Abstract
How m-calpain is activated in cells has challenged investigators because in vitro activation requires near-millimolar calcium. Previously, we demonstrated that m-calpain activation by growth factors requires extracellular signal-regulated kinase (ERK); this enables tail deadhesion and allows productive motility. We now show that ERK directly phosphorylates and activates m-calpain both in vitro and in vivo. We identified serine 50 as required for epidermal growth factor (EGF)-induced calpain activation in vitro and in vivo. Replacing the serine with alanine limits activation by EGF and subsequent cell deadhesion and motility. A construct with the serine converted to glutamic acid displays constitutive activity in vivo; expression of an estrogen receptor fusion construct produces a tamoxifen-sensitive enzyme. Interestingly, EGF-induced m-calpain activation occurs in the absence of increased intracellular calcium levels; EGF triggers calpain even in the presence of intracellular calcium chelators and in calcium-free media. These data provide evidence that m-calpain can be activated through the ERK cascade via direct phosphorylation and that this activation may occur in the absence of cytosolic calcium fluxes.
The calpain family of intracellular cysteine proteinases includes 13 known members, of which at least 2 are ubiquitously expressed (47, 48). These, m- and μ-calpain (calpain II and calpain I, respectively), are involved in cell migration and adhesion, being regulated downstream of both integrin and growth factor receptor activation (19). μ-Calpain has been implicated strongly in cell motility and adhesion primarily driven by integrin-mediated signals: calpains have been shown to be required during both cell spreading and adhesion (3, 4, 41) and for the release of the rear of migrating cells (26). On the other hand, m-calpain has been observed to be activated downstream of the epidermal growth factor (EGF) receptor (EGFR) and is required for growth factor-induced motility and deadhesion (18, 44). This effect is specific to m-calpain, as antisense down-regulation of μ-calpain did not appreciably affect growth factor-induced motility and as EGF-induced calpain activity and motility were dependent on m-calpain (18). m-Calpain affects the migration of EGF-induced fibroblasts by promoting rear release during active motility (2, 43, 44). In general, functions of calpains in motility and adhesion apparently derive from their ability to cleave components of adhesion complexes in a limited manner, altering their function and leading to increased adhesion turnover (19, 29, 39). However, the molecular mechanism by which calpain activities are regulated during these events is not understood.
The two ubiquitous isoforms, μ- and m-calpain, are presumed to be activated by intracellular calcium fluxes, since these enzymes require this divalent cation in vitro. Indeed m- and μ-calpain are named for their relative requirement for calcium, with μ-calpain requiring micromolar, and m-calpain requiring near-millimolar, concentrations of calcium (16). It has been proposed that calcium binds to four EF-hand calcium binding domains in the C terminus of the m- and μ-calpain large subunit to effect a conformational change bringing the two halves of the active cleft into functioning apposition (25, 37). The high number of calcium binding domains clearly indicates a role for this ion in regulating calpain activity. Recent findings demonstrate that limiting intracellular calcium fluxes can block activation of calpain in response to selected stimuli (9, 53). The isoform likely activated under these conditions is μ-calpain though the authors did not define the operative calpain; we find that such maneuvers can prevent the activation of μ-calpain in response to CXC chemokines (L. Satish, A. Glading, and A. Wells, submitted for publication). However, the calcium concentrations required for m-calpain activation in vitro are unattainable under normal cellular conditions (5), suggesting that there are alternate mechanisms of activation in the cell.
Other proposed mechanisms of calpain regulation include phospholipid binding, intermolecular autolysis, and interaction with activating proteins. All of these mechanisms reduce the calcium requirement for calpain in vitro, but not to the levels noted in motile cells. This suggests that these mechanisms may contribute to, but may not be solely responsible for, m-calpain activation in vivo. Autolysis, although extensively studied, is more likely a mechanism of attenuation since autolyzed calpain is rapidly degraded (22, 28) and since nonautolyzed intact and autolysis-deficient calpains retain wild-type calpain activity (14, 17). Activator proteins that copurify with calpains from various tissues and that serve to reduce the calcium requirement for activity in vitro have been discovered. However, these proteins are not well characterized at present (33-35, 42, 51).
Previously we demonstrated that m-calpain activity is required for growth factor-induced cell motility and deadhesion. EGF activation increases m-calpain activity via extracellular signal-regulated kinase (ERK) signaling rather than through phospholipase C-γ (PLCγ) and subsequent activation of inositol trisphosphate (IP3)-mediated calcium flux (18). Because ERK is a serine/threonine protein kinase, we considered multiple mechanisms of m-calpain activation, including direct phosphorylation, direct activation and inactivation by intermediary signaling molecules, and activation due to protein-protein interactions. Using a combination of in vitro and in vivo approaches, we determined that ERK directly phosphorylates, and thereby contributes to the activation of, m-calpain. We also show that phosphorylation by ERK could activate m-calpain in the absence of added calcium in vitro and in the absence of increased cytosolic calcium levels in vivo.
MATERIALS AND METHOD
Calpain cloning
Human m-calpain cDNA was obtained by reverse transcription-PCR-based cloning from human dermal fibroblasts (Hs68; American Type Culture Collection, Manassas, Va.) as described previously (43). Briefly, total RNA was collected from Hs68 cells with TRIzol (Gibco/BRL, Carlsbad, Calif.). Reverse transcription was performed with purified Hs68 total RNA by using an m-calpain-specific oligonucleotide primer (5′-CCTCGTGTCCTTTGAGAGCG-3′) and Superscript II reverse transcriptase (Gibco/BRL). To generate a polyhistidine-tagged m-calpain large subunit, cDNA sense (5′-AGCTAGCGGACCGCAGCATGG) and antisense (5′-GCCTTGCCGGCCTCAATGATGATGATGATGATGGTCAAGTACTGAGAAACAGAGCC; including the coding sequence for a six-His tag) primers were designed according to an m-calpain cDNA sequence (GenBank accession no. M23254.1) (27). Purified cDNA was amplified by PCR using sense and antisense primers and Elongase (Gibco/BRL) and cloned into a PCR II TA cloning vector (Invitrogen, Carlsbad, Calif.). After confirmation by sequencing, m-calpain cDNA was subcloned into pCEP4 (Stratagene, La Jolla, Calif.) downstream from a cytomegalovirus (CMV) promoter; a hygromycin resistance gene conferred selectability for stable expression. The CMV promoter was replaced with a murine mammary virus tumor promoter (MMTVp) to generate dexamethasone-inducible constructs (11).
PCR-based mutagenesis
Mutant m-calpain constructs were generated by PCR-based mutagenesis. Since m-calpain does not possess an ERK consensus site for phosphorylation, four possible unconventional sites were selected, and each serine or threonine was individually replaced by alanine with the QuikChange site-directed mutagenesis kit (Stratagene). The primers used to generate the mutant calpains were as follows (amino acid mutation, sense primer, antisense primer): S50A, 5′-CTCTTCCAGGACCCGGCATTCCCGGCCATCCCC, 5′-GGGGATGGCCGGGAATGCCGGGTCCTGGAAGAG; T381A, 5′-GGAACTACCCGAACGCATTCTGGATGACCCTC, 5′-GAGGGTTCATCCAGAATGCTGTGCGGTAGTTCC; S490A, 5′-TACATTCTCGTGCCTGCAACCTTCGAACCCAAC, 5′-ATTCTCGTGCCTTCCGCATTCGAACCCAACAAGA; T491A, 5′-ATTCTCGTGCCTTCCGCATTCGAACCAACAAG, 5′-CTTGTTGGGTTCGAATGCGGAAGGCACGAGAAT; ST490AA, 5′-TACATTCTCGTGCCTGCAGCATTCGAACCCAACAAG, 5′-CTTGTTGGGTTCGAATGCTGCAGGCACGAGAATGTA; S50E, 5′-CTCTTCCAGGACCCGGAATTCCCGGCCATCCCCC, 5′-GGGGATGGCCGGGAATTCCGGGTCCTGGAAGAG.
The tamoxifen-inducible human m-calpain-tamoxifen mutant murine estrogen receptor (ERTM) fusion was designed similarly to a functional tamoxifen-inducible construct (31, 32). The plasmid pCS2 ERC, containing the coding sequence for the 319-amino-acid ER hormone binding domain, ERTM, was a gift from Anne Vojtek and David Turner (University of Michigan). The 1.2-kb HindIII/SnaBI fragment encoding the ER was cloned into a HindIII and EcoRV digest of a modified pcDNA3 plasmid containing a Gateway RFA site in the polylinker, creating the plasmid pcDNA3-ERC. The plasmid pcDNA3-ERC was digested with ClaI, filled in with T4 polymerase, and subsequently digested with HindIII. The human m-calpain S50E cDNA from the construct pCEP4MMTVCANP-S50E, isolated by a HindII/ScaI digestion, was directionally cloned into the prepared plasmid. The product of the translation of the fusion construct, pcDNA3-CANPS50E-ERC, contained the human m-calpain sequence through amino acid residue V699, followed by the amino acid sequence NSRPLERSGLA from the pCS2 ERC polylinker, followed by R281 of the murine ER. The junction of the construct and reading frame was confirmed by DNA sequencing. The resulting chimeric protein has 1,031 amino acids.
