Abstract
Studies on therapeutic drug disposition in humans have shown significant alterations as the result of pregnancy. However, it is not known whether pesticide metabolic capacity changes throughout pregnancy, which could affect exposure of the developing brain. We sought to determine the effect of pregnancy on the expression of hepatic enzymes involved in the metabolism of pesticides. Livers were collected from virgin and pregnant C57BL/6 mice at gestational days (GD)7, GD11, GD14, GD17, and postpartum days (PD)1, PD15, and PD30. Relative mRNA expression of several enzymes involved in the metabolism of pesticides, including hepatic cytochromes (Cyp) P450s, carboxylesterases (Ces), and paraoxonase 1 (Pon1), were assessed in mice during gestation and the postpartum period. Compared with virgin mice, alterations in the expression occurred at multiple time points, with the largest changes observed on GD14. At this time point, the expression of most of the Cyps involved in pesticide metabolism in the liver (Cyp1a2, Cyp2d22, Cyp2c37, Cyp2c50, Cyp2c54, and Cyp3a11) were downregulated by 30% or more. Expression of various Ces isoforms and Pon1 were also decreased along with Pon1 activity. These data demonstrate significant alterations in the expression of key enzymes that detoxify pesticides during pregnancy, which could alter exposure of developing animals to these chemicals.
Introduction
Pesticides are widely used to protect crops, cattle, gardens, and households from infestations. In 2007, it was estimated that 93 million pounds of active ingredients were used in the United States, with organophosphate (OP), carbamate, and pyrethroid (PYR) insecticides the most commonly used (US EPA 2011). Although pesticides are an important tool for ensuring an adequate food supply and protecting public health, there is a risk of untoward effects in nontarget species, including humans, because target and nontarget species often possess a common molecular target of the pesticide. Of particular concern with insecticides, such as OPs and PYRs, is their targeting of the nervous system and the potential increased vulnerability of the developing brain to their neurotoxic effects (Shafer et al., 2005; Jurewicz and Hanke, 2008).
Several animal studies have consistently demonstrated that very young animals are more sensitive to the acute toxic effects of OPs and PYRs and that this increased sensitivity is likely the result of immature hepatic detoxication systems (Atterberry et al., 1997; Sheets, 2000; Anand et al., 2006; Timchalk et al., 2006). However, much less focus has been on the role of maternal detoxication systems in the vulnerability of the developing brain during pregnancy. This is a particularly important area because several studies have reported the presence of OP and PYR metabolites in urine samples from pregnant women (Fourth National Report on Human Exposure to Environmental Chemicals, 2009, http://www.cdc.gov/exposurereport/); Berkowitz et al., 2003), as well as in meconium (Whyatt et al., 2009). A recent report of biomonitoring data in pregnant women from the 2003–2004 National Health and Nutrition Examination Survey found that more than 40% of the women had detectable levels of several metabolites of OP insecticides, which have been linked to neurobehavioral alterations (Eskenazi et al., 2007), and more than 80% had detectable levels of the OP metabolite dimethylthiophosphate (Woodruff et al., 2011). Similarly, data from the 1999–2002 National Health and Nutrition Examination Survey found that 67% of pregnant women had detectable levels of the PYR metabolite 3-phenoxybenzoic acid (Castorina et al., 2010). Thus, there is widespread exposure of pregnant women to OP and PYR pesticides.
Human pregnancy is known to cause a number of physiologic changes that may alter the metabolism of drugs and toxicants, including changes that increase absorption and decrease plasma protein levels (Selevan et al., 2000). Indeed, elimination of drugs metabolized by several different isoforms of human cytochrome P450 (CYP) is altered (Dempsey et al., 2002; Anderson, 2005; Hodge and Tracy, 2007). These metabolic changes occurring in pregnant women may similarly alter the fate of environmental contaminants, such as pesticides, because many of the drug-metabolizing enzymes, such as the CYPs, carboxylesterases (CESs), and paraoxonase 1 (PON1), also function to metabolize pesticides. Thus, alteration of the activity of pesticide-metabolizing enzymes might prolong or increase exposure of the developing infant to pesticides, thereby contributing to the increased susceptibility of the developing brain.
