Abstract
Emerging evidence points to reactive glia as a pivotal factor in Parkinson’s disease (PD) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mouse model of basal ganglia injury, but whether astrocytes and microglia activation may exacerbate dopaminergic (DAergic) neuron demise and/or contribute to DAergic repair is presently the subject of much debate. Here, we have correlated the loss and recovery of the nigrostriatal DAergic functionality upon acute MPTP exposure with extensive gene expression analysis at the level of the ventral midbrain (VM) and striata (Str) and found a major upregulation of pro-inflammatory chemokines and wingless-type MMTV integration site1 (Wnt1), a key transcript involved in midbrain DAergic neurodevelopment. Wnt signaling components (including Frizzled-1 [Fzd-1] and β-catenin) were dynamically regulated during MPTP-induced DAergic degeneration and reactive glial activation. Activated astrocytes of the ventral midbrain were identified as candidate source of Wnt1 by in situ hybridization and real-time PCR in vitro. Blocking Wnt/Fzd signaling with Dickkopf-1 (Dkk1) counteracted astrocyte-induced neuroprotection against MPP+ toxicity in primary mesencephalic astrocyte–neuron cultures, in vitro. Moreover, astroglial-derived factors, including Wnt1, promoted neurogenesis and DAergic neurogenesis from adult midbrain stem/neuroprogenitor cells, in vitro. Conversely, lack of Wnt1 transcription in response to MPTP in middleaged mice and failure of DAergic neurons to recover were reversed by pharmacological activation of Wnt/β-catenin signaling, in vivo, thus suggesting MPTP-reactive astrocytes in situ and Wnt1 as candidate components of neuroprotective/neurorescue pathways in MPTP-induced nigrostriatal DAergic plasticity.
Keywords: Astroglia, Neurodegeneration, Neuroinflammation, Neuroprotection, Parkinson disease
Introduction
The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mouse model of basal ganglia injury recapitulates many of the pathogenetic processes operative in Parkinson’s disease (PD), a common neurodegenerative disorder characterized by the progressive loss of dopaminergic (DAergic) neurons in the subtantia nigra pars compacta (SNpc) and astrogliosis (Jackson-Lewis and Przedborski, 2007). The neurotoxin MPTP, converted into its active metabolite,1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPP+) in astrocytes, is selectively transported into striatal DAergic terminals via the DA transporter, DAT, where it induces oxidative stress, the opening of mitochondrial permeability transition pore (mPTP), the release of cytochrome c, and the activation of caspases (Abou-Sleiman et al., 2006; Jackson-Lewis and Przedborski, 2007). In synergy with these early events accounting for approximately 10% of DAergic neuronal death (Wu et al., 2002), glial inflammatory mechanisms are thought to contribute to nigrostriatal DAergic degeneration (Benner et al., 2004; Gao and Hong, 2008; Hu et al., 2008; Hoang et al., 2009; Hirsch and Hunot, 2009).
Astrocytes and microglia normally play neuroprotective roles; however, upon MPTP-mediated neuronal injury, activated microglia produce a panel of cytotoxic mediators including reactive oxygen and nitrogen species as well as proinflammatory cytokines that may perpetuate/exacerbate glial activation, thereby increasing neuronal vulnerability and/or promoting DAergic cell death (McNaught and Jenner, 1999a,b; Vila et al., 2001; Wu et al., 2002; Streit, 2002; Gao et al., 2003; Hauwel et al., 2005; Marchetti and Abbracchio, 2005; Griffiths et al., 2007; Hu et al., 2008; McGeer and McGeer, 2008). Astrocytes are known to secrete both inflammatory and anti-inflammatory, as well as neurotrophic and survival factors, and may play a role in modulating microglial activity, but in severe neurodegenerative conditions, they may lose their neuroprotective functions (McGeer and Mc Geer, 2008; Sandhu et al., 2009; Chen et al., 2009; L’Episcopo et al., 2010).
In response to MPTP injury, the nigrostriatal DAergic system exhibits compensatory mechanisms, but the degree of plasticity becomes reduced with age (Ricaurte et al., 1987; Hornykiewicz, 1993; Blanchard et al., 1996; Ho and Blum, 1998; Bezard and Gross, 1998; Stanic et al., 2003; Jacowec et al., 2004). Age-related alterations, including increased DAergic neuron vulnerability, increased microglia activation/dysfunction, altered glia–neuron crosstalk, and/or impaired neurogenesis represent potential contributory factors (Butovsky et al., 2006; Miller and Streit, 2007; Griffiths et al., 2009; Streit, 2010; Boger et al., 2010; Njie et al., 2010; Sharpless, 2010).
Recently, the wingless-type MMTV integration site1 (Wnt) pathway has emerged as an essential signaling cascade that regulates multiple processes in developing and adult tissues, including differentiation, neuron survival, axonal extension, synapse formation and plasticity, neurotrophin transcription, and neurogenesis (Patapoutian and Reichardt, 2000; Ciani and Salinas, 2005; Maiese et al., 2008;Inestrosa and Arenas, 2009). The extracellular Wnt molecules signal into the cell via three different pathways: the “canonical” Wnt/β-catenin and “non-canonical” Wnt/planar cell polarity (PCP) and Wnt (Ca2+) pathways (Ciani and Salinas, 2005). Common to all three pathways is binding of the Wnt ligand to the seven-pass transmembrane receptors of the Frizzled (Fzd) family. The hallmark of Wnt/β-catenin pathway is the stabilization of cytosolic β-catenin, which enters the nucleus and activates the transcription of Wnt target genes involved in cell survival, proliferation, and differentiation (Gordon and Nusse, 2006). Recent pieces of evidence clearly indicate that Wnt/β-catenin signaling plays a central role in midbrain DAergic neurodevelopment both in vivo and in vitro (Castelo-Branco et al., 2003, 2004; Prakash and Wurst, 2006; Joksimovic et al., 2009), but very little is known on Wnt1/β-catenin signaling in the adult PD midbrain.
To elucidate some of the molecular mechanism(s) underlying MPTP-induced injury and self-recovery, we used extensive gene expression analysis of a total of 92 mRNA species involved in inflammation, immunity, stemness, self-renewal, DAergic development, and DA metabolism and identified a major upregulation of pro-inflammatory transcripts and Wnt1. We herein provide pieces of evidence indicating Wnt1/β-catenin signaling and MPTP-reactive astrocytes as candidate components of neurorescue pathways involved in nigrostriatal DAergic plasticity.
Materials and methods
Mice and treatments
Eight- to ten-week-old male C57Bl/6 (Charles River, Calco, Italy) (body weight 24–26 g) received n=4 intraperitoneal (i.p.) injections of vehicle (saline, 10 ml/kg) or MPTP–HCl (20 mg kg−1 free base; Sigma) dissolved in saline, 2 h apart in 1 day, according to the acute MPTP injection paradigm (Jackson-Lewis et al., 1995). Mice were sacrificed at either early (e.g., 3, 6, 24, 72 h), mid (e.g., 5, 7, and 14 days), and late (e.g., 21, 28, 35, 42 days) time points upon MPTP administration (Table 1). MPTP was handled in accordance with the reported guidelines (Jackson-Lewis and Przedborski, 2007). Studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH) and approved by the Institutional Animal Care and Use Committee.
Table 1.
Schematic representation of MPTP time course, animal number, and experimental determinations per time point (tp).
| MPTP time course | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyses | Mice no. | −7 | 0 | +3h | +6h | +24h | +3 days | +7d | +14d | +21d | +28d | +35d | +42d |
| Gene expr. | 6/tp | + | + | + | + | + | + | + | + | + | + | + | |
| Behavior | 10/tp | + | + | + | + | + | + | + | +24h | ||||
| Neurochem. | 6/tp | + | + | + | + | + | + | + | + | ||||
| Histopathol. | 6/tp | + | + | + | + | + | + | + | + | + | |||
| Western blot | 4/tp | + | + | + | + | + | +24h | ||||||
| In situ hybrid. | 4/tp | + | + | ||||||||||
Eight- to ten-week-old male C57Bl/6 received n=4 intraperitoneal (i.p.) injections of vehicle (saline, 10 ml/kg) or MPTP–HCl (20 mg kg−1 free base; Sigma) dissolved in saline, 2 h apart in 1 day, according to the acute MPTP injection paradigm (Jackson-Lewis et al., 1995). Mice number used for the experimental protocol is indicated for each set of analyses per time point (tp). Motor behavior with the Rotarod was analyzed before (−7 days) and at the indicated time intervals after MPTP administration. Striatum and ventral midbrains tissues were processed as reported according to the different determinations. For in situ hybridization and immunohistochemical analyses, on the day of sacrifice, mice were anesthetized and transcardially perfused, the brains were processed as indicated. See Material and methods section for details.
High-performance liquid chromatography (HPLC) determination of striatal neurochemicals and high-affinity [3H]dopamine uptake assay
At the indicated time intervals, mice were sacrificed by cervical dislocation, the brains were rapidly removed and immediately placed on ice-cold saline. The right and left striata were then dissected on an ice-cold plastic dish and processed as described in full details (Serra et al., 2002). Dopamine, dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were determined by HPLC as reported in full details (Serra et al., 2002). Synaptosomial, high-affinity dopamine uptake was assessed in the presence of 10 μM mazindol, according to Morale et al. (2004).
Motor behavior analysis with the rotarod
An accelerating rotarod (five-lane accelerating rotarod; Ugo Basile, Comerio, Italy) was used to measure motor coordination in mice. Mice have to keep their balance on a horizontal rotating rod (diameter, 3 cm), and rotation speed was increased every 30 s by 4 rpm. Five mice were tested at the same time, separated by large disks. A trial starts when the mouse is placed on rotating rod, and it stops when the mouse falls down or when 5 min is completed. Falling down activates a switch that automatically stops a timer. On testing day, each mouse is submitted to 5 trials with an intertribal interval of 30 min. Mice housed five per cage were acclimated to a 12-h shift in light/dark cycle so that the exercise occurred during the animals normal wake period. Saline- and MPTP-treated mice (10/experimental group) were assessed for their Rotarod performance on day —7, 1, 7, 14, 21, 28, and 35 after MPTP injection.
