Abstract
Complications of atherosclerosis and thrombosis are leading causes of death worldwide. While experimental investigations have yielded valuable insights into key molecular and cellular phenomena in these diseases of medium- and large-sized vessels, direct visualization of relevant in vivo biological processes has been limited. However, recent developments in molecular imaging technology, specifically fluorescence imaging agents coupled with high-resolution, high-speed intravital microscopy (IVM), are now enabling dynamic and longitudinal investigations into the mechanisms and progression of many vascular diseases. Here we review recent advances in IVM that have provided new in vivo biological insights into atherosclerosis and thrombosis.
Intravital fluorescence microscopy: versatile, high-resolution, multiwavelength
Optical imaging has revolutionized our ability to probe biological processes from macro-to microscopic resolution using absorption, reflection, transmission, and fluorescence-based contrast.1, 2 In particular, intravital microscopy (IVM), which visualizes cellular and subcellular interactions as they occur in live animals, has provided unparalleled insights into the spatial and dynamic molecular aspects of vascular biologic phenomena.
Historically, IVM was first applied to study intravascular leukocyte trafficking in inflammation, and helped define the associated stepwise leukocyte adhesion response to endothelium in postcapillary venules. IVM was first described in the 19th century by Wagner, who applied brightfield microscopy to directly visualize leukocyte trafficking in translucent tissues, such as the webbed foot of a grass frog.3 In this basic technique utilizing visible light, intravascular leukocytes appeared as relatively uniform, colorless diffractive spheres, and only cells sufficiently slowed by adhesive processes could be distinguished from more rapidly flowing cells in the background.4
Fluorescence-based imaging, with its capability for high-resolution and the detection of multiple wavelength-resolved targets, is the major IVM approach in use today. Fluorescence-based IVM imaging systems have evolved substantially over the last several decades, in parallel with the development of modern optical imaging agents, which allow for subcellular resolution imaging in biological systems. Historically, epifluorescence IVM systems were first used to visualize distinct cellular populations and specific molecular targets in relatively superficial vascular beds. However, these studies were limited by relatively poor spatial resolution from out-of-plane fluorescence. For deeper tissue imaging, confocal microscopy, which better excludes out-of-focus light via point illumination and pinhole apertures, has enabled higher resolution at deeper depths than epifluorescence surface-weighted approaches. Confocal IVM can sense events up to 200 μm below the surface, although with reduced resolution at deeper depths due to sensing of out-of-focus emission light and scattering of in-focus emission light. Spinning disk confocal microscopy systems feature even faster imaging frame rates (hundreds per seconds) at the expense of deep tissue imaging, and are well suited for dynamic IVM studies of fluorescently labeled cells.5
More recently, multiphoton microscopy (MPM), a nonlinear optical method using pulsed infrared laser excitation to generate fluorescence (after a fluorophore absorbs two or more lower energy photons), has enabled deeper tissue imaging of cellular interactions, approaching 500 μm depths.6, 7,8 Compared to single photon confocal microscopy, MPM is not affected by out-of-plane fluorescence light, resulting in reduced background signal and thus improved signal-to-noise ratios. In MPM, the use of longer wavelengths above the fluorophore excitation allows for efficient light penetration due to reduced scattering and reduced light absorption by fluorophores residing above the illumination point. MPM-pulsed approaches also demonstrate reduced phototoxicity and photobleaching, thus allowing for longer imaging times.9
From its first uses in immunology, IVM has revolutionized our understanding of the cellular and molecular events critical for leukocyte recruitment, by allowing an in vivo approach. Now armed with a broad spectrum of optical microscopy techniques, IVM has emerged as a powerful approach to perform kinetic and functional biological studies in living subjects.
Molecular Specificity via Targeted and Activatable Probes
A wide array of imaging probes, including exogenous fluorophores and genetically encoded fluorescent reporters, are now available for molecular imaging of vascular diseases (Table).
TABLE.
Useful Intravital Fluorescence Microcopy Imaging Agents for Vascular Disease (Partial List)
| Agent | Excitation/Emission (nm) | Primary Target | Application |
|---|---|---|---|
| High MW TRITC-conjugated dextran | 557/576 | Biologically-inert, high molecular weight polysaccharide remains intravascular | Intravascular luminal contrast enhancement |
| FITC-conjugated dextran | 490/525 | Biologically-inert, high molecular weight polysaccharide remains intravascular | Intravascular luminal contrast enhancement |
| FITC-conjugated albumin | 490/525 | Low molecular weight protein leaks through endothelium with plasma | Plasma extravasation |
| Rhodamine 6G | 526/555 | Dye rapidly permeates cells, then sequestered | Endogenous Leukocyte labeling |
| CMTMR Calcein-AM CFSE |
541/565 495/515 495/519 |
Dye rapidly permeates cells, then sequestered | Ex vivo leukocyte and platelet labeling |
| CNA35-QD525 | 510/525 | Collagen-binding protein | Inflammation |
| Prosense750 | 750/770 | Multi-cathepsin protease activity (B, L, S), and plasmin | Inflammation |
| MMPsense680 | 680/700 | MMP 2,3,9,13, activity | Inflammation |
| Cathepsin K protease activatable agent | 674/694 | Cysteine proteinase cathepsin K | Inflammation |
| Cathepsin S protease activatable agent | 750/780 | Cysteine proteinase cathepsin S | Inflammation |
| Osteosense750 | 750/780 | Hydroxyapatite | Calcification |
| Annexin-Cy5.5, CLIO-Annexin-Cy5.5 | 674/694 | Phosphatidylserine | Apoptosis |
| RGD peptides-Cy5.5 | 674/694 | Integrin αϖβ3 | Angiogenesis |
| 2-deoxyglucose-NIR fluorochrome | 750/780 | Glut-I glucose transporter | Metabolism |
| Fluorescent nanoparticles (CLIO-AF555, CLIO-Cy5.5, CLIO-VT680, CLIO-Cy7, CLIO-VT750) | Multiple | Macrophages | Inflammation |
| VINP-28 | 680/700 | VCAM-1 | Inflammation |
| Thrombin activatable agent | 674/694 | Thrombin activity | Thrombosis |
| FXIIIa-targeted agent (A15, A14) | Multiple | Activated FXIII activity | Thrombosis |
| FTP11-Cy7 | 750/770 | Fibrin | Thrombosis |
| Green fluorescent protein (GFP) | 395/509 | Protein isolated from jellyfish, can be genetically inserted into organisms for reporter expression | Cellular labeling (in genetically modified animals or by injection of labeled cells) |
| Neptune | 600/650 | Far-red fluorescent protein suitable for deep-tissue imaging in live mammals using excitation light in the optical window | Cellular labeling, (e.g. via virus-mediated gene transfer) |
| SYTO dyes | Multiple (485–521/498–556) | Dye permeates cells, binds nucleic acids | Cellular labeling |
| Eosin | 524/545 | Elastin | Vascular integrity |
| Sulforhodamine B | 560/580 | Dye permeates cells, binds protein amino acids | Cellular proliferation |
| cNGR | Multiple | CD13 | Angiogenesis |
| Wheat germ agglutinin (WGA) | Multiple | Plasma membrane glycoconjugates | Sialic acid, glycocalyx |
| CD31 | Multiple | Endothelial cells | Angiogenesis |
| Vascular cell adhesion molecule (VCAM)-1 | Multiple | Activated endothelium | Inflammation |
| Intracellular adhesion molecule (ICAM)-1 | Multiple | Endothelial cells, leukocytes | Inflammation |
| Junctional Adhesion Molecule (JAM)-A | Multiple | Endothelial cells | Inflammation |
Injectable agents
Growth in efficient affinity ligands (peptides, antibody fragments, nanoparticles) and versatile fluorochrome attachment chemistries has led to an expansion of fluorescence reporters for molecular and cellular imaging. Historically, moderately specific fluorescent agents, such as rhodamine 6G and acridine orange, were used to investigate leukocyte kinetics.4 Alternatively, cells that are ex vivo labeled with fluorochromes (e.g. calcein AM, CMTMR, CFSE) and then injected back into the blood stream, have commonly been used for tracking cells via IVM.10
In recent years, more specific injectable fluorescence molecular imaging agents have emerged for detecting key biological processes underlying vascular diseases, including inflammation, angiogenesis, apoptosis, oxidative stress, and calcification (Table). In particular, protease-activatable near-infrared fluorescence (NIRF) reporters comprise an important class of imaging agents, and, have provided new in vivo insights into thrombin, cathepsin, and MMP activities.2 Some NIRF agents, such as indocyanine green, show translational potential for targeted imaging.11
Genetically encoded fluorescence reporter agents
Nearly two decades ago, green fluorescence protein (GFP, ex/em 395nm/504nm) revolutionized the ability to detect gene expression.12 However, due to its relatively lower excitation wavelength, GFP exhibits diminished tissue penetration and increased background autofluorescence, thus limiting its use for in vivo imaging beyond microscopy. Recently, the development of genetically encoded red and far-red proteins (ex/em 600nm/650nm) have furthered in vivo imaging of gene expression.13 The far-red and NIR windows are favorable for deep tissue imaging in vivo due to relatively lower light absorption by hemoglobin, allowing for efficient photon transmission, and diminished tissue autofluorescence, enabling higher target-to-background ratios.2 For a more detailed discussion of available genetic fluorescent reporters, the interested reader is referred to a recent review.14
IVM: A Powerful Tool in Vascular Disease Investigation
IVM studies have historically focused on rodent microvessels (e.g. arterioles, capillaries, venules), particularly within murine mesentery and cremaster muscle preparations. Microvessels are well suited for the study of leukocyte interactions, and brightfield- and fluorescence-based IVM approaches have yielded valuable insights in immunology, tumor biology, and microvascular thrombosis, largely through visualization of exogenously labeled cells.