Although human studies provide important information regarding drug-specific alterations in metabolism, direct determination of the role of pregnancy-induced alterations of pesticide metabolism is not feasible for ethical reasons. Recent studies investigated changes in rodent cytochrome P450 (Cyp) isoforms during pregnancy in mice (Zhang et al., 2008; Koh et al., 2011) and rats (He et al., 2005; Dickmann et al., 2008). However, data are lacking for some important Cyp isoforms involved in pesticide metabolism, and the regulation of hepatic Ces or Pon1 levels during pregnancy is not known. We sought to quantify hepatic expression of pesticide-metabolizing enzymes in mice during pregnancy and through the postpartum period and assess potential regulatory mechanisms underlying transcriptional changes.
Materials and Methods
Animals.
Adult virgin C57Bl/6 mice (Charles River, Wilmington, MA) were mated overnight and separated the following day [designated gestational day (GD) 0]. Dams and virgin (nonmated) mice were provided chow and water ad libitum. The room was maintained at a constant temperature, humidity, and 12-h light cycle. Tissues were collected on GD7, GD11, GD14, and GD17 and on postpartum days (PD) 1, PD15, and PD30. On each day a dam was sacrificed, an age-matched virgin mouse was also sacrificed as control, resulting in seven virgin mice and seven dam groups (n = 3–4 mice per group per time point). Offspring were weaned from lactating dams on PD21. Livers and other tissues were collected and snap-frozen in liquid nitrogen. Animals were treated according to the Animal Care and Use Policy of the University of Kansas Medical Center and Rutgers University. The Institutional Animal Care and Use Committees of both universities approved these studies.
Real-Time Quantitative Polymerase Chain Reaction (qPCR).
Quantitative polymerase chain reaction (qPCR) was performed as described previously (Richardson et al., 2006; Yacovino et al., 2013). Genes were selected on the basis of their likeliness to be involved in the biotransformation of pesticides or their potential role in regulating phase 1 enzyme transcription. Primers for the phase 1 metabolizing enzymes and the transcription factors were designed using the National Center for Biotechnology Information primer-blast application (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi) and tested on cDNA prepared from female mouse hepatic RNA. All sequences are available on request. In the event multiple splice variants existed, primers were designed to anneal within a common region when possible. Melting curve analysis was conducted, and amplification products were run on an agarose gel for amplicon size confirmation. Relative gene expression was assessed using the SYBR Green technology (Applied Biosystems, Carlsbad, CA) on an ABI7900HT Fast Real-Time PCR system (Applied Biosystems). All samples were run in duplicate; actin and gluteraldehyde-3-phosphate dehydrogenase (GAPDH) were monitored in parallel as normalizers for each gene and gave similar results. Therefore, relative expression was calculated with the ΔΔCt method using GAPDH as normalizing gene (Livak and Schmittgen, 2001). The gene isoforms, gene accession numbers, and corresponding human genes are presented in Supplemental Table 1.
Determination of Serum Pon1 Activities.
Pon1 enzyme activity toward paraoxon (paraoxonase) and chlorpyrifos-oxon (chlorpyrifosoxonase) was measured in serum from virgin and GD15 dams using spectrophotometric methods as described by Richter and Furlong (1999). Briefly, serum samples were diluted in 50 mM Tris-HCl containing 2 mM CaCl2 (pH. 7.4) and incubated at 37°C for 10 min in clear 96-well plates. The reaction was initiated by the addition of either 1.2 mM paraoxon or 320 μM chlorpyrifos-oxon, and the formation of p-nitrophenol or 3,5,6-trichloro-2-pyridinol was measured at 405 and 310 nm, respectively, for 5 min. Initial rates of hydrolysis measured in milli Optical Density Units per minute were converted to units per liter using molar extinction coefficients of 5.56 nmol/L and 3.03 nmol/L for paraoxon and chlorpyrifos-oxon, respectively. Data were expressed as percentage of virgin activities.
Statistical Analysis.
Relative expression (ΔΔCt) was computed in Excel (Microsoft Inc., 2003). The measures of central tendency, standard deviations, and standard errors were also calculated in Excel and were used for graph building. Graphs were prepared with GraphPad Prism 5 (GraphPad Software Inc., 2007, La Jolla, CA), and statistical analysis was conducted with SPSS 19 (SPSS Inc., 2010, Chicago, IL).
To assess the effect of gestational day for each gene, the ΔΔCt was calculated using the corresponding virgin group as a reference. To ensure that there were no statistically significant differences between the different virgin groups across the time points, an analysis of variance was performed for each gene. Because we observed a significant effect of gestation day using analysis of variance, unpaired t tests were used to compare each dam group to the corresponding virgin mice group. For all comparisons, α was set at 0.05. A table of the statistical outcomes is presented as Supplemental Table 2.