Gene expression analysis and real-time PCR
TaqMan Low-Density Array cards (Applied Biosystems) configured into four identical 96-gene sets, each set containing the housekeeping gene GAPDH as control, were performed onto both striatum (Str) as well as the ventral midbrain (VM, containing the substantia nigra pars compacta, SNpc) tissue samples derived from controls and MPTP-treated mice.
RNA extraction
At due time points, RNA extraction was carried out in tissue samples homogenized in 1 ml of QIAzol Lysis Reagent (Qiagen, #79306) using a rotor-stator homogenizer. Total RNA was isolated from homogenized tissue samples using RNeasy Lipid Tissue Kit (Qiagen, #74804) including Dnase digestion. At the end, RNA samples were redissolved in 30 μl of RNase-free water, and their concentrations were determined spectrophotometrically by A260 (Nanodrop-ND 1000).
Reverse transcription
cDNA synthesis was performed using Ready-To-Go You-Prime First-Strand Beads (GE Healthcare, #27-9264-01) and Random Hexamer (New England BioLabs, #S1230S) according the manufacturer’s instructions.
TLDA design and preparation
For each low-density array, there are eight separate loadings ports that feed into 48 separate wells for a total of 384 wells per card. Each 2-μl well contains specific, user-defined primers and probes, capable of detecting a single gene. The TaqMan strategies for each gene have been developed as TaqMan Gene Expression Assays by Applied Biosystems. In this study, the TLDA cards were configured into four identical 96-gene sets and each set also contains three housekeeping genes, β-actin (ACTB), glyceraldehydes-3-phosphate dehydrogenase (GAPDH), and 18S ribosomal RNA (a mandatory control designed into each array by the manufacturer). In this study, however, GAPDH was used exclusively as the housekeeping gene. For each sample, we designed a duplicate assay. Pools of cDNA from samples at the same time point and contained the same amount of RNA were prepared; 50 μl of water solution containing 0.5 μg of each pool was added to an equal volume of 2× TaqMan Universal PCR Master Mix (Applied Biosystems) and loaded into each of eight ports on the TaqMan Low-Density Array (Applied Biosystems).
Real-time PCR was performed on an ABI Prism 7700 Sequence detection system (Applied Biosystems, USA) using the TaqMan Universal PCR master mix (# 4304437); the primers and the fluorogenic probes for each target by Applied Biosystems the relative expression ratios were evaluated; for the statistical analysis, the analysis of variance (ANOVA) with repeated measures was used, as described (SAS Institute, Cary, NC, USA, www.statview.com).
Immunohistochemistry
On the day of sacrifice, mice were anesthetized and transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde in phosphate buffer (pH 7.2 at 4 °C), the brains were carefully removed and processed as described in full details (Morale et al., 2004). Tissues were frozen and stored at −80 °C until further analyses. Serial coronal sections (14 μm thick), encompassing the striatum (Bregma 1.54 to bregma −0.46) and the SNpc (Bregma −2.92 to bregma −3.8 mm) according to Franklin and Paxinos (1997) were collected, mounted on poly-l-lysine-coated slides, and processed as previously described in full details (Gallo et al., 2000; Morale et al., 2004). The following pre-absorbed primary antibodies were used: rabbit anti-tyrosine hydroxylase (TH, Chemicon International, USA), the rate-limiting enzyme in DA synthesis; rabbit anti-TH (Peel Freez Biochemicals, Rogers, AR); mouse anti-TH (Boehringer Mannheim Bioc., Philadelphia, USA), rat anti-dopamine transporter (DAT, Chemicon, Int. USA); mouse anti-neuron-specific nuclear protein (NeuN, US Biologicals, Swampscott, Massachusetts); rabbit anti-glial fibrillary acidic protein (GFAP, Dako, Cytomation, Denmark), mouse anti-glial fibrillary acidic protein (GFAP, Sigma, S. Luis MO, USA) as astrocyte-specific cell marker; goat anti-ionized calcium-binding adapter molecule 1 (IBA1, Novus Biologicals, Littleton, CO), as microglia-specific marker; rabbit anti-β-catenin (Abcam, Cambridge, UK), a key intermediate in the canonical Wnt1 signaling pathway (Logan and Nusse, 2004), rabbit anti-glycogen synthase kinase-3β (GSK-3β, S. Cruz, CA, USA); mouse anti-GSK-3β phospho-Tyr216 (BD, Biosciences). Degenerating neurons were labelled with Fluorojade C (FJC, Chemicon, USA) as described (Schmued et al., 1997). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in mounting medium (Vector Laboratory, Burlingam, CA). Sections were washed extensively and incubated with fluorochrome (FITC, CY3, CY5)-conjugated species-specific secondary antibodies for immunofluorescent detection. TH immunoreactivity was also detected using biotinylated secondary antibodies (Vector Laboratories) and diaminobenzidine (DAB, Vector Laboratories) as the developing agent as described (Gallo et al., 2000; Morale et al., 2004). Cresyl violet was used to visualize Nissl substance. In all of these protocols, blanks were processed as for experimental samples except that the primary antibodies were replaced with PBS.
Cell counts and image analysis
Quantitative analysis of DAergic neurons in the SNpc was carried out by serial section analysis of the total number of TH-positive (TH+) and NeuN-positive (NeuN+) neurons throughout the entire rostro-caudal axis of the SNpc (Franklin and Paxinos, 1997) as previously described (Morale et al., 2004; Conti et al., 2005). Total numbers of TH− and cresyl violet (CV)-stained neurons in adjacent tissue sections were estimated in parallel to validate TH+ neuron survival, using Abercrombie correction (Abercrombie, 1946). The total number of FJC-stained cells in SNpc was calculated for each side, averaged for each animal, and normalized to the number of TH+ neurons in SNpc per section.
Striatal TH and dopamine transporter (DAT)-immunoreactive (IR) fiber staining was assessed in n=3 coronal sections at three levels (bregma coordinates : + 0.5, + 0.86, and 1.1 mm, respectively) of caudate-putamen (CPu), in n=6 mice/group/time (Burke et al., 1990). Fluorescence intensity (FI) of TH and DAT-staining was above a fixed threshold using the corpus callosum for background subtraction.
Cell counts were obtained for ameboid IBA1+ microglial cells (Kreutzberg, 1996) and GFAP+ astrocytes. For SN cell counts, at least three sections were obtained from each animal representing each of the five representative planes from −2.92 to −3.8 mm relative to bregma according to the stereotaxic coordinates of Franklin and Paxinos (1997). Cell counts in striatum were assessed as above. GFAP+ astrocyte and IBA1+ microglial cell number per unit of surface area was determined in 8–10 randomly selected fields/section on both sides, the counts averaged for each animal and the mean number of cells per millimeter squared were estimated.
Fluorescence microscopy and image analysis were carried out with a confocal laser scanning microscope LEICA TCS-NT (Version 2.5, Build 1227, Leica Microsystems GmBH, Heidelberg, Germany, equipped with image analysis software), with an argon/krypton laser using 10×, 20×, and 40× and 100× (oil) immersion objectives (Morale et al., 2004; Gennuso et al., 2004). Measurements of FI were carried out by computer-assisted image analysis software (LEICA), and changes in average FI (mean ± SD) were expressed as percentage (%) of saline-injected controls.
Western blot analysis
Protein extracts were prepared for striatum and ventral midbrain (which included the SNpc) (left and right sides) at the indicated time-intervals after saline or MPTP injections (n=4 per group). The tissue samples were homogenized in lysis buffer (0.33 M sucrose/8 mM Hepes, pH 7.4 and protease inhibitors) and quantified using the BCA protein determination method (Bio-Rad, Hercules, CA). Protein samples were diluted to equivalent volumes containing 20 μg of protein and boiled in an equal volume of Laemli SDS boiling buffer (Sigma) for 10 min. Samples were loaded into a 9–12% SDS-polyacrilamide gel and separated by electrophoresis for 3 h at 100 V. Proteins were transferred to polyvinylidene difluoride membrane (Amersham Biosciences, Piscataway, NJ) for 1.5 h at 300 mA. After blocking of nonspecific binding with 5% nonfat dry milk in TBST, the membranes were then probed with the following primary antibodies: rabbit anti-TH (Chemicon), rat anti-DAT (Millipore), rabbit anti-GFAP (DAKO), rabbit anti-Wnt1 (Abcam), mouse anti-β-catenin (Transduction Labs), mouse anti-GSK-3β (Transduction Laboratories), mouse anti-GSK-3β phospho-Tyr216 (BD Biosciences), goat anti-Fzd-1 (Santa Cruz Biotechnology, Inc), β-actin (Cell Signaling). After incubation at room temperature for 1 h, membranes were washed and treated with appropriate secondary antibodies conjugated with horseradish peroxidase (HRP) and blot were exposed onto radiographic film (Hyperfilm; Amersham, Bioscience). Membranes were reprobed for β-actin immunolabeling as an internal control. The bands from the Western blots were densitometrically quantified on X-ray films using a software to determine the levels of immunoreactivity (ImageQuantity One). The data from experimental bands were normalized to β-actin. Values of GSK-3β phospho-Tyr216 were normalized for its respective control, GSK-3β, before statistical analysis of variance and values were expressed as percent (%) of saline-injected controls.
In situ hybridization
In situ hybridization was performed using FITC AntisensRNA probes (Sigma Aldrich) specific for Wnt1 and GAPDH used as the control probe. The sequences of Wnt1 and GAPDH correspond to GeneBank NM021279 Loc.2106 and NM008084 Loc. 7.
Midbrain cryosections (12 μm) at the level of the SNpc were treated with PBS for 10 min, 15 μg/ml proteinase K for 5 min at 37 °C, TEA (triethanolamine) 0,1 M PH 8 for 10 min, 1% acetic acid/0,1 M TEA for 10 min, and were then gradually dehydrated in EtOH up to 100%. Hybridization was carried out at 56 °C O/N in a humid chamber, the hybridization solution contained 50% deionized formamide, 5×SSC (standard saline citrate), 50 μg/ml yeast RNA, 1 M DTT (dithiothreitol), and 500 ng/ml RNA probe.
