Abstract
Adderall is widely prescribed for attention deficit hyperactivity disorder (ADHD) though long term neurological effects of the main ingredient d-amphetamine are not well understood. The purpose of this study was to examine effects of clinically prescribed doses of d-amphetamine and one abuse dose administered from childhood to adulthood on adult hippocampal neurogenesis and activation of the granule layer of the dentate gyrus. Beginning in early adolescence (age 28 days) to adulthood (age 71), male C57BL/6J mice were administered twice daily i.p. injections of vehicle, 0.25, 0.5 or 2 mg/kg d-amphetamine. Locomotor activity was measured in home cages by video tracking. At age 53–56, mice received BrdU injections to label dividing cells. Immunohistochemical detection of BrdU, NeuN, Doublecortin (Dcx) and Ki67 was used to measure neurogenesis and cell proliferation at age 71. ΔFosB was measured as an indicator of repeated neuronal activation. An additional cohort of mice was treated similarly except euthanized at age 58 to measure activation of granule neurons from d-amphetamine (by detection of c-Fos) and cell proliferation (ki67) at a time when the fate of BrdU cells would have been determined in the first cohort. D-amphetamine dose-dependently increased survival and differentiation of BrdU cells into neurons and increased number of Dcx cells without affecting number of Ki67 cells. Low doses of d-amphetamine decreased c-Fos and ΔFosB in the granule layer. Only the high dose induced substantial locomotor stimulation and sensitization. Results suggest both therapeutic and abuse doses of d-amphetamine increase number of new neurons in the hippocampus when administered from adolescence to adulthood by increasing survival and differentiation of cells into neurons not by increasing progenitor cell proliferation. Mechanisms for amphetamine-induced neurogenesis are unknown but appear activity-independent. Results suggest part of the beneficial effects of therapeutic doses of d-amphetamine for ADHD could be via increased hippocampal neurogenesis.
Keywords: neurogenesis, hippocampus, mouse, drugs, psychostimulant, ADHD
Introduction
Stimulant medications, such as d-amphetamine are widely prescribed for treatment of attention deficit hyperactivity disorder (ADHD) (Greenhill et al., 2002). Most properly diagnosed individuals require treatment for ADHD starting from childhood through adulthood (Angold et al., 2000). However, few studies have examined the long term neurological effects of d-amphetamine or any other approved medications for ADHD over this time period (Peterson et al., 2008). Experimental data for abuse doses of d-amphetamine suggest it can damage dopamine nerve terminals and induce cell death (Ryan et al., 1990, Cunha-Oliveira et al., 2006, Atianjoh et al., 2008). However, little is known about how lower therapeutic doses administered chronically affect the brain. One measurement of interest is adult hippocampal neurogenesis. New neurons are continuously born in the dentate gyrus of mice and humans and both the proliferation and survival of these cells is strongly influenced by environmental factors such as stress, physical activity, nutrition, drugs and medications (Gould et al., 1991, van Praag et al., 1999, Eisch et al., 2000, Lee et al., 2002a, Lee et al., 2002b, Santarelli et al., 2003). Depending on the environmental factors, the addition or subtraction of new neurons can increase or decrease the total numbers of neurons and volume of the dentate gyrus (Rhodes et al., 2003). Although the functional significance of adult neurogenesis is not known, new neurons in the hippocampus are thought to contribute to cognitive performance, learning and memory (van Praag et al., 1999, Clark et al., 2008, Mustroph et al., 2011).
Recent studies suggest that stimulant drugs decrease adult hippocampal neurogenesis under a variety of conditions in rodent models (Yamaguchi et al., 2004, Lagace et al., 2006, Mandyam et al., 2008, Noonan et al., 2010, Venkatesan et al., 2011, Yuan et al., 2011). However, only a few studies have specifically explored the effect of d-amphetamine on adult hippocampal neurogenesis. A recent study found that 2.5 mg/kg d-amphetamine injections administered for 14 days had no effect on the proliferation or survival of new neurons in the dentate gyrus of adult male Sprague-Dawley rats (Barr et al., 2010). However, in the new neuron survival group, BrdU was administered 2 weeks before amphetamine exposure. At 2 weeks of age, new neurons may already be in the process of integrating into hippocampal circuitry and could be less vulnerable to the amphetamine treatment. To the best of our knowledge, no previous study has explored the effect of chronic administration of d-amphetamine from childhood to adulthood on the survival of new neurons born and surviving in the presence of continuous d-amphetamine administration. Therefore, one of the goals of this study was to measure the effect of 2 clinically relevant d-amphetamine doses (0.25, and 0.5 mg/kg) and one abuse dose (2 mg/kg) on adult hippocampal neurogenesis.
Given that d-amphetamine at high doses is neurotoxic, (i.e., induces cell death) (Ryan et al., 1990, Atianjoh et al., 2008), one might predict that chronic d-amphetamine exposure would decrease the survival of new neurons. On the other hand, d-amphetamine is a stimulant, and generally increases the activity of neurons in the brain (Rotllant et al., 2010). If the dentate gyrus experiences increased neuronal activation in response to d-amphetamine administration, then the increased activity would be expected to increase the survival of new neurons in an activity-dependent manner (Deisseroth et al., 2004, Clark et al., 2010, Clark et al., 2011). Although many studies have examined patterns of activation in the brain from d-amphetamine using such techniques as immunohistochemical detection of c-Fos and ΔFosB (Johansson et al., 1994, Badiani et al., 1998, Engber et al., 1998, Trinh et al., 2003, Renthal et al., 2008, Rotllant et al., 2010), typically the dentate gyrus is not examined [but see (Trinh et al., 2003) who found increased c-Fos from 2 mg/kg amphetamine in the granule layer]. Acute induction of c-Fos from stimulant drugs is known to decline upon repeated stimulation, whereas ΔFosB accumulates in brain regions such as the striatum (Nestler et al., 2001, Renthal et al., 2008). Hence, another goal of this study was to determine the extent to which the granule layer of the dentate gyrus displays increased c-Fos and ΔFosB accumulation from the chronic d-amphetamine treatments in order to help interpret the results for the neurogenesis measures. Based on the limited literature, it is difficult to predict the outcome of long term administration of therapeutic doses of d-amphetamine on adult hippocampal neurogenesis. If the low doses of d-amphetamine decrease neurogenesis, then that would constitute evidence for neurotoxicity. Alternatively, if the low doses of d-amphetamine increase neurogenesis, then that would support the activity-dependent survival hypothesis. The high dose (2 mg/kg) was expected to decrease neurogenesis as this has been observed for many other drugs of abuse including methamphetamine, cocaine, morphine, heroin, and alcohol (Eisch et al., 2000, Klintsova et al., 2007, Mandyam et al., 2008, Noonan et al., 2010, Venkatesan et al., 2011, Yuan et al., 2011).
