Abstract
Purpose of review
Anterior segment dysgenesis (ASD) disorders encompass a spectrum of developmental conditions affecting the cornea, iris, and lens and are generally associated with an approximate 50% risk for glaucoma. These conditions are characterized by both autosomal dominant and recessive patterns of inheritance often with incomplete penetrance/variable expressivity. This article summarizes what is known about the genetics of ASD disorders and reviews recent developments.
Recent findings
Mutations in Collagen 4A1 (COL4A1) and Beta-1,3-glucosyltransferase (B3GALTL) have been reported in ASD patients. Novel findings in other well-known ocular genes are also presented, among which regulatory region deletions in PAX6 and PITX2 are most notable.
Summary
Although a number of genetic causes have been identified, many ASD conditions are still awaiting genetic elucidation. The majority of characterized ASD genes encode transcription factors, several factors represent extracellular matrix related proteins. All of the involved genes play active roles in ocular development and demonstrate conserved functions across species. The use of novel technologies, such as whole genome sequencing/comparative genomic hybridization, is likely to broaden the mutation spectrums in known genes and assist in the identification of novel causative genes as well as modifiers explaining the phenotypic variability of ASD conditions.
Keywords: Axenfeld-Rieger anomaly, Peters anomaly, anterior segment dysgenesis, inheritance, gene
INTRODUCTION
Anterior segment dysgenesis (ASD) disorders encompass a wide variety of developmental conditions affecting the cornea, iris, and lens. Congenital anomalies typically include, alone or in combination, corneal opacity, posterior embryotoxon, iris hypoplasia, corectopia or polycoria, and adhesions between the iris and cornea or lens and cornea. Some specific combinations have been recognized as separate diagnostic entities: Axenfeld-Rieger anomaly (ARA) is the term given to the combination of iris hypoplasia, posterior embryotoxon, iris hypoplasia, corectopia/polycoria, and/or irido-corneal adhesions while Peters anomaly is used to refer to the triad of corneal opacity, defects in the posterior layers of the cornea, and lenticulo-corneal and/or irido-corneal adhesions.
Anterior segment dysgenesis can be an isolated ocular anomaly or accompanied by systemic defects. As a whole, anterior segment anomalies are associated with an approximate 50% risk of glaucoma [1, 2]. The most common syndromes associated with ASDs are Axenfeld-Rieger syndrome (ARS), characterized by ARA of the eye and systemic anomalies (typically hypo/microdontia, redundant periumbilical skin/umbilical hernia, and craniofacial dysmorphism; Figure 1); Peters Plus syndrome (PPS) that comprises anterior chamber eye defects (primarily Peters anomaly; Figure 2) along with short stature, brachydactyly and/or rhizomelic shortening and variable other systemic anomalies; Alagille syndrome, characterized by the presence of posterior embryotoxon in the eye as well as characteristic facial appearance and abnormalities of the liver, heart, skeleton, and kidneys; SHORT syndrome (Short stature, Hyperextensibility of joints or inguinal Hernia, Ocular depression, Rieger eye anomaly, and delay in dental eruption (Teeth); Figure 3); and Pierson syndrome, also called microcoria-congenital nephrosis syndrome. Our review will focus on genes with new reports published within the past two years.
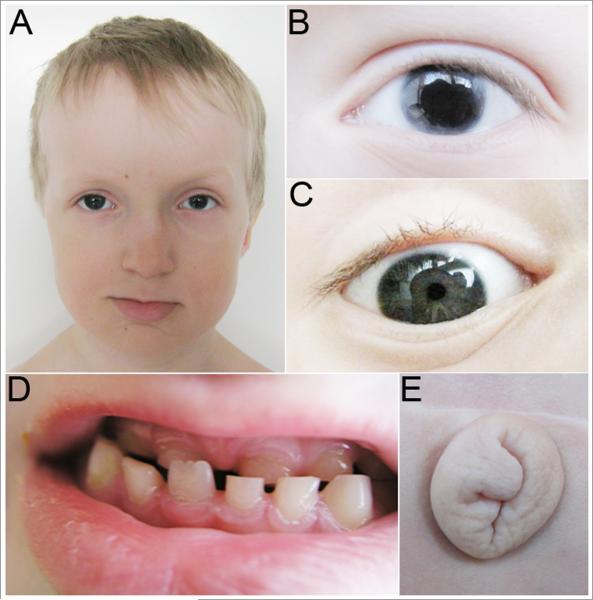
Fig 1. Photographs of Patient with PITX2 regulatory region deletion and Axenfeld-Rieger syndrome.
*Previously Published*
Legend: A. Facial photograph showing maxillary hypoplasia, thin upper lip, and broad nasal bridge. B. Left eye corectopia. C. Right eye posterior embryotoxon. D. Dental anomalies, including maxillary hypodontia. E. Redundant periumbilical skin.
Source: Figure 5 from Volkmann BA, Zinkevich NS, Mustonen A, et al. Potential novel mechanism for Axenfeld-Rieger syndrome: deletion of a distant region containing regulatory elements of PITX2. Invest Ophthalmol Vis Sci 2011, 52(3):1450-1459.
Fig. 2. Photograph of an eye affected with Peters anomaly (courtesy of Dr. Alex Levin).
Fig 3. Photographs of Patient with BMP4 deletion and SHORT syndrome.
*Previously Published*
Legend: A. Patient displays short stature, macrocephaly, decreased subcutaneous fat in upper trunk and head, prominent forehead, sunken eyes, small chin, and hypoplastic nares. B,C. Ocular anomalies include Axenfeld-Rieger anomaly, congenital glaucoma, and microcornea.
Source: Figure 1A-C from Reis LM, Tyler RC, Schilter KF, et al. BMP4 loss-of-function mutations in developmental eye disorders including SHORT syndrome. Hum Genet 2011. Epub ahead of print.
AUTOSOMAL DOMINANT ANTERIOR SEGMENT DYSGENESES
Thus far, the majority of genes associated with ASD have demonstrated autosomal dominant inheritance. Please see Table 1 for details of identified genes and associated phenotypes [3-18].
Table 1.
Summary of Major Genes Associated with Anterior Segment Dysgeneses
| Gene | OMIM | Locus | Phenotypes | Mode of inheritance |
Reference |
|---|---|---|---|---|---|
| PAX6 | 607108 | 11p13 | Aniridia, Peters anomaly, keratitis, foveal hypoplasia, congenital cataract |
AD | [3,4] |
| JAG1 | 601920 | 20p12 | Alagille syndrome with posterior embryotoxon, iris hypoplasia, small corneal diameter, iridocorneal synechiae and corectopia |
AD | [5,6] |
| PITX2 | 601542 | 4q25- q26 |
Axenfeld-Rieger syndrome, Peters anomaly, iris hypoplasia |
AD | [7] |
| FOXC1 | 601090 | 6p25 | Axenfeld-Rieger syndrome, Peters anomaly, iris hypoplasia, primary congenital glaucoma, aniridia |
AD | [8,9] |
| FOXC2 | 602402 | 16q24.3 | Lymphedema-distichiasis syndrome, ASD | AD | [10] |
| PITX3 | 602669 | 10q25 | Congenital posterior polar cataract with or without ASD |
AD | [11] |
| FOXE3 | 601094 | 1p32 | ASD with cataract, Peters anomaly, microphthalmia | AD or AR | [12] |
| BMP4 | 112262 | 14q22- q23 |
ASD with or without A/M, systemic anomalies | AD | [13] |
| COL4A1 | 120130 | 13q34 | Porencephaly/ small vessel brain disease with early- onset cataract or ASD in some |
AD | [14,15] |
| CYP1B1 | 601771 | 2p22- p21 |
Primary congenital glaucoma, Peters anomaly, other ASD, aniridia |
AR | [16] |
| LAMB2 | 150325 | 3p21 | Pierson syndrome with microcoria or other ASD | AR | [17] |
| B3GALTL | 610308 | 13q12.3 | Peters Plus syndrome | AR | [18] |
AD, Autosomal Dominant; AR, Autosomal Recessive; A/M, anophthalmia/microphthalmia; ASD, anterior segment dysgenesis
PAX6
The first gene discovered to play a role in human anterior segment disorders was PAX6 [3, 4]. The spectrum of PAX6 mutations and associated phenotypes has been extensively reviewed in several previous publications ([19-21*]. The majority of PAX6 mutations are nonsense, splicing, insertions, and deletions, and the most common associated phenotype is aniridia, with or without other ocular anomalies ([19-21*]. A few missense mutations have been reported that typically result in milder ocular phenotypes without aniridia including Peters anomaly, uveal ectropion, ectopia pupillae, cataracts, vascularized cornea (autosomal dominant kertatitis), elliptical anterior iris stromal defects and iris hypoplasia [22-25]. While PAX6 deletion was reported in one case of Axenfeld-Rieger syndrome with ocular and systemic anomalies [26], this association was recently rescinded. The authors reported subsequent testing showed normal PAX6 copy number and identification of a PITX2 mutation (c.253-11A>G) in the original patient [27**].