After hybridization, the slides were washed with 4×SSC, then treated with RNase A for 30 min at 37 °C, washed with 2× SSC for 10 min, SSC 1× for 5 min, SSC 0,1× for 30 min at 56 °C then 0.1× SSC 10 min at room temperature. The slides were then processed for GFAP immunohistochemistry, as described above.
Astrocyte cell cultures and treatments
Primary astroglial cell cultures were obtained from mouse ventral midbrain (VM), at postnatal days 2–4 (P2–P4) as described in full details (Gallo et al., 2000; Gennuso et al., 2004). The cultures were allowed to grow and differentiate until they reached confluency at which time (13–15 days in vitro, DIV) the loosely adherent microglial cells were separated by shaking for 2 h at 37 °C and 190 rpm. The attached cells were then washed with sterile PBS and incubated for 1–2 h at 37 °C incubator, at 5% CO2, before overnight shaking at 37 °C and 210 rpm. The supernatant media containing oligodendrocyte precursors and other cell types were discarded. The glial (more than 95% of the cells were GFAP-IR astrocytes) monolayers were then rinsed with sterile PBS and replated a final density of 0.4–0.6×105 cells/cm2 in poly-D-lysine (10 μg/ml)-coated 6, 12- or 24-well plates, or in insert membranes (0.4 μm polyethylene terephthalate) for indirect co-culture (BD Biosciences). Cultures of type 1 astrocytes isolated from VM of 8- to 10-week-old saline- and MPTP-treated (C57CL/J) mice were prepared according to Schwartz and Wilson (1992), as described in full details.
Astrocyte monolayers received a pretreatment with PBS (UAstro) or increasing doses (50–1000 ng/ml) of one each chemokine (CCL3, CXCL10, CXCL11, TAstro) whose mRNA was found to be hyperexpressed in MPTP mice. All treatments were carried out for only one time, and after 24 h, the cells were processed for RT-PCR.
Adult neural stem/precursor cells (NPCs) from subventricular zone (SVZ) and midbrain (MB) derivation and culture
Green fluorescent protein (GFP)-expressing adult neural/stem precursor cells (aNPCs) derived from the subventricular zone (SVZ) were obtained as previously described in full details (Pluchino et al., 2003, 2005). For aNPCs derivation from the midbrain/hindbrain, adult male C57BL/6 mice were killed by cervical dislocation and their brains were removed and placed in PBS. The midbrain and hindbrain region was microdissected using a dissection microscope and aseptically prepared (Hermann et al., 2006). The tissue was minced and then incubated for digestion at 37 °C, 5% CO2 for 40 min in Earle’s balanced salt solution containing 0.94 mg/ml papain (Worthington, Lakewood, NJ), and 0.18 mg/ml of l-cysteine and EDTA. After centrifugation at 1100 rpm for 12 min at room temperature, the tissue was mechanically dissociated by pipette trituration. Cells obtained from single-cell suspensions were plated (8000 cells/cm2) in 25-cm2 Falcon tissue-culture flasks (BD Biosciences) in NPC-culturing medium [Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (Gibco/Invitrogen) containing 2 mMl-glutamine, 0.6% glucose, 9.6 μg/ml putrescine, 6.3 ng/ml progesterone, 5.2 ng/ml sodium selenite, 0.02 mg/ml insulin, 0.1 mg/ml transferring, 2 μg/ml heparin (all from Sigma), fibroblast growth factor 2 (FGF2, human recombinant, 20 ng/ml), and epidermal growth factor (EGF, human recombinant, 20 ng/ml). The number of primary spheres was counted after 7–10 DIV. For cell amplification (8000 cells/cm2) were plated at each sub-culturing passage in untreated tissue culture flasks. After 4–5 days, neurospheres were harvested, mechanically dissociated, counted, and replated under the same culture conditions. aNPCs from either the SVZ or midbrain at passage numbers 8–12 were used in all in vitro experiments. The differentiation of aNPCs was initiated after 3–12 weeks by removal of mitogens and plating the cells on PDL (monotypic cultures), or onto astrocyte monolayers (co-cultures). For analyzing DA differentiation capacity of aNPCs, a number of experimental settings, including absence or presence of FCS (2.5–20%), direct addition of exogenous factors such as chemokines (i.e., CCL3, CXCL10, CXCL11), or DA-specific factors (i.e., GDNF, BDNF at 20 ng/ml) were tested. However, only the co-culture setting between midbrain aNPCs and VM astrocytes promoted DA neurogenesis.
Astrocyte–aNPCs co-cultures and treatments
Twenty-four hours after treatment of the glial monolayers with the different chemokines, residues of the growth medium and the chemokines were washed off by rinsing twice with fresh serum-free–N2 medium and each of the untreated or treated astrocyte cell preparation was freshly co-cultured with dissociated aNPCs from SVZ or MB for either 3 or 7 DIV. For proliferation studies, the nucleotide analogue bromodeoxyuridine (BrdU, 5 μM) was added at different time intervals and cells fixed after 24 h. For Wnt antagonism studies, the soluble Wnt inhibitor, Dikkhopf-1 (Dkk-1, R&D Systems, MN, USA, 100 ng/ml), was applied to NPCs just prior to start the co-culture with astrocytes. For DA differentiation, NPCs were grown alone, or layered on the top of u-astro or t-astro in differentiation medium containing 2.5% FCS instead of BSA, for 3 DIV, at which time the medium was changed and replaced with fresh differentiation medium (N2 medium without serum, containing 1 mg/ml BSA and 200 μM ascorbic acid), and cells were maintained for additional 4 days.
After 3 and 7 DIV, the cultures were fixed and processed for fluorescent immunocytochemistry. Briefly, cell cultures were fixed in 4% paraformaldehyde in PBS or with paraformaldehyde/PBS followed by ice-cold acidic ethanol and HCL for BrdU staining (Gennuso et al., 2004). The following markers were used: mouse anti-Tuj1, an early marker of differentiating neurons, rabbit anti-Tuj1 (both from Covanche, Richmond, CA), rabbit anti-TH (Pel-Freez, Rogers, AR), rabbit anti-TH, rabbit anti-GFAP (both from Chemicon), rat-anti BrdU (Abcam), mouse anti-BrdU (DAKO). Nuclei were counterstained with DAPI. Analyses were performed using a confocal laser microscope and computer-assisted image analysis (Leica). For quantification of the amount of cells expressing a given marker or marker combinations, the number of Tuj1+ cells was determined relative to the total number of DAPI/PI-labeled nuclei or relative to GFP+ cells; the number of TH+ cells was determined relative to the total number of Tuj1+ cells, the number of BrdU+ cells was determined relative to the total number of Tuj1+ cells or GFP+ cells, using the Leica lite Software and three-dimensional overlay to avoid false-positive/negative overlay and double counting.
Primary midbrain astroglial–neuron cultures and enriched neuronal cultures
Primary midbrain astrocyte–neuron cultures were prepared from the brain of embryonic days 13–14, as described (Gao et al., 2003). Briefly, the ventral midbrain portion of embryonic brains was dissected out under a microscope and kept in cold MEM. Mesencephalic tissues were isolated and dissociated with gentle mechanical trituration. Cells were diluted to 1.5×106/ml in maintenance medium (MEM supplemented with 10% heat-inactivated FBS, 10% heat-inactivated horse serum, 1 g/l glucose, 2 mM glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 50 U/ml penicillin, and 50 μg/ml streptomycin) and seeded into 24-well culture plates precoated with poly-D-lysine (20 μg/ml). Plates were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Seven-day-old cultures were used for treatment. The composition of the cells at the time of treatment was 53% astrocytes, 6% microglia and 41% neurons with 1 % of the neurons being TH+ neurons.
To obtain neuron enriched cultures, cytosine β-D-arabinofurano-side (Ara-c) was added to the final concentration of 6 μM 36 h after seeding the cells, to suppress glia proliferation. Cultures were changed back to maintenance medium 2 days later and were used for treatment 7 days after initial seeding. Neuronal enrichment was verified by immunocytochemistry using GFAP and TH and NeuN-Abs as described. ARA-C treatment reduced glial expression by 95%. Both purified neuronal cultures and astroglial–neuron cultures at 7 DIV received increasing doses (5, 25, and 100 μM) of MPP+. For Wnt antagonism studies, the Wnt inhibitor, Dkk-1 (R&D Systems, 100 ng/ml) was applied in purified neuronal cultures and astroglial–neuron cultures, as described. Conversely, exogenous activation of Wnt/β-catenin signaling in VM astrocyte–neuron MPP+-treated cultures was carried out with the specific GSK-3β antagonist, AR-AO14418 [N-(4-methoxybenzyl)-N′-(5-nitro-1,3-thiazol-2-yl)urea] (AR, of 5 μM Osakada et al., 2007). The specificity of astrocyte neuroprotective effect and the contribution of Wnt1 were further tested in purified neuronal cultures exposed to astrocyte inserts (indirect astrocyte–neuron co-culture). In this experimental paradigm, the inserts containing the astrocyte monolayer were added on the top of the purified neurons. These inserts allowed diffusion of factors from the glia monolayer to the mesencephalic neurons and vice versa, without direct contact between cells (Gallo et al., 2000). Wnt1 antibody (2 μg/ml) or the Fzd antagonist, Frizzled-1-cysteine-rich domain (Fzd-A, 200 ng/ml, R&D Systems, Osakada et al., 2007), that bind to the Wnt ligand preventing its interaction with the Fzd receptor were added to the neuronal cultures prior MPP+ application. The effect of an unrelated antibody (anti-prolactin polyclonal IgG, R&D Systems) was also tested and demonstrated to be without effect on TH+ neuron survival and served as control. DAergic neuron survival was estimated after 24 h, by counting the number of TH+ neurons over the DAPI-positive nuclei, and TH+ neurons expressed as percent (%) of control (-MPTP), and by determination of [3H]DA incorporation, which reflects DAergic cell count and functionality. Uptake of [3H]DA was performed essentially as previously described, by incubating the cell cultures for 20 min at 37° with 1 μM [3H]DA in Krebs-Ringer buffer (16 mM sodium phosphate, 119 mM NaCl, 4.7 mM KCl, 1.8 mM CaCl2, 1.2 mM MgSO4, 1.3 mM EDTA, and 5.6 mM glucose (PH 7.4). Non-specific DA uptake was blocked by mazindol (10 μM). Cells were then collected in 1 N NaOH after washing in ice-cold Krebs-Ringer buffer. Radioactivity was determined by liquid scintillation and specific [3H]DA uptake calculated by subtracting the mazindol counts from the wells without the uptake inhibitor.