Results
Gestational and Postpartum Changes in Hepatic Cyp mRNA Expression
Changes in the relative expression of the Cyp isoforms during pregnancy and postpartum are shown in Fig. 1. In pregnant dams, Cyp1a2 expression was decreased by about 50% at GD7, GD11, and GD14 before returning to levels similar to the virgin mice on GD17 (Fig. 1). Cyp2b10 mRNA was reduced by approximately 50% on GD 7, GD11, and GD14, but it did not quite reach statistical significance (P = 0.08–0.1). However, expression was increased by 25% on GD17 and by 100% compared with virgin mice on PD1, remaining elevated through PD30. Cyp2d22 was lower at GD11 and GD14 by about 45%, returning to near control levels by GD17 and PD1. Similarly, Cyp2c37 mRNA was lower at GD11, GD14, and GD17 by 45%–73% and returned to equivalent expression with the virgin mice on PD1. Cyp2c50 was reduced from GD7 to PD15, and Cyp2c54 mRNA was also significantly decreased by 40% at GD11, GD14, and GD17. Cyp3a11 mRNA levels were significantly decreased by 50% or more throughout the entire course of the study, lasting through PD30. Cyp3a13 expression started to rise on GD11 and was significantly higher than that in corresponding virgin mice by about 75% on GD17; expression returned to virgin levels by PD1. We also assessed the expression of the female-specific Cyp3a41a/b, Cyp3a44, and juvenile-expressed Cyp3a16, which were increased by 10 to more than 100 times, respectively, throughout the time course of the experiment (Supplemental Fig. 1).
Fig. 1.
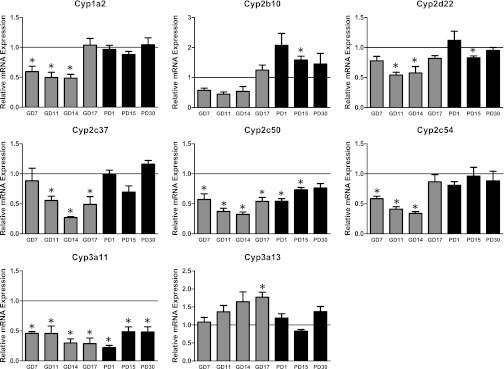
Relative mRNA expression of cytochrome P450. Mean relative mRNA ± S.E. (n = 3 or 4) at different time points during gestation (GD; gray bars) and postpartum periods (PD; black bars). mRNA data were normalized to GAPDH and compared with virgin mice by paired t tests. *Statistically significant (P < 0.05) difference between the pregnant or postpartum mice group and the corresponding age-matched control virgin mice. The lines at 1.0 represent the normalized virgin data.
Gestational and Postpartum Changes in Hepatic Ces and Pon1 mRNA Expression and Pon1 Activities
In humans, there are three major isoforms of CES—CES1, CES2, and CES3—with several isoforms contributing to these families in mice (Holmes et al., 2010). Changes in the hepatic expression of Ces isoforms and Pon1 during pregnancy are shown in Fig. 2. Ces1c expression was not significantly altered at any point of the study, although it was decreased by 30% on GD11 (P = 0.08). Ces1d was significantly reduced on GD11 by about 30% and by a similar amount on GD14, but this also did not quite reach statistical significance (P = 0.1). Ces1e was significantly reduced on GD7 by 40%. A trend was observed for the other isoforms, although it was not significant. Ces2a was downregulated from GD7 to GD14, whereas Ces2e was reduced at PD1. Ces2c was not affected by pregnancy in a statistically significant manner. Pon1 mRNA was decreased on GD14, but this did not quite reach statistical significance (P = 0.08). To determine whether changes in Pon1 mRNA were associated with changes in activity, serum Pon1 activity was determined with the substrates paraoxon and chlorpyrifos-oxon. On GD14, serum Pon1 activity toward paraoxon and chlorpyrifos-oxon was decreased by 43% and 37%, respectively.
Fig. 2.