Cell culture
The murine fibroblast line WT NR6 was used in all experiments (10). These cells express approximately 100,000 human wild-type EGFRs per cell and respond to EGF with both motility and mitogenesis. They are passaged in minimal essential medium α (MEMα) supplemented with 7.5% fetal bovine serum (FBS); 1% supplements (nonessential amino acids, 200 mM sodium pyruvate, 100 mM glutamine); and the antibiotics penicillin, streptomycin, and G418 at 350 μg/ml (all culture reagents were from Gibco/BRL). Prior to all experimentation, the cells are incubated in MEMα supplemented with 0.5% dialyzed FBS (in the absence of selection reagents) for 24 h. The human m-calpain (wild-type, S50A, and S50E) constructs were stably expressed in these cells by electroporation. Approximately 2.0 × 107 cells were electroporated (220 μF, 0.950 kV) with 20 μg of DNA in a total volume of 1 ml. Thirty-six hours after electroporation, cells were selected in complete media containing100 μg of hygromycin (Roche Diagnostics, Indianapolis, Ind.)/ml. Polyclonal lines consisting of more than 10 colonies were established. At least two independent electroporations and stably transfected lines were established and tested for each construct with identical results.
Biochemical analyses
Calpain phosphorylation in vitro was determined by incorporation of [γ-32P]ATP (0.2 μCi/μl; Amersham, Buckinghamshire, United Kingdom) into calpain in the presence of activated forms of ERK1 or MEK1 (Upstate Biotechnology). The mixture was then separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-10% PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane for autoradiography. Subsequent immunoblotting detected calpain as the control.
To demonstrate an in vivo interaction between calpains and ERK, cells were treated with the cell-permeant, homobifunctional, photoactivatable, cleavable cross-linker dimethyl-3,3′-dithiobisproprionimidate hydrochloride (DTBP; 1 mM; Pierce Chemicals, Rockford, Ill.) for 30 min. Cells were treated in the presence or absence of EGF (10 nM for 5 min; Collaborative Biomedical Products, Bedford, Mass.). Plates of adherent, treated cells were then exposed to 365-nm light to activate the cross-linker for 20 min. Cells were then lysed in 1% Triton X-100, and immunoprecipitations were performed with antibodies against ERK (pan-ERK; Cell Signaling Technologies, Beverly, Mass.), μ-calpain (SA-257; Biomol, Plymouth Meeting, Pa.), or m-calpain (AB1625; Chemicon, Temecula, Calif.). After four washes, precipitates were separated by reducing SDS-PAGE, transferred to PVDF membranes, and probed with antibodies against ERK, MEK (M17020; Transduction Laboratories, Lexington, Ky.), m-calpain (sc-7532; Santa Cruz Biotechnology), or μ-calpain.
In vivo phosphorylation of m-calpain was demonstrated by orthophosphate labeling. Cells expressing His-tagged human m-calpain were left for 24 h to become quiescent before treatment with dexamethasone (2 μM) for 18 h to induce expression of the exogenous m-calpain construct. To detect phosphorylated m-calpain, cells were incubated with [32P]orthophosphate in phosphate-free MEM for 2 h prior to PD098059 (2 μM; New England Biolabs, Beverly, Mass.) and/or EGF (10 nM) treatment for 30 min. Cells were lysed, and human m-calpain was purified by nickel affinity chromatography (Probond resin; Invitrogen). The samples were analyzed by separation through an SDS-7.5% polyacrylamide gel and transferred to a PVDF membrane. The membranes were used for autoradiography and subsequently blotted with an anti-m-calpain antibody (Santa Cruz Biotechnology).
In vivo calpain activity (Boc-LM-CMAC) assay
WT NR6 cells and cells expressing wild-type or mutant m-calpains (S50A and S50E) were plated at 50% confluence on glass coverslips for in vivo calpain activity measurement by using the t-BOC-Leu-Met-chloromethylaminocoumarin (Boc-LM-CMAC) substrate (Molecular Probes, Eugene, Oreg.) as described previously (18). To characterize the effects of the S50A and S50E mutants over the background of endogenous m-calpain expression, we utilized specific phosphothiorated antisense oligonucleotides to down-regulate the endogenous murine m-calpain (5′-TGCCCGCCATGGTAGCGATC) or the cloned mutant human m-calpain (5′-GCCATGCTGCGGTCCAAGCC). To overcome the long half-life of m-calpain, we treated the cells with EGF for 8 h in the presence of antisense oligonucleotides to stimulate endogenous m-calpain degradation (18). Cells were washed to remove the EGF, and antisense oligonucleotides were added overnight. Dexamethasone (2 μM) was added with this second dose of oligonucleotides to stimulate expression of the mutant calpains. Cells were then treated with calpain inhibitor 1 (CI-1) (5 and 25 μg/ml; Biomol) or PD098059 (2 μM; New England Biolabs) for 1 h prior to EGF (10 nM) treatment.
Calpain activity in WT NR6 cells was also measured under calcium flux-stimulating or -blocking conditions. These cells were incubated for 20 min in the presence of 50 μM Boc-LM-CMAC and treated with 10 nM EGF or 100 ng of platelet-derived growth factor BB (PDGF-BB; Peprotech, Rockyhill, N.J.)/ml for 10 min; control cells were not treated with EGF. Cells were also treated with N,N′-(1,2-ethanediylbis[oxy-2,1-phenylene])bis(N-[2-{(acetyloxy)methoxy}-2-oxoethyl])-bis([acetyloxy]methyl) ester (BAPTA-AM; 50 μM; Calbiochem, San Diego, Calif.) for 30 min prior to Boc-LM-CMAC loading (50 min total). Cells treated with PD098059 (2 μM) or CI-1 (5 μg/ml) were treated for 1 h prior to imaging. Cells treated in calcium-free media were changed from calcium-containing media to calcium-free media with two washings immediately before growth factor treatment.
Measurement of Boc-LM-CMAC fluorescence does not provide subcellular localization information due to the rapid diffusion of the fluorescent substrate coupled with rapid bleaching of the fluorophore. Thus, in vivo images are presented at a magnification which presents multiple cells for appreciation of the population characteristics rather than individual cells.
In vitro calpain activity assays. (i) AMC assay (see Fig. 2)
FIG. 2.
ERK association with m-calpain in vivo. Coimmunoprecipitation of ERK and m-calpain was performed in the presence of the cross-linking reagent DTBP. WT NR6 murine fibroblasts were treated with cross-linking reagent for 30 min, and some cells were treated with 10 mM CI-1 for 30 min, followed by EGF treatment (controls were not treated with EGF). The cross-linker was then activated with light (365 nm) for 20 min. The cells were lysed and incubated with anti-m-calpain (A and G), anti-pan-ERK (B, C, and F), anti-μ-calpain (D), anti-integrin-linked kinase 1 (ILK1; E), and nonspecific isotype immunoglobulin G (IgG; B, E, F, and G) antibodies, and then the antibody-bound proteins were removed with protein G-agarose beads. The immunoprecipitated (IP) proteins were then isolated and separated on an SDS-10% polyacrylamide gel. WT NR6 whole-cell lysates (A, C, and D) or IgG-immunoprecipitated proteins (B, E, F, and G) were run alongside as a control After transfer to a PVDF membrane, the anti-m-calpain immunoprecipitates were probed for pan-ERK (A), followed by MEK1 (A) (same blot), talin (G), and α-actinin (G) and the anti-ERK immunoprecipitates were probed for m-calpain (B), μ-calpain (C) (separate blots), and also talin (F). Anti-μ-calpain immunoprecipitates were probed unsuccessfully for ERK as an additional control (D). Shown are representative blots from at least two replicates of each.
Purified porcine m- and μ-calpains (Calbiochem) were incubated with or without calcium (2 μM or 2 mM) and with or without purified ERK1 (300 U; >100 U/mg; Upstate Biotechnology Inc. [UBI], Lake Placid, N.Y.) in a reaction mixture containing assay dilution buffer (20 mM 3-[N-morpholino]propanesulfonic acid [MOPS; pH 7.2], 25 mM β-glycerolphosphate, 1 mM sodium orthovanadate, 1 mM dithiothreitol), 100 μM ATP, and 15 mM MgCl2. Reaction mixtures without calcium additionally contained 100 μM EGTA. Reaction mixtures were incubated at 30°C for 30 min. Succinyl-Leu-Leu-Val-Tyr-aminomethylcoumarin (AMC; 50 μM) (Enzyme Systems Products, Livermore, Calif.) was then added to each reaction mixture, and fluorescence was measured immediately at an excitation wavelength of 360 nm and an emission wavelength of 460 nm for 3 min. MEK (0.5 U; UBI) was substituted for ERK as a control.
Calpain activation was validated in cell extracts with a calpain activity assay kit (Biovision, Mountain View, Calif.) according to manufacturer's protocol. In brief, confluent WT NR6 cells stably transfected with either wild-type or S50A m-calpain tagged with His were pretreated with either 20 μM murine or human m-calpain antisense oligonucleotides along with 2 μM dexamethasone (for induction of expression) and 10 nM EGF for 8 h in quiescent media. The cells were then washed with phosphate-buffered saline (PBS) and incubated with quiescent media containing 20 μM murine or human m-calpain antisense oligonucleotides for 18 h. The cells were treated with or without CI-1 (10 μM) for 30 min and then incubated with or without EGF (10 nM) for 10 min. The cells were lysed (50 mM HEPES [pH 7.4], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM sodium vanadate) on ice for 30 min, and the insoluble fraction was removed by centrifugation at 10,000 rpm for 5 min in a Fisher Microfuge. For the assay, 70 μg of protein for each sample was used. The samples were analyzed for calpain activity with the LS50B luminescence spectrometer (Perkin-Elmer, Shelton, Conn.) equipped with 400-nm excitation and 505-nm emission filters.
(ii) Tau proteolysis (see Fig. 3)
FIG. 3.