Methods
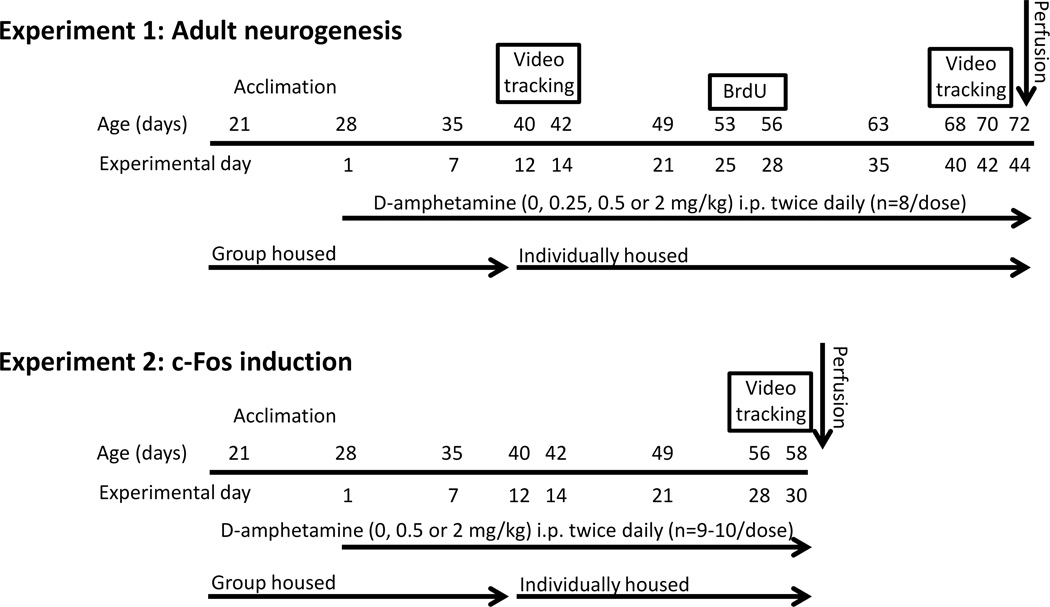
A total of 60 male C57BL/6J mice from The Jackson Laboratory (Bar Harbor, ME) arrived at our facility at 21 days of age and were placed into cages in groups of 4. The mice were left undisturbed for 1 week except for daily handling to acclimate the mice to the experimenter. At age 28 days (equivalent to late childhood, early adolescence), mice were injected intraperitoneally (i.p.) twice daily at the beginning of the dark cycle and again three hours later with either saline, 0.25, 0.5, or 2.0 mg/kg d-amphetamine. D-amphetamine sulfate (Sigma Aldrich, St. Louis, MO) was dissolved in 0.9% saline and was administered in a volume of 10 ml/kg. Doses were prepared according to the salt not the base form. After injections mice were returned to their home cages in groups of 4. At age 40, mice were individually housed as described below for experiments 1 and 2 and the twice daily injections continued (see Fig. 1). In experiment 1, animals were euthanized at 72 days of age, after having received 44 days of twice daily injections. In experiment 2, animals were euthanized at 58 days of age, after having received 30 days of twice daily injections.
Figure 1. Experimental design.
Experiment 1 was conducted to measure the effect of two low (therapeutic) doses and one abuse dose of d-amphetamine administered from childhood to adult on adult hippocampal neurogenesis. Animals were administered twice daily saline, 0.25, 0.5, or 2 mg/kg d-amphetamine (n=8 mice per group) for 44 days starting (experimental day 1) when the mice were 28 days old and continuing until they were 72 days old (experimental day 44). On experimental days 12–14 locomotor activity data were collected by video tracking. On experimental days 25 through 28 (mouse age 53–56) mice were given an additional i.p. injection of BrdU (50 mg/kg) to label dividing cells. On experimental days 40–42 (mice age 68–70 days) locomotor activity data were collected again by video tracking. On day 44 (age 72 days) mice were transcardially perfused with 4% paraformaldehyde. Experiment 2 was conducted to measure the effect of d-amphetamine on c-Fos expression in the dentate gyrus as a marker for neuronal activation at a time-point matched to experiment 1 when the fate of newly divided BrdU labeled cells in experiment 1 would have been decided. Animals were administered twice daily saline (n=9), 0.5 (n=9) or 2 (n=10) mg/kg d-amphetamine for 30 days starting when the mice were 28 days old and continuing until the mice were 58 days old. On experimental days 28–30 (mouse age 56–58 days), locomotor activity data were collected by video tracking. Exactly 90 minutes following their last injection, animals were transcardially perfused with 4% paraformaldehyde.
The two lowest doses (0.25 and 0.5 mg/kg) were chosen to represent the therapeutic range for humans. The high dose (2 mg/kg) was chosen because it is known to elicit robust locomotor stimulation and sensitization in rodent models of d-amphetamine abuse. The two daily d-amphetamine administrations were separated by 3 hours because previous studies on the brain concentrations of d-amphetamine showed levels of d-amphetamine to be almost completely negligible after 3 hours (Riffee et al., 1978). The injections were administered during the dark cycle when mice are normally awake and attentive because previous studies have indicated that some of the detrimental effects of stimulants (caffeine, methamphetamine, and modafinil) on neurogenesis could be partially due to the side effect of insomnia caused by administering stimulants during the light cycle rather than an inherent effect of the drugs (Kochman et al., 2009).
Experiment 1. Chronic effect of d-amphetamine on adult hippocampal neurogenesis
Experiment 1 included the following groups: saline, 0.25, 0.5, and 2 mg/kg d-amphetamine (n=8 mice per group). On experimental days 12–14 (mouse age 40–42 days), mice were transferred into individual custom-made home cages for video tracking (for details and dimensions of the housing see Zombeck et al., 2010a, Zombeck et al., 2010b). Animals lived in these cages continuously for 3 days during which time the drug treatments continued, i.e., twice daily injections of d-amphetamine or saline depending on the group (see Fig. 1). The movement of the animals was continuously recorded with TopScan video tracking software (CleverSys Inc., Reston, VA). At the end of this three day session, mice were individually housed in standard home cages. Mice remained individually housed for the duration of the experiment in order to prevent fighting that can occur when adult males are re-introduced back into the same cage after a period of separation. The drug treatments continued on days 15–25 in standard home cages. On experimental days 25 through 28 (mouse age 53–56), the drug treatments continued except the animals were given an additional i.p. injection of BrdU (50 mg/kg) to label dividing cells 1.5 hours after the first amphetamine (or saline) injections. On Days 29–39, the drug treatments continued. On days 40–42 (mice age 68–70 days) mice were once again moved into the custom cages for video tracking during which time the drug treatments continued. On day 44 (age 72 days) mice were transcardially perfused with 4% paraformaldehyde.
Experiment 2. Acute effect of d-amphetamine on neuronal activation of the granule layer after chronic treatment
Experiment 2 included the following groups: saline (n=9), 0.5 (n=9) and 2 (n=10) mg/kg d-amphetamine. On experimental day 12 (age 40), the mice were individually housed in standard cages. On experimental days 28–30 (mouse age 56–58 days; see Fig. 1), mice were transferred into individual custom-made home cages for video tracking. The movement of the animals was continuously recorded with TopScan video tracking software (CleverSys Inc., Reston, VA). Animals lived in these cages continuously for 3 days during which time the drug treatments continued, i.e., twice daily injections of d-amphetamine or saline depending on the group, except for the last day when the animals only received their morning injection. Exactly 90 minutes following their last injection, animals were transcardially perfused with 4% paraformaldehyde.
Immunohistochemistry
Brains were post-fixed overnight, and then transferred to 30% sucrose in PBS. The brains were then sectioned using a cryostat into 40 micrometer thick coronal sections. Sections were placed into tissue cryoprotectant in 24 well plates and stored at −20 °C. Six separate one-in-six series of these sections (i.e., series of sections throughout the rostro-caudal extent of the dentate gyrus with 240 micrometer increments separating each section, approximately nine sections) were stained in the following ways:
BrdU-DAB (Experiment 1). BrdU-DAB staining was performed to detect BrdU-positive (newly divided) cells in the dentate gyrus. Free floating sections were washed in tissue buffering solution (TBS) and then treated with 0.6% hydrogen peroxide in TBS for 30 min. To denature DNA, sections were treated for 120 min with a solution of 50% de-ionized formamide and 2X SCC buffer, rinsed for 15 min in 2X SCC buffer, then treated with 2 N hydrochloric acid for 30 min at 37 °C, then 0.1 M boric acid in TBS (pH 8.5) for 10 min at room temperature. Sections were then treated (blocked) with a solution of 0.3% Triton-X and 3% goat serum in TBS (TBS-X plus) for 30 min, and then incubated in primary antibody against BrdU made in rat at a dilution of 1:100 in TBS-X plus for 72 h at 4 °C. Sections were then washed in TBS, treated with TBS-X plus for 30 min and then incubated in biotinylated secondary antibody against rat made in goat at 1:250 in TBS-X plus for 100 min at room temperature. Sections were then treated using the ABC system and stained using diaminobenzidine as the chromogen.