There is, however, increasing interest in applying IVM to image larger vessels (e.g. carotid and femoral arteries, abdominal aorta, jugular and femoral veins) to model clinically important human vascular disease, namely atherosclerosis, macrovascular thrombosis, vasculitis, and vascular injury. With a growth of fluorescence molecular imaging reporters, there is also opportunity to track multiple cellular and molecular targets using multi-wavelength approaches. Perhaps not surprisingly, IVM of large vessel-based disease poses its own unique set of challenges. These include difficulties of surgical access to larger vessels, greater susceptibility to motion artifact from cardiac, respiratory, and blood flow sources, as well as the need for longer acquisition times due to motion compensation methods and larger field-of-views, as compared to microvascular disease models.
In the next sections, we highlight recent IVM studies featuring high-resolution cellular and molecular imaging of murine atherosclerosis and thrombosis, particularly in large vessel models. We showcase unique vascular biological insights that transcend classic ex vivo evaluations.
I. Atherosclerosis
Atherosclerosis, a chronic disease of the arterial wall with clinical sequelae including acute coronary syndromes, ischemic stroke and critical limb ischemia, remains a leading cause of morbidity and mortality worldwide.15 Current understanding of the pathophysiology of this disease centers on atherosclerotic plaques and their vulnerability to rupture and/or remodeling. Associated features of high-risk plaques include active inflammation within a necrotic lipid core with thin fibrous cap, and denuded endothelium susceptible to platelet aggregation and thrombosis. There is an increasing focus on the molecular mechanisms of lesion biology rather than on the severity of luminal stenosis.16
As such, atherosclerosis may be better understood as a systemic inflammatory process involving leukocyte-endothelial interactions, oxidized lipids, activation of inflammatory mediators (e.g. proteases), platelet activation and thrombosis, and repeated vascular injury and repair. Importantly, atherosclerosis manifests only in medium and large vessels such as the coronary arteries, carotid arteries, and aorta.15 Thus, in order to study atherosclerosis, traditional IVM methods focusing on the microvasculature have required adaptations for optimal visualization of those vascular beds. For instance, higher speed image capture has been utilized to overcome motion artifact.
Leukocyte-endothelial interaction in aortic inflammation
In 2000, Eriksson et al. reported a novel model and approach for direct visualization of leukocyte-endothelium interactions in a large animal vessel in vivo using epi-illumination IVM.17 The mouse aorta was stimulated locally with the inflammatory cytokines IL-1β and TNF-α to induce leukocyte rolling and adhesion. Circulating leukocytes were then endogenously labeled via intravenous injection of rhodamine 6G (ex/em 526/555 nm). Images of the anterior aspect of the abdominal aorta were obtained using an intravital microscope with a water-immersion objective, and epi-illumination fluorescence was performed through a cooled infrared-filtered lamp. Leukocyte rolling on aortic endothelium was shown to be cytokine-induced, modulated by E-selectin and α4 integrin, dependent on P-selectin, and sensitive to wall shear stress. High shear stress decreased rolling leukocyte flux and the rate of firm adhesion, with interesting implications for leukocyte infiltration in atherosclerotic plaques, which preferentially occurs in areas of low shear stress.18, 19
In 2001, this model was employed to visualize recruitment of leukocytes, primarily neutrophils, to aortic atheroma in vivo.20 Following rhodamine 6G injection, epi-illumination IVM was used to detect leukocyte-endothelial rolling in aortic and iliac artery plaques of ApoE−/− and ApoE−/−/LDLR−/− mice that were fed high cholesterol diets. Of note, leukocyte tethering, rolling and firm adhesion were readily observed in atherosclerotic mice, but virtually absent in matched control mice on regular diets. Notably, most rolling interactions were transient, and visualization of advanced atherosclerotic lesions was limited by the increased thickness of the diseased arterial wall. Also, tethering of leukocytes in advanced atherosclerotic lesions was seen primarily at the periphery (mostly downstream) of the plaque rather than diffusely along injured areas of early lesions. Post-mortem morphological data in humans suggest that coronary plaque rupture occurs commonly at the edge, or “shoulder,” of eccentric plaques,21 though the mechanisms for this are not fully understood.22,23,24 To further enable visualization of distinct leukocyte subsets in atherosclerosis, Rotzius et al. used epi-illumination IVM to image leukocyte trafficking into plaques in the abdominal aorta of ApoE−/−/LysozymeEGFP/EGFP mice,25 which have genetically-encoded GFP-fluorescent neutrophils and monocytes. Individual fluorescent neutrophils and/or monocytes were readily visible within the atherosclerotic lesion and rolling transiently along the endothelium at different velocities.
As an adjunct to arterial endothelial-leukocyte interactions, microvessels associated with atherosclerotic lesions may play a role in the delivery of leukocytes to plaque, especially as plaques progress. In 2011, Eriksson utilized fluorescence IVM to study directly microvascular recruitment of rhodamine 6G-labeled leukocytes in advanced atherosclerotic lesions.26 Using histology and transmission electron microscopy, the author showed presence of predominantly adventitial rather than intimal microvessels in advanced plaques in mice, with leukocyte-endothelial interactions occurring within lesion venules. In some studies, specialized antibody-derivatized fluorescent beads were prepared and injected for detection of mouse P-selectin. Microvessel density in aortic plaques of ApoE−/− mice fed a high fat diet correlated with the size of lesions, and larger, thicker plaques contained more microvessels. Leukocyte rolling was largely P-selectin dependent, with residual rolling also mediated by L-selectin and endothelial P-selectin glycoprotein ligand 1. Interestingly, the density of firmly arrested leukocytes was significantly higher in plaque microvenules compared with arterioles or capillaries, as was the distribution of fluorescent myelomonocytic cells (ApoE−/−/lysMEGFP/EGFP). It is well known that venous endothelium provides an efficient recruitment pathway for leukocytes to sites of inflammation,27 and these findings support a role for microvenules as a site of entry for leukocytes into atherosclerotic plaque.