S50A mutant m-calpain resistance to ERK-induced activation and activity in low and high calcium concentrations. (A) The activity of in vitro-transcribed and -translated wild-type and mutant (S50A) m-calpains was measured against that of recombinant m-calpain (Calbiochem) to determine their ability to cleave the calpain substrate Tau (0.5 μg). Addition of ERK to the in vitro-translated wild type, the T381A mutant m-calpain, and recombinant m-calpain showed equivalent abilities to cleave Tau within 10 min, as shown by loss of the Tau band at 65 kDa. On the other hand, the S50A mutant m-calpain exhibits a significant reduction in ERK activation, as shown by reduced loss of Tau (n = 3). Of the m-calpains tested none were shown to be able to cleave Tau alone (NT) or in the presence of 2 μM calcium (Ca++) during a 10-min incubation. (B) Recombinant m-calpain is activated by 2 μM calcium over time. Early time points do not demonstrate noticeable levels of Tau cleavage; however, by 60 min all Tau is cleaved. Wild-type in vitro-translated m-calpain demonstrated a similar time course of calcium-induced activity (not shown). In vitro-translated mutant S50A m-calpain was also activated by calcium, but only at a 100-fold-higher level (200 μM); at 2 μM calcium no loss of Tau was noted over the 60-min time period (data not shown) (n = 3).
Mutations in m-calpain were screened for those that produced changes in ERK-induced calpain activity by an in vitro assay for Tau cleavage (23). Briefly, wild-type and mutant calpains were cloned into T7-containing bacterial expression vector pCR3.1 (Invitrogen) and translated in vitro with the TNT T7 quick-coupled transcription/translation system (Promega, Madison, Wis.). Five microliters of TNT product or 1 μg of recombinant rat m-calpain (Calbiochem; roughly equivalent as seen by Western blotting) was incubated with 0.5 μg of recombinant Tau protein (Panvera, Madison, Wis.) with or without calcium (2 μM) and ERK (300 U) for 10 min (see Fig. 3A). To measure calpain activity over a time course of calcium treatment, the recombinant or in vitro-translated protein (0.5 μg and 5 μl, respectively) was incubated in the presence of calcium (2 μM and 200 μM, respectively) for 0, 15, 20, 30, 45, and 60 min (S50A construct also for 75 min). The reaction mixture was then separated by SDS-10% PAGE, transferred to a PVDF membrane, and probed for Tau (Zymed, South San Francisco, Calif.). Loss of the Tau band (65 kDa) was indicative of calpain activity.
Cell motility and deadhesion assays
The cell motility and deadhesion functional assays were performed as previously described (10, 18). EGF-induced migration was assessed by determining the ability of the cells to move into an acellular area in a two-dimensional in vitro “wound-healing” assay (10). In brief, WT NR6 cells expressing either wild-type or S50A m-calpain were plated on a 12-well plastic dish and grown to confluence. The cells were incubated with 20 μM antisense oligonucleotides against either murine or human m-calpain diluted in quiescent media (0.5% dialyzed FBS-MEMα media) containing 2 μM dexamethasone and then treated with EGF (10 nM) for 8 h. The cells were washed with quiescent media and further incubated in quiescent media containing 2 μM dexamethasone and 20 μM antisense oligonucleotides against either murine or human m-calpain for 24 h. The cells were denuded by a rubber policeman at the center of the plate and then treated with or without EGF (10 nM) and incubated at 37°C; antisense oligonucleotides against murine or human m-calpain (20 μM) were added at the same time as EGF. Photographs were taken at 0 and 24 h, and the relative area reclaimed by the cells in the acellular area was determined.
Cell-substratum adhesiveness was quantitated by an inverted centrifugation detachment assay (18). In brief, WT NR6 cells expressing either wild-type or S50A m-calpain were plated at the concentration of 3.0 × 104 cells/well in a 24-well tissue culture plate for 12 h at 37°C. The medium was removed, and the cells were incubated in quiescent media containing dexamethasone (2 μM) for 24 h at 37°C in the presence of antisense oligonucleotides against the endogenous murine m-calpain (20 μM). For the last 30 min EGF (10 nM), ALLN (CI-I; 5 μg/ml), or diluent was added. Plates were filled with MEMα with 1% bovine serum albumin and 25 mM HEPES. Then plates were sealed with enzyme-linked immunosorbent assay sealing tape (Corning, Cambridge, Mass.) and centrifuged inverted for 5 min at 3,000 rpm at 37°C with a Beckman CS6R plate centrifuge. Before and after the centrifugation the cells on the plates were counted by phase-contrast microscopy.
Antisense oligonucleotide down-regulation of m-calpains enabled us to isolate the biological effects of the expressed constructs (18, 43). Briefly, cells were treated with 20 μM phosphothiorated oligonucleotide (IDT Inc.) throughout the cell motility assay or prior to deadhesion testing. The oligonucleotides were designed to hybridize with the initiation codon for each species of m-calpain (5′-TGCCCGCCATGGTAGCGATC-3′ for endogenous murine m-calpain and 5′-GCCATGCTGCGGTCCAAGCC-3′ for exogenously encoded human m-calpain [the sequences across the initiation codon are identical for wild-type and S50A m-calpain]), with the two oligonucleotides serving as controls for each other. Calpain activation by EGF was assessed after overnight treatment of the cells with antisense oligonucleotides.
Calcium imaging
Murine WT NR6 fibroblast cells were plated at 60% confluence on 31-mm-diameter glass coverslips. The cells were incubated in 1% dialyzed FBS-containing media for 18 to 24 h. The cells were then loaded with 5 μM 5-oxazolecarboxylic acid, 2-(6-[bis{2-([acetyloxy]-methoxy)-2-oxoethyl}amino]-5-[2-{2-(bis[2-{(acetyloxy)methoxy}-2-oxoethyl]amino)-5-methylphenoxy}ethoxy]-2-benzofuranyl), (acetyloxy)methyl (Fura-2-AM; Molecular Probes) for 30 to 45 min at room temperature. The coverslips were placed in a perfusion chamber mounted on an Olympus BW50XI microscope. With a water immersion ×40 lens, a field of cells (three to nine cells per field) was identified and a differential interference contrast image was obtained. This image was used to define the borders of the individual cells and areas of background to be used in interpreting the results. The fluorescence of the Fura-2 dye within the borders of the identified cells was then measured at an image acquisition rate of 0.2 Hz for 10 to 15 min under various treatments (see below), and the ratio of fluorescence intensity following excitation at 340 or 380 nm was obtained (21). Treatments were as follows. Cells were perfused (5 ml/min) with HEPES-buffered salt solution (HBSS; 20 mM HEPES, 137 mM NaCl, 5 mM KCl, 10 mM NaHCO3, 5.5 mM glucose, 0.6 mM KH2PO4, 0.6 mM Na2HPO4, 1.4 mM CaCl2, 0.9. mM MgSO4, pH 7.4) for 1 to 2 min. The cells were then perfused with HBSS containing EGF (10 nM), PDGF (100 ng/ml), or bradykinin (1 μM; Sigma-Aldrich, St. Louis, Mo.). Cells treated with BAPTA-AM were loaded with 50 μM BAPTA-AM for 30 min prior to Fura-2 loading. Cells treated with calcium-free buffer were perfused with HBSS buffer without calcium and with EGTA (20 μM). Perfusion was stopped while the HBSS buffer and treatment were in the perfusion chamber, and measurement was continued for an additional 10 min. At this time, the perfusion was restarted and the treatment was washed out.
Experimental analysis
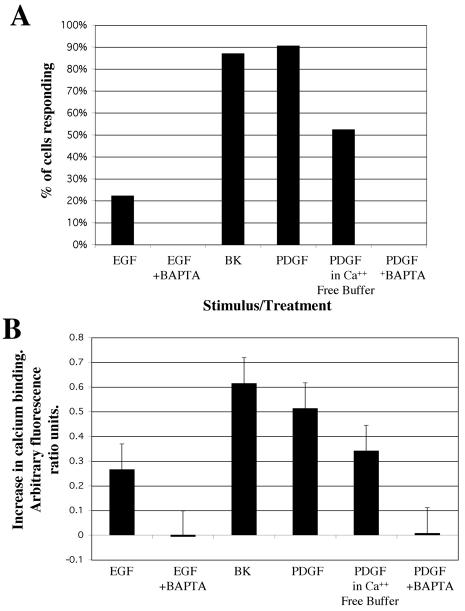
Background changes in fluorescence were subtracted from the ratios obtained for individual identified cells over the entire measurement period. The resulting ratios were used to classify cells as responding or nonresponding. Responding cells were classified as those whose Fura-2 ratios rose by ≥0.1 ratio units during incubation with treatment. Of those cells that responded, the mean increase was calculated. The maximum level of calcium increase in responding cells was estimated at 500 nM by comparison with ionophore-induced calcium flux. Measurement and analysis of raw data were done with Simple PCI imaging software (Compix, Cranberry, Pa.). The numbers of cells analyzed were as follows: EGF, 90 cells from seven independent experiments; EGF plus BAPTA-AM, 13 cells from one experiment; bradykinin, 23 cells from one experiment; PDGF, 74 cells from seven independent experiments; PDGF in Ca2+-free buffer, 42 cells from four independent experiments; PDGF plus BAPTA-AM, 28 cells from three independent experiments.