Double fluorescent label BrdU and NeuN (Experiment 1). Double fluorescent staining was performed to estimate the proportion of BrdU-positive cells in the granule layer that had differentiated into neurons as indicated by co-expression of NeuN (neuronal nuclear protein, a mature neuronal marker). The BrdU-DAB procedure was repeated except that a cocktail was used for the primary antibody step. Rat anti-BrdU (1:100, catalog number OBT0030; AbD Serotec) was combined with mouse anti-neuronal nuclear protein (NeuN) (1:50, catalog number MAB377; Millipore, Billerica, MA, USA) for 72 h at 4°C. Secondary antibodies were conjugated with fluorescent markers (Cy2-green anti-mouse, and Cy3-red anti-rat, catalog numbers 115-225-166 and 112-165-167; Jackson ImmunoResearch, West Grove, PA, USA, respectively) at a dilution of 1:250, and also delivered as a cocktail.
Ki67-DAB (Experiment 1 and Experiment 2). Ki67-DAB staining was performed to estimate total number of cells in the subgranule layer of the dentate gyrus in the process of dividing at the time when the animals were euthanized (i.e., marker of cell proliferation). The immunohistochemistry proceeded similar to BrdU-DAB except omitting the DNA denaturing steps. The Primary antibody was rabbit anti-Ki67 (1:500; catalog number ab15580; Abcam, Cambridge, MA); secondary antibody was biotinylated rabbit anti goat (1:250; Vector Laboratories, Burlingame, CA).
Doublecortin-DAB (DCX-DAB; Experiment 1). DCX-DAB staining was conducted to estimate total numbers of immature neurons in the granule cell layer of the dentate gyrus at the time when the animals were euthanized. The immunohistochemistry proceeded similar to BrdU-DAB except omitting the DNA denaturing steps. The Primary antibody was goat anti-DCX (1:1000; catalog number sc-8066; Santa Cruz Biotechnology, Santa Cruz, CA); secondary antibody was biotinylated donkey anti goat (1:200; Santa Cruz Biotechnology, Santa Cruz, CA).
ΔFosB-DAB (Experiment 1). ΔFosB-DAB staining was performed to estimate the total number of granule neurons in the dentate gyrus that had experienced repeated neuronal activation sufficient to accumulate ΔFosB in the nucleus. The immunohistochemistry proceeded similar to BrdU-DAB except omitting the DNA denaturing steps. The Primary antibody was rabbit anti ΔFosB (1:500; catlog number 9890S; Cell Signaling Technology, Danvers, MA); secondary antibody was biotinylated goat anti rabbit 1:200; Vector Laboratories, Burlingame, CA).
c-Fos-DAB (Experiment 2). c-Fos-DAB staining was performed to estimate the total number of neurons that had experienced acute activation sufficient to accumulate c-Fos within approximately 90 min prior to euthanasia. The total number of c-Fos positive neurons in the dentate gyrus and number of c-Fos positive neurons in a sampled region of the paraventricular thalamic nucleus were estimated. The paraventricular thalamic nucleus was included as a positive control, i.e., based on previous literature it was expected to display increased c-Fos from acute amphetamine. The immunohistochemistry proceeded similar to BrdU-DAB except omitting the DNA denaturing steps. The primary antibody was rabbit anti-c-Fos (1:20000; catalog number PC38; EMD Millipore, Billerica, MA); secondary antibody was biotinylated goat anti-rabbit (1:200; Vector Laboratories, Burlingame, CA).
Image analysis
BrdU-DAB, Ki67-DAB, DCX-DAB, ΔFosB-DAB, c-Fos-DAB (dentate gyrus)
The entire granule layer of the dentate gyrus (bilateral), represented in the one-in-six series, was photographed by systematically advancing the field of view of the Zeiss brightfield light microscope, and taking multiple photographs, via video camera interfaced to computer, under 10X or 20X (total 100X or 200X) magnification. The number of DAB-positive cells was then hand-counted for each image. Cells in the top plane of the sections were discounted. Total number of cells was multiplied by 6 to account for the space between the one-in-six series of sections.
Volume of the dentate gyrus
For all the DAB stained sections analyzed above for cell counts in the dentate gyrus, we also estimated the area of the granule layer in those sections by carefully outlining the structure using ImageJ software at 100 X total magnification. The software determined the total number of pixels within the outlined area. The total number of pixels was then converted to square microns using the internal calibration within Axiovision (Zeiss software), and checked using a micrometer. This area was then multiplied by 40 microns between sections to estimate volume in cubic microns.
c-Fos-DAB (paraventricular thalamic nucleus)
The paraventricular thalamic niucleus was analyzed to serve as a positive control for induction of c-Fos from acute d-amphetamine. This brain region occurs in the same coronal sections as the dentate gyrus and displays robust c-Fos induction from stimulant drugs and drug-paired stimuli (Rhodes et al., 2005, Pasumarthi and Fadel, 2008). Three coronal sections starting at stereotaxic coordinates −1.3 mm relative to bregma with 240 microns separating posterior sections were analyzed for numbers of c-Fos positive cells within the paraventricular thalamic nucleus. Within these three sections, the paraventricular thalamic nucleus was photographed under 20X (total 200X) magnification. The number of DAB-positive cells was automatically counted within a hand-drawn border around the nucleus using ImageJ software.
Double fluorescent BrdU/NeuN
A Leica SP2 laser scanning confocal microscope (40X oil objective; pinhole size, 81.35 lm in diameter) was used to determine the proportion of dentate gyrus BrdU-positive cells that differentiated into neurons (NeuN). Dentate gyrus BrdU-positive cells in the granule layer were identified as either co-expressing NeuN or not. Each BrdU-positive cell (represented in the one-in-six series) was analyzed by focusing through the tissue in the z-axis to establish co-labeling with NeuN. The number of new neurons per mouse was calculated as the total number of BrdU cells multiplied by the average proportion of BrdU cells co-expressing NeuN for the designated group.
Statistical analysis
Data were analyzed with SAS (version 9.2) and R (version 2.13.1) statistical software. In all analyses, P ≤ 0.05 was considered to be statistically significant. Numbers of BrdU, Ki67, DCX, ΔFosB, and c-Fos positive cells, and volume of the dentate gyrus were analyzed by 1-way ANOVA with dose as the factor (saline, 0.25, 0.5, 2 mg/kg). These data were also analyzing using polynomial regression with dose entered as a continuous variable. Cumulative distance traveled 1 hour after injections was analyzed by repeated measures ANOVA with dose as the between-subjects factor, and day as the within-subjects factor. The first day of each 3 day test was omitted due to increased activity from the novelty of recently being moved into the new cage. Hence, for experiment 1 there were 4 days of locomotor data analyzed, days 2 and 3 of the first test, and days 2 and 3 of the second test. Posthoc comparisons of means were conducted using Tukey tests. The proportion of BrdU cells co-labeled with NeuN was analyzed using logistic regression with dose as the factor (saline, 0.25, 0.5, 2 mg/kg), and with dose entered as a continuous variable. For these analyses, the deviance is reported in place of the F statistic.