Multiphoton laser-scanning microscopy (MPLSM) techniques are being developed for 3-dimensional imaging of atheroma-inflammatory cell interactions in vascular structures. Using MPLSM, Maffia et al. visualized infiltration of fluorescently labeled, adoptively transferred lymphocytes within the adventitia of isolated carotid arteries of ApoE−/− mice.8 In 2008, Megens et al. utilized two-photon laser scanning microscopy (TPLSM) for subcellular visualization of collagen and inflammatory cells during plaque progression in mounted carotid arteries of ApoE−/− mice.28, 29 In 2010, Megens et al. went further to demonstrate in vivo imaging of large arteries at subcellular resolution.30 Using TPLSM for accelerated image acquisition triggered on cardiac and respiratory cycles to minimize motion artifact, the authors used fluorescently labeled collagen-binding protein (CNA35-QD525) to visualize subendothelial collagen (~1 μm) exposed following mechanical damage to mouse carotid endothelium in vivo. As noted by Millington et al., there are currently few MPLSM systems that provide the tuneable excitation laser, accurate scan head and sensitive detection system required for in vivo imaging,31 and research is ongoing with customized microscope and protocol systems.32
Platelet activation in atheroma
Using stroboscopic epifluorescence illumination IVM on ApoE−/− mouse carotid arteries, Huo et al. presented direct evidence that circulating activated platelets contribute to the formation of atherosclerotic plaques.33 Thrombin-activated, calcein AM-labeled platelets were administered intravenously to mice, along with rhodamine 6G to label leukocytes. Activated platelets and platelet-leukocyte aggregates were shown to interact with atherosclerotic lesions and promote leukocyte binding to inflamed endothelium. Fluorescently labeled platelets tethered, rolled and arrested (often transiently) on atherosclerotic endothelium, mostly along early lesions or the “shoulder” of established ones. Injection of activated wild type, but not P-selectin-deficient, platelets increased leukocyte interactions with atherosclerotic endothelium, and co-injection of EGFP-expressing monocytes led to increased monocyte arrest on the surface of atherosclerotic lesions in ApoE−/− mice.
Reitsma et al. utilized IVM to investigate the role of endothelial glycocalyx (EG) thickness and platelet-vessel wall interactions in atherogenesis.34 The EG, the carbohydrate-rich luminal surface of endothelial cells, serves as a barrier between endothelium and blood, and its disruption is associated with vascular dysfunction. In this study, circulating blood platelets were fluorescently labeled with intravenous acridine red (ex/em 455–600/560–680 nm). Significantly higher levels of platelet adhesion were seen in carotid bifurcations of ApoE−/− mice as compared to control, and this correlated with thinner EG in the same regions, as assessed via two-photon laser scanning microscopy ex vivo. Whereas the EG of control mice increased in thickness with age, that of ApoE−/− mice did not change.
Recently, Gandhi et al. found that the antithrombotic enzyme ADAMTS13 reduces vascular inflammation and development of early atherosclerosis in mice.35 ADAMTS13, a metalloproteinase, prevents spontaneous microvascular thrombosis by cleaving large, hyperactive von Willebrand factor (VWF) multimers into smaller, less active ones. Using IVM of rhodamine 6G-labeled leukocytes, the authors observed excessive leukocyte adhesion and accelerated plaque formation at the carotid sinus of ApoE−/−/Adamts13−/− mice, as compared to ApoE−/− mice fed a high fat diet.
Macrophages
Numerous investigations in vitro have highlighted the role of the macrophage as a central inflammatory mediator in atherosclerotic plaques.15 Pande et al. went further to directly image macrophage activity in vivo in a murine model of atherosclerosis using laser scanning IVM and a macrophage-targeted, near-infrared magnetofluorescent nanoparticle (MFNP).36 Dextran-coated nanoparticles conjugated to the near-infrared fluorochrome cyanine 5.5 (ex/em 674/694 nm) were injected intravenously and internalized by macrophages. Focal NIRF signal with high plaque-to-target ratios was detected from MFNP-enhanced carotid atheroma of ApoE−/− mice, and colocalized with macrophages as determined by immunohistochemistry ex vivo. Benefits of the near-infrared fluorescence (NIRF) preparation include lower absorption of photons by hemoglobin and water, facilitating tissue depth penetration; reduced tissue autofluorescence, allowing higher sensitivity to exogenous fluorochromes; and application in a wide array of in vivo microscopic platforms, including epifluorescence and laser scanning fluorescence.37
Extending this imaging approach, McCarthy et al. reported a novel multimodal light-activated theranostic (i.e. therapeutic and diagnostic) nanoagent for targeted macrophage ablation in inflammatory atherosclerosis.38 ApoE−/− mice on high-cholesterol diets were injected with a NIRF- and photosensitized-modified magnetic nanoagent or a fluorescence-matched nonphototoxic control nanoagent 24 hours prior to exposure of carotid arteries. IVM was used to visualize macrophage-rich atherosclerotic lesions, and exposed carotids were irradiated with 650 nm laser light to excite the macrophage-targeted photosensitizer. After 24 hours, histology and biochemical analysis showed significant presence of apoptotic cells in treated vs. control preparations (55% vs. <1% plaque areas, respectively), and these colocalized with cells expressing macrophage markers. This nanoagent can thus function not only as a multimodal imaging agent for macrophage-rich atherosclerotic lesions, but also as a novel paradigm for light-activated inflammatory cell ablation, with implications for management of atherosclerosis.
Inflammatory protease activity
Proteases, via degradation of extracellular matrix, have been implicated in the progression of atherosclerosis and plaque rupture. Cathepsin K (CatK), a lysosomal cysteine protease and a potent elastase, localizes in rupture-prone areas, including the fibrous cap and plaque shoulder.39,40 To image CatK activity in vivo, Jaffer et al. developed a novel NIRF probe that, quenched at baseline, generated strong NIRF signal upon cleavage by CatK.41 Multichannel laser scanning IVM of atheroma in exposed carotids of ApoE−/− mice revealed nearly two-fold NIRF signal increases over that seen with an uncleavable control probe, with intravascular location confirmed via coinjection of a vascular NIRF agent. Focal NIRF signal was detected at plaque shoulders and along the intimal-medial border, and colocalized with CatK detected by immunohistochemistry.
Notably, NIRF signal reflecting CatK activity was more selective than immuno-histochemical stain depicting CatK presence, and colocalized nearly exclusively within the vicinity of plaque macrophages, rather than along smooth muscle cells and macrophages. The authors note that currently available antibodies do not distinguish active CatK from zymogen precursors lacking proteolytic ability. This finding suggests that macrophages, but not smooth muscle cells, are associated with active CatK in atherosclerotic lesions, and illustrates the power of this technique over static approaches to uncover functional and mechanistic information.