Interestingly, several groups have reported deletions of the downstream regulatory region of PAX6 which leave the coding region intact yet result in an aniridia phenotype [28-31]. Array-based comparative genomic hybridization (aCGH) has recently identified deletions downstream of PAX6 in three additional families and multiplex ligation-dependent probe amplification (MLPA) analysis identified a fourth. These deletions resulted in aniridia in three families [32*,33*,34*] and atypical aniridia which was initially diagnosed as Axenfeld-Rieger anomaly in the other [35**]. These new reports confirm the importance of the 3’ regulatory region and provide further evidence of disease causing mutations outside the coding region of a gene.
Several recent publications have focused on the role of PAX6 in the developing brain, reporting mutations associated with absence of the pineal gland and interhemispheric brain anomalies [36*], autism [37*], and developmental delay [38*]. A number of other papers published in the past year report novel PAX6 mutations with expected phenotypes or new occurrences of previously reported mutations [39*,40*,41*,42*].
JAG1
Alagille syndrome, a highly variable developmental disorder involving liver, heart, ocular, and vertebral anomalies along with characteristic facial features, is associated with mutations in or deletions of the JAG1 gene [5, 6]. Bile duct paucity, chronic cholestasis, heart murmur due to pulmonary stenosis or structural intracardiac disease, and characteristic facial features are seen in almost all affected individuals [43]. Butterfly vertebrae and kidney disease also commonly seen [43]. Ocular anomalies are seen in 78-95% of patients, primarily posterior embryotoxon, although other ASDs such as iris hypoplasia and small corneal diameters are also common and irido-corneal synechiae and corectopia have been occasionally reported [43-45]. Abnormalities affecting the posterior segment of the eye are also seen in most patients, including pigmentary retinopathy, abnormal retinal vessels, and optic disc anomalies, particularly optic disc drusen [44-45].
PITX2 and FOXC1
Both PITX2 [7] and FOXC1 [8, 9] disruption via intragenic mutation or deletion are associated with Axenfeld-Riger anomaly with or without systemic anomalies. The spectrum of phenotypes associated with mutations in these genes was recently reviewed [46**]. While other phenotypes including Peters anomaly (PITX2 and FOXC1), isolated iris hypoplasia/iridogoniodysgenesis (PITX2 and FOXC1), ring dermoid of the cornea (PITX2), primary congenital glaucoma (FOXC1), and aniridia (FOXC1) have been associated with mutations in these genes, PITX2 disruption is primarily associated with ARS with typical ocular anomaly accompanied by dental and umbilical defects and FOXC1 disruption primarily results in ARS with heart or hearing defects or an isolated ocular phenotype [46]. Duplication of FOXC1 also results in ASD including iris hypoplasia with glaucoma [47, 48], microcornea [49], Peters anomaly [50], and iridogoniodysgenesis [51].
A recent screening of 80 probands with ASD with or without systemic anomalies provided the first thorough screening of PITX2 and FOXC1 for both nucleotide mutations and copy number changes. Intragenic mutations or deletions were detected in 32 (40%) [52**]. Eleven of the 13 probands with PITX2 disruption had ocular, dental and umbilical anomalies, one had ocular and dental anomalies with umbilicus not examined, and no details were available for the final case [52**]. Details regarding systemic features was provided for 14 of the 19 probands with FOXC1 disruption: six had isolated ocular (with minor craniofacial features in three), six had ocular and hearing loss (with dental anomalies in four), and two had ocular and dental anomalies with normal hearing [52**].
Similar to PAX6, aCGH identified deletion of an upstream regulatory region of PITX2 in a patient with ARS (Figure 1) [53**]. Array CGH also identified a deletion of FOXC1 in an infant with partial aniridia, congenital glaucoma, heart defect, and bilateral club feet [35]. Two additional papers presented new cases of intragenic PITX2 mutations: a novel missense mutation was reported in a patient with Peters anomaly, persistent ocular fetal vasculature, and unilateral microphthalmia [54*]. A previously reported missense mutation was identified in a three-generation pedigree with dental and craniofacial features in affected family members [55*]. These reports further highlight the variable expressivity of PITX2 mutations, including both ocular and craniofacial features.
FOXC2
Ocular examination of patients with lymphedema-distichiasis syndrome and mutations in FOXC2, another member of the forkhead family, identified mild ASD, including partial iris hypoplasia, corectopia, reduced corneal diameter, and localized corneal opacification, in those with mutations within the forkhead domain [10]. No subsequent studies have investigated the role of FOXC2 in anterior segment dysgenesis.
PITX3
Heterozygous mutations in PITX3, another member of the PITX family, have been reported in 11 families with congenital posterior polar cataract with ASD in some individuals [11, 56-60]. The most common mutation is a recurrent 17-bp insertion (c.657_673dup17) in the C-terminal region seen in all but three families. In one family, two individuals inherited homozygous PITX3 mutations resulting in bilateral microphthalmia, corneal opacification, and severe developmental delay [59]. The paucity of reported cases as well as two recent studies reporting a lack of mutation in 27 probands with isolated congenital cataract [41]and a large family with autosomal recessive history of cataract and mental retardation [61*] suggests that mutations in PITX3 do not seem to be a major cause of congenital cataract. A recent review summarized what is known about the Pitx3 molecule and the role of PITX3 in human disease [62*]. New investigations suggest that PITX3 may play a role in Parkinson’s disease as well [63, 64*] .
FOXE3
While homozygous/compound heterozygous mutations in FOXE3 result in microphthalmia with primary aphakia/sclerocornea, heterozygous mutations have been reported in six families with ASD with or without cataract [12, 41, 65-67*]. The first mutation identified and three subsequent heterozygous mutations disrupt the distal part of the gene (three in stop codon 320 and one in residue 315) and result in extension of the FOXE3 protein with the addition of 72-117 erroneous amino acids [12, 41, 66, 67**]. These mutations primarily resulted in anterior segment dysgenesis with cataract, one proband demonstrated colobomatous microphthalmia in addition to ASD, and one had isolated congenital cataract. The first missense mutation, p.R90L in the DNA-binding domain, resulted in familial Peters anomaly with or without cataract [65]. A second missense mutation, p.G49A, was reported in a proband with microphthalmia/coloboma and family members with cataract [66]; although, a more recent study suggested that p.G49A may be a polymorphic variant as it was seen in 3% of African American controls [68]. Interestingly, carrier relatives of individuals with homozygous/compound heterozygous FOXE3 mutations are almost universally unaffected [66, 68-71], suggesting an alternate mechanism for mutations which result in dominant versus recessive disease. The possibility of a dominant-negative mechanism for heterozygous mutations has been raised, but one group recently challenged this hypothesis [67**].
BMP4 and BMP7
Disruption of BMP4 by deletion or mutation is associated with ocular, digit (polydactyly), brain/neurological (abnormal structure, hypotonia/delay) and craniofacial (dysmorphic facial features, macrocephaly) anomalies as well as poor growth [72**]. While most individuals with BMP4 disruption exhibit clinical anophthalmia/microphthalmia with or without ASD [13, 72**], anterior segment anomalies with normal eye size has also been reported [73]. Most recently, a patient with SHORT syndrome was found to have a 2.3-Mb deletion of BMP4 and 13 other genes [72**]; ocular features included ARA, congenital glaucoma and microcornea (Figure 3).
Another member of the BMP family, BMP7, has been recently evaluated in patients with anophthalmia, microphthalmia, and coloboma with mutations not seen in controls (but inherited from unaffected or mildly affected mothers) identified in two patients with anophthalmia and systemic defects [74*]. Screening of patients with ASD phenotypes is indicated to determine whether or not this gene plays a role in other ocular disorders since, similar to Bmp4, Bmp7 has also been implicated in the development of anterior segment structures in mice [75].