Results
Experiment 1
Locomotor activity
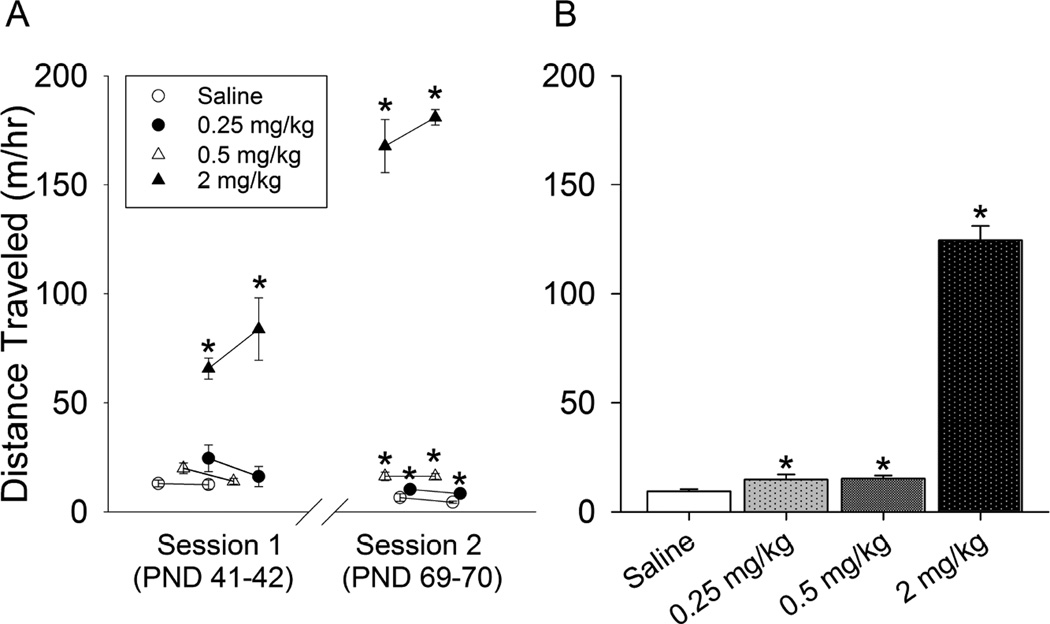
Distance traveled 1 hour after injections was not significantly different during the first and second tests (separated by three hour intervals) within each day. Therefore, the two tests were averaged to produce a single measurement of distance traveled post injection per individual per day. The repeated measures ANOVA revealed a significant effect of dose (F3,84=241.3, P<0.0001), day (F3,84=25.5, P<0.0001) and the interaction of dose and day (F9,84=42.7, P<0.0001; Fig. 2A). On average across test days, distance traveled after the 2 mg/kg dose was 8-fold greater than the two lower doses, and 13-fold greater than the saline group (all doses significantly different from saline; Fig. 2B). However, the two low doses only showed significant dose-dependent differences from saline during the second locomotor testing session at the end of the experiment (all pairwise differences, P<0.05; Fig. 2A). During the first session, levels of locomotor activity were similar for saline and the two low doses of d-amphetamine. Distance traveled after saline and 0.25 mg/kg tended to decrease across days (all pairwise differences between the first and second sessions, P<0.05), whereas at the 0.5 mg/kg dose, distance remained relatively similar on all 4 test days (Fig. 2A). Locomotor activity increased on successive days for the 2 mg/kg dose of d-amphetamine indicating locomotor sensitization had occurred (all pairwise differences between the first and second sessions, P<0.05; Fig. 2A).
Figure 2. Locomotor activity.
A) Average distance traveled (m) the first hour after injection of saline (open circles), 0.25 mg/kg (closed circles), 0.5 mg/kg (open triangles), and 2 mg/kg (closed triangles) d-amphetamine (n=8 per group). Data are shown for PND 41 and 42 (session 1) and PND 69 and 70 (session 2). Data were collected using continuous video tracking in home cages after animals had acclimated to their cages. During the first session, distance was significantly elevated in the 2 mg/kg group relative to the other groups. In the second session, all three doses were elevated as compared to saline. Only the 2 mg/kg dose displayed significant sensitization, i.e., elevated stimulation in session 2 relative to session 1. B) Average distance traveled (m) across all 4 days of data shown in panel A. Standard error bars are shown. *indicates significantly different from saline.
Adult hippocampal neurogenesis
D-amphetamine dose-dependently increased the proportion of BrdU-positive cells that differentiated into neurons (Deviance=9.9, p=0.02). In the saline group, 74% (± 5.3 SE) of BrdU labeled cells co-expressed NeuN, whereas 88% (± 4.3), 91% (± 3.7) and 91% (± 3.8) co-labeled with NeuN in the 0.25, 0.5 and 2 mg/kg groups, respectively. When dose was entered as a continuous variable and analyzed by logistic regression, the proportion of BrdU labeled cells co-expressing NeuN displayed a significant positive slope in relation to dose (Deviance=4.5, P=0.03). The polynomial term was negative and also statistically significant indicating an outward curve in the dose response, i.e., curve toward a plateau or peak within the dose range tested (Deviance=5.2, P=0.02).
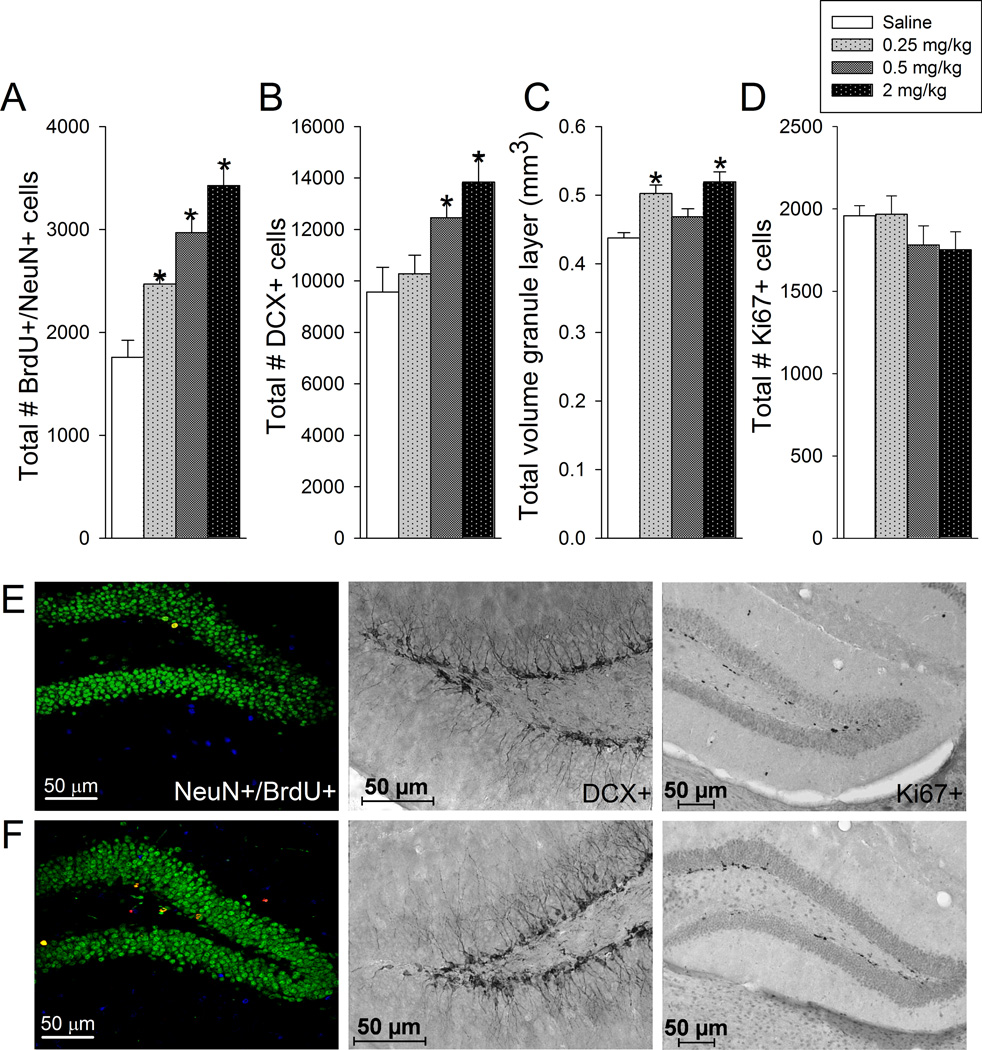
D-amphetamine dose-dependently increased the total number of BrdU-positive cells (F3,28=9.3, P=0.0002; means ± standard errors were 2,377 ± 222, 2806 ± 85, 3264 ± 200, 3766 ± 239 for saline, 0.25,0.5, and 2 mg/kg, respectively), number of new neurons (F3,28=18.1, P<0.0001; Fig. 3A), and DCX-positive cells (F3,28=5.1, P=0.006; Fig. 3B) in the granule layer. D-amphetamine also increased volume of the granule layer, at least for the 0.25 and 2 mg/kg groups (F3,27=9.6, P=0.0002; Fig. 3C). D-amphetamine treatment did not significantly affect number of Ki67-positive cells (Fig. 3D). Posthoc analysis revealed the following results. For BrdU, saline was different from 0.5 and 2 mg/kg, and 0.25 was different from 2 mg/kg at P<0.05 level. For number of new neurons, all pairwise differences were significant except 0.25 versus 0.5 mg/kg and 0.5 versus 2.0 mg/kg. For volume, saline was different from 0.25 and 2 mg/kg, and 2 mg/kg was different from 0.5 mg/kg. For DCX, saline and 0.25 mg/kg were different from 2 mg/kg. When dose was entered as a continuous variable and analyzed by polynomial regression, all the outcome variables, BrdU cells, DCX cells, number of new neurons and volume displayed a significant positive slope in relation to dose (all p<0.05). For number of new neurons, the polynomial (second order) term was also statistically significant and indicated an outward curve in the dose-response relationship toward a plateau or peak.