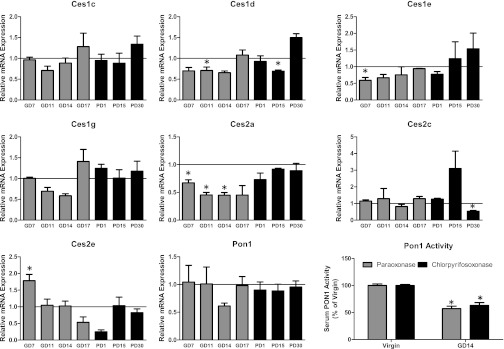
Relative mRNA expression of carboxylesterases and paraoxonase 1. Mean relative mRNA ± S.E. (n = 3 or 4) at different time points during gestation (GD; gray bars) and postpartum periods (PD; black bars). mRNA data normalized to GAPDH and serum Pon1 activities as percent of virgin activities were compared with virgin mice by paired t tests. *Statistically significant (P < 0.05) difference between the pregnant or postpartum mice group and the corresponding age-matched control virgin mice. The lines at 1.0 represent the normalized virgin data.
Gestational Changes in Hepatic Nuclear Receptors and Transcription Factors mRNA Expression
In general, the expression of nuclear receptors and transcription factors involved in regulating expression of these phase 1 enzymes did not appear to be affected by gestation or lactation in a statistically significant manner (Table 1). However, peroxisome proliferator-activated receptor α (Pparα) mRNA tended to be reduced by 23%–45% from GD7-PD1 and was significantly reduced at GD17 and PD1. Although not statistically significant (P = 0.054–0.075), CAR (the constitutive androstane receptor) was consistently reduced through the course of pregnancy by 25%–58% but did not quite reach statistical significance (Supplemental Table 2).
TABLE 1.
Hepatic transcription factor and nuclear receptor relative mRNA expression during gestation and postpartum in dams compared with age-matched virgin mice [relative expression (S.E.)]
| Nuclear Receptor | Virgins | GD7 | GD11 | GD14 | GD17 | PD1 | PD15 | PD30 |
|---|---|---|---|---|---|---|---|---|
| Hnf4α | 1 | 1.14(0.11) | 1.28(0.16) | 1.13(0.26) | 1.34(0.21) | 1.02(0.11) | 1.20(0.07) | 1.10(0.10) |
| Pparα | 1 | 0.77(0.09) | 0.73(0.07) | 0.78(0.07) | 0.68(0.06)* | 0.56(0.09)* | 0.83(0.10) | 0.97(0.08) |
| Gr | 1 | 1.08(0.14) | 0.90(0.14) | 1.38(0.36) | 0.96(0.10) | 1.07(0.06) | 0.65(0.10) | 1.08(0.11) |
| Pxr | 1 | 1.16(0.19) | 0.86(0.07) | 1.11(0.15) | 1.04(0.13) | 0.83(0.03) | 1.02(0.08) | 0.74(0.10) |
| CAR | 1 | 1.10(0.20) | 0.48(0.09) | 0.42(0.07) | 0.70(0.09) | 0.75(0.21) | 0.85(0.07) | 0.87(0.14) |
| Ahr | 1 | 1.42(0.32) | 1.21(0.22) | 0.86(0.05) | 1.29(0.20) | 0.81(0.05) | 0.84(0.03) | 0.96(0.06) |
| Rxrα | 1 | 1.08(0.11) | 1.11(0.08) | 0.77(0.15) | 0.98(0.04) | 1.03(0.15) | 0.87(0.08) | 1.00(0.05) |
| Fxr | 1 | 1.85(0.33) | 1.04(0.16) | 0.86(0.12) | 1.05(0.04) | 0.91(0.18) | 0.44(0.05) | 1.24(0.06) |
| Lxr | 1 | 1.12(0.14) | 0.97(0.09) | 0.94(0.10) | 0.81(0.04) | 1.04(0.05) | 0.63(0.09) | 0.92(0.07) |
AhR, Aryl Hydrocarbon Receptor; CAR, constitutive androstane receptor; FXR, Farnesoid X Receptor; Gr, Growth Hormone receptor; GD, gestational day; Hnf, hepatocyte nuclear factor; Lxr, Liver X Receptor; PD, postpartum day; Pparα, peroxisome proliferator-activated receptor α; PXR, Pregnane X Receptor; RXRalpha, Retinoid X Receptor.
P < 0.05.