COL4A1
One of the most recently reported genes associated with ASD is COL4A1, which encodes a collagen chain important in the formation of basement membranes. Mouse Col4a1 is expressed in the anterior segment of the eye [76]. Mutations in COL4A1 were initially identified as a cause of porencephaly and brain small vessel disease with stroke and retinal arteriolar tortuosity in some individuals [14, 15] and then also found to be associated with anterior segment ocular anomalies (Table 2) including early-onset cataract [77-79], ARA, corneal opacities, congenital cataract, microcornea, elevated intraocular pressure, and/or glaucoma [80, 81**]. The range of neurological features was recently reviewed [82*]. Brain MRI often reveals anomalies in mutation carriers even in the absence of neurological symptoms.
Table 2.
Anterior Segment Anomalies Described in Patients with COL4A1 mutations
|
COL4A1 mutation |
Patient identifier |
Age | Anterior segment anomaliesa | Other ocular featuresa |
Neurological symptoms |
Brain anomalies |
Reference |
|---|---|---|---|---|---|---|---|
| p.G1236R | Patient 1 | 54 | cataract (age 52) | exotropia, mild retinal arteriolar tortuosity |
Yes | Yes | [77] |
| p.G1236R | Patient 2 | 33 | “polar” cataract (age 33) | mild esotropia | Yes | Yes | [77] |
| p.G1236R | Patient 3 | 30 | nuclear cataract (age 30) | mild exotropia | Yes | Yes | [77] |
| p.G755R | Proband | 14 | dot-like cataracts (age 9) | Yes | Yes | [78] | |
| p.G755R | Mother | NR | cataracts (age mid-30s) | Yes | Yes | [78] | |
| p.G755R (presumed) |
Maternal grandmother |
NR | cataracts (age 9) | Yes | Yes | [78] | |
| p.G755R | Case | 21 | unilateral posterior “capsular” cataract (age 21) |
infantile strabismus | No | Yes | [79] |
| p.G720D | Case II.2 | 58 | iridogoniodysgenesis, iris hypoplasia, microcornea, congenital cataract, elevated IOP (age 55) |
high myopia | Yes | Yes | [80] |
| p.G720D | Case III.2 | 37 | congenital cataracts, congenital glaucoma, microcornea, peripheral opacities |
unilateral retinal detachment |
Yes | Yes | [80] |
| p.G720D | Case III.3 | 32 | congenital cataract, juvenile glaucoma, unilateral polycoria, |
high myopia | No | Yes | [80] |
| p.G720D | Case III.4 | 29 | microcornea, cataract | No | Yes | [80] | |
| p.G720D | Case IV.1 | 8 | congenital cataract, iris hypoplasia, microcornea |
Yes | Yes | [80] | |
| p.G2159A | A.I.1 | 58 | Iridogoniodysgenesis, iridocorneal synechiae, iris hypoplasia, microcornea, congenital cataract, elevated IOP |
high myopia, macular hemorrhages |
No | Yes | [81**] |
| p.G2159A | A.II.1 | 38 | congenital cataract, iris hypoplasia, high IOP (age 23), microcornea, peripheral corneal opacities, corectopia |
myopia, severe amblyopia, unilateral retinal detachment |
Yes | Yes | [81**] |
| p.G2159A | A.II.2 | 35 | microcornea, congenital cataract, juvenile glaucoma (age 32), corneal opacities, corneal neovascularization, iridocorneal synechiae, unilateral corectopia and polycoria |
high myopia, unilateral retinal detachment |
No | Yes | [81**] |
| p.G2159A | A.II.3 | 29 | microcornea, cataract | strabismus | No | Yes | [81**] |
| p.G2159A | A.III.1 | 8 | congenital cataract, iris hypoplasia, microcornea |
strabismus | Yes | Yes | [81**] |
| p.G755R | B.I.1 | 47 | lens opacities | severe hyperopia | Yes | Yes | [81**] |
| p.G755R | B.II.1 | 10 | congenital cataract, posterior embyotoxon, microcornea |
No | Yes | [81**] |
Bilateral unless specified; IOP = intraocular pressure
AUTOSOMAL RECESSIVE ANTERIOR SEGMENT DYSGENESES
Other identified loci are mainly associated with autosomal recessive inheritance of ocular disease (Table 1).
CYP1B1
Homozygous/compound heterozygous mutations in CYP1B1 are typically associated with primary congenital glaucoma [16]. Several reports have identified mutations in patients with isolated Peters or Axenfeld-Rieger anomaly, typically in association with glaucoma [83-86]. Three out of 11 patients with anterior segment defects had only a single heterozygous mutation identified, suggesting that there may be a second mutation in a CYP1B1 regulatory region that is yet to be identified, or a mutation in a second gene which contributes to the phenotype, as has been reported for CYP1B1 and MYOC in primary congenital and juvenile onset glaucoma [87-89].
Two novel anterior segment phenotypes were recently linked to CYP1B1 mutations. Compound heterozygous CYP1B1 mutations were identified in a patient with Axenfeld-Rieger syndrome including protruding umbilicus and malaligned teeth [90**]. This is the first association of CYP1B1 with the systemic features of ARS. Homozygous or compound heterozygous mutations were identified in eight probands with mild ectropion uveae, partial aniridia, and congenital glaucoma [91*], further expanding the ocular phenotype associated with CYP1B1 mutations.
LAMB2
Mutations in LAMB2 result in Pierson syndrome, characterized by congenital nephrotic syndrome and ocular defects along with neurologic anomalies in some [17]. The primary ocular feature is miosis. Other eye defects that are occasionally observed include iris hypoplasia, ectropion uveae, microcornea, glaucoma, cataract, posterior embryotoxon, microphthalmia, posterior lenticonus, microspherophakia, cloudy or enlarged corneas, and generalized anterior segment dysgenesis [92]. The full spectrum of mutations and associated phenotypes was recently reviewed [93**]. The majority of mutations are truncating; while missense mutations are typically associated with later onset of renal disease and lack of neurologic abnormalities, phenotypic variability is not perfectly correlated to LAMB2 genotype. Recently, the first two Asian patients with two truncation mutations have been reported with typical ocular findings but milder renal involvement, suggesting that race may play a role in phenotypic variability [94*, 95]. Finally, a novel homozygous missense mutation was seen in nine members of a Mennonite family affected with a variant phenotype consisting of infantile nephrotic syndrome, chorioretinal pigmentary changes, and retinal detachments in some individuals, but no microcoria or neurologic anomalies [96*]. Results from a screening of 30 patients suggested that LAMB2 mutations explain all cases of typical Pierson syndrome [93**].
B3GALTL
Peters Plus syndrome (PPS) is a rare congenital disorder characterized by anterior chamber eye defects (primarily Peters anomaly) and variable systemic anomalies primarily including short stature, brachydactyly and/or rhizomelic shortening, developmental delay, dysmorphic facial features, cleft lip and/or palate, and heart, genitourinary, or ear anomalies [97]. The role of B3GALTL in PPS was recently identified with splicing mutations/deletions identified in 20 of 20 patients [18], including a recurrent c.660+1G>A mutation. Subsequent screens revealed mutations/deletions in nine additional patients with typical PPS [98-101*,102*]; splicing mutations are the most commonly seen. Review of these previous reports suggests that the presence of ASD, short stature, brachydactyly, and characteristic facial features is predictive of B3GALTL mutations while no mutations in B3GALTL were identified in eight patients with atypical PPS (some but not all of these typical features) (Table 3) [99, 101*, 104*]. However, recent screening of B3GALTL in two siblings whose phenotype closely matched PPS failed to identify mutations: the siblings were affected with bilateral corneal opacity, small hands/feet, short stature, and similar facial features along with growth hormone deficiency and hypoplastic pituitary gland in one (Table 3) [103**,].
Table 3.