Pharmacological activation of Wnt/β-catenin signaling in middle-aged mice
In order to link Wnt signaling pathway to the recovery process, in vivo, we took advantage of the lack of recovery of middle-aged mice observed after MPTP acute exposure (Ho and Blum, 1998). Middle-aged (9–11 months of age) C57Bl/6 (Charles River, Calco, Italy) male mice were then used to address (i) the effect of age in Wnt/β-catenin signaling in response to MPTP and (ii) the effect of pharmacological activation of Wnt/β-catenin signaling in nigrostriatal neurorescue. In order to exogenously activate Wnt/β-catenin signaling, we used the specific GSK-3β inhibitor, AR-AO14418, as above (Wang et al., 2007). AR (10 mg/kg twice a day) was systemically (i.p.) injected starting 72 h after MPTP discontinuance for 3 weeks (i.e., during the temporal window of Wnt1 expression observed in younger mice). Groups of age-matched MPTP mice received physiologic saline, while groups of saline-injected mice received AR. Mice were sacrificed 3, 14, and 42 after MPTP and processed as described for gene expression, neurochemical and histopathological determinations.
Data analysis
Statistical significance between means ± SEM was analyzed by a two-way analysis of variance (ANOVA). Experimental series performed on different days were compared by the Student–Newman–Keuls t-test. A value of p<0.05 was considered to be statistically significant.
Results
The model
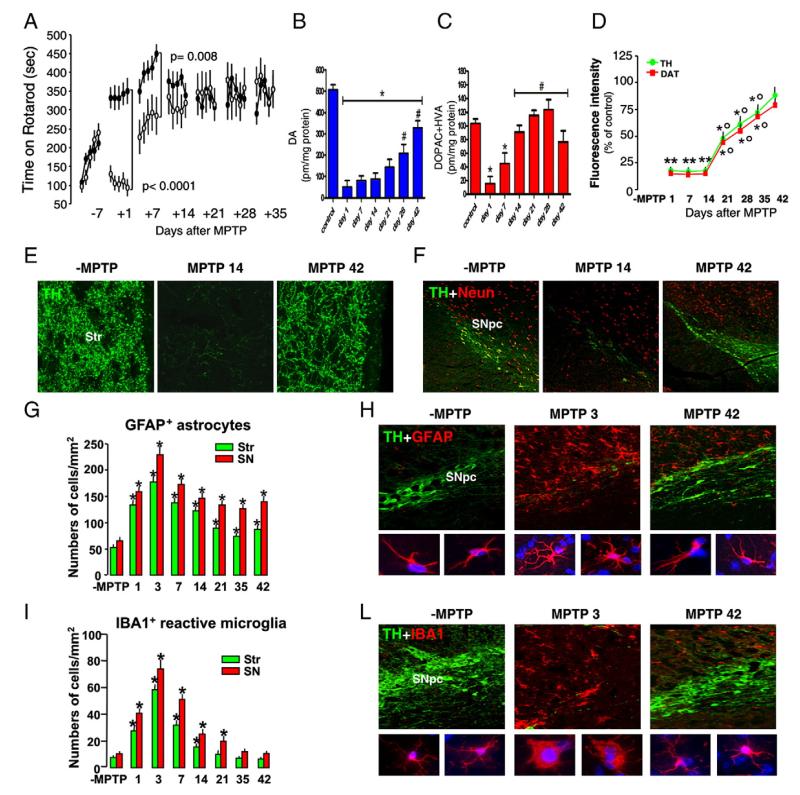
In order to elucidate some of the molecular mechanism(s) underlying the MPTP-induced injury of nigrostriatal DA neurons, we first confirmed that the acute MPTP-dose regimen resulted in the expected markers of DAergic toxicity and glia activation. Spatiotemporal analyses in both striatum (Str) and ventral midbrain (VM) supported the early and profound nigrostriatal impairment followed by a substantial recovery. Hence, after a significant motor deficit assessed with the rotarod (Fig. 1A), MPTP-treated mice performed as well as the control mice starting from 14 days on. At Str level, MPTP induced the recognized early and long-lasting decrease of dopamine (Fig. 1B) and its metabolites (DOPAC + HVA, Fig. 1C), compared with saline-injected controls (p<0.05) for all the duration of the experiment. At a functional level, high-affinity synaptosomial DA uptake underwent comparable decreases 1–14 dpt (Supplementary Fig. 1A). With time, however, the degree of dopamine (B) and DOPAC + HVA (C) depletion observed at both 28 dpt and 42 dpt was significantly (p<0.05) reduced as compared with 1–7 dpt. Likewise striatal DA uptake levels gradually returned to pre-MPTP levels starting from 21 dpt (Supplementary Fig. 1). At a protein level, after the recognized decreases of TH and DAT-IR measured 1–14 dpt (>−80%), a gradual return to almost control levels was reached by 42 dpt (Fig. 1D and Supplementary Fig. 1B–D). Within the VM, at the SNpc level, dual staining with TH+ and NeuN+ confirmed the significant loss of DAergic cell bodies (Fig. 1F). Estimation of the total number of TH+ Nissl+ neurons (Supplementary Fig. 1D) showed >60% loss 14 dpt (from 8120±439 of saline to 3015±290 of MPTP mice estimated by 14 dpt), whereas an approximate 40% decrease was estimated by 42 dpt (4810±310 of TH+ Nissl+), indicating an attenuation of TH+ neuron decrease by this time. The recognized increases in hypertrophic GFAP+ astrocytes (G–H) and ameboid-shaped IBA1+ microglia (I–L) were restricted to both Str and SNpc levels, starting as early as 1 dpt and reaching a peak at 3 dpt. After this time, GFAP+ astrocytes, slightly decreased, but remained still significantly greater in numbers until 42 dpt (G–H), whereas activated IBA1+ cells started to subside, to reach control levels after 21dpt (I–L), thereby supporting the site-restricted and time-specific MPTP-induced glial reaction.
Fig. 1.
MPTP-induced injury and spontaneous recovery of nigrostriatal DAergic endpoints and glia activation. (A) Motor performances on Rotarod of saline- (black dots) and MPTP-treated (white dots) mice (n=10/group). Time of permanence on revolving bars (ordinate) is plotted against pre- and post-treatment days (5 trials/day) during which experiments were performed. Mean and SEM values are reported. Establishment of a motor deficit (1–7 dpt) is followed by recovery from motor impairment by 14 dpt. (B–C) HPLC analysis of dopamine (B), its metabolites DOPAC + HVA (C) in the striatum (Str) of saline and MPTP mice (n=6/time point). Differences were analyzed by ANOVA followed by Newman–Keuls test and considered significant when p<0.05. (B) *vs. saline; #vs. 1–14 dpt; (C) *vs. saline; #vs. 1dpt. (D) TH and DAT-IR in striatum (Str) assessed by immunofluorescent staining and image analysis by confocal laser microscopy in saline and MPTP mice (n=6/time point). Fluorescence intensity values (FI, means ± SEM) are expressed as percent (%) of saline. **p<0.05 vs. ct, °p<0.05 vs. 1–14 dpt. (E) Representative confocal images show loss TH-IF (revealed by FITC, green) in Str of MPTP mice at 14 days and a substantial recovery of TH-IF by 42 dpt. (F) DAergic cell bodies in SNpc. Representative confocal images of dual staining with TH+ and NeuN + of coronal midbrain sections at the level of the SNpc showing the recognized loss of TH+ NeuN +cell bodies 14 dpt and a partial recovery observed by 42 dpt. (G, I) Astrocyte and microglial cell numbers at different time intervals after saline and MPTP injection (n=4/experimental group) in Str and SNpc. (H) Representative confocal images of dual staining with GFAP+ (red) and TH (green) in saline, 3 and 42 dpt. (L) Representative confocal images of dual staining with IBA1+ (red) and TH (green) in SNpc. Nuclei are counterstained with DAPI (blue, insets). Differences were analyzed by ANOVA followed by Newman–Keuls test and considered significant when p<0.05. **p<0.05 vs. saline.
MPTP-dependent regulation of mRNA species in DAergic injury and recovery uncovers Wnt1 upregulation
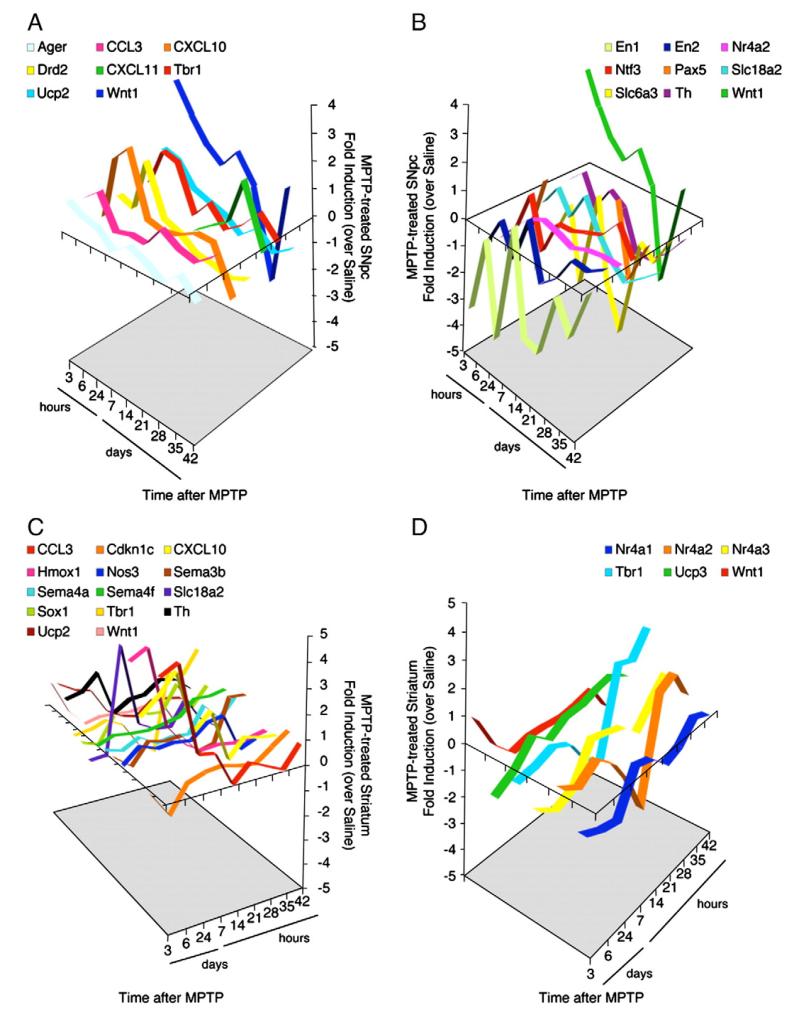
Wide TaqMan® Low-Density-based Array (TLDA)-based gene expression profiling was carried out in the acutely dissociated VM and striata of MPTP- and saline-treated mice at the indicated time points, from MPTP insult (Fig. 2). Ninety-three different mRNA species involved in stemness, inflammation, immunity, self-renewal, neural differentiation, migration, and DA metabolism were measured (see Supplementary Information, gene card).