RESULT
ERK activates and phosphorylates calpain directly
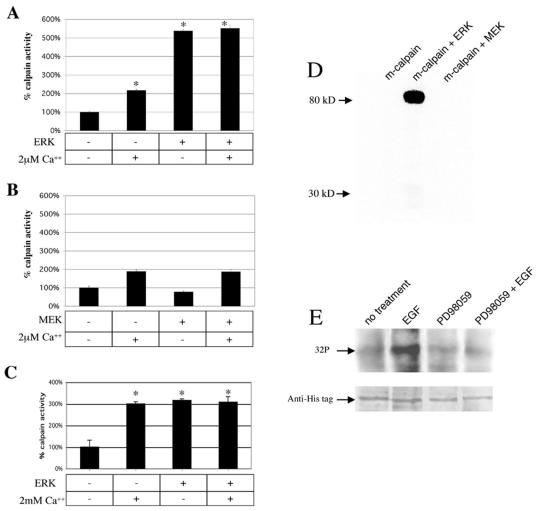
We have reported that EGF-induced m-calpain activation is blocked by MEK1 inhibitors (PD98059 and U0126) and down-regulation of ERK (18). To study potential mechanisms by which ERK mediates growth factor-induced m-calpain activity (18), we incubated purified activated ERK (or MEK), purified porcine m-calpain, and the synthetic substrate succinyl-LLVY-AMC in the presence or absence of calcium chloride (2 μM). Calcium alone induced an approximate twofold increase in m-calpain cleavage of the substrate. However, the addition of active ERK enzyme increased the activation of m-calpain by over fivefold in both the presence and absence of calcium (Fig. 1A). A constitutively active form of the upstream trigger of ERK, MEK1, did not affect m-calpain (Fig. 1B). This failure of MEK1 to activate m-calpain is not surprising, as antisense down-regulation of ERK in addition to MEK1 inhibitors blocks EGF-induced m-calpain activation, strongly suggesting that MEK1 activates m-calpain in vivo via ERK (18). At low levels of calcium (some twice what can be attained in calcium sparks [24]), ERK appears to activate m-calpain to a greater extent than calcium. In the presence of 2 mM calcium, used for maximal in vitro activation, both ERK and calcium activate m-calpain to the same extent, without additivity (Fig. 1C). Thus, it appears that m-calpain can be equivalently activated by both calcium and ERK.
FIG. 1.
ERK activation and phosphorylation of m-calpain in vitro and in vivo. (A) Purified activated ERK (300 U) and calpain (10 μg) enzymes were incubated together with or without calcium chloride (2 μM). The presence of ERK increased m-calpain activity over fivefold (*, P < 0.01). This occurred in both the presence and absence of calcium (error bars, standard errors of the means; n = 4). (B) As a control, the upstream enzyme MEK was substituted for ERK and had no effect on m-calpain activity (n = 4). (C) The in vitro activation assay was repeated in the presence or absence of ERK (300 U) and calcium chloride (2 mM). The presence of ERK or calcium increased m-calpain activity over threefold (*, P < 0.01), but there was no difference between these states (n = 4). This series of experiments were performed with different batches of m-calpain, and this may be the reason for higher levels of ERK-induced activation in panel A. (D) Adding [γ-32P]ATP to the mixture of ERK and calpain enzymes caused radiolabeling of the large subunit of m-calpain, whereas MEK did not cause radiolabeling (n = 3). (E) In vivo, [32P]orthophosphate labeling of WT NR6 murine fibroblasts demonstrates increased radiolabeling of histidine-tagged m-calpain upon EGF stimulation. This increase was eliminated by pretreatment with the MEK inhibitor PD98059 (2 μM), suggesting that the increase in phosphorylation seen with EGF treatment is mediated by ERK (n = 3). An antihistidine blot is shown to demonstrate equal protein levels. In panels D and E representative blots are shown.
Addition of [γ-32P]ATP to the reaction mixture resulted in the labeling of the large subunit of m-calpain (Fig. 1D). The stoichiometry of labeling was approximately 1 (n = 3). MEK, on the other hand, did not phosphorylate m-calpain (Fig. 1D). Both the activation and phosphorylation of m-calpain were seen when rat recombinant m-calpain was substituted for purified porcine m-calpain (data not shown). The ability of ERK to directly activate m-calpain in the absence of other proteins indicates that ERK may interact directly with m-calpain.
Phosphorylation in vitro does not necessarily correlate with phosphorylation in vivo but rather provides only a proof of principle. Therefore, we loaded cells expressing wild-type histidine-tagged human m-calpain with radiolabeled orthophosphate, exposed them to EGF, purified the calpain by nickel affinity chromatography, and analyzed the incorporation of 32P by SDS-PAGE and autoradiography (Fig. 1E). This demonstrated EGF-stimulated phosphorylation of m-calpain in vivo, which was blocked by the MEK inhibitor PD98059 (Fig. 1E), demonstrating that this occurs via the proposed EGFR/ERK signaling cascade.
m-Calpain interacts with ERK in vivo
The in vitro results suggested a direct phosphorylation of m-calpain by ERK as no other proteins were added to the reaction. To determine whether EGF stimulation-induced phosphorylation of m-calpain in vivo is due to direct or indirect phosphorylation by ERK, we analyzed the ERK-m-calpain interaction. Immunoprecipitation of m-calpain or ERK from Triton X-100 lysates failed to pull down other proteins. However, as there is precedent for weak or transient interactions between kinases and their targets, the cross-linking reagent DTBP was added before lysis. Under these conditions, immunoprecipitation of m-calpain coprecipitated ERK but not the upstream enzyme MEK (Fig. 2A). Immunoprecipitation of ERK in the presence of DTBP yielded m-calpain (Fig. 2B) but not μ-calpain (Fig. 2C). As an additional control, immunoprecipitation of μ-calpain did not coprecipitate ERK (Fig. 2D). This bidirectional coprecipitation demonstrates a protein-protein association in vivo.
Further demonstrating the specificity of this ERK coprecipitation with m-calpain, we investigated interactions with other focal adhesion constituents. We found that m-calpain did not coprecipitate with integrin-linked kinase or α-actinin, constituents of focal adhesions which have not been shown to be cleaved by calpain (Fig. 2E). Nor did ERK coprecipitate with talin (Fig. 2F), further suggesting that the interactions were direct. However, m-calpain did coprecipitate with talin in an activation-dependent manner (Fig. 2G). The failure to detect association of either upstream kinase MEK or the related proteinase μ-calpain demonstrates a specific interaction, suggesting a direct phosphorylation of m-calpain by ERK.
Serine 50 is a putative ERK regulatory site on m-calpain
m-Calpain does not possess a known ERK phosphorylation consensus sequence. However, examination of the murine and human amino acid sequences revealed four sites that possessed similarities to the phosphorylation consensus sequence Pro-X-Ser/Thr-Pro (47, 48). The following sequences were chosen to investigate further: Pro-Ser50*-Phe-Pro, Pro-Asn-Thr381*-Phe-Trp-Met-Asn-Pro, and Pro-Ser490*-Thr491*-Phe-Glu-Pro. m-Calpain was cloned from human dermal fibroblasts by reverse transcription-PCR, and constructs in which these potential kinase sites were replaced with alanines were made (S50A, T381A, S490A, and T491A, respectively). These mutant proteins were expressed by in vitro transcription and translation, the latter using rat reticulocytes, which do not contain endogenous m-calpain (27a). Products of the predicted molecular weights were recognized by immunoblotting for m-calpain (data not shown). The in vitro-translated products were incubated with 0.5 μg of purified Tau protein, a known calpain substrate, with loss of Tau on immunoblotting signifying calpain activity. Tau cleavage was increased when either recombinant rat m-calpain or wild-type in vitro-translated calpain was incubated with active ERK (Fig. 3A). During the short incubation period used to demonstrate ERK-induced Tau cleavage (Fig. 3A), calcium-induced cleavage was too limited to be detected; longer incubations are required (Fig. 3B). As seen in Fig. 3A, only the S50A mutant m-calpain proteins exhibited reduced ERK-induced cleavage of Tau. The recombinant wild-type and the in vitro-translated S50A m-calpains were still activable by calcium (Fig. 3B, shown for recombinant rat m-calpain and the S50A mutant calpain; wild-type m-calpain was similar to recombinant m-calpain [data not shown]). Longer incubation of S50A with ERK did not increase Tau degradation (data not shown). The other constructs demonstrated ERK-induced Tau degradation indistinguishable from that of wild-type calpain (Fig. 3A; results for mutants S490A and T491A were similar to those for T381A m-calpain; data not shown). The presence of calcium can overcome at least partly the loss of ERK-induced activity in the S50A mutant calpain. This strongly suggests that the S50A mutation did not globally destroy m-calpain functioning, but only the ability to be activated by ERK.
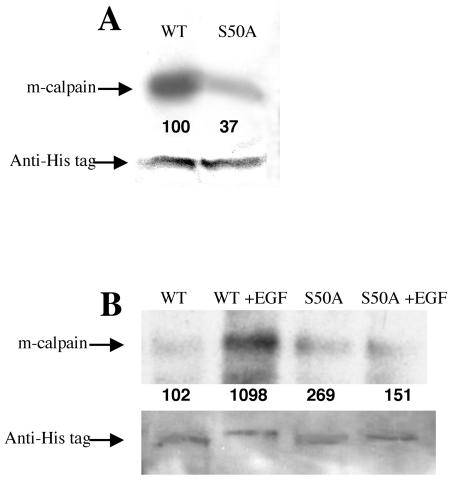
To determine whether the serine at amino acid 50 might be the site of ERK phosphorylation, the wild type and S50A construct were cloned into a dexamethasone-inducible eukaryotic expression vector; this strategy was used because constitutive overexpression of wild-type m-calpain is toxic (43). WT NR6 murine fibroblasts were transfected by electroporation with the plasmid pCEP4-MMTV-CANP-His (wild type) or pCEP4-MMTV-CANP S50A-His (S59A), both of which encode a polyhistidine tag at the carboxy terminus of m-calpain. The in vivo-expressed wild-type and S50A His-tagged m-calpain proteins were purified with a nickel column. These proteins were subjected to in vitro phosphorylation from ERK. S50A m-calpain was phosphorylated to a lesser extent than wild-type m-calpain (Fig. 4A). Interestingly, some low-level ERK phosphorylation was still detectable on the S50A protein; this may indicate that ERK is, to a much lesser extent, able to phosphorylate other sites. However, in vivo, EGF treatment does not result in increased S50A m-calpain phosphorylation (Fig. 4B). This supports the contention that S50 is the major, if not the only, relevant site of ERK phosphorylation.