Comparison of Patients with and without mutations in B3GALTL
| Publication |
B3GALTL mutations |
Peters Anomaly |
Anterior Chamber Anomaly |
Short Stature (<=3rd) |
Brachy- dactyly |
Develop mental Delay |
Cleft Lip/ Palate |
Heart Anomaly |
Genitourinary anomaly |
Ear Anomaly |
Brain anomaly |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lesnik Oberstein, et al. (2006) [18] |
20/20 | 15/19 | 20/20 | 20/20 | NR | 15/19 | 9/20 | 5/19 | renal 5/20 | NR | NR |
| Kapoor et al. (2008) [98] |
1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 | 0/1 | 0/1 | 1/1 | 0/1 |
| Reis, et al. (2008) [99] |
4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 3/3 | 4/4 | 3/4 | 2/4 | 3/4 | 2/3 hypoplasia of corpus callosum and enlarged ventricles (2) |
| Halderman-Englert et al. (2009) [100] |
1/1 | NR | 1/1 | 1/1 | 1/1 | NR | 0/1 | 1/1 | 1/1 | 1/1 | 1/1 -hypoplasia of corpus callosum and enlarged ventricles |
| Dassie-Ajdid et al. (2009) [101*] |
2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 0/2 | 2/2 | 0/2 | 0/2 | NR |
| Aliferis et al. (2010) [102*] |
1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 | 0/1 | 1/1 | 1/1 | 1/1 hypoplasia of corpus callosum and enlarged ventricles |
|
Total Mutation
Positive |
29/29
(100%) |
21/27
(78%) |
29/29
(100%) |
29/29
(100%) |
9/9
(100%) |
22/26
(85%) |
13/29
(45%) |
11/29
(38%) |
9/29 (31%) |
6/9
(67%) |
4/6
(67%) |
| Reis et al. (2008) [99*] |
0/4 | 3/4 | 4/4 | 1/4 | 1/3 | 2/3 | 2/4 | 1/4 | 3/4 | 4/4 | 3/4 |
| Dassie-Ajdid et al. (2009) [101*] |
0/2 | 2/2 | 2/2 | 0/2 | 0/2 | 2/2 | 0/2 | 0/2 | 0/2 | 0/2 | NR |
| Al-Gazali et al. (2009) [103**] |
0/2 | 2/2 | 2/2 | 2/2 | 1/2 | 2/2 | 0/2 | 1/2 | 0/2 | 0/2 | 2/2 hypoplasia of corpus callosum (1), hypoplastic pituitary gland (1) |
| Shimizu et al. (2010) [104*] |
0/1 | 0/1 | 1/1 | 0/1 | 1/1 | NR | 1/1 | 1/1 | 0/1 | 1/1 | NR |
|
Total Mutation
Negative |
0/9 |
7/9
(78%) |
9/9
(100%) |
3/9
(33%) |
3/8
(38%) |
6/7
(86%) |
3/9
(33%) |
3/9
(33%) |
3/9
(33%) |
5/9
(56%) |
5/6
(83%) |
NR= Not Reported
CONDITIONS AND GENES OCCASIONALLY ASSOCIATED WITH ANTERIOR SEGMENT DYSGENESIS
Oculo-dental-digital dysplasia, affecting the eye, dentition, digits of the hands and toes, with facial features, and neurological and hearing deficits, is caused by mutations in the GJA1 gene [105]. While microphthalmia and microcornea are the primary ocular phenotype, increased risk of glaucoma is also seen and a proportion of patients have iris anomalies [106]. Mutations in SOX2 are primarily associated with SOX2 Anophthalmia syndrome characterized by clinical anophthalmia/microphthalmia and variable brain, pituitary, genitourinary, and gastroesophageal defects [107, 108]. One individual with isolated iris hypoplasia and a family member with isolated microcornea were reported with a missense mutation in SOX2 [109*]. Subsequent screening of 28 probands with various ASDs failed to identify any mutations in SOX2 [110*].
Anterior segment anomalies were first reported with mutations in FGFR2 in three patients with Pfeiffer or Crouzon syndrome (craniosynostosis syndromes), including two with Peters anomaly [111]. In another family, the proband was affected with ARA and right microcornea along with premature fusion of the sagittal suture. His mother carried the same FGFR2 mutation and was found to have mild iris hypoplasia and posterior embryotoxon with mild exorbitism but no craniosynostosis [112]. Most recently, corectopia, limbal scleralization, microcornea, and glaucoma were reported in a patient with Pfeiffer syndrome [113*].
NOVEL GENOMIC REGIONS ASSOCIATED WITH ANTERIOR SEGMENT DYSGENESIS
Several new cases of chromosomal anomalies associated with ASD have been reported. Linkage to chromosome 2 was identified in a large family affected with microcornea, atypical iris coloboma, congenital cataract, and microphthalmia [114]. A proband with ARA, global delay, dysmorphic features, and retinoblastoma was found to have del(13)(q12;q22) including the RB1 gene at 13q14 [115*]and overlapping the 13q deletions reported in two previous cases of ARA, one of whom also exhibited retinoblastoma and severe developmental delay [116, 117]. A complex chromosomal rearrangement, inv(2)(p22.3q12.1)t(2;16)(q12.1;q12.2), was detected in another patient with aniridia, aphakia, corneal anomalies, and congenital glaucoma. The authors hypothesized that the separation of the IRXB gene cluster from its regulatory region at 16q12.2 is the cause of the observed anterior segment dysgenesis [118*]. Finally, aCGH identified a 326-kb deletion at 9p22.31 in a patient with an atypical PPS phenotype consisting of unilateral Peters anomaly and microphthalmia, intrauterine growth retardation, clinodactyly, dsymorphic facial features, agenesis of the right lung, and heart, gastrointestinal, and genitourinary defects [119]. This deletion encompasses 8 of the 9 exons of the ROR2 gene, previously associated with an autosomal recessive form of Robinow syndrome, a condition that sometimes involves infantile glaucoma (Dr. Alex Levin, personal communication).
CONCLUSION
Identification of the genetic etiology of ASDs has revealed multiple genetic loci. While the primary phenotype associated with ASD genes is typically distinct, most genes are involved in multiple related ASD phenotypes with a high degree of inter- and intrafamilial variable phenotypic expressivity. Novel technologies including whole genome/exome sequencing and aCGH have greatly facilitated identification of the genetic etiology of these conditions [120*] and are likely to broaden the spectrum of mutations associated with known ASD genes as well as to assist in identification of new causative and modifier loci. Array CGH has identified several deletions of regulatory regions in ASDs, highlighting a novel disease mechanism. Genetic interactions between known factors are beginning to be elucidated and are likely to provide insight into the basis of the extreme variability of what are currently known as single-gene conditions.
KEY POINTS.
Anterior segment dysgeneses demonstrate different modes of inheritance and a high degree of inter- and intrafamilial phenotypic variability. Molecular genetic analysis and examination of family members can assist in providing genetic counseling.
A significant overlap among phenotypes attributed to mutations in different ASD genes is well recognized. Functional studies have suggested that several of the ASD causative genes may function within a common pathway.
Identification of deletions downstream of PAX6 and upstream of PITX2 highlight the importance of regulatory region mutations in human ocular disease and suggest that aCGH data should be analyzed for possible deletions of upstream and downstream regions of other genes.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health awards EY020134 and DC010912 (EVS) as well as support from the Children’s Research Institute at the Children’s Hospital of Wisconsin and the Medical College of Wisconsin.