Fig. 2.
MPTP administration induces expression of pro-inflammatory chemokines, DA transporters, neurogenic patterning factors, and homeobox genes responsible for survival and identity of telencephalic precursor cells in both ventral midbrain (containing the SNpc) (A–B) and striatum (C–D). Groups of 6 mice/time interval received intraperitoneal injections of MPTP–HCl, 20 mg/kg, 4 times in a day at 2-h intervals. Mice were sacrificed on the indicated days after MPTP treatment. Real-time PCR was used for semi-quantitative analysis of selected mRNAs. In SNpc, 8 selected genes showed significant (fold induction≥ 1) upregulation (A) and 9 selected genes significant (fold induction≤−1) downregulation (B) at any time point in comparison to saline-treated controls. Wnt1 gene resulted upregulated up to day 21, and from day 35 to day 42, but downregulated from day 21 to day 35. In the striatum, 14 selected genes showed significant upregulation (C) and 6 selected genes significant downregulation (D) at any time point in comparison to saline-treated controls. Wnt1 was downregulated, with the exception of 3-h time point.
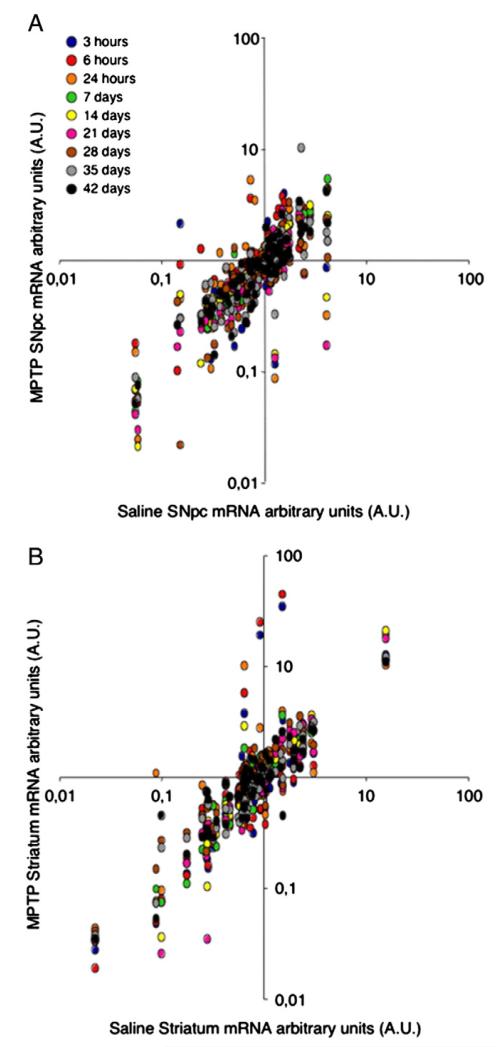
In the VM of MPTP-treated mice, differences of gene expression with saline-treated mice were mostly seen starting at 3 and 6 h and peaking at 24 h post-MPTP (r2=0.38; r2=0.54, and r2=0.15, respectively), when compared to 7 and 42 days post-MPTP (r2=0.90 and r2=0.70, respectively) (Fig. 3A).
Fig. 3.
Systemic administration of MPTP induces pro-inflammatory chemokines, DA transporters, and neurogenic transcription factors in the SNpc and corpus striatum at mRNA level. Bivariate scatter plot analysis of gene expression levels of 96b format TaqMan® low-density-based array (TLDA) performed onto acutely dissociated SNPc (A) and striatum (B) from MPTP-treated mice (y-axis) compared to saline-treated controls (x-axis). Differences in gene expression were seen in the SNpc of MPTP-treated mice starting at 3 and 6 h and peaking at 24 h post-MPTP (r2=0.38; r2=0.54 and r2=0.15, respectively), when compared to 7 and 42 days post-MPTP (r2=0.90 and r2=0.70, respectively). In the striata, differences were seen in MPTP-treated mice again starting at 3 h post-MPTP and peaking at 6 h post-MPTP (r2=0.10 and r2=0.05, respectively), then slightly recovering at 24 h post-MPTP (r2=0.46), when compared to time points at which differences in striatal gene expression were only minor, such as from 7 days post-MPTP on (r2=0.97) Data are expressed as mRNA arbitrary units (AU) over whole adult healthy mouse brain.
In the striata, differences of gene expression with saline-treated mice were again mostly seen as early as 3 h while peaking at 6 h post-MPTP (r2=0.10 and r2=0.05, respectively) and then slightly – though progressively – recovering at 24 h post-MPTP (r2=0.46), when compared to later time points at which differences in striatal gene expression were only minor, such as from 7 days post-MPTP on (r2=0.97) (Fig. 3B).
The gene expression analysis in the VM showed that 4.3% (4/93) of screened genes were not expressed, while 59.1% (55/93) of genes did not change their levels of expression at any time point. Only 7.5% (7/93) of VM genes were upregulated – compared to saline-treated mice – very early after the administration of MPTP (namely 3–24 h post-MPTP), among which we identified at least two major pro-inflammatory chemokines, such as CXCL10/IP-10 and CCL3/MIP-1a the advanced glycosylation end product-specific receptor (ager) member of neural cell adhesion molecules (N-CAM L1), the mitochondrial carrier uncoupling protein (ucp) 2, the dopamine receptor (Dr) 2, the T-box brain gene (Tbr) 1 responsible for instructing immature neural progenitors trough dorsal telencephalic identity.
Of special interest, a major and long-lasting upregulation of the midbrain-patterning factor Wnt-1 (Castelo-Branco et al., 2003) was observed (Fig. 2A). The VM gene expression profile of both CXCL10/IP-10 and Wnt-1 turned to be bi-fasic with a trend towards downregulation at very late (e.g., 42 days) time point post-MPTP. A single gene out of 93 (1%) – the interferon (IFN) γ-inducible pro-inflammatory chemokine CXCL11/I-TAC – show 4-fold upregulation very late (35 days post-MPTP) upon the acute administration of MPTP (Fig. 2A). By contrast, 9.6% (9/93) of VM genes showed persistent downregulation – compared to saline-treated mice – at most screened post-MPTP time points. Among these latter genes, we identified the two homeobox transcription factors Engrailed (En) 1 and 2 – which have been shown to be required for the survival of mesencephalic dopaminergic neurons (Simon et al., 2001) – and the two DA transporters Slc6a3 and Slc18a2. The steroid nuclear hormone receptor Nr4a2/Nurr1 and the neurotrophin (NT) 3 were downregulated only very early post-MPTP (e.g., 3–24 h), while the transcription factor Paired box gene (Pax) 5 and the tyrosine hydroxylase (TH) showed late (e.g., from 21 days on) peak of downregulation (Fig. 2B). Interestingly enough, Wnt-1 was also found to be significantly 7-fold downregulated at 35 days post-MPTP (Fig. 2B).
On the other hand, the 16.1% (15/93) of genes were not expressed in the striatum, while 41.9% (39/93) of striatal genes did not change their levels of expression at any time point. Fifteen percent (14/93) of striatal mRNAs were upregulated – compared to saline-treated mice – very early after the administration of MPTP (namely 3–24 h post-MPTP), among which we identified the very same pro-inflammatory chemokines – CXCL10/IP-10 and CCL3/MIP-1a – as in the SNpc, the heme oxygenase (Hmox) 1 promoting oxidative mitochondrial damage (Song et al., 2006), the DA transporter Slc18a2, the mitochondrial carrier ucp 2, and TH being significantly upregulated very early after MPTP. Other mRNA species, such as Tbr1, the transcription factor Sox 1 specific for the telencephalic neurons of the ventral striatum (Ekonomou et al., 2005), and the three semaphorins (Sema) 3b, 4a, and 4f showed a profile of expression which turned to upregulation at later (e.g., from 21 to 28 days post-MPTP on) time points (Fig. 2C). Only 6.4% (6/93) of striatal genes showed remarkable downregulation, compared to saline-treated mice. We identified again Tbr1 and the three steroid nuclear hormone receptors Nr4a1, Nr4a2, and Nr4a3 (Fig. 2D). Finally, the striatal levels of Wnt-1 reached a peak of upregulation early after MPTP and then turned to downregulation at all later time points (Fig. 2D).
MPTP-induced dynamic changes in prototypic elements of canonical Wnt signaling during DAergic injury and glial activation
Accumulating evidence clearly indicates a vital role for Wnt signaling pathway in neuronal cell survival and homeostasis (Inestrosa and Arenas, 2009; Maiese et al., 2008).The highly significant and sustained upregulation of Wnt1 transcript levels among 92 mRNA species studied associated to the sharp increase of pro-inflammatory chemokines prompted us to address MPTP-regulation of Wnt1 signaling during DAergic injury and glia activation.