Figure 3. Adult hippocampal neurogenesis.
A) Total number of BrdU positive cells in the granule layer of the dentate gyrus co-labeled with NeuN mature neuronal marker. B) Total number of cells in the granule layer of the dentate gyrus expressing doublecortin (DCX) marker of immature neurons. C) Total volume of the granule layer of the dentate gyrus. D) Total number of cells in the subgranule layer of the dentate gyrus expressing Ki67 mitotic marker. Averages and standard error bars are shown for each treatment group: saline, 0.25, 0.5, and 2 mg/kg d-amphetamine (n=8 per group). *indicates significantly different from saline. E) photographs of representative sections through the dentate gyrus from a saline treated animal immunohistochemically stained for BrdU and NeuN (left), DCX (middle), and Ki67 (right). F) photographs of representative sections through the dentate gyrus from an animal treated with 2 mg/kg d-amphetamine stained as described in E.
Number of BrdU-positive cells, new neurons, DCX-positive cells, and Ki67-positive cells were significantly correlated with the volume of the granule layer (R2=0.36, 0.30, 0.16, 0.18, respectively; all P<0.05). Number of new neurons and number of BrdU cells were significantly correlated with number of DCX positive cells (R2=0.34, 0.31, respectively; all P<0.05). Number of Ki67 cells was not significantly correlated with new neurons, BrdU cells or DCX cells.
ΔFosB marker of repeated neuronal activation
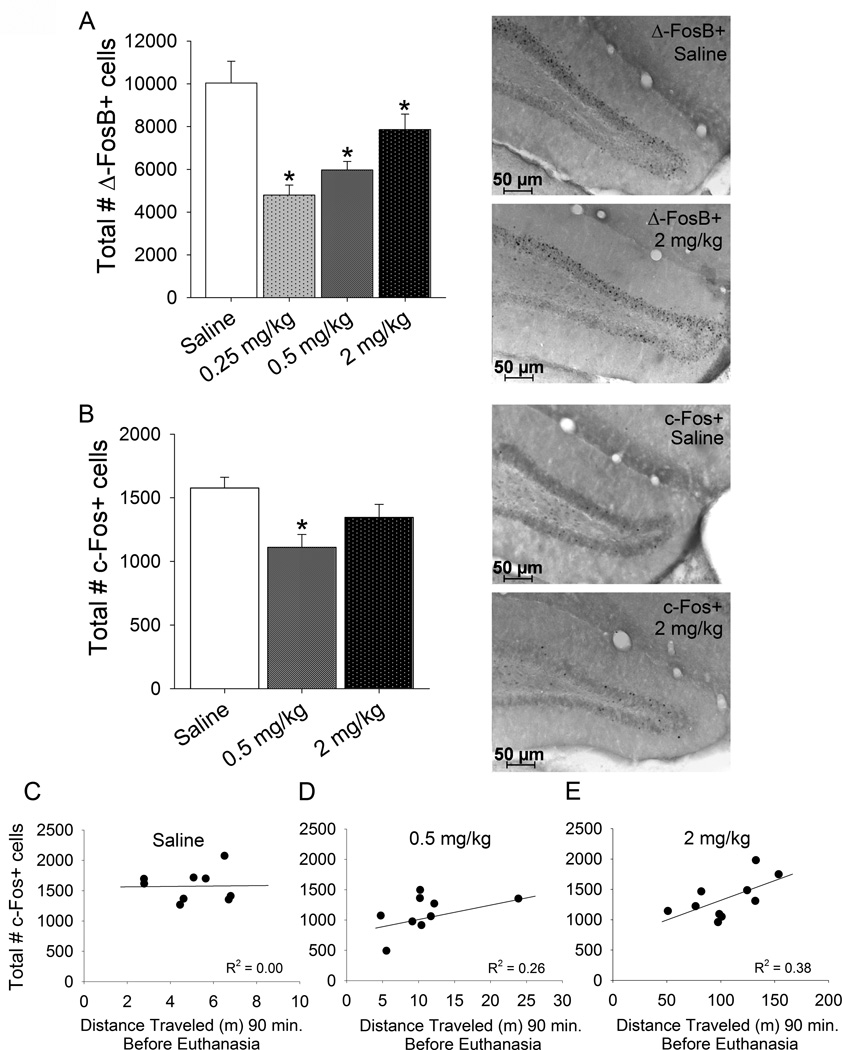
D-amphetamine decreased ΔFosB-positive cells (F3,27=12.3, P<0.0001), with greater decreases caused by lower doses of d-amphetamine (Fig. 4A). All posthoc pairwise differences were significant (P<0.05) except 0.25 versus 0.5 mg/kg and 0.5 versus 2.0 mg/kg. When dose was entered as a continuous variable, no linear relationship between dose and ΔFosB-positive cells was detected. However, the polynomial regression indicated a significant negative first order term (F1,28=14.0, P<0.0001) and positive second order term (F1,28=15.0, P<0.0001) indicating a significant U-shape curve in the dose response. Number of ΔFosB-positive cells was not significantly correlated with number of new neurons, DCX-positive cells, Ki67-positive cells or volume of the granule layer.
Figure 4. Neuronal activation markers.
A) Total number of ΔFosB positive cells in the granule cell layer of the dentate gyrus in animals from experiment 1. Averages and standard errors shown for each treatment group: saline, 0.25, 0.5, and 2 mg/kg d-amphetamine (n=8 per group). *indicates significantly different from saline. Photographs to the right of the graph show representative sections through the dentate gyrus immunohistochemically stained for ΔFosB from a saline-treated animal (top) and an animal treated with 2 mg/kg d-amphetamine (bottom). B) Total number of c-Fos positive cells in the granule layer of the dentate gyrus in animals from experiment 2. Averages and standard errors shown for each treatment group: saline (n=9), 0.5 mg/kg (n=9), and 2 mg/kg (n=10) d-amphetamine. *indicates significantly different from saline. Photographs to the right show representative sections through the dentate gyrus immunohistochemically stained for c-Fos from a saline treated animal (top) and an animal treated with 2 mg/kg d-amphetamine (bottom) C) Total number of c-Fos positive cells plotted against the distance traveled (m) 90 min preceding euthanasia for the saline treatment group. D) same as C except for the 0.5 mg/kg d-amphetamine group. E) same as C except for the 2 mg/kg d-amphetamine group. Simple linear regression lines and R2 values are shown in each plot.
