 effect.
Figure 5. IGF-1 influences SBP2-GPX1 mRNA complex formation.
(A) Western blot analysis for SBP2 expression in hAECs. Exposure to 0–100 ng/mL IGF-1 for 24 hr did not alter SBP2 levels. (B) After 18 hr of IGF-1 exposure (100 ng/mL), polysome fraction was extracted and subjected to immunoprecipitation using anti-SBP2 monoclonal antibody. Precipitated SBP2 was visualized by Western blot. IGF-1 did not alter immunoprecipitable SBP2 levels. (C) Levels of GPX1 mRNA associated with SBP2 were determined by quantitative RT-PCR in immunoprecipitates using anti-SBP2 monoclonal antibody. *P<0.01, n=8. (D) Levels of GPX4 mRNA associated with SBP2 were determined by quantitative RT-PCR in immunoprecipitates using anti-SBP2 monoclonal antibody. The difference was no statistically significant. n=8.
3.4 A PI3k-dependent signaling pathway is required for IGF-1 mediated upregulation of GPX1 expression and activity
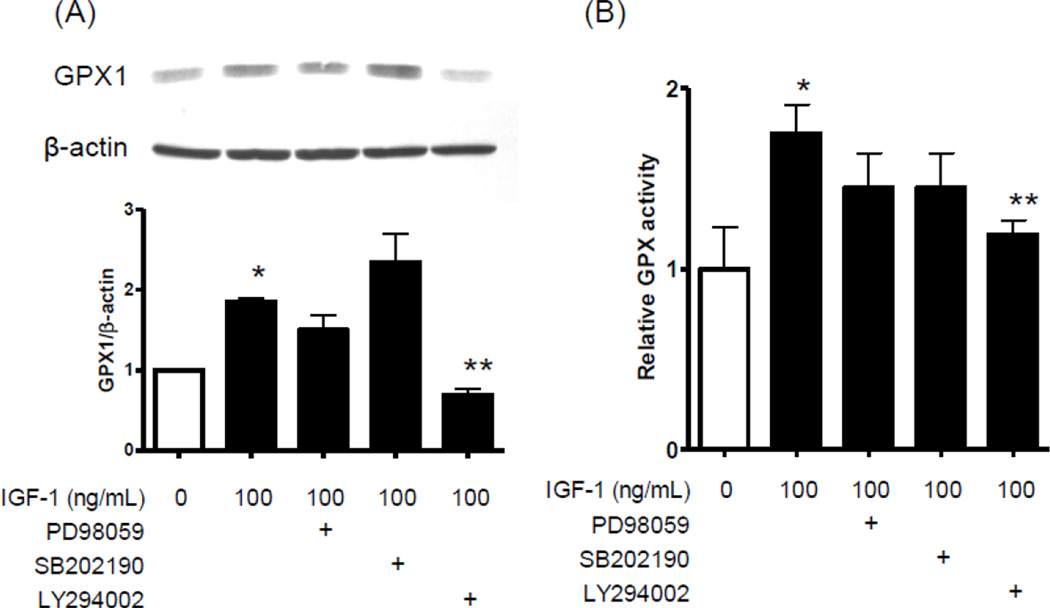
We tested inhibitors of the major IGF-1 signaling pathways to gain insights into mechanisms whereby IGF-1 regulated GPX1 expression and activity (Fig 6). Whereas ERK (PD98059) and p38 MAP kinase (SB202190) inhibitors did not alter IGF-1’s effect, the phosphatidylinositide 3-kinase (PI3k) inhibitor, LY294002, significantly suppressed IGF-1 upregulation of GPX1 (Fig 6A). IGF-1 upregulation of GPX activity was also significantly suppressed by LY294002 but not by PD98059 or SB202190 (Fig 6B), confirming that IGF-1 regulation of GPX was PI3k dependent.
Figure 6. LY294002, PI3k inhibitor, attenuates IGF-1 upregulation of GPX1 and GPX activity.
Human AECs were exposed to PD98059 (MEK1 inhibitor, 10 µM), SB202190 (p38 MAP kinase inhibitor, 10 µM), and LY294002 (PI3k inhibitor, 50 µM) for 30 min prior to IGF-1 exposure, and were also included in the subsequent 24 hr exposure with IGF-1. (A) Western blot analysis for GPX1 protein levels and (B) GPX activity assay were performed. *P<0.01 vs 0 ng/mL IGF-1, n=4. **P<0.01 vs 100 ng/mL IGF-1 with no inhibitor, n=4.