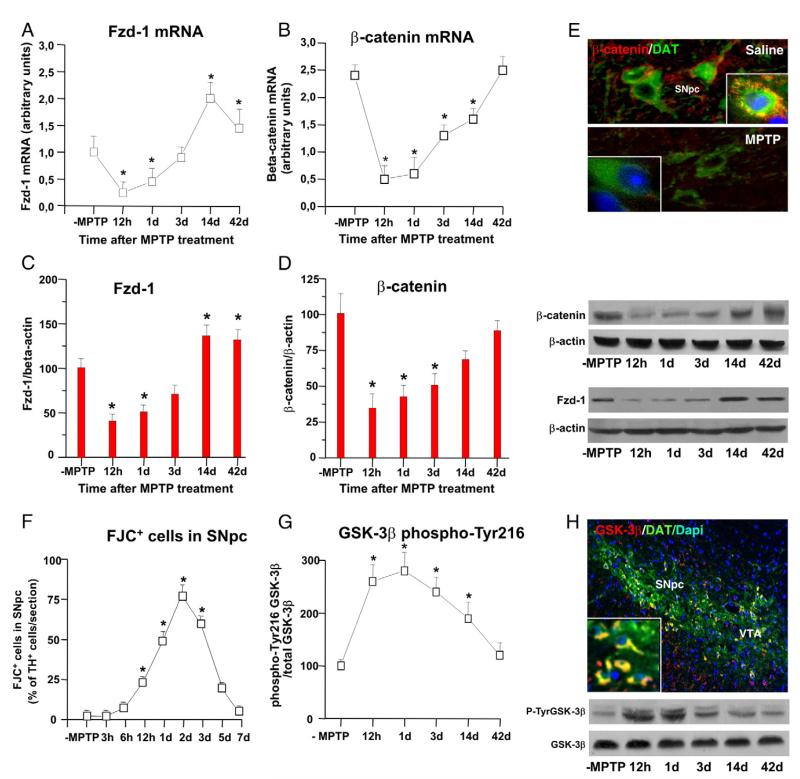
Freezled receptor-1 (Fzd-1)
Given that the first step in Wnt signal transduction is binding of the Wnt ligand to its receptors, the seven-pass transmembrane Fzd proteins (Gordon and Nusse, 2006), we used real-time PCR and identified Fzd-1 gene expression within the adult VM. Temporal analysis in MPTP mice showed an early and sharp downregulation of Fzd-1 receptor transcript at 12 h and 1 dpt (Fig. 4A). After this time, Fzd-1 transcript showed a recovery by 3 dpt, and upregulation at 14 dpt. Western blotting showed comparable down- and up-modulation of Fzd-1 protein levels (Fig. 4C), as indicated by the early decrease 12 h and 1 dpt, followed by a significant increase observed by 14 days. Thus, Fzd-1 was upregulated within the temporal window of Wnt1 mRNA expression in the injured VM of MPTP mice.
Fig. 4.
MPTP-induced dynamic changes in Wnt signaling components during DAergic degeneration and glial activation. (A–B) Temporal analysis of Fzd-1 and β-catenin mRNA expression in the VM of saline and MPTP mice (n=4/group) was carried out by real-time quantitative PCR performed using TaqMan™ Assay Reagents on an Step One Detection System (Applied Biosystems) according to manufacturer’s protocol with specific primers for Fzd-1 receptor (assay ID: Mm00445405_s1) and β-catenin (ID: Mm00483039-m1). The housekeeping gene, β-actin, was used as normalizer and embrionic mouse brain as calibrator. Results are expressed as arbitrary units (AU) and represent mRNA levels detected in VM tissue samples from saline (-MPTP) and 12 h, 1, 3, 14, and 42 dpt. *p<0.05 vs. -MPTP. (C–D) Temporal analysis of Fzd-1 and β-catenin protein levels within the VM of saline and MPTP-treated mice (n=4/time point) by Western blot (wb), showing comparable downregulation of Fzd-1 and β-catenin starting 12 hpt, whereas a gradual trend towards a recovery is observed from 3 dpt on. Data from the experimental bands were normalized to beta-actin, and values were expressed as percent (%) of saline-injected controls. *p<0.05 vs. -MPTP. (E) Dual staining with β-catenin and the dopamine transporter, DAT, in the SNpc showing loss of β-catenin-IR signal 1 day post-MPTP, as opposed to β-catenin-IR signal localized abundantly beneath the cell nucleus of saline-injected controls. (F) Degeneration of SNpc neurons. The total number of Fluorojade C (FJC)-stained cells in SNpc was calculated for each side, averaged for each animal (n=4/time point) and normalized to the number of TH+ neurons in SNpc per section. *p<0.05 vs. -MPTP. (G) Active GSK-3β (GSK-3β phospho-Tyr216) protein levels (n=4/time point) as determined by wb are maximally upregulated by 12 h–3dpt, paralleling DAergic degeneration (D). Data from the experimental bands were normalized to beta-actin, as above, values expressed as percent (%) of saline-injected controls. *p<0.05 vs. -MPTP. (G) Dual staining with GSK-3β and DAT indicated a sharp increase of active GSK-3β-IF signal in DAT-IF neurons within the SNpc already 6 h after the 2nd MPTP injection, thereby preceding SNpc degeneration.
β-Catenin
Fzd-1 receptor provides the best discrimination of the Wnt1/β-catenin pathway (Gordon and Nusse, 2006), spatio-temporal analyses were next conducted in order to correlate β-cateninby real-time PCR (Fig. 4B), Western blotting (Fig. 4D), and immunohistochemical analyses (Fig. 4E). Gene expression analysis indicated an early and sharp downregulation of β-catenin transcript 12 h and 1 day after MPTP, which was followed by a gradual recovery starting from 3 dpt on (Fig. 4B). Dual immunofluorescent (IF) staining with β-catenin and DAT indicated a severe decrease in β-catenin-IF signal as early as 1 dpt, as opposed to β-catenin-IF signal localized abundantly beneath the cell nucleus of saline-injected controls (Fig. 4E). Western blotting (wb) further supported loss of β-catenin protein levels at both 12 h and 1 dpt (Fig. 4D), thus within the temporal window of the active degeneration phase, as revealed by the selective increase in the percentage of Fluorojade C- (FJC)-stained cells within the SNpc (Fig. 4F), starting 12 hpt, reaching a peak 2 dpt, and then subsiding by 5 dpt. On the other hand, starting by 3 dpt on, thus within the temporal window of Wnt1 upregulation and Fzd-1 recovery, a gradual return of β-catenin mRNA (Fig. 4B) and protein (Fig. 4D), reaching control levels by 42 dpt, was observed in the MPTP-injured VM. Given the critical role of β-catenin in cell survival and neuroprotection (Li et al., 2007), these results, therefore, suggested β-catenin down-regulation as an early signal linked to SNpc degeneration, whereas its recovery might contribute to limit MPTP-induced DAergic degeneration.
Glycogen synthase kinase-3β (GSK-3β)
In the absence of Wnt ligand, β-catenin is constantly phosphorylated by a destruction complex consisting besides others, of GSK-3β, thereby targeting it for ubiquitination and subsequent degradation via the ubiquitin-proteasome system (Aberle et al., 1997). Here, MPTP-induced activation of GSK-3β (Fig. 4G and H), evidenced by increased tyrosine phosphorylation at residue 216 (p-Tyr216-GSK-3β), preceded β-catenin loss within SNpc DAT-neurons, starting already 6 h after the second MPTP injection, when dual staining with DAT indicated a sharp increase of active GSK-3β-IF signal in DAT-IF neurons within the SNpc (Fig. 4H). Western blotting indicated increased active GSK-3β protein levels 12 h after MPTP discontinuation (Fig. 4E), paralleling DAergic degeneration (Fig. 4G) and the time course of microglial activation (Fig. 1I). On the other hand, between 3 and 14 dpt, a significant decline of active GSK-3β levels (Fig. 4G) was measured, when ameboid IBA1+ microglial cells in Str and SNpc (Fig. 1I) were significantly reduced, whereas an initial recovery of β-catenin was observed (Fig. 4B and D).
Together these results indicated MPTP-induced GSK-3β activation and β-catenin downregulation within the temporal window of active DAergic degeneration and microglial activation.
MPTP-dependent Wnt1 expression in the injured VM and VM astrocytes
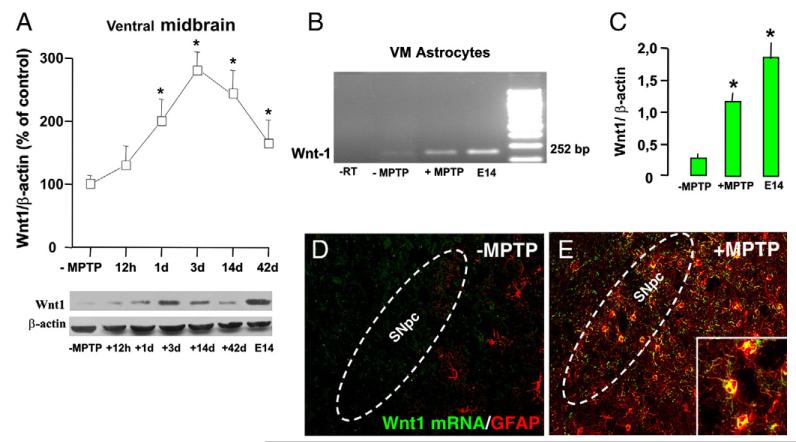
We thus correlated Wnt1 mRNA expression with Wnt1 protein levels in VM, using embryonic (E-14) brain as a control tissue (Fig. 5A and B), and the recombinant Wnt1 protein as an internal control (not shown), only a very faint band corresponding to the expected size for Wnt1 (41 kDa) was revealed in VM of saline-injected controls as opposed to the significantly higher amount of the endogenous protein present in E-14 brain (Fig. 5B). Temporal analysis of Wnt1 protein in the VM of MPTP mice showed a significant increase in Wnt1-IR band detected already 1 dpt with a peak reached 3 dpt. Wnt1-IR band still remained significantly higher at both 7 and 14 dpt, thereby within the temporal window of initial β-catenin recovery (Fig. 4C and D). Thus, Wnt1 expression appeared associated to the temporal window of GSK-3β inhibition (Fig. 4G), Fzd-1 upregulation (Fig. 4A), and β-catenin recovery (Fig. 4C and D), namely within the temporal window of astrocyte activation (Fig. 1G).
Fig. 5.