Inflammatory protease activity: matrix metalloproteinases
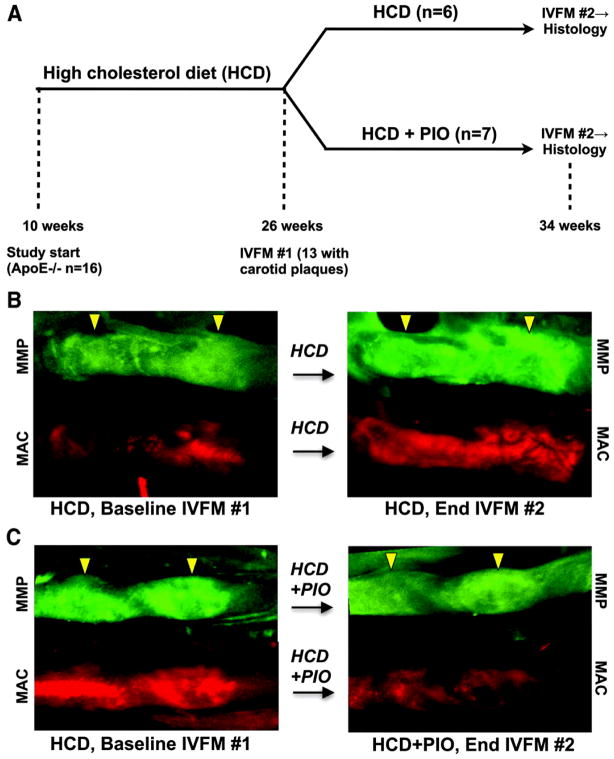
Deguchi et al. extended the NIRF protease reporter portfolio by validating a gelatinase MMP-9 and MMP-2 NIRF activity sensor (MMPSense680, ex/em 680/700nm) in atherosclerosis.42 Recently, Chang et al. used this MMP sensor with serial IVM to show that pioglitazone, a peroxisome proliferator-activated receptor (PPAR)-γ agonist used as an anti-diabetic agent, reduces murine carotid plaque inflammation, as measured by matrix metalloproteinase (MMP) and macrophage phagocytic activity.43 ApoE−/− mice on high-cholesterol diet were fed pioglitazone 13 mg/kg dailyfor 8 weeks and injected with a MMP-activatable fluorescent probe and a spectrally-distinct macrophage-avid fluorescent magnetic nanoparticle for imaging with IVM before and after intervention. As compared to ApoE−/− high-cholesterol diet controls that showed 42–43% increase in carotid plaque MMP activity and macrophage signals, mice treated with pioglitazone showed 13% reduction in plaque MMP activity and 19% reduction in macrophage uptake, which correlated with decreased histological and biochemical measures of inflammation (Figure 1).
Figure 1.
Serial fluorescence IVM demonstrates that pioglitazone, a peroxisome proliferator-activated receptor (PPAR) γ agonist used as an anti-diabetic agent, reduces murine carotid plaque inflammation, as measured by matrix metalloproteinase (MMP) (colored green) and macrophage phagocytic (colored red) activity. ApoE−/− mice on high-cholesterol diet were fed pioglitazone or control diets for 8 weeks, and injected with spectrally distinct MMP-activatable and macrophage-avid magnetic nanoparticle fluorescent probes for imaging with IVM before and after intervention. (B) ApoE−/− diet controls showed significant increases in carotid plaque MMP activity and macrophage signal. (C) ApoE−/− mice treated with pioglitazone showed significant reductions in plaque MMP activity and macrophage uptake. Representative B and C images were processed identically. IVFM=intravital fluorescence microscopy, HCD=high-cholesterol diet, MAC=macrophage. Reprinted with permission from Lippincott Williams & Wilkins, Chang et al. (2010).43
Quillard et al. found that selective inhibition of matrix metalloproteinase-13 (MMP-13) collagenase increased collagen content in mouse atheroma.44 A NIRF activatable probe preferentially cleaved by MMP-13 (MMPSense680) and macrophage-avid fluorescent iron oxide nanoparticles were detected with multichannel fluorescence IVM and ex vivo fluorescence reflectance imaging of carotid plaque in ApoE−/− mice. After 10 weeks of treatment with an orally available MMP-13 inhibitor, there was reduced plaque MMP-13 activity, but similar macrophage content. Assessment of interstitial collagen using picrosirius red revealed substantially thicker collagen fibers in the intima and fibrous cap of plaques of animals treated with MMP-13 inhibitor, as compared to vehicle-treated controls.
Inflammation and calcification
Vascular calcification is associated with atherosclerosis and is predictive of cardiovascular events, but the mechanism for this remains unclear, with studies investigating the role of inflammation in calcification limited largely to in vitro work.45, 46 In 2001, Zaheer et al. synthesized a NIRF bisphosphonate derivative (ex/em 771/796 nm) with rapid, specific binding to hydroxyapatite, a byproduct of osteoblasts required for bone homeostasis.47 This allowed, for the first time, optical detection of osteoblastic activity in vivo, specifically of bony structures via intraperitoneal injection of hairless nu/nu mice using epi-fluorescence microscopy.
Using multichannel IVM, Aikawa et al. investigated the relationship of macrophage burden with osteogenic activity in early stage atherosclerosis, providing a tool to identify preclinical microcalcifications in vivo.48 A bisphosphonate-conjugated NIRF imaging agent (OsteoSense750, ex/em 750/780 nm) visualized osteogenic activity in ApoE−/− mouse aorta that was otherwise undetectable by x-ray computed tomography. Flow cytometry validated the target specifically in osteoblast-like cells. The in-plane resolution of 5 × 5 μm achieved by confocal fluorescence IVM approached the ability to resolve in vivo single macrophages (~10 to 30 μm diameter), with the availability of multiple optical channels to resolve distinct anatomical spaces for optimal structural definition. Co-injection of a macrophage-targeted, spectrally-distinct, near-infrared nanoparticle (CLIO-VT680, ex/em 673/694 nm) allowed simultaneous imaging of plaque macrophages, and IVM of ApoE−/− mouse carotid arteries revealed concomitant increase of macrophage burden and osteogenesis during plaque progression, which decreased after statin treatment (Figure 2). IVM revealed that in early stage atherosclerotic plaques, macrophage infiltration preceded calcification, and macrophage burden and osteogenic activity colocalized primarily in regions of high mechanical stress, including the lesser curvature of the aortic arch, the aortic root, the inominate artery, the carotid bifurcation, and the aortic valve.
Figure 2.
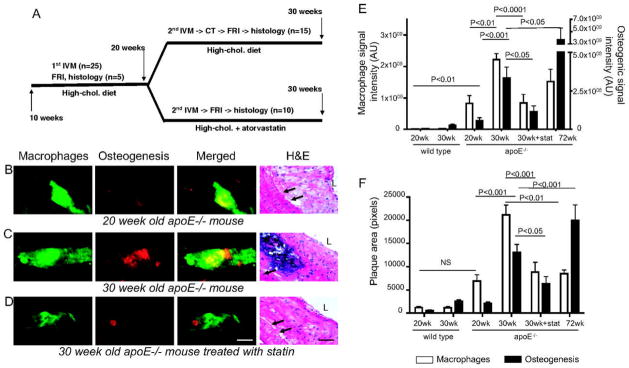
Molecular IVM of vascular calcification. Serial multichannel IVM shows that atorvastatin, a HMG-CoA reductase inhibitor used to lower cholesterol, reduces murine carotid plaque inflammation and calcification in early stage atherosclerosis, as measured by macrophage accumulation (colored green) and osteogenic activity (colored red). ApoE−/− mice on high-cholesterol diets were randomized to atorvastatin or control diets for 10 weeks, and injected with spectrally-distinct bisphosphonate-conjugated NIRF or macrophage-targeted nanoparticles before (B) and after (C, D) intervention, and compared with histology. (C, E, F) ApoE−/− high-cholesterol diet controls showed a significant increase in carotid plaque macrophage and osteogenic signals. (D, E, F) ApoE−/− high-cholesterol mice treated with atorvastatin showed a significant decrease in plaque inflammation and osteogenesis. Reprinted with permission from Lippincott Williams & Wilkins, Aikawa et al. (2007).48
To further explore mechanisms underlying calcification in atherosclerosis, Aikawa et al. used dual-channel confocal IVM to provide direct in vivo evidence that the potent elastase cathepsin S (catS) aggravates arterial and valvular calcification in a mouse model of chronic renal disease (CRD).49 A novel peptide-based cathepsin S-activatable probe (ex/em 750/780 nm) was used to detect elastolytic activity; a bisphosphonate-conjugated probe (OsteoSense680, ex/em 680/700 nm) was used to detect osteogenic activity; and macrophages were imaged using a cross-linked iron oxide (CLIO-gly) fluorescent nanoparticle (ex/em 750/780 nm). CRD was induced by left hemi-nephrectomy followed by right total nephrectomy 1 week later, procedures known to exacerbate atherosclerosis.50,51 CRD ApoE−/− mice with and without endogenous cathepsin S (ApoE−/− catS−/−) were injected simultaneously with spectrally-distinct imaging agents (activatable catS and bisphosphonate probes, or bisphophonate and CLIO-gly agents) and two-channel IVM imaging was serially performed. IVM co-registered strong osteogenic and elastolytic signals in carotid plaques of ApoE−/−/catSwt mice, with decreased osteogenic (and no elastolytic) signal in ApoE−/−/catS−/− mice. Histological analysis corroborated the IVM imaging results, with advanced calcification and alkaline phosphatase activity found to be notably higher in the ApoE−/−/catSwt group.