Discussion
The goal of the present study was to determine whether pregnancy and lactation in mice altered the mRNA expression of hepatic enzymes involved in pesticide metabolism, particularly for OP and PYR insecticides. In general, the expression of several P450 isoforms was reduced, particularly during the time of the brain growth spurt in utero (GD11–17). Likewise, there was a general reduction in the expression of most of the Ces isoforms during this period and a decrease in Pon1 expression and activity on GD14. Overall, the results of the present study suggest a lower hepatic metabolic capacity for insecticide metabolism in dams during the period corresponding to the murine embryonic development phase. Although it remains to be established whether these changes result in alteration of pesticide disposition during pregnancy in mice, these data suggest that there may be a reduced clearance of pesticides, which would translate into higher internal exposure for the dam and fetuses.
CYP-mediated oxidative metabolism of OPs and PYRs is complex and involves several pathways, as different CYP isoforms can metabolize the same compound (e.g., chlorpyrifos) and different compounds (e.g., deltamethrin) to different degrees (Mutch and Williams 2006; Godin et al., 2007; Scollon et al., 2009). However, when the relative abundance of the different isoforms is taken into account, the predominant hepatic Cyp enzymes (Cyp1a2, Cyp2c50, and Cyp3a11) are all considerably reduced during the course of gestation, especially at GD14. The findings with Cyp1a2 and 3a11 are in agreement with two other studies (Koh et al., 2011; Walker et al., 2011) but also extend them by demonstrating decreased expression of Cyp3a11 out to PD30. Functional data in humans indicate the human orthologs of Cyp1a2 (CYP1A2) and Cyp2c50 and 54 (CYP2C19) enzymes are also likely decreased during pregnancy, which parallels our findings (Anderson 2005). However, our data and that from Koh and coworkers (2011) found that Cyp2d22 is decreased throughout gestation, in contrast to the increased metabolism of CYP2D6 substrates in pregnant women (Anderson, 2005; Hodge and Tracy, 2007). This discrepancy might be explained by the fact that several isoforms in mice are orthologous with CYP2D6 (Nelson et al., 2004), which we did not measure. Alternatively, it could suggest that the pregnant mouse is not a good model for human pregnancy and compounds metabolized by CYP2D6. Additional functional experiments are required to address this issue.
We also found that Cyp2b10 mRNA is decreased during early and midgestation and increases expression over that of virgin mice starting at GD17 and continuing through PD15. This pattern is consistent with the decreased CAR expression observed on GD11 and 14. To our knowledge, data regarding the regulation of the human ortholog CYP2B6 during pregnancy are still lacking. However, two recent articles found that high concentrations of estradiol induce 2B6 expression and activity in hepatocytes (Dickmann et al., 2008; Koh et al., 2012). Given this finding and the fact that estradiol levels are highest in mice at 18 to 19 days of gestation (McCormack and Greenwald, 1974), this may provide a plausible mechanism for the increased 2b10 expression observed at PD1. The estrogen surge may also be relevant to the induction of female-specific Cyp3a41a/b and Cyp3a44, as well as the juvenile Cyp3a16 isoform we observed, confirming results previously reported (Zhang et al., 2008), although glucocorticoids and growth hormone may also play a role in the induction of these isoforms (Sakuma et al., 2008). Cyp3a11, Cyp3a16, Cyp3a41a/b, and Cyp3a44 are orthologs of human CYP3A4 and 3A7. Thus, although we observed a profound decrease in Cyp3a11, it is possible that these other isoforms are responsible for the observed increased metabolism of CYP3A4 substrates in humans (Anderson, 2005) and mice (Zhang et al., 2008). Because of these multiple Cyp3A orthologs in rodents and the shift from constitutive to inducible forms (female-specific and juvenile), it will be important in the future to determine functionally whether hepatic P450-mediated metabolism of OPs and PYRs is increased or decreased during pregnancy in the mouse. However, the potential alterations of Ces and Pon1 are also likely to play an important role in the disposition of OPs and PYRs in pregnancy.
Ces stoichiometrically degrades active OP metabolites, including chlorpyrifos-oxon, whereas Pon1 actively hydrolyzes chlorpyrifos-oxon (Pond et al., 1995). Thus, the general decreased expression of some of the Ces isoforms during early gestation observed here may increase exposure to chlorpyrifos-oxon. Although the expression of CES has not been assessed in pregnancy, bioactivation of the antiviral drug oseltamivir, a CES1 substrate, is decreased in pregnant women, suggesting reduced CES1 activity (Beigi et al., 2011). We also observed reduced expression of Pon1 at GD14, but this did not quite reach statistical significance. However, PON1 activity has been documented to decrease through the course of pregnancy in humans (Ferre et al., 2006). To address this issue, we measured Pon1 activity in the serum toward two substrates, paraoxon and chlorpyrifos-oxon. Similar to that observed in humans (Ferre et al., 2006), we measured significant decreases in serum Pon1 activity toward these two substrates on GD14 compared with virgin mice. This decreased activity is consistent with the observation of increased parathion toxicity in pregnant mice (Weitman et al., 1983) and demonstrates a functional correlation between hepatic mRNA expression of Pon1 and enzymatic activity.