Wnt1 expression in MPTP-injured VM and VM astrocytes. (A) Western blot analysis of Wnt1 protein in saline (-MPTP) and at different time intervals after MPTP (n=4/time point). Embryonic (E-14) brain was used as a control tissue. Data from the experimental bands were normalized to beta-actin, as above, values expressed as percent (%) of saline-injected controls. *p<0.05 vs. -MPTP. (B–C) Wnt1 mRNA transcription in astrocytes derived ex vivo from MPTP-injured VM (3 dpt). Total RNA was extracted and processed as described, and 250 ng of cDNA was used for semi-quantitative polymerase chain reaction (RT-qPCR) using specific primer pairs for Wnt1 (F: ccgagaaacagcgttcatct; R: gcctcgttgttgtgaaggtt; amplicon: 252 bp) and β-actin (F: cttttccagccttccttctt; R: tcaggaggagcaatgatctt; amplicon: 220 bp). Embryonic (E-14) brain was used as a control tissue. Samples from PCR reactions were separated electrophoretically on 2% agarose gel containing 0.2 μg/ml of ethidium bromide. Fluorescent bands of amplified gene products were captured by using Gel Logic 200 Imaging System (Kodak). Note the sharp increase in Wnt1 mRNA transcription (B, C) in samples derived from VM astrocytes of MPTP mice as opposed to controls (-MPTP). (D–E) In situ hybridization with Wnt1 probe was carried out in midbrain sections (at the level of the SNpc) overnight as described, using FITC antisense RNA probe (Sigma Aldrich) specific for Wnt1. GFAP-Ab was then applied for dual localization, in saline (-MPTP) and 2 dpt (n=4/group). Wnt1 signal (green) was not revealed in uninjured VM or GFAP+ astrocytes (red, D), whereas after MPTP (E) Wnt1 mRNA signal (green) and GFAP+ astrocyte signal (red), colocalized (orange–yellow).
The question therefore arose as to the potential source of Wnt1 expression in the MPTP-injured VM. While little is known on the source Wnts in adult brain, recent findings indicate the expression of Wnt’s components in adult astrocytes (Lie et al., 2005; Cahoy et al., 2008). We next conducted Wnt1 mRNA analysis in astrocytes derived ex vivo from MPTP-injured VM (3 dpt) and found increased Wnt1 mRNA transcription by RT-PCR, compared to Wnt1 transcription of uninjured VM astrocytes (Fig. 5B and C). In situ hybridization histochemistry further confirmed colocalization of Wnt1 with reactive GFAP+ astrocytes within the MPTP-injured VM (Fig. 5D and E), thereby implicating, MPTP-reactive astrocytes as candidate elements of Wnt/β-catenin signaling.
Activated astrocytes and Wnt1/β-catenin signaling rescue mesencephalic DAergic neurons from MPP+-induced TH+ neuron toxicity
All together these informations raised the possibility that astroglial-derived Wnt1 might provide a compensatory mechanism to limit the degenerative process and/or activate the spontaneous SNpc self-repair program.
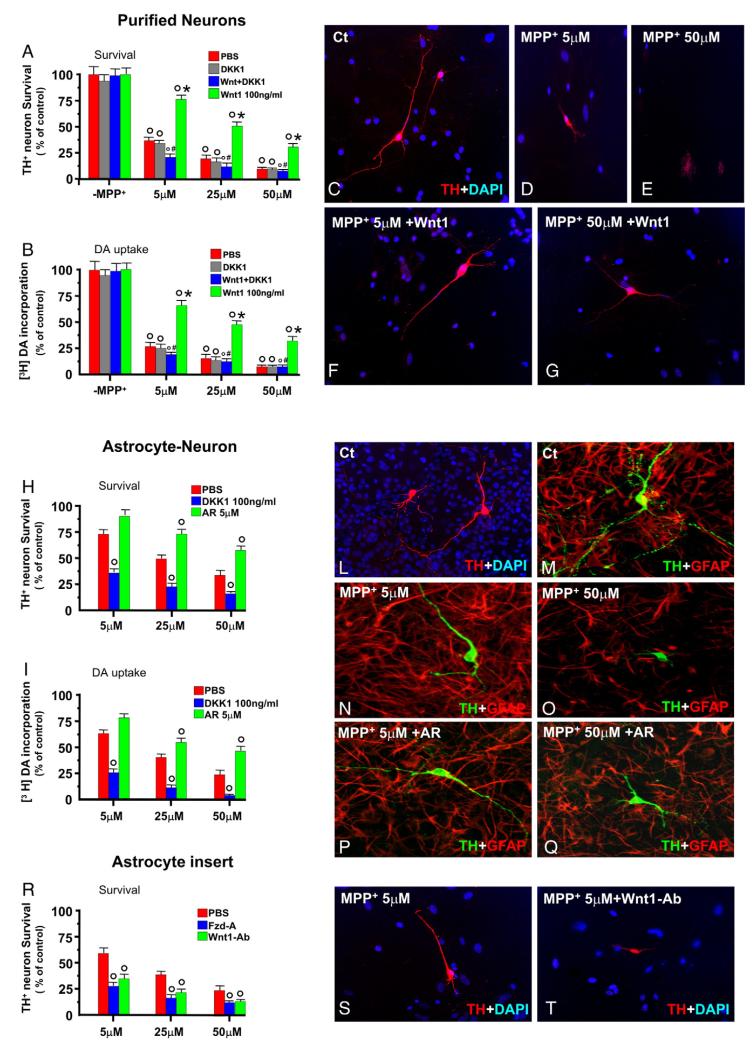
Indeed, astrocytes represent a vital source of survival and neurotrophic factors for several types of neurons, including DAergic neurons (Engele and Bohn, 1991; Takeshima et al., 1994; Gallo et al., 2000; Castelo-Branco et al., 2006; Blackburn et al., 2009). We thus investigated the ability of VM astrocytes to afford DAergic neuroprotection against MPP+ through Wnt/β-catenin activation. To this end, we took advantage of cell culture systems to study the toxic effect of MPP+ in purified primary mesencephalic neurons as opposed to astrocyte–neuron cultures, in vitro. In enriched primary mesencephalic neuronal cultures at 7 DIV, MPP+ induced a significant time-dependent loss of β-catenin protein levels preceded by a sharp increase of active GSK-3β starting 0.5 h following MPP+ treatment, with levels remaining elevated for 2 h (not shown). MPP+ induced the recognized DAergic toxicity as demonstrated by the dose-dependent decrease in the number TH+ neurons paralleled by inhibition of [3DA] incorporation (Fig. 6A–E). By contrast, when MPP+ was applied to astrocyte–neuron cultures, a significant degree of protection was observed at MPP+ dose range of 5–25 μM, whereas at 50 μM dose of MPP+, astroglial neuroprotective effect was sharply impaired (Fig. 6H–O). By contrast, blocking Wnt/Fzd signaling with the prototypic inihibitor of canonical Wnt signaling, Dkk1 (100 ng/ml) sharply antagonized VM astrocyte-induced neuroprotection, as demonstrated by the significant decrease in the number of TH+ neurons and [3DA] incorporation (Fig. 6H and I). On the other hand, exogenous activation of Wnt/β-catenin signaling in VM astrocyte–neuron MPP+-treated cultures by the specific GSK-3β antagonist, AR-14418 (5 μM), significantly magnified astrocyte neuroprotective effect at all MPP+ doses tested (Fig. 6H, I, P, Q). Consistently, direct application of Wnt1 (100 ng/ml) to purified mesencephalic neurons 1 h before MPP+ resulted in a significant protection against MPP+ toxicity (Fig. 6F and G), whereas Dkk1 application, while inactive by itself, efficiently antagonized Wnt1-induced DAergic neuroprotection (Fig. 6A). Likewise, in the indirect co-culture paradigm, addition of increasing MPP+ doses to purified mesencephalic neurons exposed to astrocyte inserts, increased by about 2-fold TH+ neuron survival (Figs. 6R and S) as opposed to purified neurons alone (Fig. 6A, D, E). On the other hand, the addition to the purified neurons of a blocking antibody against Wnt1 (Wnt1-Ab, 2 μg/ml), or a Wnt/Fzd antagonist (Fzd-A, 200 ng/ml), which binds to the Wnt ligand preventing its interaction with the Fzd receptor, while inactive per se (not shown), reduced TH+ neuron survival upon MPP+ application (Fig. 6R and T), indicating that the protective effect of astrocyte inserts is at least in part induced by the Wnt ligand and not by another component. Together, these results suggested that activated astroglial cells and Wnt1 activation of Wnt/β-catenin signaling are critical contributors of VM astrocyte-induced protection of mesencephalic DAergic neurons against MPP+ toxicity.
Fig. 6.