Experiment 2
c-Fos marker of acute neuronal activation in the granule cell layer of the dentate gyrus
The medium dose of d-amphetamine (0.5 mg/kg) decreased total number of c-Fos positive cells in the granule cell layer of the dentate gyrus (F2,25=5.6, P=0.01; Fig. 4B). Posthoc comparisons indicated that the 0.5 mg/kg group displayed significantly lower c-Fos than saline. No other pairwise differences were significant. When dose was entered as a continuous variable, no linear relationship between dose and c-Fos-positive cells was detected. However, the polynomial regression indicated a non-significant negative first order term and a significant positive second order term (F1,25=10.5, P=0.003) indicating a significant U-shape curve in the dose response. Within the saline group, number of c-Fos positive cells was not significantly correlated with cumulative distance traveled 90 min after injection (Fig. 4C). However, in the 0.5 mg/kg group a trend for a positive correlation was detected (R2=0.26, P=0.16; Fig. 4D) and in the 2 mg/kg group, a significant positive correlation was detected (R2=0.38, P=0.05; Fig. 4E). Collapsed across groups, no significant correlation was observed.
c-Fos marker of acute neuronal activation in the paraventricular thalamic nucleus
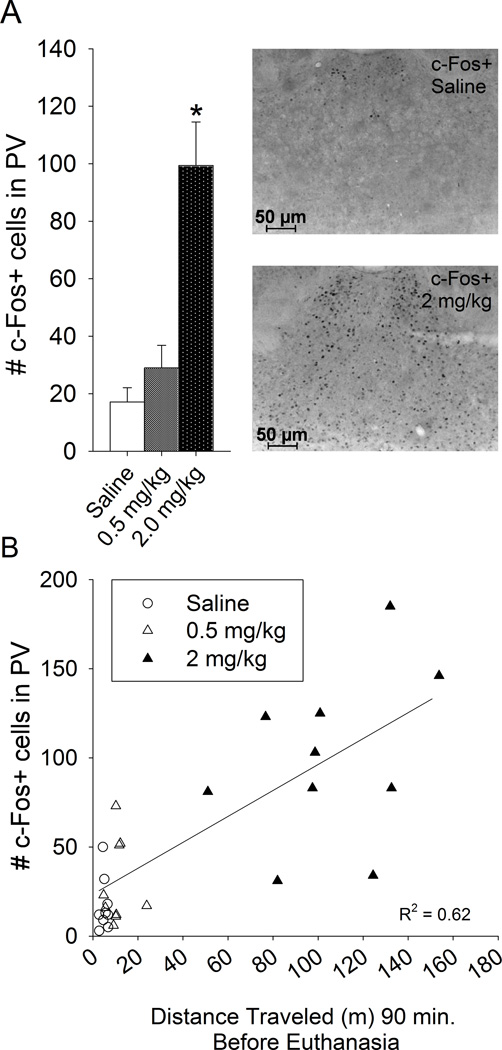
Upon inspection of the c-Fos-DAB stained tissue, many brain regions displayed increased c-Fos from the 2 mg/kg dose of d-amphetamine relative to saline including most of the cortex, striatum and thalamus, similar to previous reports (Johansson et al., 1994, Badiani et al., 1998, Engber et al., 1998, Trinh et al., 2003, Rotllant et al., 2010). As a representative positive control for c-Fos induction from d-amphetamine in the brain, the paraventricular thalamic nucleus was quantified (Pasumarthi and Fadel, 2008, Rotllant et al., 2010). The high dose (2 mg/kg) d-amphetamine increased number of c-Fos positive cells in the paraventricular thalamic nucleus (F2,25=17.8, P<0.0001; Fig. 5A). Posthoc comparisons indicated that the 2 mg/kg group displayed significantly higher c-Fos than saline or 0.5 mg/kg. Saline and 0.5 mg/kg were not significantly different from each other. Number of c-Fos positive cells was significantly correlated with cumulative distance traveled 90 min after injection across groups (R2=0.62, P<0.0001; Fig. 5B), but not within groups.
Figure 5. Induction of c-Fos from d-amphetamine in the paraventricular thalamic nucleus as a representative positive control.
A) Total number of c-Fos positive cells in the sampled region of the paraventricular thalamic nucleus in animals from experiment 2. Averages and standard errors shown for each treatment group: saline (n=9), 0.5 mg/kg (n=9), and 2 mg/kg (n=10) d-amphetamine. *indicates significantly different from saline. Photographs to the right show representative sections through the paraventricular thalamic nucleus immunohistochemically stained for c-Fos from a saline treated animal (top) and an animal treated with 2 mg/kg d-amphetamine (bottom) B) Total number of c-Fos positive cells in the sampled region of the paraventricular thalamic nucleus plotted against the distance traveled (m) 90 min preceding euthanasia. Simple linear regression line and R2 value is shown.
Cell proliferation in the subgranule layer of the dentate gyrus
Numbers of Ki67 positive cells were counted in the subgranule layer of the dentate gyrus using the same method as described in Experiment 1. Consistent with results in Experiment 1, number of Ki67 cells did not differ among the three experimental groups (data not shown).
Discussion
The main discovery of the study is that chronic d-amphetamine treatment starting in childhood to adulthood increases adult hippocampal neurogenesis in male C57BL/6J mice (Fig. 3). The dose-dependent increase in neurogenesis was supported by 3 different independent measures of neurogenesis including counts of total numbers of BrdU-labeled cells in the granule layer, the proportion of BrdU cells differentiated as neurons as indicated by co-labeling with NeuN (mature neuron marker), and counts of doublecortin (DCX) positive cells, a marker of immature neurons. Moreover, increased neurogenesis was correlated with increased volume of the granule layer suggesting that the new neurons were not simply replacing dead cells, but instead were adding neurons to the granule layer. The increased BrdU and DCX cell numbers together with no change in Ki67 cell numbers suggests d-amphetamine increased numbers of new neurons by increasing the survival and differentiation of new cells into neurons, not by increasing proliferation. This is similar to other environmental factors that increase neurogenesis such as wheel running (Clark et al., 2010).
D-amphetamine-induced neurogenesis is a novel discovery with mixed support from the literature. No previous study that we are aware of except for Barr et al. (2010) has examined effects of repeated administration of therapeutic doses of d-amphetamine on adult hippocampal neurogenesis. In Barr et al. (2010), 2.5 mg/kg d-amphetamine administered for 14 days had no effect on the proliferation or survival of new neurons in the granule layer. However, in the new neuron survival group, animals were injected with BrdU 2 weeks before the amphetamine treatment began. It is possible that 2-week old neurons were already integrated into the circuit and could no longer be influenced by d-amphetamine. In our study the BrdU was administered starting on day 25 of twice daily amphetamine treatments and animals were euthanized on day 44 (see Fig. 1) to measure the survival of new neurons born and surviving in the presence of amphetamine. Barr et al. (2010) also examined a group euthanized 4 weeks after the amphetamine treatment ceased which displayed decreased neurogenesis, but this group has marginal relevance for our study because it was measuring neurogenesis during amphetamine withdrawal (Barr et al., 2010).
A few related studies have been conducted with methylphenidate. Schaefers et al. (2009) found that 5 mg/kg methylphenidate administered from post natal day (PND) 30–60 in gerbils had no effect on numbers of BrdU positive cells in the granule layer on PND 90, 7 days after a single BrdU injection. However, Lagace et al. (2006) found that 2 mg/kg methylphenidate administered from PND 20–35 decreased BrdU positive cells in the granule layer on PND 112, 27 days after single BrdU injection. One major difference between our study and the studies of Schaefers et al. (2009) and Lagace et al. (2006), is that in our study, neurogenesis was measured during a continuous amphetamine treatment from childhood to adult, whereas in Schaefers et al. (2009) and Lagace et al. (2006), neurogenesis was measured long after the amphetamine treatment had ended, 30 days in Schaefers et al. (2009) and 77 days in Lagace et al. (2006).
Based on the available literature on abuse doses of other stimulants such as methamphetamine (Teuchert-Noodt et al., 2000, Mandyam et al., 2008, Venkatesan et al., 2011, Yuan et al., 2011) and cocaine (Yamaguchi et al., 2004, Noonan et al., 2010), we expected d-amphetamine to reduce adult hippocampal neurogenesis, particularly when given at the 2 mg/kg dose. On the other hand, some authors have found no effect (Mustroph et al., 2011) or increased neurogenesis from repeated cocaine treatment (Lloyd et al., 2010). Moreover, self-administration of methamphetamine for one hour, twice weekly for approximately 50 days increased numbers of immature neurons as indicated by DCX in rats (Mandyam et al., 2008). Hence, our finding that d-amphetamine increases adult hippocampal neurogenesis is not without precedent. However, the functional significance of amphetamine-induced neurogenesis and the mechanisms by which d-amphetamine increased neurogenesis in the hippocampus remain elusive. The hypothesis that amphetamine-induced neurogenesis results from increased neuronal activation of the granule layer was not supported by the data because both c-Fos and ΔFosB data indicated decreased activation of granule neurons from amphetamine at low doses and no effect at the 2 mg/kg dose (Fig. 4). Taken together, the results support the novel hypothesis that d-amphetamine increases adult hippocampal neurogenesis via an activity-independent process.