Abbreviations
- (ASD)
Anterior segment dysgenesis
- (ARS)
Axenfeld-Rieger syndrome
- (ARA)
Axenfeld-Rieger anomaly
- (PPS)
Peters Plus syndrome
- (aCGH)
Array-based comparative genomic hybridization
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review (2009-2010), have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Alward WL. Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol. 2000;130:107–115. doi: 10.1016/s0002-9394(00)00525-0. [DOI] [PubMed] [Google Scholar]
- 2.Sowden JC. Molecular and developmental mechanisms of anterior segment dysgenesis. Eye (Lond) 2007;21:1310–1318. doi: 10.1038/sj.eye.6702852. [DOI] [PubMed] [Google Scholar]
- 3.Ton CC, Hirvonen H, Miwa H, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 4.Jordan T, Hanson I, Zaletayev D, et al. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1992;1:328–332. doi: 10.1038/ng0892-328. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 6.Oda T, Elkahloun AG, Pike BL, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 7.Semina EV, Reiter R, Leysens NJ, et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- 8.Mears AJ, Jordan T, Mirzayans F, et al. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. Am J Hum Genet. 1998;63:1316–1328. doi: 10.1086/302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura DY, Swiderski RE, Alward WL, et al. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat Genet. 1998;19:140–147. doi: 10.1038/493. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann OJ, Tuft S, Brice G, et al. Novel anterior segment phenotypes resulting from forkhead gene alterations: evidence for cross-species conservation of function. Invest Ophthalmol Vis Sci. 2003;44:2627–2633. doi: 10.1167/iovs.02-0609. [DOI] [PubMed] [Google Scholar]
- 11.Semina EV, Ferrell RE, Mintz-Hittner HA, et al. A novel homeobox gene PITX3 is mutated in families with autosomal-dominant cataracts and ASMD. Nat Genet. 1998;19:167–170. doi: 10.1038/527. [DOI] [PubMed] [Google Scholar]
- 12.Semina EV, Brownell I, Mintz-Hittner HA, et al. Mutations in the human forkhead transcription factor FOXE3 associated with anterior segment ocular dysgenesis and cataracts. Hum Mol Genet. 2001;10:231–236. doi: 10.1093/hmg/10.3.231. [DOI] [PubMed] [Google Scholar]
- 13.Bakrania P, Efthymiou M, Klein JC, et al. Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways. Am J Hum Genet. 2008;82:304–319. doi: 10.1016/j.ajhg.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breedveld G, de Coo IF, Lequin MH, et al. Novel mutations in three families confirm a major role of COL4A1 in hereditary porencephaly. J Med Genet. 2006;43:490–495. doi: 10.1136/jmg.2005.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould DB, Phalan FC, van Mil SE, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 16.Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet. 1997;6:641–647. doi: 10.1093/hmg/6.4.641. [DOI] [PubMed] [Google Scholar]
- 17.Zenker M, Aigner T, Wendler O, et al. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13:2625–2632. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 18.Lesnik Oberstein SA, Kriek M, White SJ, et al. Peters Plus syndrome is caused by mutations in B3GALTL, a putative glycosyltransferase. Am J Hum Genet. 2006;79:562–566. doi: 10.1086/507567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prosser J, van Heyningen V. PAX6 mutations reviewed. Hum Mutat. 1998;11:93–108. doi: 10.1002/(SICI)1098-1004(1998)11:2<93::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Tzoulaki I, White IM, Hanson IM. PAX6 mutations: genotype-phenotype correlations. BMC Genet. 2005;6:27. doi: 10.1186/1471-2156-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Kokotas H, Petersen MB. Clinical and molecular aspects of aniridia. Clin Genet. 2010;77:409–420. doi: 10.1111/j.1399-0004.2010.01372.x. Review of PAX6 associated aniridia including common mutations and disease mechanisms.
- 22.Hanson IM, Fletcher JM, Jordan T, et al. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters’ anomaly. Nat Genet. 1994;6:168–173. doi: 10.1038/ng0294-168. [DOI] [PubMed] [Google Scholar]
- 23.Azuma N, Yamada M. Missense mutation at the C terminus of the PAX6 gene in ocular anterior segment anomalies. Invest Ophthalmol Vis Sci. 1998;39:828–830. [PubMed] [Google Scholar]
- 24.Hanson I, Churchill A, Love J, et al. Missense mutations in the most ancient residues of the PAX6 paired domain underlie a spectrum of human congenital eye malformations. Hum Mol Genet. 1999;8:165–172. doi: 10.1093/hmg/8.2.165. [DOI] [PubMed] [Google Scholar]
- 25.Hingorani M, Williamson KA, Moore AT, et al. Detailed ophthalmologic evaluation of 43 individuals with PAX6 mutations. Invest Ophthalmol Vis Sci. 2009;50:2581–2590. doi: 10.1167/iovs.08-2827. [DOI] [PubMed] [Google Scholar]
- 26.Riise R, Storhaug K, Brondum-Nielsen K. Rieger syndrome is associated with PAX6 deletion. Acta Ophthalmol Scand. 2001;79:201–203. doi: 10.1034/j.1600-0420.2001.079002201.x. [DOI] [PubMed] [Google Scholar]
- **27.Riise R, D’haene B, De Baere E, et al. Rieger syndrome is not associated with PAX6 deletion: a correction to Acta Ophthalmol Scand 2001. Acta Ophthalmol. 2009;79:201–203. doi: 10.1111/j.1755-3768.2009.01696.x. Original patient reported with ARS and PAX6 deletion was found to have PITX2 mutation and normal PAX6 copy number.
- 28.Lauderdale JD, Wilensky JS, Oliver ER, et al. 3′ deletions cause aniridia by preventing PAX6 gene expression. Proc Natl Acad Sci U S A. 2000;97:13755–13759. doi: 10.1073/pnas.240398797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Elia AV, Pellizzari L, Fabbro D, et al. A deletion 3′ to the PAX6 gene in familial aniridia cases. Mol Vis. 2007;13:1245–1250. [PubMed] [Google Scholar]
- 30.Davis LK, Meyer KJ, Rudd DS, et al. Pax6 3′ deletion results in aniridia, autism and mental retardation. Hum Genet. 2008;123:371–378. doi: 10.1007/s00439-008-0484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redeker EJ, de Visser AS, Bergen AA, et al. Multiplex ligation-dependent probe amplification (MLPA) enhances the molecular diagnosis of aniridia and related disorders. Mol Vis. 2008;14:836–840. [PMC free article] [PubMed] [Google Scholar]
- *32.Bayrakli F, Guney I, Bayri Y, et al. A novel heterozygous deletion within the 3′ region of the PAX6 gene causing isolated aniridia in a large family group. J Clin Neurosci. 2009;16:1610–1614. doi: 10.1016/j.jocn.2009.03.022. Use of a chromosome 11-specific aCGH identified a deletion ~140kb downstream of PAX6 in a large family with aniridia; the deletion cosegregates with disease phenotype.
- *33.Cheng F, Song W, Kang Y, et al. A 556 kb deletion in the downstream region of the PAX6 gene causes familial aniridia and other eye anomalies in a Chinese family. Mol Vis. 2011;17:448–455. Another report of a deletion ~123-kb downstream of PAX6 which cosegregated with disease phenotype (aniridia and other anterior segment defects) identified by whole genome aCGH.
- *34.Zhang X, Zhang Q, Tong Y, et al. Large novel deletions detected in Chinese families with aniridia: correlation between genotype and phenotype. Mol Vis. 2011;17:548–557. Clinical and genetic details of two large families are reported, one with deletion of PAX6 and one with a deletion downstream of PAX6.
- **35.Balikova I, de Ravel T, Ayuso C, et al. High Frequency Of Submicroscopic Chromosomal Deletions in Patients with Idiopathic Congenital Eye Malformations. Am J Ophthalmol. 2011 doi: 10.1016/j.ajo.2010.11.025. Use of array CGH to screen 37 patients with various ocular defects identified causal deletions in 13%, including deletion of FOXC1 in a patient with partial aniridia and congenital glaucoma and a regulatory deletion downstream of PAX6 in a patient with atypical aniridia.
- *36.Abouzeid H, Youssef MA, ElShakankiri N, et al. PAX6 aniridia and interhemispheric brain anomalies. Mol Vis. 2009;15:2074–2083. Investigation of three families with PAX6 mutations and aniridia revealed structural brain anomalies in all three probands, with normal neurological examinations.
- *37.Maekawa M, Iwayama Y, Nakamura K, et al. A novel missense mutation (Leu46Val) of PAX6 found in an autistic patient. Neurosci Lett. 2009;462:267–271. doi: 10.1016/j.neulet.2009.07.021. Screening of 285 autistic patients identified a PAX6 missense mutation in one individual with autism, reduced vision, photophobia, and ptosis, but without aniridia.
- *38.Chien YH, Huang HP, Hwu WL, et al. Eye anomalies and neurological manifestations in patients with PAX6 mutations. Mol Vis. 2009;15:2139–2145. This study reports PAX6 mutations identified in 5 of 6 patients with aniridia, 4 of whom had developmental delay. No PAX6 mutations were identified in one patient with other eye anomalies (including microphthalmia and microcornea).
- *39.Cai F, Zhu J, Chen W, et al. A novel PAX6 mutation in a large Chinese family with aniridia and congenital cataract. Mol Vis. 2010;16:1141–1145. A novel 17-bp deletion in exon 5 of PAX6 was identified in a large family with aniridia and congenital cataract.