II. Thrombosis
To limit bleeding from blood vessel injury, the human body rapidly forms clot comprised of platelets and fibrin at sites of endothelial injury, a process known as hemostasis. This adaptive response must be regulated to prevent pathologic vascular clotting, or thrombosis, which can restrict distal blood flow directly or via dissociated emboli. Thromboembolic disease, particularly within the macrocirculation, may manifest clinically as acute myocardial infarction, stroke, or deep venous thrombosis with pulmonary embolism, and is a leading cause of mortality. The biological mechanisms underlying the balance between hemostasis and thrombosis are complex, and models of the interplay between coagulation reactions, platelet activation and fibrin assembly are of interest. In particular, IVM provides a powerful tool for studying these multipart cascades as they occur in vivo. We begin by highlighting a few key works visualizing thrombus formation in the microcirculation, and focus on more recent studies probing thrombogenesis in the macrocirculation.
Microcirculation
Disordered blood coagulation can result in thrombosis, atherosclerosis and hemorrhage. Most studies of platelet thrombus formation have used in vitro systems with purified components or anticoagulated blood to study this highly regulated, complex process. In contrast, IVM allows for real-time visualization and characterization of early events in thrombus formation in vivo. In 2000, Ni et al. used IVM to visualize ferric-chloride-induced injury and thrombosis in exteriorized mesenteric arterioles of mice with and without the platelet ligands von Willebrand factor (vWF) and fibrinogen (Fg).52 Ferric chloride treatment led to denudation of endothelium and the deposition of previously-injected, fluorescently-labeled platelets, but the characteristics of these platelet plugs varied significantly among knockout mice. In vWF−/− mice, few platelet-vessel wall interactions were seen early on, but thrombus formation eventually occurred. In Fg−/− mice, thrombi grew efficiently, but were unstable and often embolized distally. In vWF−/−/Fg−/− mice, thrombus formation still occurred, but it was delayed and associated with increased embolization, as well as platelet accumulation of fibronectin.
Falati et al. developed a new approach for real-time imaging of thrombus formation in the mouse microcirculation using laser-mediated endothelial injury to induce thrombi. Imaging was performed with a custom-designed intravital high-speed confocal and widefield microscopy system.53 This novel system allowed near-simultaneous fluorescence imaging in three separate channels, along with a brightfield image to provide morphological information. The authors demonstrated real-time accumulation of fluorescently-labeled circulating platelets, tissue factor and fibrin within vascular arterioles of the mouse cremaster muscle (Figure 3). Since thrombus formation following laser injury occurred within 1 to 3 minutes, high-speed digital capture of fluorescent images with short exposure times were required.
Figure 3.
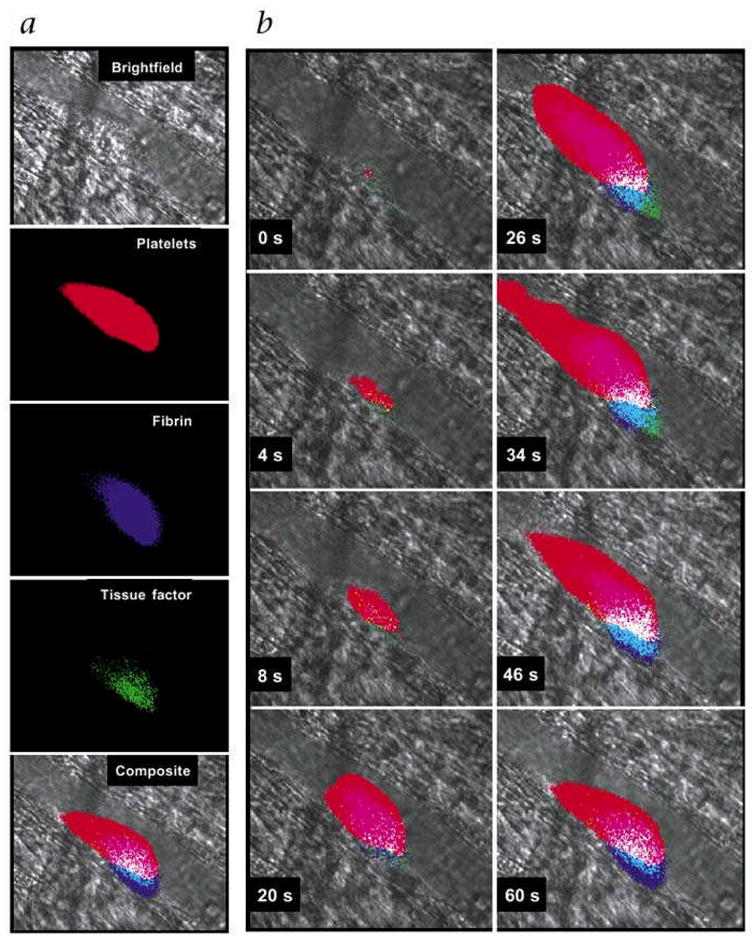
Multichannel IVM detection of real-time thrombus formation in the mouse microcirculation. Near-simultaneous imaging in 4 separate brightfield and fluorescence channels allowed visualization of accumulating platelets (colored red, Alexa 660-conjugated CD41 Fab fragments), fibrin (colored purple, Alexa 350-conjugated mouse antibodies against human fibrin), and tissue factor (colored green, Alexa 488-conjugated sheep antibody against tissue factor) within developing thrombus in vascular arterioles of mouse cremaster muscle exposed to laser endothelial injury. Blood flow is from right to left. Reprinted by permission from Macmillan Publishers Ltd., Falati et al (2002).53
Recently, Kamocka et al. modified the protocol described above by using two-photon IVM to image thrombus development in mouse mesenteric microvasculature.54 Fibrinogen and platelets were tagged using intravenously-injected, fluorochrome-labeled antibodies. The plasma was illuminated with MW 70,000 tetramethylrhodamine-lableddextran, which allowed for detection of slow-moving, unlabeled blood cells as “black hole” silhouettes within the plasma. Images were acquired in two modes: (1) continuous acquisition along a single optical plane to record dynamic movies of thrombus evolution, and (2) stepwise collection of vertical “Z stacks” to generate high-resolution, 3-dimensional reconstructions of developing thrombus. The temporal resolution was approximately one minute. Incorporation of platelets, fibrin and unlabeled blood cells led to perturbation of flow around the developing thrombus.
Macrocirculation
As with initial studies using IVM to visualize inflammation and leukocyte-endothelial interactions, most studies of in vivo thrombus formation have focused on the microvasculature. As advances in optical hardware have resulted in higher signal-to-noise ratios and higher image capture rates, imaging of thrombosis in larger vessels, requiring larger fields-of-view, is increasingly more approachable.2 This is a particularly relevant area of translational study, as thrombi in large vessels more faithfully recapitulate common clinical cardiovascular diseases. In addition, the, altered kinetics of macrothrombi formation and flow dynamics at thrombus edges hold substantial translational relevance for studies of fibrinolytic resistance.