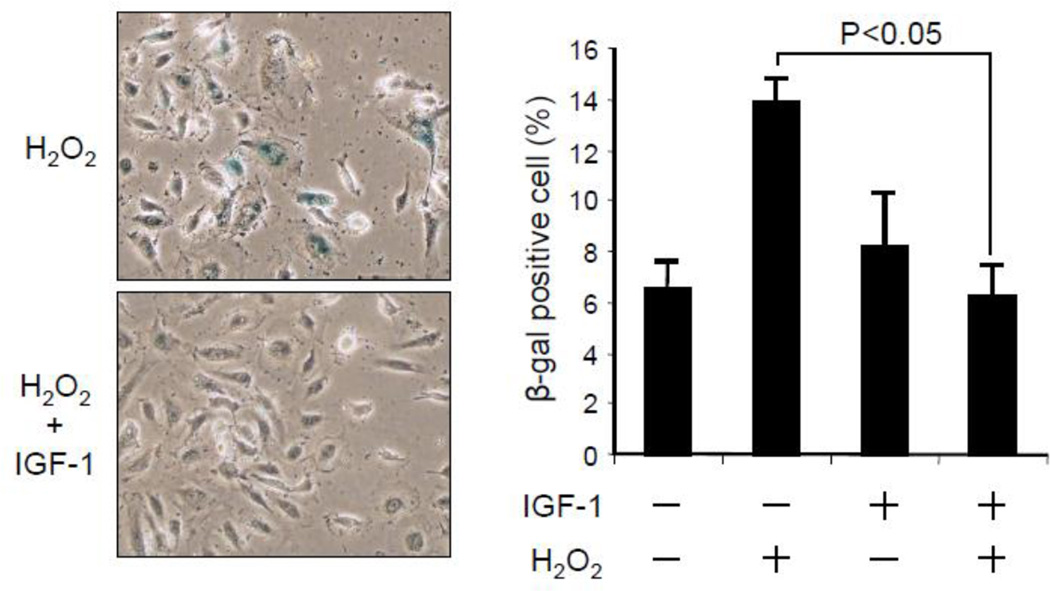
3.5 IGF-1 attenuates hydrogen peroxide-induced premature cell senescence
One of the consequences of oxidative stress in vascular endothelium is premature cell senescence[32]. To gain insights into the biological significance of GPX upregulation by IGF-1, hAECs were exposed to 100 µM hydrogen peroxide for an hour and then replated and cultured to sub-confluence, followed by in situ staining for senescence associated β-galactosidase activity (Fig 7). Incubation with 100 ng/mL IGF-1 for 24 hours prior to hydrogen peroxide exposure significantly reduced expression of β-galactosidase activity (Fig 7), indicating that the enhanced antioxidant activity in response to IGF-1 counteracted oxidative stress induced premature cell senescence.
Figure 7. IGF-1 prevented oxidative stress induced premature cell senescence.
hAECs were exposed to 100 ng/mL IGF-1 for 24 hr, subsequently exposed to 100 µM hydrogen peroxide for 1 hr. Cells were replated and cultured to sub-confluence. Cell number positive for senescence-associated β-galactosidase expression was counted using Image J software. IGF-1 suppressed hydrogen peroxide-induced β-galactosidase expression (P<0.05, n=3). The data represents 3 independent experiments.
4. Discussion
GPX1 is a major component of cellular antioxidant systems and is ubiquitously expressed throughout a variety of tissues including the vasculature. Systemic deficiency of GPX1 has been shown to accelerate atherosclerosis and is associated with elevated oxidative stress in the vascular wall[33, 34], indicating that the antioxidant activity of GPX1 is essential to protect the vasculature from oxidative stress evoked complications. Based on our previous studies demonstrating atheroprotective effects of IGF-1 in Apoe−/− mice[6, 7], we tested potential antioxidant effects of IGF-1 in endothelial cells. Our data showed that IGF-1 reduces endothelial oxidative stress in a dose-dependent and time-dependent manner (Fig 1). Among the tested antioxidant systems, IGF-1 did not upregulate superoxide dismutase or catalase but upregulated glutathione peroxidase activity (Fig 2C). IGF-1 consistently upregulated endothelial GPX1 protein expression levels (Fig 3A), which should, at least in part, account for the antioxidant effect of IGF-1. Furthermore, IGF-1 markedly inhibited oxidant stress induced endothelial cell senescence, providing a potential important and novel mechanism whereby IGF-1 may exert anti-atherosclerotic effects in vivo.