- *40.Jia X, Guo X, Jia X, et al. A novel mutation of PAX6 in Chinese patients with new clinical features of Peters’ anomaly. Mol Vis. 2010;16:676–681. A novel missense mutation was identified in a patient with Peters anomaly, microphthalmia, abnormal rod and cone function, opaque vitreous, and abnormal optic nerve.
- *41.Bremond-Gignac D, Bitoun P, Reis LM, et al. Identification of dominant FOXE3 and PAX6 mutations in patients with congenital cataract and aniridia. Mol Vis. 2010;16:1705–1711. Report of a novel mutation in the stop codon of FOXE3 in a proband with isolated congenital cataract. Also reports three new cases of previously reported PAX6 mutations in probands with aniridia and cataract.
- *42.Beby F, Dieterich K, Calvas P. A [c.566-2A>G] heterozygous mutation in the PAX6 gene causes aniridia with mild visual impairment. Eye (Lond) 2011 doi: 10.1038/eye.2010.234. This letter describes a rare PAX6 missense splice site mutation in a patient with aniridia but relatively preserved visual function.
- 43.Emerick KM, Rand EB, Goldmuntz E, et al. Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology. 1999;29:822–829. doi: 10.1002/hep.510290331. [DOI] [PubMed] [Google Scholar]
- 44.Kim BJ, Fulton AB. The genetics and ocular findings of Alagille syndrome. Semin Ophthalmol. 2007;22:205–210. doi: 10.1080/08820530701745108. [DOI] [PubMed] [Google Scholar]
- 45.Hingorani M, Nischal KK, Davies A, et al. Ocular abnormalities in Alagille syndrome. Ophthalmology. 1999;106:330–337. doi: 10.1016/S0161-6420(99)90072-6. [DOI] [PubMed] [Google Scholar]
- **46.Tümer Z, Bach-Holm D. Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet. 2009;17:1527–1539. doi: 10.1038/ejhg.2009.93. A good review of PITX2 and FOXC1 mutations, phenotypes, and function.
- 47.Lehmann OJ, Ebenezer ND, Jordan T, et al. Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am J Hum Genet. 2000;67:1129–1135. doi: 10.1016/s0002-9297(07)62943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chanda B, Asai-Coakwell M, Ye M, et al. A novel mechanistic spectrum underlies glaucoma- associated chromosome 6p25 copy number variation. Hum Mol Genet. 2008;17:3446–3458. doi: 10.1093/hmg/ddn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehmann OJ, Ebenezer ND, Ekong R, et al. Ocular developmental abnormalities and glaucoma associated with interstitial 6p25 duplications and deletions. Invest Ophthalmol Vis Sci. 2002;43:1843–1849. [PubMed] [Google Scholar]
- 50.Nishimura DY, Searby CC, Alward WL, et al. A spectrum of FOXC1 mutations suggests gene dosage as a mechanism for developmental defects of the anterior chamber of the eye. Am J Hum Genet. 2001;68:364–372. doi: 10.1086/318183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strungaru MH, Dinu I, Walter MA. Genotype-phenotype correlations in Axenfeld-Rieger malformation and glaucoma patients with FOXC1 and PITX2 mutations. Invest Ophthalmol Vis Sci. 2007;48:228–237. doi: 10.1167/iovs.06-0472. [DOI] [PubMed] [Google Scholar]
- **52.D’haene B, Meire F, Claerhout I, et al. Expanding the spectrum of FOXC1 and PITX2 mutations and copy number changes in patients with anterior segment malformations. Invest Ophthalmol Vis Sci. 2011;52:324–333. doi: 10.1167/iovs.10-5309. This manuscript describes the first large-scale screening of 80 ASD patients for intragenic and copy number mutations in PITX2 and FOXC1 and identifies mutations in 40% of cases. Virtually all patients with PITX2 disruption displayed ASD, dental anomalies and umbilical anomalies. Patients with FOXC1 disruption were more likely to have isolated ASD or ASD with hearing loss.
- **53.Volkmann BA, Zinkevich NS, Mustonen A, et al. Potential novel mechanism for Axenfeld-Rieger syndrome: deletion of a distant region containing regulatory elements of PITX2. Invest Ophthalmol Vis Sci. 2011;52(3):1450–1459. doi: 10.1167/iovs.10-6060. This study reports the first ARS patient with a genomic deletion upstream of PITX2. The authors identify conserved regulatory elements in this upstream region and show that these elements direct pitx2 expression in zebrafish.
- *54.Arikawa A, Yoshida S, Yoshikawa H, et al. Case of novel PITX2 gene mutation associated with Peters’ anomaly and persistent hyperplastic primary vitreous. Eye (Lond) 2010;24:391–393. doi: 10.1038/eye.2009.114. The authors report a missense mutation near the end of the PITX2 protein in a patient with Peters, persistent fetal vasculature, and unilateral microphthalmia.
- *55.Dressler S, Meyer-Marcotty P, Weisschuh N, et al. Dental and Craniofacial Anomalies Associated with Axenfeld-Rieger Syndrome with PITX2 Mutation. Case Report Med. 2010;2010:621984. doi: 10.1155/2010/621984. This paper presents detailed evaluation of craniofacial and dental anomalies in four family members with a mutation in PITX2 and reviews the literature regarding dental defects in ARS.
- 56.Berry V, Yang Z, Addison PK, et al. Recurrent 17 bp duplication in PITX3 is primarily associated with posterior polar cataract (CPP4) J Med Genet. 2004;41:e109. doi: 10.1136/jmg.2004.020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finzi S, Li Y, Mitchell TN, et al. Posterior polar cataract: genetic analysis of a large family. Ophthalmic Genet. 2005;26:125–130. doi: 10.1080/13816810500229124. [DOI] [PubMed] [Google Scholar]
- 58.Burdon KP, McKay JD, Wirth MG, et al. The PITX3 gene in posterior polar congenital cataract in Australia. Mol Vis. 2006;12:367–371. [PubMed] [Google Scholar]
- 59.Bidinost C, Matsumoto M, Chung D, et al. Heterozygous and homozygous mutations in PITX3 in a large Lebanese family with posterior polar cataracts and neurodevelopmental abnormalities. Invest Ophthalmol Vis Sci. 2006;47:1274–1280. doi: 10.1167/iovs.05-1095. [DOI] [PubMed] [Google Scholar]
- 60.Summers KM, Withers SJ, Gole GA, et al. Anterior segment mesenchymal dysgenesis in a large Australian family is associated with the recurrent 17 bp duplication in PITX3. Mol Vis. 2008;14:2010–2015. [PMC free article] [PubMed] [Google Scholar]
- *61.Chograni M, Chaabouni M, Chelly I, et al. Absence of PITX3 mutation in a Tunisian family with congenital cataract and mental retardation. Mol Vis. 2010;16:582–585. This paper reports screening of a family with autosomal recessive congenital cataracts and mental retardation that failed to identify mutations in PITX3.
- *62.Medina-Martinez O. Focus on Molecules: Pitx3. Exp Eye Res. 2010 doi: 10.1016/j.exer.2010.08.023. A brief review of what is known about the Pitx3 molecule and the role of PITX3 in human disease.
- 63.Fuchs J, Mueller JC, Lichtner P, et al. The transcription factor PITX3 is associated with sporadic Parkinson’s disease. Neurobiol Aging. 2009;30:731–738. doi: 10.1016/j.neurobiolaging.2007.08.014. [DOI] [PubMed] [Google Scholar]
- *64.Bergman O, Hakansson A, Westberg L, et al. PITX3 polymorphism is associated with early onset Parkinson’s disease. Neurobiol Aging. 2010;31:114–117. doi: 10.1016/j.neurobiolaging.2008.03.008. This association study identified a new polymorphisms in PITX3 which was significantly more common in individuals with early-onset Parkinson disease, but failed to replicate a previous association with a different polymorphism in the PITX3 promoter region.