Thrombin activity
Thrombin is the principal protease of the coagulation cascade, and plays a central role in the activation of several coagulation factors, including the conversion of fibrinogen to fibrin, and the activation and aggregation of platelets.55 In 2002, Jaffer et al. used epifluorescence IVM and a novel thrombin-activatable NIRF molecular probe to image in vivo thrombin activity in a murine model of large vein thrombosis.56 Injection of the thrombin-activatable probe (ex/em 675/694 nm) into the circulation of mice just prior to tail vein amputation led to a large detectable NIRF signal from the resultant hematoma, as assessed by surface-weighted fluorescence reflectance imaging. Next, injection of the probe after application of ferric chloride to exposed femoral veins led to focal, specific NIRF detection by epifluorescence IVM in multiple areas of venous thrombi at early and late time point of thrombus formation. The signal was present from occlusive as well as nonocclusive thrombi, and highest around luminal margins. Advantages of this technique include (1) the ability of a single enzymatically-active thrombin molecule to cleave multiple probe substrates, allowing for amplification of the NIRF signal in the relatively short life of thrombin, and (2) preferential detection of biologically active thrombi, that is, acute thrombi with high thrombin activity, potentially allowing for assessment of thrombus age in vivo.
Recently, an intermediate-level magnified image capture system was developed for evaluation of in vivo large-vessel arterial and venous thrombosis, with the capability for temporal and spatial quantification as well as normalization for inter-animal comparisons.57 The IVM system provided 2×2 μm in-plane spatial resolution with an expanded field of view of 2.4 × 3.2 mm. Thrombosis was induced in murine carotid arteries and femoral veins in a controlled, reproducible manner by iron-based electrolytic injury to a focused spot on the vessel surface. Thrombi were visualized using targeted spectrally-distinct fluorescent agents (e.g. for platelets, fibrin, clotting factors), and excited under narrow bandwidths using shuttered laser array for uniform field illumination. Use of fluorophores activated by longer wavelengths allowed for their excitation and detection within larger thrombi.
To modulate thrombus formation, the clinical antithrombotic agents heparin, hirudin, aspirin and clopidogrel, were separately administered following vessel injury. Iron-based electrolytic vessel wall injury led to nonocclusive accumulation of platelets and polymerizing fibrin in a manner similar to, but slower than, that observed with ferric chloride treatment. Clot growth started immediately after thrombus induction, and peaked at greater than ten minutes in both arteries and veins, with both vessel types showing prominent accumulation of platelets and fibrin, without predominance of any one component (i.e. “white” platelet-rich thrombi in arteries versus “red” fibrin-rich thrombi in veins), as has been previously described.58, 59 Platelets accumulated in a homogeneous mass at the clot induction site, whereas fibrin-specific labeled antibodies localized along a perimeter of the injury zone. Coagulation enzyme complexes colocalized with fibrin deposition. Whereas arterial thrombi often underwent massive embolization and regrowth, venous thrombi showed gradual shedding of microemboli.
Administration of heparin and hirudin, which primarily inhibit thrombin activity, led to a 10-fold reduction of fibrin in arteries and veins. In contrast, aspirin and clopidogrel, potent inhibitors of platelet activation and aggregation, reduced platelet accumulation in both vessel types. Interestingly, a transgenic mouse with mutant factor V (analogous to factor V Leiden mutation in humans, which confers risk for deep vein thrombosis via ineffective inhibition by activated protein C) showed larger clots with more fibrin and platelets57.
Blood transglutaminase (FXIII) activity
The ability to specifically detect acute thrombi using NIRF probes was further highlighted in an additional study that targeted FXIIIa activity.60 Activated factor XIII (FXIIIa) mediates fibrinolytic resistance and is a marker of newly formed thrombi. A novel NIRF peptide (based on the amino terminus of alpha-2-antiplasmin, a substrate that is cross-linked to fibrin by FXIIIa) was fluorescently labeled with a NIR fluorochrome (ex/em 679/702 nm, termed A15) and imaged by IVM to detect FXIIIa activity in exposed murine femoral vessels treated with ferric chloride. Significant signal enhancement was detected in acute, but not older (>24 hrs) intravascular thrombi (both arterial and venous), particularly along the thrombus-vessel interface. Results were confirmed by correlative ex vivo fluorescence microscopy and histopathology. In contrast to the thrombin-activatable NIRF agent,56 the FXIIIa-targeted agent covalently binds fibrin, and is therefore not subject to signal loss from washout.
Fibrin
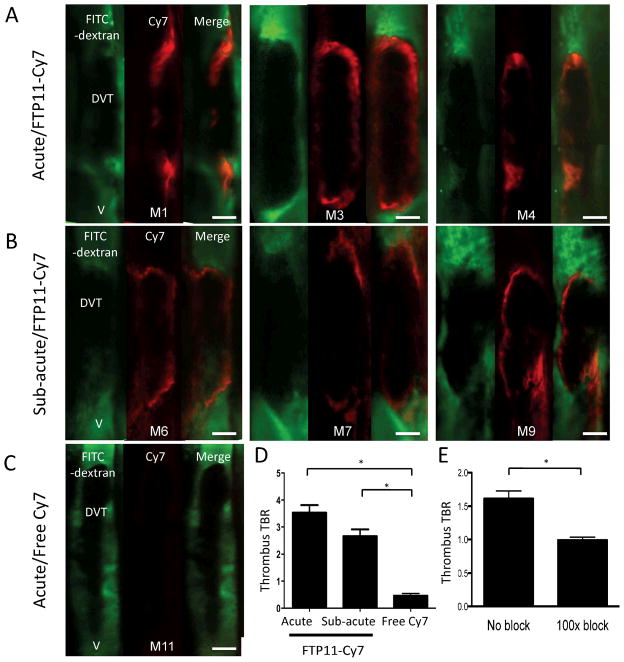
Fibrin provides the critical scaffold for thrombus formation, but limited sensors for fibrin are available for in vivo fluorescence imaging. To address this need, Hara and colleagues recently developed a new peptide-based NIRF sensor for in vivo optical imaging of fibrin.61 The authors modified a previous fibrin peptide derived from phage display and tested clinically in a phase II MRI trial. After coupling the fibrin-targeted peptide (FTP11) to an NIR fluorochrome, IVM was performed in a ferric chloride-induced model of murine femoral deep vein thrombosis (DVT). Facilitated by the agent’s short half-life, fibrin deposition was rapidly detected by IVM, and localized to the margins of both acute and subacute DVT (Figure 4). Control agents did not significantly enhance venous thrombi. The availability of this agent coupled to IVM offers the potential to dynamically track fibrin accumulation and dissipation in vivo.
Figure 4.
IVM molecular imaging of fibrin deposition in murine femoral deep vein thrombosis (DVT). A fibrin-targeted peptide coupled to a NIR fluorochrome (FTP11-Cy7, red) allowed visualization of fibrin in a topical ferric chloride-induced model of acute (A, D) and subacute (B, D) DVT. Control agents did not enhance the thrombi (C, D). Signal deposition in acute thrombi was blocked by pre-injection of 100-fold excess unlabeled FTP11 (E). Reprinted with permission from Elsevier, Hara et al. (2012).61
Atherothrombosis
Many models of thrombosis involve perturbation of uncharacteristically healthy vessels. In contrast, Kuijpers and Nergiz-Unal et al. used focused ultrasound (10 seconds at 6 kHz) to rupture carotid arterial plaques in ApoE−/− mice and simultaneously monitor subsequent atherothrombosis with IVM.62, 63 Specifically, CFSE-labeled platelets were injected into mice treated with a reversible platelet P2Y(12) receptor antagonist, such as ticagrelor, which abolishes ADP- and collagen-induced platelet aggregation. This led to significantly reduced platelet accumulation induced by carotid plaque rupture, with the appearance of only single-layered fluorescent platelets and loose thrombi, as compared to bright fluorescent thrombi in vehicle-treated mice. These findings confirm the role for P2Y(12)-dependent stabilization of thrombus formation at high shear conditions.