The activity-independent hypothesis is only supported to the extent that c-Fos and ΔFosB were good indicators of neuronal activation in the granule layer. It is possible that d-amphetamine increased neuronal activation of the granule layer without inducing c-Fos and ΔFosB (Dragunow and Faull, 1989). However, this possibility is unlikely. Previous studies from our laboratory have shown strong induction of c-Fos in the granule layer from wheel running, even after 50 days of running, demonstrating c-Fos is a good indicator of neuronal activation of the granule layer (Clark et al., 2010). Moreover, in previous studies, we have observed strong correlations between numbers of c-Fos-positive cells in the granule layer and adult hippocampal neurogenesis induced from wheel running demonstrating that c-Fos in the granule layer can predict neurogenesis (Clark et al., 2010, Clark et al., 2011). In the current study, we confirmed that d-amphetamine indeed increased c-Fos in the paraventricular thalamic nucleus, as a positive control (Fig. 5). However, in the dentate gyrus, results were more complicated. The relationship between dose of d-amphetamine and c-Fos was U-shape, with 0.5 mg/kg reducing c-Fos and 2 mg/kg having no effect relative to saline (Fig. 4B). Within the 2 mg/kg group, a positive correlation between locomotor activity and c-Fos was observed (Fig. 4E) indicating that locomotor stimulation was associated with increased c-Fos in the granule layer. Average levels of c-Fos for 2 mg/kg were similar to saline even though 2 mg/kg displayed substantial locomotor stimulation (Fig. 2). These results suggest 2 mg/kg d-amphetamine reduced c-Fos, and that levels were recovered to baseline because of the increased locomotor stimulation. Taken together, c-Fos appeared to reflect neuronal activation accurately in the granule layer, was reduced from d-amphetamine and uncoupled with neurogenesis.
We reasoned that if neuronal activation of the granule layer was decreased or not changed as a direct acute response to d-amphetamine, then perhaps the granule layer was excited during withdrawal or other drug-free periods to account for the increased neurogenesis (Recinto et al., 2012). To that end, we also examined ΔFosB which accumulates upon repeated neuronal stimulation (Nestler et al., 2001, Renthal et al., 2008). If the granule layer were repeatedly activated at some other time besides during the acute response to d-amphetamine, then ΔFosB should reflect that repeated activation. However, the ΔFosB data showed the same U-shape dose response as c-Fos with low doses decreasing ΔFosB, providing another piece of evidence that amphetamine-induced neurogenesis is activity-independent.
The molecular mechanisms supporting activity independent neurogenesis from d-amphetamine observed in our study remain unclear. A previous study in rats found that d-amphetamine increased levels of brain-derived neurotrophic factor (BDNF) in the hippocampus (Griesbach et al., 2008), and BDNF is required for both baseline levels of adult hippocampal neurogenesis and increased neurogenesis from environmental enrichment (Rossi et al., 2006) and dietary restriction (Lee et al., 2002a) in mice. Hence, it is possible that BDNF contributed to the increased neurogenesis induced from d-amphetamine observed in our study. However, we did not measure BDNF, so the role of BDNF or any other molecules known to influence neurogenesis remain viable candidate mechanisms.
The implication of amphetamine-induced neurogenesis for behavior is not known and will be the topic of future investigation. Although adult neurogenesis is widely believed to have functional significance for cognitive performance and learning and memory (van Praag et al., 1999, Shors et al., 2001, Kempermann et al., 2004, Sahay et al., 2011), we did not test cognitive performance in the animals in this study so the cognitive outcome cannot be determined. It is important to note that increased neurogenesis is not sufficient for enhanced learning and memory performance in rodents (Rhodes et al., 2003). Moreover, the literature is mixed about the functional significance of new neurons in learning and memory (Shors et al., 2001, Meshi et al., 2006, Clark et al., 2008, Jaholkowski et al., 2009). D-amphetamine at low or moderate doses tends to enhance learning and memory via its stimulant and attention-enhancing properties (e.g., Brown et al., 2000). Therefore, it may be tempting to conclude that amphetamine-induced neurogenesis could contribute to some of these pro-cognitive effects of amphetamine. However, most of the cognitive benefits of amphetamine are thought to be due to the stimulant properties of the drug and are acute effects, i.e., they occur on first exposure to amphetamine, whereas it takes weeks to months for new neurons to integrate into the circuit in a way that could affect learning. Therefore, it seems unlikely that amphetamine-induced neurogenesis contributes to any of the acute cognitive benefits observed from low doses of amphetamine in the literature (e.g., Kulkarni and Job, 1967, Martinez et al., 1980, Brown et al., 2000, Fenu and Di Chiara, 2003, Breitenstein et al., 2004).
The data in our study suggest that the d-amphetamine increased neurogenesis by increasing survival and differentiation of new cells into neurons rather than by increasing proliferation of new cells. This is because of the group differences in the proportion of BrdU cells co-labeled with NeuN, numbers of BrdU positive cells and Dcx positive cells without any change in Ki67 cells. However, it is still possible that cell proliferation contributed to the increased BrdU and Dcx cells if d-amphetamine shortened the length of the cell cycle (Brandt et al., 2010, Yoshinaga et al., 2010). In that case, Ki67 would be expressed for a shorter time for each cell undergoing division and total Ki67 positive cells could remain unchanged if the total number of cell divisions increased. Future analysis is needed to confirm that cell proliferation rates in the adult mouse hippocampus are not affected by d-amphetamine.
In conclusion, results demonstrate that d-amphetamine increases adult hippocampal neurogenesis in mice when administered at low doses from childhood to adulthood. The increased neurogenesis appears to result from increased survival and differentiation of new cells into neurons rather than increased proliferation of progenitor cells. The c-Fos and ΔFosB data suggest that d-amphetamine-induced neurogenesis was unrelated to neuronal activation of the granule layer. Future studies are needed to determine the functional significance of amphetamine-induced neurogenesis.
Highlights.
D-amphetamine dose dependently increases adult hippocampal neurogenesis.
D-amphetamine enhances survival and differentiation of new hippocampal neurons.
D-amphetamine-induced neurogenesis appears to be activity independent.