The effects of pregnancy on the Cyps and Ces is also likely to have a profound influence on the metabolism of pyrethroid insecticides, as metabolism by CYPs and CESs serve to inactivate PYR pesticides (Soderlund et al., 2002). PYR use has increased significantly over the past 10 years, particularly for residential purposes (Horton et al., 2011). Although data are limited (Shafer et al., 2005), there is increasing concern over the potential developmental neurotoxicity of PYRs since developing animals are more sensitive to their neurotoxic effects (Sheets, 2000). As with chlorpyrifos, this increased susceptibility is hypothesized to be the result of immature detoxication enzymes, including various CYPs and CESs (Tornero-Velez et al., 2010). Various CYP isoforms are capable of contributing to the metabolism of a wide variety of PYR, including CYP2C19, which was the most active human isoform against several PYRs (Godin et al., 2007). Based on the data presented here, the mouse orthologs of CYP2C19, 2c50, and 2c54 were decreased significantly throughout pregnancy, which might suggest that pregnant women may have reduced capacity to metabolize certain PYRs. Hydrolysis of PYRs by CES enzymes has been demonstrated by the urinary metabolite profile following low-dose administration in human volunteers (Eadsforth et al., 1988, Woollen et al., 1992) and in human purified CES (Godin et al., 2007). Human CES1 and 2 hydrolyze the central ester bond to produce two moieties, each of which has no known neurotoxic properties (Gaughan et al., 1976). In our study, the mRNA expression of Ces 1d, 1e, and 1g were decreased by 25% to 30% from GD7 to GD14, although most of the results did not quite reach statistical significance. Likewise, Ces2a was significantly reduced at early time points, whereas Ces2e decreased later in pregnancy. The lack of statistical significance may reflect the low number of mice per group and variability in Ces expression in the virgin mice. Additional studies to determine the functional relevance of these changes during pregnancy on the metabolism of PYRs are currently ongoing.
Overall, our results suggest that gestation in mice generally decreases the expression of a number of pesticide-metabolizing enzymes, which may increase maximal insecticide concentration and duration of exposure. Most of the changes in phase 1 enzymes reported in the present study are consistent with those presented elsewhere (Koh et al., 2011), as well as with the functional studies conducted in humans (Anderson, 2005). Additionally, this study provides novel information regarding the expression of Ces isoforms Pon1, Cyp2c50, and Cyp2c54 during pregnancy in mice, which has not previously been reported. However, additional studies of OP and PYR metabolism in pregnant mice are needed to determine the functional relevance of altered mRNA expression. In turn, these data will provide important information to quantify properly the risks for the pregnant women and their unborn children.
Supplementary Material
Acknowledgments
The authors thank Dr. Curtis Klaassen for providing livers for the mRNA time course experiments.
Abbreviations
- CAR
constitutive androstane receptor
- Cyp
mouse cytochrome P450
- CYP
human cytochrome P450
- Ces
mouse carboxylesterase
- CES
human carboxylesterase
- GD
gestational day
- OP
organophosphate
- PCR
polymerase chain reaction
- PD
postpartum day
- Pon1
mouse paraoxonase
- Pparα
peroxisome proliferator-activated receptor α. PYR, pyrethroid
- qPCR
quantitative PCR
Authorship Contributions
Participated in research design: Fortin, Aleksunes, Richardson.
Conducted experiments: Fortin, Richardson.
Performed data analysis: Fortin, Richardson.
Wrote or contributed to the writing of the manuscript: Fortin, Aleksunes, Richardson.
Footnotes
This work was supported in part by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK080774] and National Institutes of Health National Institute of Environmental Health Sciences [Grants ES015991, ES020522, ES007148, and ES005022].The content of this manuscript does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institute of Environmental Health Sciences.
 This article contains supplemental material available at dmd.aspetjournals.org.
This article contains supplemental material available at dmd.aspetjournals.org.