Clinical Translation
The clinical translatability of IVM to human subjects remains unclear given its invasive nature. However, intravascular NIRF catheter reflectance approaches are emerging for clinical molecular imaging of human coronary arteries.64–66 In addition, promising fluorescence probes with diagnostic potential are emerging for translational investigation, including: 1) a folate receptor-α-targeted fluorescent agent recently evaluated in humans,67 2) a cysteineprotease-activatable NIRF agent planned for clinical trials,2 and 3) indocyanine green, an FDA-approved NIR fluorochrome, which shows promise for targeted atherosclerosis NIRF imaging.11
Concluding Remarks
High-resolution vascular molecular imaging using epifluorescence, confocal and multi-photon techniques are now revolutionizing our ability to probe biological processes in vivo. Catalyzed by parallel growths in fluorescent reporter technology and the availability of versatile IVM imaging systems, IVM-based molecular imaging is rapidly becoming an essential technology for investigating the biology of large-vessel vascular diseases in vivo. In the next several years, IVM molecular imaging is poised to provide unparalleled insights into the genesis and evolution of atherosclerosis, thrombosis, vasculitis, and vascular injury and repair.
Supplementary Material
Insight, Innovation, Integration.
In vivo molecular imaging of specific cells and molecules may provide unparalleled insights into how vascular diseases evolve, and reveal new opportunities for targeted therapeutics. In the last decade, integration of fluorescence molecular imaging agents with high-resolution intravital microscopy (IVM) has enabled in vivo, serial, and dynamic observations of vascular biology. This represents a significant advance over static ex vivo methodologies. Here we showcase applications of fluorescence IVM-based molecular imaging of clinically relevant diseases of medium- and large-sized vessels, specifically, atherosclerosis and thrombosis.
Acknowledgments
Dr. Jaffer’s laboratory is funded by the National Institutes of Health (NIH HL 108229), American Heart Association Scientist Development Grant #0830352N, Howard Hughes Medical Institute Career Development Award, and MGH SPARK Award.
Footnotes
Disclosures: None
References
- 1.Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010;7:603–614. doi: 10.1038/nmeth.1483. [DOI] [PubMed] [Google Scholar]
- 2.Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: insights into atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1017–1024. doi: 10.1161/ATVBAHA.108.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner R. Erlauterungstaflen zur Physiologie und Entwicklungsgeschichte. Leipzig, Germany: Leopold Voss; 1839. [Google Scholar]
- 4.Mempel TR, Scimone ML, Mora JR, von Andrian UH. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr Opin Immunol. 2004;16:406–417. doi: 10.1016/j.coi.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest. 2008;118:710–721. doi: 10.1172/JCI33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Zandvoort M, Engels W, Douma K, Beckers L, Oude Egbrink M, Daemen M, Slaaf DW. Two-photon microscopy for imaging of the (atherosclerotic) vascular wall: a proof of concept study. J Vasc Res. 2004;41:54–63. doi: 10.1159/000076246. [DOI] [PubMed] [Google Scholar]
- 7.Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002;2:872–880. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maffia P, Zinselmeyer BH, Ialenti A, Kennedy S, Baker AH, McInnes IB, Brewer JM, Garside P. Images in cardiovascular medicine. Multiphoton microscopy for 3-dimensional imaging of lymphocyte recruitment into apolipoprotein-E-deficient mouse carotid artery. Circulation. 2007;115:e326–328. doi: 10.1161/CIRCULATIONAHA.106.658492. [DOI] [PubMed] [Google Scholar]
- 9.Bullen A. Microscopic imaging techniques for drug discovery. Nat Rev Drug Discov. 2008;7:54–67. doi: 10.1038/nrd2446. [DOI] [PubMed] [Google Scholar]
- 10.Park EJ, Peixoto A, Imai Y, Goodarzi A, Cheng G, Carman CV, von Andrian UH, Shimaoka M. Distinct roles for LFA-1 affinity regulation during T-cell adhesion, diapedesis, and interstitial migration in lymph nodes. Blood. 2010;115:1572–1581. doi: 10.1182/blood-2009-08-237917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinegoni C, Botnaru I, Aikawa E, Calfon MA, Iwamoto Y, Folco EJ, Ntziachristos V, Weissleder R, Libby P, Jaffer FA. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci Transl Med. 2011;3:84ra45. doi: 10.1126/scitranslmed.3001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 13.Lin MZ, McKeown MR, Ng HL, Aguilera TA, Shaner NC, Campbell RE, Adams SR, Gross LA, Ma W, Alber T, Tsien RY. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem Biol. 2009;16:1169–1179. doi: 10.1016/j.chembiol.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pittet MJ, Weissleder R. Intravital imaging. Cell. 2011;147:983–991. doi: 10.1016/j.cell.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 16.Libby P, DiCarli M, Weissleder R. The vascular biology of atherosclerosis and imaging targets. J Nucl Med. 2010;51 (Suppl 1):33S–37S. doi: 10.2967/jnumed.109.069633. [DOI] [PubMed] [Google Scholar]
- 17.Eriksson EE, Werr J, Guo Y, Thoren P, Lindbom L. Direct observations in vivo on the role of endothelial selectins and alpha(4) integrin in cytokine-induced leukocyte-endothelium interactions in the mouse aorta. Circ Res. 2000;86:526–533. doi: 10.1161/01.res.86.5.526. [DOI] [PubMed] [Google Scholar]
- 18.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 19.Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Direct viewing of atherosclerosis in vivo: plaque invasion by leukocytes is initiated by the endothelial selectins. FASEB J. 2001;15:1149–1157. doi: 10.1096/fj.00-0537com. [DOI] [PubMed] [Google Scholar]
- 21.Richardson PD, Davies MJ, Born GV. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989;2:941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- 22.Cheng GC, Loree HM, Kamm RD, Fishbein MC, Lee RT. Distribution of circumferential stress in ruptured and stable atherosclerotic lesions. A structural analysis with histopathological correlation. Circulation. 1993;87:1179–1187. doi: 10.1161/01.cir.87.4.1179. [DOI] [PubMed] [Google Scholar]
- 23.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 24.Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–1569. [PubMed] [Google Scholar]
- 25.Rotzius P, Soehnlein O, Kenne E, Lindbom L, Nystrom K, Thams S, Eriksson EE. ApoE(−/−)/lysozyme M(EGFP/EGFP) mice as a versatile model to study monocyte and neutrophil trafficking in atherosclerosis. Atherosclerosis. 2009;202:111–118. doi: 10.1016/j.atherosclerosis.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson EE. Intravital microscopy on atherosclerosis in apolipoprotein e-deficient mice establishes microvessels as major entry pathways for leukocytes to advanced lesions. Circulation. 2011;124:2129–2138. doi: 10.1161/CIRCULATIONAHA.111.030627. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson EE, Karlof E, Lundmark K, Rotzius P, Hedin U, Xie X. Powerful inflammatory properties of large vein endothelium in vivo. Arterioscler Thromb Vasc Biol. 2005;25:723–728. doi: 10.1161/01.ATV.0000157578.51417.6f. [DOI] [PubMed] [Google Scholar]
- 28.Megens RT, oude Egbrink MG, Merkx M, Slaaf DW, van Zandvoort MA. Two-photon microscopy on vital carotid arteries: imaging the relationship between collagen and inflammatory cells in atherosclerotic plaques. J Biomed Opt. 2008;13:044022. doi: 10.1117/1.2965542. [DOI] [PubMed] [Google Scholar]
- 29.Megens RT, Oude Egbrink MG, Cleutjens JP, Kuijpers MJ, Schiffers PH, Merkx M, Slaaf DW, van Zandvoort MA. Imaging collagen in intact viable healthy and atherosclerotic arteries using fluorescently labeled CNA35 and two-photon laser scanning microscopy. Mol Imaging. 2007;6:247–260. [PubMed] [Google Scholar]