FIG. 4.
S50A m-calpain phosphorylation in vitro and in vivo. Expression of histidine-tagged m-calpains (wild type and S50A) was induced with dexamethasone. (A) Wild-type (WT) and S50A mutant calpains were purified by nickel affinity chromatography. Equal amounts of the purified proteins (as shown by antihistidine blotting) were incubated with activated ERK (300 U) in the presence of [γ-32P]ATP. S50A m-calpain was radiolabeled to a lesser extent than wild-type m-calpain (n = 2). (B) Quiescent cells expressing wild-type and S50A histidine-tagged calpains were labeled with [32P]orthophosphate for 2 h prior to EGF treatment. The exogenously encoded calpains were purified by nickel affinity chromatography, and phosphorylated m-calpains were detected by autoradiography. EGF induced phosphorylation of wild-type m-calpain but not S50A m-calpain in vivo (n = 2). Shown are representative antihistidine blots to demonstrate equal loading. The numbers below the bands are relative densities (B) or percentages of wild-type levels (A) that were determined with National Institutes of Health Image.
S50A m-calpain-expressing cells lack EGF-induced calpain activity
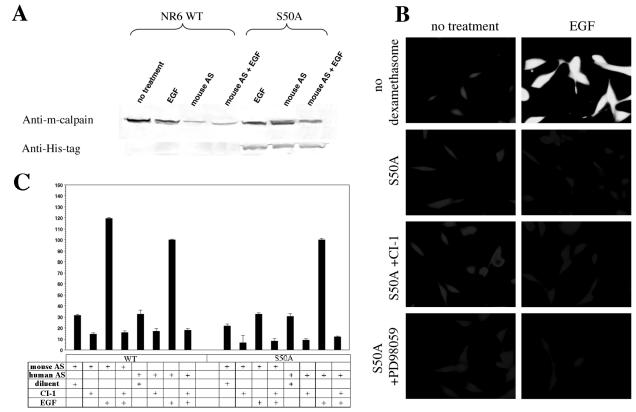
The above findings suggested that S50 is an activation site for ERK. For in vivo biological confirmation of the S50A construct, the endogenous murine m-calpain, which would obscure a failure to activate S50A m-calpain, was suppressed with an antisense oligonucleotide against murine m-calpain. The antisense suppression (18) down-regulated endogenous murine m-calpain, while S50A human m-calpain expression was induced with dexamethasone (Fig. 5A). While murine antisense treatment down-regulated m-calpain, cells expressing S50A m-calpain showed a smaller decrement of total m-calpain. The blot was reprobed with an antihistidine antibody to demonstrate mutant calpain expression. Calpain activity in the S50A-expressing cells (with endogenous m-calpain activity absent) was measured with the cell-permeable synthetic substrate Boc-LM-CMAC (Fig. 5B); that in cell extracts was measured with the substrate Ac-LLY-AFC (BioVision) (Fig. 5C). The S50A cells lacked EGF-induced calpain activity, whereas cells that were transfected but neither induced to express mutant protein nor subjected to antisense down-regulation of endogenous m-calpain demonstrated normal EGF-induced calpain activity. CI-1 and the MEK inhibitor PD98059 (inhibits activation of ERK) had no additional effects, as expected, since there was no calpain activity to inhibit (Fig. 5B).
FIG. 5.
EGF-induced calpain activity in S50A m-calpain-expressing cells. (A) Activity of S50A m-calpain was measured in the face of down-regulation of endogenous murine m-calpain. Antisense oligonucleotides were designed to be specific for murine m-calpain. These did not block the expression of S50A m-calpain, which is human in origin. Antisense treatment in the presence of EGF for 12 h was adequate to down-regulate endogenous m-calpain (lanes 3 and 4 [from the left]), and exogenous expression of S50A was not blocked (lanes 5 to 7; n = 3). (B) Calpain activity was measured in cells expressing S50A m-calpain with the substrate Boc-LM-CMAC (5 μM). Cells that possessed the S50A plasmid but that were not induced to express it were used as a control (no dexamethasone). Control cells were not treated with murine m-calpain antisense oligonucleotides. All other cells were induced with dexamethasone (2 μM) for 18 to 24 h and treated with murine m-calpain antisense oligonucleotides for the same time period. This produced conditions under which the activity of the S50A m-calpain construct could be measured. Control cells show normal EGF-induced calpain activity (indicated by increased fluorescence, equivalent to lightness of tone in micrographs). S50A-expressing cells do not exhibit EGF-induced calpain activity, and CI-1 and the MEK inhibitor PD98059 have no additional effects (images are representative; n = 3). (C) Calpain activity was determined by a calpain activity assay kit (BioVision). WT NR6 cells expressing wild-type (WT) or S50A human m-calpain-His were pretreated for 24 h with either antisense oligonucleotides against murine (mouse AS) or human (human AS) m-calpain to down-regulate either the endogenous murine or the exogenous human m-calpain and then treated with or without CI-1 and/or EGF. The cells were lysed, and the lysates were analyzed for calpain activity. The results from this assay correlate with those from the Boc assay showing that the mutation of serine 50 to alanine significantly reduces EGF-induced m-calpain activity (P < 0.05 for endogenous mouse antisense down-regulation compared to human antisense down-regulation). Cells treated with diluent were incubated with PBS, the vehicle for EGF.
S50E m-calpain presents constitutive calpain activity
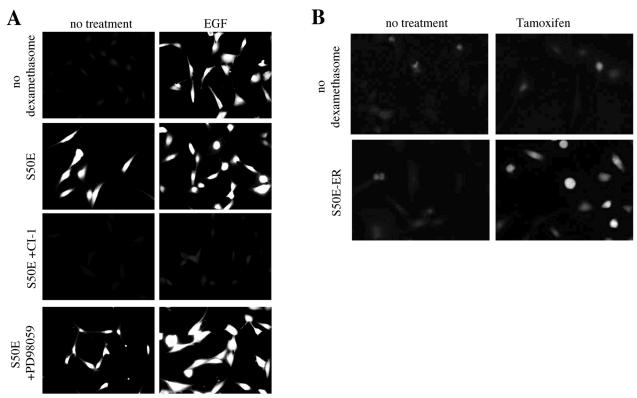
Protein phosphorylation effects can often be mimicked by an acidic amino acid. Therefore we generated a construct containing glutamic acid in place of serine 50. S50E-expressing cells presented constitutive Boc-LM-CMAC cleavage activity even in the absence of EGF stimulation (Fig. 6A). That this was due to mutant calpain activity was demonstrated by inhibition by CI-1, though at slightly higher concentrations (25 μg/ml) than normally required to block EGF-induced Boc-LM-CMAC cleavage (5 μg/ml), indicating that the mutation did not have a direct effect on the active site. However, the MEK inhibitor PD98059, which was previously shown to block EGF-induced calpain activity, had no effect on S50E-expressing cells, demonstrating that S50E expression is not dependent on ERK activity. A highly retracted morphology suggested that the S50E calpain was functional in decreasing the attachment to the substratum (data not shown).
FIG. 6.
Calpain activity in S50E m-calpain-expressing cells. (A) As in Fig. 5, control cells possessed the S50E plasmid but were not induced to express it (no dexamethasone) and were not treated with murine m-calpain antisense oligonucleotides. Cells induced to express S50E m-calpain in the presence of endogenous m-calpain down-regulation show increased calpain activity even in the absence of EGF (indicated by increased fluorescence). CI-1 blocks the activity of S50E m-calpain. However, the MEK inhibitor PD98059, which would normally block EGF-induced calpain activity, has no effect (images are representative; n = 3). (B) Cells expressing the S50E-ER hybrid construct were examined for calpain activity. In the absence of induction, tamoxifen (1 μM) did not trigger cleavage of Boc-LM-CMAC. After induction of the S50E-ER construct, tamoxifen was required to note fluorescence. Shown are images taken 60 min after addition of tamoxifen to the cells (images are representative; n = 3).
It was possible that the constitutive Boc-LM-CMAC cleavage activity noted was due to cellular changes secondary to expression of this mutant protein. To visualize directly acute cleavage of the substrate, the S50E construct was fused to the ER regulatory domain as described in Materials and Methods. After stable transfection Boc-LM-CMAC cleavage was not observed, either in the presence or absence of tamoxifen (1 μM) (Fig. 6B). Even after dexamethasone induction, fluorescence was not seen. However, upon exposure to tamoxifen, Boc fluorescence is seen within 60 min. This strongly supports the contention that S50E is active in the absence or EGFR signaling.
S50A m-calpain-expressing cells lack EGF-induced motility and deadhesion
Inhibition of the activation mechanism of m-calpain should eliminate the m-calpain-dependent cell responses, namely, EGF-induced cell motility and deadhesion from the substratum. EGF enhanced cell motility, as determined by measuring the decrease in the denuded area in a monolayer, in cells expressing the wild-type human m-calpain. Down-regulation of either the endogenous murine m-calpain or the exogenous human wild-type m-calpain had no effect on EGF-induced motility (Fig. 7A). This is expected, as both calpains should function equivalently. However, in cells expressing the S50A m-calpain, EGF failed to promote wound closure in the absence of the endogenous murine wild-type m-calpain (Fig. 7A). In the presence of antisense oligonucleotides directed against murine m-calpain, the wound closure induced by EGF was not significantly different from results with no EGF. This was dramatically different from the EGF-induced response in the presence of antisense oligonucleotides directed against human m-calpain (P < 0.01). Unregulated activation of calpain, present in the WT NR6 cells in which S50E m-calpain is induced, is detrimental to cell functioning over extended periods, and thus the motility of these cells could not be consistently enumerated. However, these cells were noted to be limited in their movement (data not shown).