References
- Anand SS, Kim KB, Padilla S, Muralidhara S, Kim HJ, Fisher JW, Bruckner JV. (2006) Ontogeny of hepatic and plasma metabolism of deltamethrin in vitro: role in age-dependent acute neurotoxicity. Drug Metab Dispos 34:389–397 [DOI] [PubMed] [Google Scholar]
- Anderson GD. (2005) Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44:989–1008 [DOI] [PubMed] [Google Scholar]
- Atterberry TT, Burnett WT, Chambers JE. (1997) Age-related differences in parathion and chlorpyrifos toxicity in male rats: target and nontarget esterase sensitivity and cytochrome P450-mediated metabolism. Toxicol Appl Pharmacol 147:411–418 [DOI] [PubMed] [Google Scholar]
- Beigi RH, Han K, Venkataramanan R, Hankins GD, Clark S, Hebert MF, Easterling T, Zajicek A, Ren Z, Mattison DR,, et al. (2011) Pharmacokinetics of oseltamivir among pregnant and nonpregnant women. Am J Obstet Gynecol 204:S84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, Landrigan PJ, Wolff MS. (2003) Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect 111:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorina R, Bradman A, Fenster L, Barr DB, Bravo R, Vedar MG, Harnly ME, McKone TE, Eisen EA, Eskenazi B. (2010) Comparison of current-use pesticide and other toxicant urinary metabolite levels among pregnant women in the CHAMACOS cohort and NHANES. Environ Health Perspect 118:856–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D, Jacob P, 3rd, Benowitz NL. (2002) Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther 301:594–598 [DOI] [PubMed] [Google Scholar]
- Dickmann LJ, Tay S, Senn TD, Zhang H, Visone A, Unadkat JD, Hebert MF, Isoherranen N. (2008) Changes in maternal liver Cyp2c and Cyp2d expression and activity during rat pregnancy. Biochem Pharmacol 75:1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadsforth CV, Bragt PC, van Sittert NJ. (1988) Human dose-excretion studies with pyrethroid insecticides cypermethrin and alphacypermethrin: relevance for biological monitoring. Xenobiotica 18:603–614 [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. (2007) Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect 115:792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré N, Camps J, Fernández-Ballart J, Arija V, Murphy MM, Marsillach J, Joven J. (2006) Longitudinal changes in serum paraoxonase-1 activity throughout normal pregnancy. Clin Chem Lab Med 44:880–882 [DOI] [PubMed] [Google Scholar]
- Gaughan LC, Unai T, Casida JE. (1976) Permethrin metabolism in rats. J Agric Food Chem 25:9–17 [DOI] [PubMed] [Google Scholar]
- Godin SJ, Crow JA, Scollon EJ, Hughes MF, DeVito MJ, Ross MK. (2007) Identification of rat and human cytochrome p450 isoforms and a rat serum esterase that metabolize the pyrethroid insecticides deltamethrin and esfenvalerate. Drug Metab Dispos 35:1664–1671 [DOI] [PubMed] [Google Scholar]
- He XJ, Ejiri N, Nakayama H, Doi K. (2005) Effects of pregnancy on CYPs protein expression in rat liver. Exp Mol Pathol 78:64–70 [DOI] [PubMed] [Google Scholar]
- Holmes RS, Wright MW, Laulederkind SJ, Cox LA, Hosokawa M, Imai T, Ishibashi S, Lehner R, Miyazaki M, Perkins EJ, et al. (2010) Recommended nomenclature for five mammalian carboxylesterase gene families: human, mouse, and rat genes and proteins. Mamm Genome 21:427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge LS, Tracy TS. (2007) Alterations in drug disposition during pregnancy: implications for drug therapy. Expert Opin Drug Metab Toxicol 3:557–571 [DOI] [PubMed] [Google Scholar]
- Horton MK, Jacobson JB, McKelvey W, Holmes D, Fincher B, Quantano A, Diaz BP, Shabbazz F, Shepard P, Rundle A,, et al. (2011) Characterization of residential pest control products used in inner city communities in New York City. J Expo Sci Environ Epidemiol 21:291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Hanke W. (2008) Prenatal and childhood exposure to pesticides and neurobehavioral development: review of epidemiological studies. Int J Occup Med Environ Health 21:121–132 [DOI] [PubMed] [Google Scholar]
- Koh KH, Jurkovic S, Yang K, Choi SY, Jung JW, Kim KP, Zhang W, Jeong H. (2012) Estradiol induces cytochrome P450 2B6 expression at high concentrations: implication in estrogen-mediated gene regulation in pregnancy. Biochem Pharmacol 84:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KH, Xie H, Yu AM, Jeong H. (2011) Altered cytochrome P450 expression in mice during pregnancy. Drug Metab Dispos 39:165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- McCormack JT, Greenwald GS. (1974) Progesterone and oestradiol-17beta concentrations in the peripheral plasma during pregnancy in the mouse. J Endocrinol 62:101–107 [DOI] [PubMed] [Google Scholar]