Acknowledgments
This work was supported by NIH grants MH 083807 and DA027487.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angold A, Erkanli A, Egger HL, Costello EJ. Stimulant treatment for children: a community perspective. J Am Acad Child Adolesc Psychiatry. 2000;39:975–984. doi: 10.1097/00004583-200008000-00009. discussion 984–994. [DOI] [PubMed] [Google Scholar]
- Atianjoh FE, Ladenheim B, Krasnova IN, Cadet JL. Amphetamine causes dopamine depletion and cell death in the mouse olfactory bulb. Eur J Pharmacol. 2008;589:94–97. doi: 10.1016/j.ejphar.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine-induced behavior, dopamine release, and c-fos mRNA expression: modulation by environmental novelty. J Neurosci. 1998;18:10579–10593. doi: 10.1523/JNEUROSCI.18-24-10579.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr JL, Renner KJ, Forster GL. Withdrawal from chronic amphetamine produces persistent anxiety-like behavior but temporally-limited reductions in monoamines and neurogenesis in the adult rat dentate gyrus. Neuropharmacology. 2010;59:395–405. doi: 10.1016/j.neuropharm.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt MD, Maass A, Kempermann G, Storch A. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. Eur J Neurosci. 2010;32:1256–1264. doi: 10.1111/j.1460-9568.2010.07410.x. [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Wailke S, Bushuven S, Kamping S, Zwitserlood P, Ringelstein EB, Knecht S. D-amphetamine boosts language learning independent of its cardiovascular and motor arousing effects. Neuropsychopharmacology. 2004;29:1704–1714. doi: 10.1038/sj.npp.1300464. [DOI] [PubMed] [Google Scholar]
- Brown RW, Bardo MT, Mace DD, Phillips SB, Kraemer PJ. D-amphetamine facilitation of morris water task performance is blocked by eticlopride and correlated with increased dopamine synthesis in the prefrontal cortex. Behav Brain Res. 2000;114:135–143. doi: 10.1016/s0166-4328(00)00225-4. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Rhodes JS. Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience. 2011;184:16–27. doi: 10.1016/j.neuroscience.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Haferkamp EH, Rhodes JS. Adult hippocampal neurogenesis and c-Fos induction during escalation of voluntary wheel running in C57BL/6J mice. Behav Brain Res. 2010;213:246–252. doi: 10.1016/j.bbr.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Rego AC, Cardoso SM, Borges F, Swerdlow RH, Macedo T, de Oliveira CR. Mitochondrial dysfunction and caspase activation in rat cortical neurons treated with cocaine or amphetamine. Brain Res. 2006;1089:44–54. doi: 10.1016/j.brainres.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engber TM, Koury EJ, Dennis SA, Miller MS, Contreras PC, Bhat RV. Differential patterns of regional c-Fos induction in the rat brain by amphetamine and the novel wakefulness-promoting agent modafinil. Neurosci Lett. 1998;241:95–98. doi: 10.1016/s0304-3940(97)00962-2. [DOI] [PubMed] [Google Scholar]
- Fenu S, Di Chiara G. Facilitation of conditioned taste aversion learning by systemic amphetamine: role of nucleus accumbens shell dopamine D1 receptors. Eur J Neurosci. 2003;18:2025–2030. doi: 10.1046/j.1460-9568.2003.02899.x. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313:479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Benson RS, Bukstein O, Kinlan J, McClellan J, Rue D, Shaw JA, Stock S. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41:26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F, Sutton RL. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154:530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, Blecharz-Klin K, Figiel I, Lioudyno V, Widy-Tyszkiewicz E, Wilczynski GM, Lipp HP, Kaczmarek L, Filipkowski RK. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Mem. 2009;16:439–451. doi: 10.1101/lm.1459709. [DOI] [PubMed] [Google Scholar]
- Johansson B, Lindstrom K, Fredholm BB. Differences in the regional and cellular localization of c-fos messenger RNA induced by amphetamine, cocaine and caffeine in the rat. Neuroscience. 1994;59:837–849. doi: 10.1016/0306-4522(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31:2073–2082. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Kochman LJ, Fornal CA, Jacobs BL. Suppression of hippocampal cell proliferation by short-term stimulant drug administration in adult rats. Eur J Neurosci. 2009;29:2157–2165. doi: 10.1111/j.1460-9568.2009.06759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AS, Job WM. Facilitation of avoidance learning by d-amphetamine. Life Sci. 1967;6:1579–1587. doi: 10.1016/0024-3205(67)90167-1. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Yee JK, Bolanos CA, Eisch AJ. Juvenile administration of methylphenidate attenuates adult hippocampal neurogenesis. Biol Psychiatry. 2006;60:1121–1130. doi: 10.1016/j.biopsych.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002a;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002b;80:539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- Lloyd SA, Balest ZR, Corotto FS, Smeyne RJ. Cocaine selectively increases proliferation in the adult murine hippocampus. Neurosci Lett. 2010;485:112–116. doi: 10.1016/j.neulet.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL, Jr, Vasquez BJ, Rigter H, Messing RB, Jensen RA, Liang KC, McGaugh JL. Attenuation of amphetamine-induced enhancement of learning by adrenal demedullation. Brain Res. 1980;195:433–443. doi: 10.1016/0006-8993(80)90077-3. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Mustroph ML, Stobaugh DJ, Miller DS, DeYoung EK, Rhodes JS. Wheel running can accelerate or delay extinction of conditioned place preference for cocaine in male C57BL/6J mice, depending on timing of wheel access. Eur J Neurosci. 2011;34:1161–1169. doi: 10.1111/j.1460-9568.2011.07828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi RK, Fadel J. Activation of orexin/hypocretin projections to basal forebrain and paraventricular thalamus by acute nicotine. Brain Res Bull. 2008;77:367–373. doi: 10.1016/j.brainresbull.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl) 2008;197:1–11. doi: 10.1007/s00213-007-0996-4. [DOI] [PubMed] [Google Scholar]
- Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology. 2012;37:1275–1287. doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, 3rd, Truong HT, Alibhai I, Kumar A, Montgomery RL, Olson EN, Nestler EJ. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Ryabinin AE, Crabbe JC. Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci. 2005;119:759–771. doi: 10.1037/0735-7044.119.3.759. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr, Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Riffee WH, Ludden TM, Wilcox RE, Gerald MC. Brain and plasma concentrations of amphetamine isomers in mice. J Pharmacol Exp Ther. 1978;206:586–594. [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Rotllant D, Marquez C, Nadal R, Armario A. The brain pattern of c-fos induction by two doses of amphetamine suggests different brain processing pathways and minor contribution of behavioural traits. Neuroscience. 2010;168:691–705. doi: 10.1016/j.neuroscience.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Linder JC, Martone ME, Groves PM. Histological and ultrastructural evidence that D-amphetamine causes degeneration in neostriatum and frontal cortex of rats. Brain Res. 1990;518:67–77. doi: 10.1016/0006-8993(90)90955-b. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schaefers AT, Teuchert-Noodt G, Bagorda F, Brummelte S. Effect of postnatal methamphetamine trauma and adolescent methylphenidate treatment on adult hippocampal neurogenesis in gerbils. Eur J Pharmacol. 2009;616:86–90. doi: 10.1016/j.ejphar.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Teuchert-Noodt G, Dawirs RR, Hildebrandt K. Adult treatment with methamphetamine transiently decreases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm. 2000;107:133–143. doi: 10.1007/s007020050012. [DOI] [PubMed] [Google Scholar]
- Trinh JV, Nehrenberg DL, Jacobsen JP, Caron MG, Wetsel WC. Differential psychostimulant-induced activation of neural circuits in dopamine transporter knockout and wild type mice. Neuroscience. 2003;118:297–310. doi: 10.1016/s0306-4522(03)00165-9. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A, Uzasci L, Chen Z, Rajbhandari L, Anderson C, Lee MH, Bianchet MA, Cotter R, Song H, Nath A. Impairment of adult hippocampal neural progenitor proliferation by methamphetamine: role for nitrotyrosination. Mol Brain. 2011;4:28. doi: 10.1186/1756-6606-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci. 2004;1025:351–362. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- Yoshinaga Y, Kagawa T, Shimizu T, Inoue T, Takada S, Kuratsu J, Taga T. Wnt3a promotes hippocampal neurogenesis by shortening cell cycle duration of neural progenitor cells. Cell Mol Neurobiol. 2010;30:1049–1058. doi: 10.1007/s10571-010-9536-6. [DOI] [PubMed] [Google Scholar]
- Yuan CJ, Quiocho JM, Kim A, Wee S, Mandyam CD. Extended access methamphetamine decreases immature neurons in the hippocampus which results from loss and altered development of neural progenitors without altered dynamics of the S-phase of the cell cycle. Pharmacol Biochem Behav. 2011;100:98–108. doi: 10.1016/j.pbb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Lewicki AD, Patel K, Gupta T, Rhodes JS. Patterns of neural activity associated with differential acute locomotor stimulation to cocaine and methamphetamine in adolescent versus adult male C57BL/6J mice. Neuroscience. 2010a;165:1087–1099. doi: 10.1016/j.neuroscience.2009.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Swearingen SP, Rhodes JS. Acute locomotor responses to cocaine in adolescents vs. adults from four divergent inbred mouse strains. Genes Brain Behav. 2010b;9:892–898. doi: 10.1111/j.1601-183X.2010.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]