FIG. 7.
Expression of the S50A m-calpain mutant inhibits EGF-induced motility and deadhesion in fibroblasts. (A) WT NR6 cells expressing the S50A mutant m-calpain were analyzed for their ability to migrate into a denuded space. The cells were pretreated for 24 h with either antisense oligonucleotides against murine (mouse AS) or human (human AS) m-calpain to down-regulate either the endogenous murine or the exogenous human m-calpain. A central swath was denuded in the confluent layer of cells, which were then treated with or without EGF (10 nM) in the presence of antisense oligonucleotides (20 μM) and incubated for 24 h. The area of the wound was measured, and the decreased area from the control (mouse antisense treatment only) was subtracted as background. The decreased area from the cells treated with human antisense oligonucleotides and EGF was set to 100%, and results for the other treatments are percentages of this value. The exogenous wild-type (WT) human m-calpain demonstrated the same EGF-induced motility as the endogenous murine m-calpain (P was not significant). However, the S50A mutant m-calpain showed a significant decrease in motility compared to the endogenous murine m-calpain (P < 0.05; n = 3). (B) WT NR6 cells stably expressing wild-type (black bars) or the S50A (striped bars) human m-calpain were incubated with antisense oligonucleotides against murine m-calpain and then treated with or without CI-1 and/or EGF. The cells were then inverted and centrifuged (1,643 × g) for 5 min at 37°C. The adherent cells were counted before and after centrifugation. The number of remaining cells are expressed as a percentage of the starting number of cells. Cells expressing wild-type human m-calpain and treated with EGF showed an enhanced ability to detach from the substratum compared to the S50A-expressing cells (P < 0.01; n = 6). Cells treated with diluent or not treated were incubated with PBS, the vehicle for EGF.
Deadhesion from the substratum depends on m-calpain activation (18). Thus, if activation of this protease in cells requires phosphorylation at serine 50, then cells expressing the S50A mutant protein should not undergo deadhesion. EGF reduced the adhesiveness of the WT NR6 cells expressing wild-type human m-calpain (Fig. 7B). However, EGF had no effect on the adhesiveness of WT NR6 cells expressing the S50A m-calpain. Antisense oligonucleotides against the endogenous murine m-calpain provided a clean system in which the effects of expressed human clones could be assessed. The fact that the calpain inhibitor ALLN (CI-1) eliminated the deadhesion, when evident, confirmed that this was due to calpain-mediated events. The S50E-expressing cells were retracted, with some randomly undergoing deadhesion even in the absence of centripetal forces rendering quantitation difficult; however, the repeated qualitative result was severely diminished adhesiveness (data not shown).
EGF-induced calpain activity is not dependent on increased intracellular calcium
The finding that ERK phosphorylation activates m-calpain still leaves open the question of whether calcium fluxes contribute to EGFR-mediated activation of this proteinase. We investigated increases in intracellular calcium following perfusion of adherent WT NR6 cells with EGF (Fig. 8A). Fura-2-AM was loaded into cells in a perfusion chamber, and real-time changes in calcium levels were measured via the changes in fluorescence ratio between calcium-free and calcium-bound forms of the dye. Interestingly, although EGF triggers IP3 production via PLCγ, EGF stimulation did not produce a consistent increase in intracellular calcium. In fact, only 20% of the cells studied (n = 90 over multiple experiments) exhibited a calcium increase, whereas over 95% of cells demonstrated Boc-LM-CMAC cleavage. Bradykinin consistently stimulated increased calcium, even in cells that did not respond to EGF, demonstrating that these cells possessed normal calcium transport mechanisms. Although this result supported our hypothesis that ERK-mediated calpain activation occurs in the absence of increased calcium, independent agonist confirmation was sought.
FIG. 8.
Effect of intracellular calcium chelation by BAPTA-AM and removal of medium calcium on EGF- and PDGF-induced increases in intracellular calcium. (A) Percentage of cells that demonstrated an increase in intracellular calcium in response to stimulus (i.e., EGF, PDGF, or bradykinin). This response does not occur in cells pretreated with BAPTA-AM, and the response is attenuated in cells perfused in calcium-free buffer. (B) Average increases in intracellular calcium level in response to stimulus (arbitrary fluorescence ratio units) for cells that demonstrated a rise in intracellular calcium levels ± standard errors of the means. The maximum response (bradykinin) was estimated at 500 nM by comparison to ionophore treatment (∼1.3 ratio units; data not shown). The intracellular calcium concentration of BAPTA-AM-treated cells is shown for comparison, although none of these cells were classified as responding. Numbers of cells and numbers of experiments for the different treatments are provided in Materials and Methods (“Experimental analysis”).
To show that calcium fluxes do not play a role in ERK-induced calpain activation, one needs a situation in which such fluxes are eliminated, for which we used a calcium-free buffer and intracellular calcium chelation system. Because internal calcium fluxes and other pitfalls may complicate interpretation of these results, this system was tested by using the growth factor PDGF, which had been shown to stimulate robust calcium fluxes in Swiss 3T3 fibroblasts (36). Importantly, PDGF stimulates calpain activity through activation of ERK, similar to EGF (44). Therefore, we used PDGF as a substitute stimulus to provide conditions in which we could consistently trigger calcium flux and calpain activity (Fig. 7B). PDGF-stimulated calcium flux was blocked partially by incubation of the cells in calcium-free buffer and was blocked completely by preloading the cells with BAPTA-AM, an intracellular calcium chelator. These treatments also completely blocked any detectable calcium flux in EGF-stimulated cells. Replicating the conditions under which intracellular calcium increases were totally blocked, we measured calpain activity using the synthetic substrate Boc-LM-CMAC. Blocking calcium flux with either BAPTA-AM or calcium-free medium or both together did not prevent EGF or PDGF activation of calpain (Fig. 9). This demonstrates that EGF-mediated calpain activity is not dependent on high intracellular levels of calcium.
FIG. 9.
Growth factor-induced calpain activity under conditions that do not permit increased intracellular calcium. WT NR6 murine fibroblasts were incubated with growth factors (EGF and PDGF) in the presence and absence of BAPTA-AM or calcium free buffer. These treatments were identical to those under which intracellular calcium levels were measured. To measure calpain activity, the cells were subsequently loaded with the substrate Boc-LM-CMAC. Calpain activity is indicated by an increase in fluorescence (images are representative; n = 3).
DISCUSSION
The mechanisms by which calpain is regulated during cell motility and adhesion have only been tentatively outlined. Herein we demonstrate a novel mechanism of m-calpain activation, via direct phosphorylation by ERK. Although such phosphorylation cascades are common regulatory mechanisms in various signaling pathways, phosphorylation has long been dismissed as a potential regulatory mechanism for calpain due to early reports that calpains were not phosphorylated in vivo. Unusual properties of calpains make it difficult to detect calpain phosphorylation. Adachi et al., using [32P]phosphate loading and calpain purification, found that, after a 24-h incubation, purified calpain was not significantly phosphorylated (1). This was likely to occur for two reasons: m- and μ-calpain have long half-lives of more than 5 days (54), so that shorter-term labeling would be substoichiometric, and further no external stimuli were applied to trigger phosphorylation by various kinases. Recent reports suggest that calpain is highly phosphorylated. There are at least three sites each for tyrosine, serine, and threonine phosphorylation that undergo posttranslational phosphorylation even under constitutive conditions. Of interest, and confounding for earlier studies, it appears that each site is only fractionally phosphorylated (D. Goll, personal communication), suggesting active regulation of these sites. We have reported that some sites may be phosphorylated upon external stimulation; ELR-negative CXC chemokines activate cyclic AMP-dependent protein kinase A to phosphorylate the serine at amino acid 369, inhibiting growth factor-induced m-calpain activation (43, 46). Herein, we report a second phosphorylation site, one that promotes activation: the serine at amino acid 50. Activation of m-calpain by ERK and subsequent cell deadhesion and motility are dependent on this site, and replacement of the serine with glutamic acid, which can mimic phosphorylation, confers EGFR-independent activity on m-calpain. The physiological relevance of this phosphorylation is demonstrated by the fact that cells expressing the S50A substitution mutant protein fail to undergo EGF-induced motility and deadhesion, coupled with the fact that cells expressing the constitutively active S50E m-calpain readily detach from the substratum. This is the first evidence of phosphorylation as an activating mechanism for m-calpain.
Activation of m-calpain by phosphorylation could be either alternative to, or coordinate with, the earlier proposed activation by calcium fluxes. Our first hint that ERK phosphorylation was an alternative pathway of activation was the apparent calcium independence of in vitro activation and the fact that activation by ERK was greater than that induced by calcium alone at a 2 μΜ concentration, which approximates the highest levels attainable in motile cells. Conditions that prevent calcium increases and activation of μ-calpain (9, 53; Satish et al., submitted) demonstrated normal growth factor-induced calpain activation. This suggests that calcium fluxes play little to no role in vivo in growth factor-induced m-calpain activation. These findings do not, and cannot, address the question of whether ambient calcium is required for a basal preactivation process. The presence of multiple calcium-binding sites in m-calpain argues strongly for such a process (25, 37). However, that elevated calcium levels are not absolutely required for activation is a novel finding. No previous reports have determined that calpain can be activated at physiologic intracellular calcium concentrations.