- Mutch E, Williams FM. (2006) Diazinon, chlorpyrifos and parathion are metabolised by multiple cytochromes P450 in human liver. Toxicology 224:22–32 [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14:1–18 [DOI] [PubMed] [Google Scholar]
- Pond AL, Chambers HW, Chambers JE. (1995) Organophosphate detoxication potential of various rat tissues via A-esterase and aliesterase activities. Toxicol Lett 78:245–252 [DOI] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Wang M, Dean ED, Pennell KD, Miller GW. (2006) Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB J 20:1695–1697 [DOI] [PubMed] [Google Scholar]
- Richter RJ, Furlong CE. (1999) Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics 9:745–753 [PubMed] [Google Scholar]
- Sakuma T, Bhadhprasit W, Hashita T, Nemoto N. (2008) Synergism of glucocorticoid hormone with growth hormone for female-specific mouse Cyp3a44 gene expression. Drug Metab Dispos 36:878–884 [DOI] [PubMed] [Google Scholar]
- Scollon EJ, Starr JM, Godin SJ, DeVito MJ, Hughes MF. (2009) In vitro metabolism of pyrethroid pesticides by rat and human hepatic microsomes and cytochrome p450 isoforms. Drug Metab Dispos 37:221–228 [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. (2000) Identifying critical windows of exposure for children’s health. Environ Health Perspect 108 (Suppl 3):451–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer TJ, Meyer DA, Crofton KM. (2005) Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environ Health Perspect 113:123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets LP. (2000) A consideration of age-dependent differences in susceptibility to organophosphorus and pyrethroid insecticides. Neurotoxicology 21:57–63 [PubMed] [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. (2002) Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology 171:3–59 [DOI] [PubMed] [Google Scholar]
- Timchalk C, Poet TS, Kousba AA. (2006) Age-dependent pharmacokinetic and pharmacodynamic response in preweanling rats following oral exposure to the organophosphorus insecticide chlorpyrifos. Toxicology 220:13–25 [DOI] [PubMed] [Google Scholar]
- Tornero-Velez R, Mirfazaelian A, Kim KB, Anand SS, Kim HJ, Haines WT, Bruckner JV, Fisher JW. (2010) Evaluation of deltamethrin kinetics and dosimetry in the maturing rat using a PBPK model. Toxicol Appl Pharmacol 244:208–217 [DOI] [PubMed] [Google Scholar]
- US EPA (United States Environmental Protection Agency) (2011) Pesticide Industry Sales and Usage 2006 and 2007 Market Estimates. Office of Chemical Safety and Pollution Prevention, Washington DC EPA 733-R-11-001.
- Walker AA, Dickmann L, Isoherranen N. (2011) Pregnancy decreases rat CYP1A2 activity and expression. Drug Metab Dispos 39:4–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman SD, Vodicnik MJ, Lech JJ. (1983) Influence of pregnancy on parathion toxicity and disposition. Toxicol Appl Pharmacol 71:215–224 [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Garfinkel R, Hoepner LA, Andrews H, Holmes D, Williams MK, Reyes A, Diaz D, Perera FP, Camann DE, et al. (2009) A biomarker validation study of prenatal chlorpyrifos exposure within an inner-city cohort during pregnancy. Environ Health Perspect 117:559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. (2011) Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119:878–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollen BH, Marsh JR, Laird WJ, Lesser JE. (1992) The metabolism of cypermethrin in man: differences in urinary metabolite profiles following oral and dermal administration. Xenobiotica 22:983–991 [DOI] [PubMed] [Google Scholar]
- Yacovino LL, Gibson CJ, Aleksunes LM. (2013) Down-regulation of brush border efflux transporter expression in the kidneys of pregnant mice. Drug Metab Dispos 41:320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wu X, Wang H, Mikheev AM, Mao Q, Unadkat JD. (2008) Effect of pregnancy on cytochrome P450 3a and P-glycoprotein expression and activity in the mouse: mechanisms, tissue specificity, and time course. Mol Pharmacol 74:714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