We observed GPX1 upregulation by IGF-1 with no change in GPX1 mRNA levels, suggesting post-transcriptional/translational regulation. Transcriptional control of GPX1 gene has not been well characterized; however its post-transcriptional regulation has been extensively studied. GPX1 is one of the proteins referred to as a selenoprotein, which is characterized by a selenocysteine residue(s) in the primary structure. There is no corresponding mRNA codon to be translated to selenocysteine; instead, an UGA codon, which regularly functions as a termination codon, is decoded to selenocysteine. The UGA-codon decoding process requires multiple trans-acting factors, including SBP2 and eukaryotic elongation factor selenocysteine-tRNA specific, as well as a cis-acting element in the mRNA defined as SECIS[25–27]. The decoding process is still not fully understood; however evidence suggests that the selenocysteine biosynthesis and incorporation mechanisms represent a major regulatory point for GPX1 synthesis[25–27]. SBP2 is indispensable for the selenocysteine decoding process by directly binding to the SECIS element in mRNAs of selenoproteins, thereby recruiting other necessary proteins to the selenocysteine-decoding complex[35]. IGF-1 did not alter SBP2 expression levels (Fig 5A); however assessment of SBP2 binding to GPX1 mRNA yielded the surprising result that IGF-1 decreased GPX1 mRNA levels associated with SBP2 (Fig 5C). Of note, the antibody used for immunoprecipitation was a monoclonal antibody raised against a recombinant protein corresponding to a region near the N-terminus of SBP2 of human origin; thus it is possible that the antibody binding to the SBP2 - GPX1 mRNA complex is inhibited because of potential modifications to the protein-mRNA complex as a consequence of IGF-1 effects (e.g. recruitment of other proteins and binding to the mRNA-SBP2 complex may interfere with antibody access to the epitope). Intriguingly, there was no change in GPX4 mRNA levels in the anti-SBP2 immunoprecipitates (Fig 5D). This result is consistent with our finding that GPX4 protein levels were not altered by IGF-1. How IGF-1 signaling regulates the selenocysteine-decoding machinery specifically for GPX1, not for GPX4, would be an intriguing subject for future study. It is important to point out that a hierarchy has been reported for SBP2 binding to mRNAs of different selenoproteins, i.e. SBP2 preferentially stimulates selenocysteine incorporation directed by GPX4 SECIS elements over GPX1, especially when availability of selenium is limited[35, 36].
The oxidation hypothesis of atherosclerosis, which has been updated and thus evolved from its original premise, is currently the most widely accepted mechanism for the development and progression of atherosclerotic vascular disease[37]. Elevated oxidative stress in the endothelium has been linked to development of endothelial dysfunction[38–41]. Endothelial dysfunction leads to upregulation of adhesion molecules[42, 43], inflammatory cell recruitment[44, 45], reduced NO bioavailability, and reduced endothelial-dependent vasodilation[38, 41]. Oxidative stress has also been shown to alter endothelium function by inducing premature senescence[32]. In fact, our data demonstrated that a brief exposure to hydrogen peroxide (100 µM for 1 hour) induced senescence-associated β-galactosidase activity in hAECs; and notably, preconditioning with IGF-1 significantly attenuated it (Fig 7), strongly suggesting that IGF-1’s antioxidant effect can prevent premature senescence in the endothelium. Since endothelial dysfunction is a hallmark of early stages of vascular complications including atherosclerosis, our findings that IGF-1 exerts an antioxidant effect on the endothelium suggests a potential mechanism for the anti-atherogenic effect of IGF-1.
Recently it has been shown that the acceleration of atherosclerosis that occurs with aging, which correlates with failure to upregulate antioxidant genes[46]. It is well known that levels of circulating IGF-1 decline with aging[47–50], and intriguingly our previous study performed using Apoe−/− mice showed that an about 20 % reduction in circulating IGF-1 levels was accompanied by a significant increase in aortic atherosclerotic lesion size[51]. In animal models of growth hormone (GH) deficiency (Ames dwarf mice and Lewis dwarf rats), GH and IGF-1 deficiencies were accompanied by high oxidative stress in the vasculature, potentially leading to endothelial dysfunction[52, 53]. GH and IGF-1 availabilities have been associated with expression and activity of nuclear factor erythroid 2-like 2 (NRF2)[20], a transcription factor known to regulate expression of genes important for regulation of redox homeostasis [54]. Thus a failure of NRF2-dependent gene regulation due to the loss of GH/IGF-1 signaling potentially accounts for diminished antioxidant activity in GH-deficient animals[20, 53]. Our data showed that IGF-1 regulates GPX1 expression by post-transcriptional mechanisms, providing another potentially important mechanism regulating antioxidant activity in the endothelium. One can speculate that the decline in circulating IGF-1 levels with aging leads to decreased GPX expression and antioxidant activity in the endothelium, resulting in an elevated risk for atherogenesis. Future studies will be required to test this hypothesis.