- 30.Megens RT, Reitsma S, Prinzen L, oude Egbrink MG, Engels W, Leenders PJ, Brunenberg EJ, Reesink KD, Janssen BJ, ter Haar Romeny BM, Slaaf DW, van Zandvoort MA. In vivo high-resolution structural imaging of large arteries in small rodents using two-photon laser scanning microscopy. J Biomed Opt. 2010;15:011108. doi: 10.1117/1.3281672. [DOI] [PubMed] [Google Scholar]
- 31.Millington OR, Brewer JM, Garside P, Maffia P. Imaging interactions between the immune and cardiovascular systems in vivo by multiphoton microscopy. Methods Mol Biol. 2010;616:193–206. doi: 10.1007/978-1-60761-461-6_13. [DOI] [PubMed] [Google Scholar]
- 32.Megens RT, Kemmerich K, Pyta J, Weber C, Soehnlein O. Intravital imaging of phagocyte recruitment. Thromb Haemost. 2011;105:802–810. doi: 10.1160/TH10-11-0735. [DOI] [PubMed] [Google Scholar]
- 33.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 34.Reitsma S, Oude Egbrink MG, Heijnen VV, Megens RT, Engels W, Vink H, Slaaf DW, van Zandvoort MA. Endothelial glycocalyx thickness and platelet-vessel wall interactions during atherogenesis. Thromb Haemost. 2011;106:939–946. doi: 10.1160/TH11-02-0133. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi C, Khan MM, Lentz SR, Chauhan AK. ADAMTS13 reduces vascular inflammation and the development of early atherosclerosis in mice. Blood. 2012;119:2385–2391. doi: 10.1182/blood-2011-09-376202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pande AN, Kohler RH, Aikawa E, Weissleder R, Jaffer FA. Detection of macrophage activity in atherosclerosis in vivo using multichannel, high-resolution laser scanning fluorescence microscopy. J Biomed Opt. 2006;11:021009. doi: 10.1117/1.2186337. [DOI] [PubMed] [Google Scholar]
- 37.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy JR, Korngold E, Weissleder R, Jaffer FA. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small. 2010;6:2041–2049. doi: 10.1002/smll.201000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novinec M, Grass RN, Stark WJ, Turk V, Baici A, Lenarcic B. Interaction between human cathepsins K, L, and S and elastins: mechanism of elastinolysis and inhibition by macromolecular inhibitors. J Biol Chem. 2007;282:7893–7902. doi: 10.1074/jbc.M610107200. [DOI] [PubMed] [Google Scholar]
- 41.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 42.Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 43.Chang K, Francis SA, Aikawa E, Figueiredo JL, Kohler RH, McCarthy JR, Weissleder R, Plutzky J, Jaffer FA. Pioglitazone suppresses inflammation in vivo in murine carotid atherosclerosis: novel detection by dual-target fluorescence molecular imaging. Arterioscler Thromb Vasc Biol. 2010;30:1933–1939. doi: 10.1161/ATVBAHA.110.206342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quillard T, Tesmenitsky Y, Croce K, Travers R, Shvartz E, Koskinas KC, Sukhova GK, Aikawa E, Aikawa M, Libby P. Selective inhibition of matrix metalloproteinase-13 increases collagen content of established mouse atherosclerosis. Arterioscler Thromb Vasc Biol. 31:2464–2472. doi: 10.1161/ATVBAHA.111.231563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, Nishizawa Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ Res. 2002;91:9–16. doi: 10.1161/01.res.0000026421.61398.f2. [DOI] [PubMed] [Google Scholar]
- 47.Zaheer A, Lenkinski RE, Mahmood A, Jones AG, Cantley LC, Frangioni JV. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat Biotechnol. 2001;19:1148–1154. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 48.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 49.Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, Fukuda D, Kohler RH, Shi GP, Jaffer FA, Weissleder R. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119:1785–1794. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massy ZA, Ivanovski O, Nguyen-Khoa T, Angulo J, Szumilak D, Mothu N, Phan O, Daudon M, Lacour B, Drueke TB, Muntzel MS. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J Am Soc Nephrol. 2005;16:109–116. doi: 10.1681/ASN.2004060495. [DOI] [PubMed] [Google Scholar]
- 51.Bro S, Bentzon JF, Falk E, Andersen CB, Olgaard K, Nielsen LB. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J Am Soc Nephrol. 2003;14:2466–2474. doi: 10.1097/01.asn.0000088024.72216.2e. [DOI] [PubMed] [Google Scholar]
- 52.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8:1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 54.Kamocka MM, Mu J, Liu X, Chen N, Zollman A, Sturonas-Brown B, Dunn K, Xu Z, Chen DZ, Alber MS, Rosen ED. Two-photon intravital imaging of thrombus development. J Biomed Opt. 2010;15:016020. doi: 10.1117/1.3322676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 56.Jaffer FA, Tung CH, Gerszten RE, Weissleder R. In vivo imaging of thrombin activity in experimental thrombi with thrombin-sensitive near-infrared molecular probe. Arterioscler Thromb Vasc Biol. 2002;22:1929–1935. doi: 10.1161/01.atv.0000033089.56970.2d. [DOI] [PubMed] [Google Scholar]
- 57.Cooley BC. In vivo fluorescence imaging of large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol. 2011;31:1351–1356. doi: 10.1161/ATVBAHA.111.225334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman M, Van den Bovenkamp GJ. Role of thrombus in plaque formation in the human diseased coronary artery. Br J Exp Pathol. 1966;47:550–557. [PMC free article] [PubMed] [Google Scholar]
- 59.Sevitt S. Organization of valve pocket thrombi and the anomalies of double thrombi and valve cusp involvement. Br J Surg. 1974;61:641–649. doi: 10.1002/bjs.1800610812. [DOI] [PubMed] [Google Scholar]
- 60.Jaffer FA, Tung CH, Wykrzykowska JJ, Ho NH, Houng AK, Reed GL, Weissleder R. Molecular imaging of factor XIIIa activity in thrombosis using a novel, near-infrared fluorescent contrast agent that covalently links to thrombi. Circulation. 2004;110:170–176. doi: 10.1161/01.CIR.0000134484.11052.44. [DOI] [PubMed] [Google Scholar]
- 61.Hara T, Bhayana B, Thompson B, Kessinger CW, Khatri A, McCarthy JR, Weissleder R, Lin CP, Tearney GJ, Jaffer FA. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging. 2012;5:607–615. doi: 10.1016/j.jcmg.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuijpers MJ, Gilio K, Reitsma S, Nergiz-Unal R, Prinzen L, Heeneman S, Lutgens E, van Zandvoort MA, Nieswandt B, Egbrink MG, Heemskerk JW. Complementary roles of platelets and coagulation in thrombus formation on plaques acutely ruptured by targeted ultrasound treatment: a novel intravital model. J Thromb Haemost. 2009;7:152–161. doi: 10.1111/j.1538-7836.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 63.Nergiz-Unal R, Cosemans JM, Feijge MA, van der Meijden PE, Storey RF, van Giezen JJ, oude Egbrink MG, Heemskerk JW, Kuijpers MJ. Stabilizing role of platelet P2Y(12) receptors in shear-dependent thrombus formation on ruptured plaques. PLoS One. 2010;5:e10130. doi: 10.1371/journal.pone.0010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, Ntziachristos V, Libby P, Weissleder R. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118:1802–1809. doi: 10.1161/CIRCULATIONAHA.108.785881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaffer FA, Calfon MA, Rosenthal A, Mallas G, Razansky RN, Mauskapf A, Weissleder R, Libby P, Ntziachristos V. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011;57:2516–2526. doi: 10.1016/j.jacc.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoo H, Kim JW, Shishkov M, Namati E, Morse T, Shubochkin R, McCarthy JR, Ntziachristos V, Bouma BE, Jaffer FA, Tearney GJ. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat Med. 2011;17:1680–1684. doi: 10.1038/nm.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJ, van der Zee AG, Bart J, Low PS, Ntziachristos V. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.