- 65.Ormestad M, Blixt A, Churchill A, et al. Foxe3 haploinsufficiency in mice: a model for Peters’ anomaly. Invest Ophthalmol Vis Sci. 2002;43:1350–1357. [PubMed] [Google Scholar]
- 66.Iseri SU, Osborne RJ, Farrall M, et al. Seeing clearly: the dominant and recessive nature of FOXE3 in eye developmental anomalies. Hum Mutat. 2009;30:1378–1386. doi: 10.1002/humu.21079. [DOI] [PubMed] [Google Scholar]
- **67.Doucette L, Green J, Fernandez B, et al. A novel, non-stop mutation in FOXE3 causes an autosomal dominant form of variable anterior segment dysgenesis including Peters anomaly. Eur J Hum Genet. 2010 doi: 10.1038/ejhg.2010.210. Another report of a heterozygous missense mutation in a stop codon leading to anterior segment defects. Study of cDNA from lymphoblasts of an affected family member did not identify the mutant allele, suggesting that the phenotype may be related to haploinsufficiency rather than an extended FOXE3 protein.
- 68.Reis LM, Tyler RC, Schneider A, et al. FOXE3 plays a significant role in autosomal recessive microphthalmia. Am J Med Genet A. 2010 doi: 10.1002/ajmg.a.33257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valleix S, Niel F, Nedelec B, et al. Homozygous nonsense mutation in the FOXE3 gene as a cause of congenital primary aphakia in humans. Am J Hum Genet. 2006;79:358–364. doi: 10.1086/505654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ali M, Buentello-Volante B, McKibbin M, et al. Homozygous FOXE3 mutations cause non- syndromic, bilateral, total sclerocornea, aphakia, microphthalmia and optic disc coloboma. Mol Vis. 2010;16:1162–1168. [PMC free article] [PubMed] [Google Scholar]
- 71.Anjum I, Eiberg H, Baig SM, et al. A mutation in the FOXE3 gene causes congenital primary aphakia in an autosomal recessive consanguineous Pakistani family. Mol Vis. 2010;16:549–555. [PMC free article] [PubMed] [Google Scholar]
- **72.Reis LM, Tyler RC, Schilter KF, et al. BMP4 loss-of-function mutations in developmental eye disorders including SHORT syndrome. Hum Genet. 2011 doi: 10.1007/s00439-011-0968-y. This is the first report of BMP4 deletion in a proband with SHORT syndrome. The authors also identified loss-of-function mutations in three other families with clinical anophthalmia/microphthalmia with anterior segment defects.
- 73.Hayashi S, Okamoto N, Makita Y, et al. Heterozygous deletion at 14q22.1-q22.3 including the BMP4 gene in a patient with psychomotor retardation, congenital corneal opacity and feet polysyndactyly. Am J Med Genet A. 2008;146A:2905–2910. doi: 10.1002/ajmg.a.32519. [DOI] [PubMed] [Google Scholar]
- *74.Wyatt AW, Osborne RJ, Stewart H, et al. Bone morphogenetic protein 7 (BMP7) mutations are associated with variable ocular, brain, ear, palate, and skeletal anomalies. Hum Mutat. 2010;31:781–787. doi: 10.1002/humu.21280. This paper reports screening of BMP7 in a cohort of 279 patients with anophthalmia, microphthalmia, or coloboma. Two mutations were identified that were not seen in controls. One mutation was seen in a control. All were inherited from unaffected or mildly affected mothers.
- 75.Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- 76.Saito K, Yonezawa T, Minaguchi J, et al. Distribution of alpha(IV) collagen chains in the ocular anterior segments of adult mice. Connect Tissue Res. 2011;52:147–156. doi: 10.3109/03008207.2010.492062. [DOI] [PubMed] [Google Scholar]
- 77.van der Knaap MS, Smit LM, Barkhof F, et al. Neonatal porencephaly and adult stroke related to mutations in collagen IV A1. Ann Neurol. 2006;59:504–511. doi: 10.1002/ana.20715. [DOI] [PubMed] [Google Scholar]
- 78.Shah S, Kumar Y, McLean B, et al. A dominantly inherited mutation in collagen IV A1 (COL4A1) causing childhood onset stroke without porencephaly. Eur J Paediatr Neurol. 2010;14:182–187. doi: 10.1016/j.ejpn.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 79.Rouaud T, Labauge P, Tournier Lasserve E, et al. Acute urinary retention due to a novel collagen COL4A1 mutation. Neurology. 2010;75:747–749. doi: 10.1212/WNL.0b013e3181eee440. [DOI] [PubMed] [Google Scholar]
- 80.Sibon I, Coupry I, Menegon P, et al. COL4A1 mutation in Axenfeld-Rieger anomaly with leukoencephalopathy and stroke. Ann Neurol. 2007;62:177–184. doi: 10.1002/ana.21191. [DOI] [PubMed] [Google Scholar]
- **81.Coupry I, Sibon I, Mortemousque B, et al. Ophthalmological features associated with COL4A1 mutations. Arch Ophthalmol. 2010;128:483–489. doi: 10.1001/archophthalmol.2010.42. This manuscript describes ASD features in two families with COL4A1 mutations and suggests that brain MRI may be indicated in patients with apparently isolated ARA.
- *82.Vahedi K, Alamowitch S. Clinical spectrum of type IV collagen (COL4A1) mutations: a novel genetic multisystem disease. Curr Opin Neurol. 2011;24:63–68. doi: 10.1097/WCO.0b013e32834232c6. This paper reviews the clinical features reported in patients with COL4A1 mutations including brief discussion of ocular anomalies.
- 83.Vincent A, Billingsley G, Priston M, et al. Phenotypic heterogeneity of CYP1B1: mutations in a patient with Peters’ anomaly. J Med Genet. 2001;38:324–326. doi: 10.1136/jmg.38.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edward D, Al Rajhi A, Lewis RA, et al. Molecular basis of Peters anomaly in Saudi Arabia. Ophthalmic Genet. 2004;25:257–270. doi: 10.1080/13816810490902648. [DOI] [PubMed] [Google Scholar]
- 85.Vincent A, Billingsley G, Priston M, et al. Further support of the role of CYP1B1 in patients with Peters anomaly. Mol Vis. 2006;12:506–510. [PubMed] [Google Scholar]
- 86.Chavarria-Soley G, Michels-Rautenstrauss K, Caliebe A, et al. Novel CYP1B1 and known PAX6 mutations in anterior segment dysgenesis (ASD) J Glaucoma. 2006;15:499–504. doi: 10.1097/01.ijg.0000243467.28590.6a. [DOI] [PubMed] [Google Scholar]
- 87.Vincent AL, Billingsley G, Buys Y, et al. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70:448–460. doi: 10.1086/338709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaur K, Reddy AB, Mukhopadhyay A, et al. Myocilin gene implicated in primary congenital glaucoma. Clin Genet. 2005;67:335–340. doi: 10.1111/j.1399-0004.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 89.Geyer O, Wolf A, Levinger E, et al. Genotype/phenotype correlation in primary congenital glaucoma patients from different ethnic groups of the Israeli population. Am J Ophthalmol. 2011;151:263–71.e1. doi: 10.1016/j.ajo.2010.08.038. [DOI] [PubMed] [Google Scholar]
- **90.Tanwar M, Dada T, Dada R. Axenfeld-Rieger Syndrome Associated with Congenital Glaucoma and Cytochrome P4501B1 Gene Mutations. Case Report Med. 2010;2010:212656. doi: 10.1155/2010/212656. Epub 2010 Aug 9. First report of CYP1B1 mutations associated with ARS with systemic anomalies (protruding umbilicus and malaligned teeth).
- *91.Khan AO, Aldahmesh MA, Al-Abdi L, et al. Molecular Characterization Of Newborn Glaucoma Including A Distinct Aniridic Phenotype. Ophthalmic Genet. 2011 doi: 10.3109/13816810.2010.544365. First report of CYP1B1 mutations in eight patients with mild iris ectropion and partial aniridia in addition to congenital glaucoma, providing further support for the role of CYP1B1 in anterior segment development.
- 92.Bredrup C, Matejas V, Barrow M, et al. Ophthalmological aspects of Pierson syndrome. Am J Ophthalmol. 2008;146:602–611. doi: 10.1016/j.ajo.2008.05.039. [DOI] [PubMed] [Google Scholar]
- **93.Matejas V, Hinkes B, Alkandari F, et al. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31:992–1002. doi: 10.1002/humu.21304. The authors present evidence that LAMB2 mutations explain all cases of classic Pierson syndrome based on their screening of 30 cases and review all previously reported mutations/ phenotypes.
- *94.Zhao D, Ding J, Wang F, et al. The first Chinese Pierson syndrome with novel mutations in LAMB2. Nephrol Dial Transplant. 2010;25:776–778. doi: 10.1093/ndt/gfp563. This is a case report of a Chinese child with Pierson syndrome with later onset renal disease and novel truncating LAMB2 mutations.