In summary, we found that IGF-1 has potent antioxidant effects in vascular endothelial cells, which is at least in part mediated by upregulation of GPX activity. IGF-1 upregulated GPX1 protein levels by post-transcriptional mechanisms, potentially by modulating the selenocysteine decoding complex formation on Gpx1 mRNA. The observed antioxidant effect of IGF-1 may contribute to maintaining vascular integrity by counteracting oxidative stress, thereby limiting atherosclerosis development.
Supplementary Material
Highlights.
Potential antioxidant effects of IGF-1 were tested in vascular endothelial cells.
IGF-1 upregulated GPX activity via a phosphoinositide-3 kinase dependent pathway.
IGF-1 modulated selenocysteine-incorporation complex formation on GPX1 mRNA.
IGF-1 blocked oxidant-induced premature senescence in vascular endothelial cells.
Acknowledgements
This work was supported by grants from the National Institute of Health R01HL070241 and R01HL080682.
Abbreviations
- hAECs
human aortic endothelial cells
- oxLDL
oxidized low-density lipoprotein
- nLDL
native low-density lipoprotein
- GPX
glutathione peroxidase
- IGF-1
insulin-like growth factor 1
- Apoe
apolipoprotein e
- ROS
reactive oxygen species
- L-NAME
NG-nitro-L-arginine methyl ester
- D-NAME
NG-nitro-D-arginine methyl ester
- SECIS
selenocysteine insertion sequence
- SBP2
SECIS-binding protein 2
- PI3k
phosphatidylinositide 3-kinase
- GH
growth hormone
- NRF2
nuclear factor erythroid 2-like 2
- H2DCFDA
2’,7’-dichlorodihydrofluorescein diacetate
- DHE
dihydroethidium
- CHX
cycloheximide
- eIF4E
eukaryotic initiation factor 4E
- 4EBP1
eukaryotic initiation factor 4E binding protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 3.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 6.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 7.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe−/− mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vasc Biol. 2010;30:1916–1924. doi: 10.1161/ATVBAHA.110.210831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papp LV, Lu J, Striebel F, Kennedy D, Holmgren A, Khanna KK. The redox state of SECIS binding protein 2 controls its localization and selenocysteine incorporation function. Molecular and cellular biology. 2006;26:4895–4910. doi: 10.1128/MCB.02284-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higashi Y, Peng T, Du J, Sukhanov S, Li Y, Itabe H, Parthasarathy S, Delafontaine P. A redox-sensitive pathway mediates oxidized LDL-induced downregulation of insulin-like growth factor-1 receptor. J Lipid Res. 2005;46:1266–1277. doi: 10.1194/jlr.M400478-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Tenenbaum SA, Lager PJ, Carson CC, Keene JD. Ribonomics: identifying mRNA subsets in mRNP complexes using antibodies to RNA-binding proteins and genomic arrays. Methods. 2002;26:191–198. doi: 10.1016/S1046-2023(02)00022-1. [DOI] [PubMed] [Google Scholar]
- 11.Peritz T, Zeng F, Kannanayakal TJ, Kilk K, Eiriksdottir E, Langel U, Eberwine J. Immunoprecipitation of mRNA-protein complexes. Nat Protoc. 2006;1:577–580. doi: 10.1038/nprot.2006.82. [DOI] [PubMed] [Google Scholar]
- 12.Zmijewski JW, Moellering DR, Le Goffe C, Landar A, Ramachandran A, Darley-Usmar VM. Oxidized LDL induces mitochondrially associated reactive oxygen/nitrogen species formation in endothelial cells. Am J Physiol Heart Circ Physiol. 2005;289:H852–H861. doi: 10.1152/ajpheart.00015.2005. [DOI] [PubMed] [Google Scholar]
- 13.Maziere C, Morliere P, Massy Z, Kamel S, Louandre C, Conte MA, Maziere JC. Oxidized low-density lipoprotein elicits an intracellular calcium rise and increases the binding activity of the transcription factor NFAT. Free Radic Biol Med. 2005;38:472–480. doi: 10.1016/j.freeradbiomed.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 16.Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 18.Stielow C, Catar RA, Muller G, Wingler K, Scheurer P, Schmidt HH, Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem Biophys Res Commun. 2006;344:200–205. doi: 10.1016/j.bbrc.2006.03.114. [DOI] [PubMed] [Google Scholar]
- 19.Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech Ageing Dev. 2005;126:389–398. doi: 10.1016/j.mad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-Specific Knockdown of IGF-1 Decreases Vascular Oxidative Stress Resistance by Impairing the Nrf2-Dependent Antioxidant Response: A Novel Model of Vascular Aging. J Gerontol A Biol Sci Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen WH, Boyle DW, Wisniowski P, Bade A, Liechty EA. Insulin and IGF-I stimulate the formation of the eukaryotic initiation factor 4F complex and protein synthesis in C2C12 myotubes independent of availability of external amino acids. J Endocrinol. 2005;185:275–289. doi: 10.1677/joe.1.06080. [DOI] [PubMed] [Google Scholar]