One caveat is that we have not directly determined the site of ERK phosphorylation either in vivo or in vitro. In vivo determination was not attempted, as only a minor fraction of m-calpain, which resides in the periplasma membrane space, is activated by EGF (20). This, coupled with the multiple partial phosphorylations unrelated to EGF stimulation, would have made analysis very complex. Despite considerable effort, mapping the phosphorylated site in vitro was not successful. Calpain is resistant to consistent complete proteolytic digestion, thus yielding multiple overlapping fragments, and, even for recombinant m-calpain, substoichiometric complex phosphorylations confound analyses (D. Goll, personal communication). However, the mutational approach shows that S50 is a site of ERK phosphorylation and is probably the major one controlling m-calpain activation. Because substitution at this site also changes the calcium dependence by a factor of ∼100, however, it is still possible that other sites or protein interactions which contribute to the observed phenotype of this mutant are also dependent on calcium. The diminution in ERK phosphorylation and the elimination of EGF-induced calpain activity in the S50A construct strongly support this conclusion. Still, the fact that ERK weakly phosphorylates S50A, at least in vitro, may portend a second site. This second site would probably be a minor one, however, as judged by our in vivo findings that EGF induced by and large only m-calpain (18) and that there was a lack of EGF-induced calpain activity and cell responses in cells expressing only the S50A construct.
One might speculate how phosphorylation of S50 might lead to activation of m-calpain. Recent work has produced crystal structures of m-calpain with indications as to the physical requirements for an activating mechanism (25, 49). In the calcium-free structure, the active site, which is formed by the juxtaposition of two globular catalytic domains, is too far apart and misaligned. Any activating mechanism would have to release the physical constraints present in this molecule and close the active-site cleft. In this context, the structural implications of phosphorylation at serine 50 are as yet unclear. This site resides in a loop region following the first alpha helix in the first catalytic domain. That this site would effect the formation of the active site or release other structural constraints to enhance this transformation is not, a priori, evident from study of the sequence or crystal structure (Z. Jia, personal communication). It is also possible that phosphorylation at this site alters interaction with target molecules and allows positioning for subsequent cleavage; further investigations are required to investigate this proposition. Regardless of the molecular mechanism, comparisons of the primary amino acid sequences of calpains provide some support for S50 being a key site for activation. The muscle-specific calpain isoform (calpain III or p94), which may have calcium-independent activity, presents a glutamic acid at the S50 position while conserving the adjacent amino acids (6). μ-Calpain (calpain I) does not have a phosphorylatable amino acid at this site (RCL50QS) (National Center for Biotechnology Information accession no. AAH08751) and is not activated by either growth factors in vivo (18) or ERK in vitro (data not shown). In fact, our complementary data demonstrate that μ-calpain can be activated by a calcium flux in response to chemokines in keratinocytes (Satish et al., submitted). Thus, the results presented here provide experimental impetus to study the modification of this residue from a structural standpoint to highlight the mechanisms by which most calpains might become active.
The finding that ERK directly phosphorylates and activates m-calpain raises the question of how an often-activated and pleiotropic signaling nexus such as ERK can be restricted to only triggering m-calpain in response to growth factor signaling. We propose that this is due to temporal and spatial restrictions. We had earlier reported that EGF-induced calpain activation occurred only when both EGFR and ERK were plasma membrane associated (20). This finding was attractive as the putative targets for calpain-mediated deadhesion are components of adhesion complexes, including β-integrin cytoplasmic tails, talin, paxillin, and focal adhesion kinase (7, 8, 15, 40, 52). However, there have been no literature reports in which the subcellular location of active calpain has been successfully described. Even whether calpains are activated at the cell front during spreading or at the rear during detachment has only been addressed indirectly (3, 26, 41, 43, 45). It is clear that a standing challenge in the calpain field is how to localize active calpain, which has been an elusive target. Currently, this is beyond our technical reach, as antibodies specific for active calpains or substrates that can be constrained to, or identified within, subcellular compartments are not available. Currently, only the distribution of total calpain protein has been demonstrated and is dependent on cell type (4, 30, 38). In our cell system, m- and μ-calpain proteins appear to be distributed evenly throughout the cell, as seen by immunofluorescence (data not shown). As we had previously localized the upstream molecules ERK and EGFR to the plasma membrane, the problem in colocalizing the calpain-activating complex and its putative targets was whether calpain was, in fact, activated at this locale or whether there were intermediaries between ERK and m-calpain. The present data answer this in showing a direct interaction with, and activation by, ERK. This strongly suggests that the activation of m-calpain occurs at the plasma membrane. The fact that activated calpain is quickly degraded (18, 22, 23) would then likely prevent this molecule from attacking proteins distant from the site of activation and thus limit the biological outcome of membrane-associated activation.
How m-calpain is associated with the membrane is another unsettled question, though only a small fraction of the enzyme appears to be located on the membrane (20). Recent identification of the putative C2 domain has led to studies that report an interaction of m-calpain with phosphoinositides, though confirmatory demonstration and in vivo verification remain to be accomplished (50). This model is attractive as it may also provide for the transcellular asymmetry that would be needed if m-calpain is the switch that controls rear deadhesion during growth factor-induced motility (19, 44). Phosphoinositides are rapidly turned over at the leading edge of motile cells, as active PLCγ functions preferentially at the front of the cell (12, 13). Thus, in forming a stable interaction with the membrane through phosphoinositides, m-calpain may accumulate at trailing edges. In conclusion, our data support an emerging model of phosphoinositide-bound m-calpain localized to the cytoplasmic face of adhesion complexes, where it is activated at a key site.
Acknowledgments
We thank John Hempel, Valery Thompson, Douglas Lauffenburger, Jeff Chou, Jareer Kassis, and Jon Marsh for technical help and helpful discussions. Support was provided by the Indiana University Vector Production Facility.
This work was supported by grants from the National Institutes of General Medical Sciences, Aging, and Arthritis and Musculoskeletal Diseases (NIH), the DoD/VA Combat Casualty Program, the Department of Veterans Affairs, Indiana University Thoracic Oncology Program, and the Clarian Values Foundation.
REFERENCE
- 1.Adachi, Y., N. Kobayashi, T. Murachi, and M. Hatanaka. 1986. Ca2+-dependent cysteine proteinase, calpains I and II are not phosphorylated in vivo. Biochem. Biophys. Res. Commun. 136:1090-1096. [DOI] [PubMed] [Google Scholar]
- 2.Allen, F. D., C. F. Asnes, P. Chang, E. L. Elson, D. A. Lauffenburger, and A. Wells. 2002. EGF-induced matrix contraction is modulated by calpain. Wound Repair Regen. 10:67-76. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt, A., I. Kaverina, C. Otey, and A. Huttenlocher. 2002. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J. Cell Sci. 115:3415-3425. [DOI] [PubMed] [Google Scholar]
- 4.Bialkowska, K., S. Kulkarni, X. Du, D. E. Goll, T. C. Saido, and J. E. Fox. 2000. Evidence that β3 integrin-induced Rac activation involves the calpain-dependent formation of integrin clusters that are distinct from the focal complexes and focal adhesions that form as Rac and RhoA become active. J. Cell Biol. 151:685-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bootman, M. D., P. Lipp, and M. J. Berridge. 2001. The organisation and functions of local Ca2+ signals. J. Cell Sci. 114:2213-2222. [DOI] [PubMed] [Google Scholar]
- 6.Branca, D., A. Gugliucci, D. Bano, M. Brini, and E. Carafoli. 1999. Expression, partial purification and functional properties of the muscle-specific calpain isoform p94. Eur. J. Biochem. 265:839-846. [DOI] [PubMed] [Google Scholar]
- 7.Carragher, N. O., V. J. Fincham, D. Riley, and M. C. Frame. 2001. Cleavage of focal adhesion kinase by different proteases during SRC-regulated transformation and apoptosis. Distinct roles for calpain and caspases. J. Biol. Chem. 276:4270-4275. [DOI] [PubMed] [Google Scholar]
- 8.Carragher, N. O., B. Levdau, R. Ross, and E. W. Raines. 1999. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125FAK, paxillin, and talin. J. Cell Biol. 147:619-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhuri, P., S. M. Colles, D. S. Damron, and L. M. Graham. 2003. Lysophosphatidylcholine inhibits endothelial cell migration by increasing intracellular calcium and activating calpain. Arterioscler. Thromb. Vasc. Biol. 23:218-223. [DOI] [PubMed] [Google Scholar]