- 95.Choi HJ, Lee BH, Kang JH, et al. Variable phenotype of Pierson syndrome. Pediatr Nephrol. 2008;23:995–1000. doi: 10.1007/s00467-008-0748-7. [DOI] [PubMed] [Google Scholar]
- *96.Mohney BG, Pulido JS, Lindor NM, et al. A Novel Mutation of LAMB2 in a Multigenerational Mennonite Family Reveals a New Phenotypic Variant of Pierson Syndrome. Ophthalmology. 2011 doi: 10.1016/j.ophtha.2010.10.009. This paper reports a homozygous missense mutation in nine family members with nephrotic syndrome and retinal abnormalities, but without microcorea or neurologic anomalies.
- 97.Maillette de Buy Wenniger-Prick LJ, Hennekam RC. The Peters’ plus syndrome: a review. Ann Genet. 2002;45:97–103. doi: 10.1016/s0003-3995(02)01120-6. [DOI] [PubMed] [Google Scholar]
- 98.Kapoor S, Mukherjee SB, Arora R, et al. Peters plus syndrome. Indian J Pediatr. 2008;75:635–637. doi: 10.1007/s12098-008-0122-6. [DOI] [PubMed] [Google Scholar]
- 99.Reis LM, Tyler RC, Abdul-Rahman O, et al. Mutation analysis of B3GALTL in Peters Plus syndrome. Am J Med Genet A. 2008;146A:2603–2610. doi: 10.1002/ajmg.a.32498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haldeman-Englert CR, Naeem T, Geiger EA, et al. A 781-kb deletion of 13q12.3 in a patient with Peters plus syndrome. Am J Med Genet A. 2009;149A:1842–1845. doi: 10.1002/ajmg.a.32980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *101.Dassie-Ajdid J, Causse A, Poidvin A, et al. Novel B3GALTL mutation in Peters-plus Syndrome. Clin Genet. 2009;76:490–492. doi: 10.1111/j.1399-0004.2009.01253.x. This paper reports B3GALTL mutations, including the first missense mutation, identified in 2 patients with typical PPS but not in 2 patients with Peters anomaly and developmental delay only.
- *102.Aliferis K, Marsal C, Pelletier V, et al. A novel nonsense B3GALTL mutation confirms Peters plus syndrome in a patient with multiple malformations and Peters anomaly. Ophthalmic Genet. 2010;31:205–208. doi: 10.3109/13816810.2010.512355. Report of a novel homozygous mutation in B3GALTL identified in a patient with classic Peters Plus syndrome.
- **103.Al-Gazali L, Shather B, Kaplan W, et al. Anterior segment anomalies of the eye, growth retardation associated with hypoplastic pituitary gland and endocrine abnormalities: Jung syndrome or a new syndrome? Am J Med Genet A. 2009;149A:251–256. doi: 10.1002/ajmg.a.32626. This is the first report of a patient with ASD, brachydactyly, and short stature without a mutation in B3GALTL. Other features present included hypoplastic pituitary gland and growth hormone deficiency.
- *104.Shimizu R, Saito R, Hoshino K, et al. Severe Peters Plus syndrome-like phenotype with anterior eye staphyloma and hypoplastic left heart syndrome: proposal of a new syndrome. Congenit Anom (Kyoto) 2010;50:197–199. doi: 10.1111/j.1741-4520.2010.00282.x. This paper reports lack of B3GALTL mutations in a patient with atypical Peters Plus syndrome.
- 105.Paznekas WA, Boyadjiev SA, Shapiro RE, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Paznekas WA, Karczeski B, Vermeer S, et al. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- 107.Fantes J, Ragge NK, Lynch SA, et al. Mutations in SOX2 cause anophthalmia. Nat Genet. 2003;33:461–463. doi: 10.1038/ng1120. [DOI] [PubMed] [Google Scholar]
- 108.Schneider A, Bardakjian T, Reis LM, et al. Novel SOX2 mutations and genotype-phenotype correlation in anophthalmia and microphthalmia. Am J Med Genet A. 2009;149A:2706–2715. doi: 10.1002/ajmg.a.33098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *109.Mihelec M, Abraham P, Gibson K, et al. Novel SOX2 partner-factor domain mutation in a four- generation family. Eur J Hum Genet. 2009;17:1417–1422. doi: 10.1038/ejhg.2009.79. The first report of a SOX2 mutations resulting in anterior segment anomalies (iris hypoplasia, microcornea) without microphthalmia. Other family members had variable clinical anophthalmia/ microphthalmia with anterior segment defects in some.
- *110.Reis LM, Tyler RC, Schneider A, et al. Examination of SOX2 in variable ocular conditions identifies a recurrent deletion in microphthalmia and lack of mutations in other phenotypes. Mol Vis. 2010;16:768–773. Screening of 28 probands with ASD did not identify any mutations in SOX2.
- 111.Okajima K, Robinson LK, Hart MA, et al. Ocular anterior chamber dysgenesis in craniosynostosis syndromes with a fibroblast growth factor receptor 2 mutation. Am J Med Genet. 1999;85:160–170. doi: 10.1002/(sici)1096-8628(19990716)85:2<160::aid-ajmg11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 112.McCann E, Kaye SB, Newman W, et al. Novel phenotype of craniosynostosis and ocular anterior chamber dysgenesis with a fibroblast growth factor receptor 2 mutation. Am J Med Genet A. 2005;138A:278–281. doi: 10.1002/ajmg.a.30944. [DOI] [PubMed] [Google Scholar]
- *113.Barry GP, Ny BM, Zackai EH, et al. A case report of a patient with Pfeiffer syndrome, an FGRF2 mutation (Trp290Cys) and unique ocular anterior segment findings. Ophthalmic Genet. 2010;31:193–195. doi: 10.3109/13816810.2010.505225. Report of the rare association of anterior segment defects, including microcornea, corectopia, and glaucoma, in a patient with Pfeiffer syndrome caused by mutation in FGFR2
- 114.Abouzeid H, Meire FM, Osman I, et al. A new locus for congenital cataract, microcornea, microphthalmia, and atypical iris coloboma maps to chromosome 2. Ophthalmology. 2009;116:154–162.e1. doi: 10.1016/j.ophtha.2008.08.044. [DOI] [PubMed] [Google Scholar]
- *115.Roche A, Mora J, Perez Mdel M, et al. Axenfeld-Rieger ocular anomaly and retinoblastoma caused by constitutional chromosome 13q deletion. Pediatr Blood Cancer. 2010;54:480–482. doi: 10.1002/pbc.22354. A new report of ARA and retinoblastoma associated with a deletion of 13(q12q22) including the RB1 gene.
- 116.Akazawa K, Yamane S, Shiota H, et al. A case of retinoblastoma associated with Rieger’s anomaly and 13q deletion. Jpn J Ophthalmol. 1981;25:321–325. [Google Scholar]
- 117.Stathacopoulos RA, Bateman JB, Sparkes RS, et al. The Rieger syndrome and a chromosome 13 deletion. J Pediatr Ophthalmol Strabismus. 1987;24:198–203. doi: 10.3928/0191-3913-19870701-12. [DOI] [PubMed] [Google Scholar]
- *118.Chaabouni M, Etchevers H, De Blois MC, et al. Identification of the IRXB gene cluster as candidate genes in severe dysgenesis of the ocular anterior segment. Invest Ophthalmol Vis Sci. 2010;51:4380–4386. doi: 10.1167/iovs.09-4111. The authors provide analysis of a very complicated chromosomal rearrangement in a patient with aniridia, aphakia, corneal anomalies, and congenital glaucoma. They suggest that disregulation of the IRXB gene cluster is responsible for the phenotype.
- 119.Hanna NN, Eickholt K, Agamanolis D, et al. Atypical Peters plus syndrome with new associations. J AAPOS. 2010;14:181–183. doi: 10.1016/j.jaapos.2010.02.003. [DOI] [PubMed] [Google Scholar]
- *120.Kuhlenbaumer G, Hullmann J, Appenzeller S. Hum Mutat. 2011;32:144–151. doi: 10.1002/humu.21400. Not specific to ocular disorders, but provides a nice review of the new genomic technologies and applications to understanding genetic diseases.