- 22.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Molecular and cellular biology. 1999;19:1871–1880. doi: 10.1128/mcb.19.3.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyronnet S, Imataka H, Gingras AC, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J, Holmgren A. Selenoproteins. J Biol Chem. 2009;284:723–727. doi: 10.1074/jbc.R800045200. [DOI] [PubMed] [Google Scholar]
- 26.Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 27.Driscoll DM, Copeland PR. Mechanism and regulation of selenoprotein synthesis. Annu Rev Nutr. 2003;23:17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- 28.Copeland PR, Fletcher JE, Carlson BA, Hatfield DL, Driscoll DM. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. EMBO J. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small-Howard A, Morozova N, Stoytcheva Z, Forry EP, Mansell JB, Harney JW, Carlson BA, Xu XM, Hatfield DL, Berry MJ. Supramolecular complexes mediate selenocysteine incorporation in vivo. Molecular and cellular biology. 2006;26:2337–2346. doi: 10.1128/MCB.26.6.2337-2346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang HD, Lee TY, Tzeng SW, Horng JT. Kinase Phos: a web tool for identifying protein kinase-specific phosphorylation sites. Nucleic Acids Res. 2005;33:W226–W229. doi: 10.1093/nar/gki471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Du Y, Lu M, Li T. ASEB: a web server for KAT-specific acetylation site prediction. Nucleic Acids Res. 2012;40:W376–W379. doi: 10.1093/nar/gks437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torzewski M, Ochsenhirt V, Kleschyov AL, Oelze M, Daiber A, Li H, Rossmann H, Tsimikas S, Reifenberg K, Cheng F, Lehr HA, Blankenberg S, Forstermann U, Munzel T, Lackner KJ. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 34.Lewis P, Stefanovic N, Pete J, Calkin AC, Giunti S, Thallas-Bonke V, Jandeleit-Dahm KA, Allen TJ, Kola I, Cooper ME, de Haan JB. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- 35.Low SC, Grundner-Culemann E, Harney JW, Berry MJ. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 2000;19:6882–6890. doi: 10.1093/emboj/19.24.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behne D, Hilmert H, Scheid S, Gessner H, Elger W. Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim Biophys Acta. 1988;966:12–21. doi: 10.1016/0304-4165(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 37.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 38.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 39.Wind S, Beuerlein K, Armitage ME, Taye A, Kumar AH, Janowitz D, Neff C, Shah AM, Wingler K, Schmidt HH. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–497. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 41.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 42.Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, Medford RM. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993;92:1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal JF, Michel JB. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–651. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- 44.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109:III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 45.Vanhoutte PM. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J. 2009;73:595–601. doi: 10.1253/circj.cj-08-1169. [DOI] [PubMed] [Google Scholar]
- 46.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 47.Hammerman MR. Insulin-like growth factors and aging. Endocrinol Metab Clin North Am. 1987;16:995–1011. [PubMed] [Google Scholar]
- 48.D'Costa AP, Ingram RL, Lenham JE, Sonntag WE. The regulation and mechanisms of action of growth hormone and insulin-like growth factor 1 during normal ageing. J Reprod Fertil Suppl. 1993;46:87–98. [PubMed] [Google Scholar]
- 49.Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol. 1991;46:B180–B187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- 50.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 51.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Rosen CJ, Delafontaine P. Low circulating insulin-like growth factor I increases atherosclerosis in ApoE-deficient mice. Am J Physiol Heart Circ Physiol. 2011;300:H1898–H1906. doi: 10.1152/ajpheart.01081.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–H1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ungvari Z, Gautam T, Koncz P, Henthorn JC, Pinto JT, Ballabh P, Yan H, Mitschelen M, Farley J, Sonntag WE, Csiszar A. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats, The journals of gerontology. Series A. Biological sciences and medical sciences. 2010;65:1145–1156. doi: 10.1093/gerona/glq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.