- 10.Chen, P., K. Gupta, and A. Wells. 1994. Cell movement elicited by epidermal growth factor receptor requires kinase and autophosphorylation but is separable from mitogenesis. J. Cell Biol. 124:547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, P., H. Xie, and A. Wells. 1996. Mitogenic signaling from the EGF receptor is attenuated by a motility-associated phospholipase C-γ/protein kinase C feedback mechanism. Mol. Biol. Cell 7:871-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou, J., D. Beer-Stolz, N. Burke, S. C. Watkins, and A. Wells. 2002. Distribution of gelsolin and phosphoinositol 4,5-bisphosphate in lamellipodia during EGF-induced motility. Int. J. Biochem. Cell Biol. 34:776-790. [DOI] [PubMed] [Google Scholar]
- 13.Chou, J., N. A. Burke, A. Iwabu, S. C. Watkins, and A. Wells. 2003. Directional motility induced by EGF requires cdc42. Exp. Cell Res. 287:47-56. [DOI] [PubMed] [Google Scholar]
- 14.Cong, J., D. E. Goll, A. M. Peterson, and H. P. Kapprell. 1989. The role of autolysis in activity of the Ca2+-dependent proteinases (μ-calpain and m-calpain). J. Biol. Chem. 264:10096-10103. [PubMed] [Google Scholar]
- 15.Cooray, P., Y. Yuan, S. M. Schoenwaelder, C. A. Mitchell, H. H. Salem, and S. P. Jackson. 1996. Focal adhesion kinase (pp125FAK) cleavage and regulation by calpain. Biochem. J. 318:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croall, D. E., and G. N. DeMartino. 1991. Calcium activated neutral protease (calpain) system: structure, function, and regulation. Physiol. Rev. 71:813-847. [DOI] [PubMed] [Google Scholar]
- 17.Fujitani, K., J. Kambayashi, M. Sakon, S. I. Ohmi, S. Kawashima, M. Yukawa, Y. Yano, H. Miyoshi, M. Ikeda, N. Shinoki, and M. Monden. 1997. Identification of μ-, m-calpains and calpastatin and capture of μ-calpain activation in endothelial cells. J. Cell. Biochem. 66:197-209. [DOI] [PubMed] [Google Scholar]
- 18.Glading, A., P. Chang, D. A. Lauffenburger, and A. Wells. 2000. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J. Biol. Chem. 275:2390-2398. [DOI] [PubMed] [Google Scholar]
- 19.Glading, A., D. A. Lauffenburger, and A. Wells. 2002. Cutting to the chase: calpain proteases in cell migration. Trends Cell Biol. 12:46-54. [DOI] [PubMed] [Google Scholar]
- 20.Glading, A., F. Uberall, S. M. Keyse, D. A. Lauffenburger, and A. Wells. 2001. Membrane proximal ERK signaling is required for M-calpain activation downstream of EGF receptor signaling. J. Biol. Chem. 276:23341-23348. [DOI] [PubMed] [Google Scholar]
- 21.Grynkiewicz, G., M. Poenie, and R. Y. Tsien. 1985. A new generation of Ca+2 indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440-3445. [PubMed] [Google Scholar]
- 22.Guttmann, R. P., J. S. Elce, P. D. Bell, J. C. Isbell, and G. V. Johnson. 1997. Oxidation inhibits substrate proteolysis by calpain I, but not autolysis. J. Biol. Chem. 272:2005-2012. [DOI] [PubMed] [Google Scholar]
- 23.Guttmann, R. P., and G. V. W. Johnson. 2000. Measurement of calpain activity in vitro and in situ using a fluorescent compound and tau as substrates. Methods Mol. Biol. 144:143-150. [DOI] [PubMed] [Google Scholar]
- 24.Hirose, K., S. Kadowaki, M. Tanabe, T. Takeshima, and M. Iino. 1999. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science 284:1527-1530. [DOI] [PubMed] [Google Scholar]
- 25.Hosfield, C. M., J. S. Elce, O. K. Davies, and Z. Jia. 1999. Crystal structure of calpain reveals the structural basis for Ca2+-dependent protease activity and a novel model of enzyme activation. EMBO J. 18:6880-6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huttenlocher, A., S. P. Palecek, Q. Lu, W. Zhang, R. L. Mellgren, D. A. Lauffenburger, M. H. Ginsburg, and A. F. Horwitz. 1997. Regulation of cell migration by the calcium-dependent protease calpain. J. Biol. Chem. 272:32719-32722. [DOI] [PubMed] [Google Scholar]
- 27.Imajoh, S., K. Aoki, S. Ohno, Y. Emori, H. Kawasaki, H. Sugihara, and K. Suzuki. 1988. Molecular cloning of the cDNA for the large subunit of the high-Ca2+-requiring form of human Ca2+-activated neutral protease. Biochemistry 27:8122-8128. [DOI] [PubMed] [Google Scholar]
- 27a.Inomata, M., M. Nakamura, S. Imajoh-Ohmi, and S. Kawashima. 1993. A variety of calpain/calpastatin systems in mammalian erythrocytes. Biochim. Biophys. Acta 1178:207-214. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, G. V. W., and R. P. Guttmann. 1997. Calpains: intact and active? Bioessays 19:1011-1018. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni, S., D. E. Goll, and J. E. Fox. 2002. Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J. Biol. Chem. 277:24435-24441. [DOI] [PubMed] [Google Scholar]
- 30.Lane, R. D., D. M. Allan, and R. L. Mellgren. 1992. A comparison of the intracellular distribution of μ-calpain, m-calpain, and calpastatin in proliferating human A431 cells. Exp. Cell Res. 203:5-16. [DOI] [PubMed] [Google Scholar]
- 31.Littlewood, T. D., D. C. Hancock, P. S. Danielian, M. G. Parker, and G. I. Evans. 1995. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Research 23:1686-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyon, J. J., and R. J. Watson. 1996. Interference of Myb transactivation activity by a conditional dominant negative protein: functional interference in a cytotoxic T-cell line results in G1 arrest. Gene 182:123-128. [DOI] [PubMed] [Google Scholar]
- 33.Melloni, E., M. Averna, F. Salamino, B. Sparatore, R. Minafra, and S. Pontermoli. 2000. Acyl-CoA-binding protein is a potent m-calpain activator. J. Biol. Chem. 275:82-86. [DOI] [PubMed] [Google Scholar]
- 34.Melloni, E., M. Michetti, F. Salamino, and S. Pontermoli. 1998. Molecular and functional properties of a calpain activator protein specific for μ-isoforms. J. Biol. Chem. 273:12827-12831. [DOI] [PubMed] [Google Scholar]
- 35.Michetti, M., P. L. Viotti, E. Melloni, and S. Pontremoli. 1991. Mechanism of action of the calpain activator protein in rat skeletal muscle. Eur. J. Biochem. 202:1177-1180. [DOI] [PubMed] [Google Scholar]
- 36.Miyakawa, T., M. Kojima, and M. Ui. 1998. Differential routes of Ca2+ influx in Swiss 3T3 fibroblasts in response to receptor stimulation. Biochem. J. 329:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moldoveanu, T., C. M. Hosfield, D. Lim, L. S. Elce, Z. Jia, and P. L. Davies. 2002. A Ca2+ switch aligns the active site of calpain. Cell 108:649-660. [DOI] [PubMed] [Google Scholar]
- 38.Neuberger, T., A. K. Chakrabarti, T. Russell, G. H. DeVries, E. L. Hogan, and N. L. Banik. 1997. Immunolocalization of cytoplasmic and myelin m-calpain in transfected Schwann cells. I. Effect of treatment with growth factors. J. Neurosci. Res. 47:521-530. [PubMed] [Google Scholar]
- 39.Perrin, B. J., and A. Huttenlocher. 2002. Calpain. Int. J. Biochem. Cell Biol. 34:722-725. [DOI] [PubMed] [Google Scholar]
- 40.Pfaff, M., X. Du, and M. H. Ginsberg. 1999. Calpain cleavage of integrin β cytoplasmic domains. FEBS Lett. 460:17-22. [DOI] [PubMed] [Google Scholar]
- 41.Potter, D. A., J. S. Tirnauer, R. Janssen, D. E. Croall, C. N. Hughes, K. A. Fiacco, J. W. Mier, M. Maki, and I. M. Herman. 1998. Calpain regulates actin remodeling during cell spreading. J. Cell Biol. 141:647-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salamino, F., R. DeTullio, P. Mengotti, P. L. Viotti, E. Melloni, and S. Pontremoli. 1993. Site-directed activation of calpain is promoted by a membrane-associated natural activator protein. Biochem. J. 290:191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiraha, H., A. Glading, J. Chou, Z. Jia, and A. Wells. 2002. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol. Cell. Biol. 22:2716-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiraha, H., K. Gupta, A. Glading, and A. Wells. 1999. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J. Cell Biol. 146:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shuster, D. B., and I. M. Herman. 1995. Indirect association of ezrin with F-actin: isoform specificity and calcium sensitivity. J. Cell Biol. 128:837-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, S. D., Z. Jia, K. K. Huynh, A. Wells, and J. S. Elce. 2003. Glutamate substitutions at a PKA consensus site are consistent with inactivation of calpain by phosphorylation. FEBS Lett. 542:115-118. [DOI] [PubMed] [Google Scholar]
- 47.Sorimachi, H., S. Ishura, and K. Suzuki. 1997. Structure and physiological function of calpains. Biochem. J. 328:721-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorimachi, H., and K. Suzuki. 2001. The structure of calpain. J. Biochem. 129:653-664. [DOI] [PubMed] [Google Scholar]
- 49.Strobl, S., C. Fernandez-Catalan, M. Braun, R. Huber, H. Masumoto, K. Nakagawa, A. Irie, H. Sorimachi, G. Bourenkow, H. Bartunik, K. Suzuki, and W. Bode. 2000. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc. Natl. Acad. Sci. USA 97:588-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tompa, P., Y. Emori, H. Sorimachi, K. Suzuki, and P. Friedrich. 2001. Domain III of calpain is a Ca+2-regulated phospholipid-binding domain. Biochem. Biophys. Res. Commun. 280:1333-1339. [DOI] [PubMed] [Google Scholar]
- 51.Tompa, P., Z. Mucsi, G. Orosz, and P. Friedrich. 2002. Calpastatin subdomains A and C are activators of calpain. J. Biol. Chem. 277:9022-9026. [DOI] [PubMed] [Google Scholar]
- 52.Tranqui, L., and M. R. Block. 1995. Intracellular processing of talin occurs within focal adhesions. Exp. Cell Res. 217:149-156. [DOI] [PubMed] [Google Scholar]
- 53.Upadhya, G. A., S. A. Topp, R. S. Hotchkiss, J. Anagli, and S. M. Strasberg. 2003. Effect of cold preservation on intracellular calcium concentration and calpain activity in rat sinusoidal endothelial cells. Hepatology 37:313-323. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, W., R. D. Lane, and R. L. Mellgren. 1996. The major calpain isozymes are long-lived proteins. Design of an antisense strategy for calpain depletion in cultured cells. J. Biol. Chem. 271:18825-18830. [DOI] [PubMed] [Google Scholar]