Astrocytes and Wnt1/β-catenin signaling rescue mesencephalic DAergic neurons from MPP+-induced TH+ neurotoxicity. Enriched mesencephalic neuronal cultures at 7 DIV, prepared as described, received increasing doses of MPP+ and the number of TH+ neurons (A) and [3H]DA incorporation (B) analyzed after 24 h, the data expressed as percent (%) of control values (see Materials and methods). Wnt1 (100 ng/ml, A–B, F) or the Wnt antagonist, Dkk1 (100 ng/ml), were applied either alone or in combination. In this case, application of Dkk1 preceded Wnt1 administration. (G–L) Differences were analyzed by ANOVA followed by Newman–Keuls test and considered significant when p<0.05. °p<0.05 vs. -MPP+ within each respective group; *p<0.05 vs. PBS, within each respective group; #p<0.01 vs. Wnt1, within each respective group. (C–E) Representative immunocytochemical images showing purified mesencephalic TH+ neurons (red) in control (Ct) conditions (-Mpp+, C) and 24 h after MPP+ at 5 μM (D) or 50 μM (E). Nuclei were counterstained with DAPI (blue). Note the marked neuroprotection afforded by Wnt1 treatment at both doses (F–G). (H–I) Astrocyte–neuron cultures at 7–9 DIV received increasing doses of MPP+ and DAergic toxicity analyzed as above both in the absence or the presence of a concomitant treatment with the specific GSK-3β antagonist AR (5 μM, Osakada et al., 2007). For Wnt antagonism, Dkk1 (100 ng/ml) was applied prior MPP+ application. (L–Q) Representative immunocytochemical images showing astrocyte–neuron cultures at 7–9 DIV. (L) Control culture stained for TH+ (red) and counterstained with DAPI (blue). Differences were analyzed by ANOVA followed by Newman–Keuls test. °p<0.05 vs. PBS, within each respective group; (M–Q) representative confocal images of dual staining with TH+ (green) and GFAP (red) of astrocyte–neuron cultures without (M) and 24 h after 5 (N) or 50 μM (O) of MPP+. Note the increased branching and varicosities of control TH+ neurons in the presence of astrocytes (M–N), and the significant protection afforded by astrocytes at 5 μM (N) and 25 μM (H–I) MPP+ doses. In addition, exogenous activation of Wnt signaling with the GSK-3 inhibitor sharply magnified astrocyte neuroprotective effect (P–Q). Note the increased process length and branching of TH+ neurons even at the highest (50 μM) MPP+ dose (Q). (R–T) Purified mesencephalic neurons were exposed to astrocyte inserts (i.e., indirect co-culture paradigm) and increasing doses of MPP+ were applied, both in the absence and the presence of a blocking antibody against Wnt1 (Wnt1-Ab, 2 μg/ml) or the Wnt/Fzd antagonist, Fzd-A (200 ng/ml). Differences were analyzed by ANOVA followed by Newman–Keuls test. °p<0.05 vs. PBS, within each respective group. Note the ability of astrocyte inserts to increase by twofold TH+ neuron survival (R, S) as opposed to purified neurons alone (A, D, E), whereas this effect is counteracted by Wnt1 blocking antibody (T), or Fzd-A.
Chemokine-activated astrocytes and Wnt1 promote neurogenesis and DA neurogenesis from NPCs of the adult midbrain (MB)
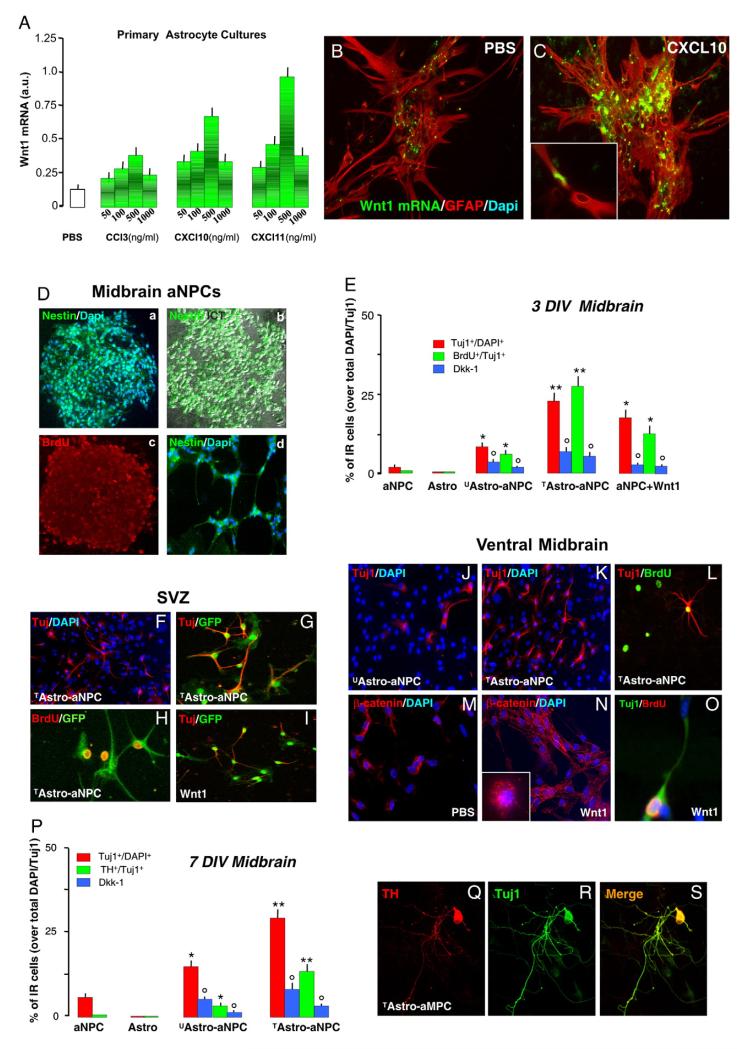
In view of the delayed SNpc neurorecovery herein observed in vivo upon acute MPTP injury, and given the critical role of Wnt/β-catenin in regulating the proliferation and differentiation of midbrain DAergic neuroprogenitors during development (Castelo-Branco et al., 2003), we thus investigated a potential role of VM astrocytes and Wnt/β-catenin signaling in promoting adult midbrain neurogenesis. Indeed, emerging evidence clearly indicates that astrocyte-derived Wnts have instructive effects to induce adult neurogenesis (Lie et al., 2005; Yu et al., 2006). To mimic the in vivo inflammatory condition, we first tested the modulation of Wnt1 expression in VM astrocyte cultures by various inflammatory factors and found that the pro-inflammatory chemokines over-expressed in MPTP-injured VM, i.e., CCL3, CXCL10, and CXCL11, dose-dependently stimulated Wnt1 expression, both by real-time PCR (Fig. 7A) as well as by in situ hybridization, (Fig. 7B and C).
Fig. 7.
Chemokine-activated astrocytes express Wnt1 and promote dopaminergic neurogenesis from adult midbrain neural stem/precursor cells (NPCs) via Wnt/β-catenin activation. (A) Expression levels of Wnt1 mRNA in untreated (UAstro) or treated (TAstro) ventral midbrain (VM) astrocyte cultures. VM astrocytes grown as described received a pretreatment with PBS (UAstro) or increasing doses (50–1000 ng/ml) of one the chemokines (CCL3, CXCL10 or CXCL11, TAstro). Treatments were carried out only one time and cells processed 24 hpt for real time PCR. (B–C) In situ hybridization with Wnt1 probe was carried in U/TAstro, as described, using FITC antisense RNA probe specific for Wnt1. GFAP-Ab was then applied for dual localization, in control (pbs) UAstro (B) and 24 h after CXCL10 treatment (C). In control cultures, only a slight Wnt1 signal (green) was revealed in GFAP+ astrocytes (red), whereas after CXCL10 (D), a robust Wnt1 mRNA signal (green) colocalized with GFAP+ astrocyte signal (red). (D) Morphology and marker expression of aNPCs derived from midbrain/hindbrain (MB) during in vitro expansion. Spheres were cultured for 2–3 h to allow attachment and then stained with nestin (panel Da, b) and BrdU (c).The spheres were cultured in the presence of 5 μM BrdU for 24 h before plating. Nuclei were counterstained with DAPI. The respective marker protein is expressed in nearly all cells within the neurosphere and after withdrawal of mitogens (d). (E) Effect of co-culture of U/TAstro with aNPCs derived from MB (E, and J–L) or SVZ (F–I). Tuj1+ cells was determined relative to the total number of DAPI+ nuclei (E, %Tuj1+/DAPI+); the number of BrdU+ cells was determined relative to the total number of Tuj1+ cells (E, %BrdU+/Tuj1). Within 24 h of BrdU pulse, approximately 25% of the Tuj1+ cells were labeled, an effect counteracted by Dkk1 treatment. Direct application of recombinant Wnt1 protein (100 ng/ml), significantly increased the number of Tuj1+ cells (see M, N, O), and the proportion of BrdU+ out of the total number of Tuj1+ cells in aNPC derived from MB (O) and SVZ (I). (P) VM astrocytes promote DA neurogenesis in MB aNPCs. After 7 DIV, TAstro produced 3–4 times more TH+ cells out of the total number of Tuj1+, as compared to UAstro (<3%). (Q–S) Confocal images of a Tuj1+ TH+ neuron extending long TH+ processes with numerous varicosities. Differences between means were analyzed by ANOVA and considered significant when p<0.05. *p<0.05 vs. NPCs alone; **p<0.05 vs. UAstro; °p<0.05 vs. U/TAstro, within each respective group.
We next addressed whether untreated (UAstro) or chemokine-treated (TAstro) VM astrocytes and Wnt1 might promote adult neurogenesis and DA neurogenesis from NPCs derived from mouse midbrain/hindbrain (MB). For comparative studies, green fluorescent protein (GFP)-expressing NPCs derived from the SVZ (Pluchino et al., 2003, 2005) were treated in parallel.
By culturing NPCs derived from adult midbrain/hindbrain (MB, Hermann et al., 2006), using the neurosphere culturing technique similar to those used for propagation of NPCs derived from the SVZ (Pluchino et al., 2003, 2005), we found that the phenotype of these MB NPCs was similar to those derived from the SVZ. Accordingly, nestin, a marker for precursor cells in adult brain, and BrdU, a marker for cell proliferation, were expressed during in vitro clonal expansion of NPCs from the adult MB (Fig. 7D). In NPCs cultures from both MB (E) and SVZ (F), grown in PDL alone in N2 medium, and in the absence of exogenous factors, only rare Tuj1+ neurons could be detected after only 3 DIV as compared to direct co-culture of NPCs with UAstro (Fig. 7E and J), which produced a significant increase in the number Tuj1+ cells. This finding was in accordance with earlier studies carried out with adult SVZ precursors cultured on type 1 astrocyte monolayers (Lim and Alvarez-Buylla, 1999) and further indicated the ability of VM astrocytes to promote neurogenesis from NPCs derived from adult midbrain.
On the other hand, direct co-culture of NPCs with TAstro significantly increased the proportion of Tuj1+ cells out of the DAPI+ nuclei, as compared to co-cultures with UAstro (Fig. 7E, K and F, G), indicating the ability of TAstro to sharply increase the neurogenic potential of NPCs, whereas UAstro or TAstro cultured alone for either 3 or 7 DIV failed to induce any Tuj1+ cells (Fig. 7E and P). We next looked at changes in proliferation and found that in co-culture of NPCs isolated from the MB and SVZ, with U/TAstro, within 24 h of BrdU pulse, a significant increase in the number of Tuj1+ labeled with BrdU was observed, indicating the proliferative origin for a certain proportion of adult-derived Tuj1+ neurons (Fig. 7E, H, L). By contrast, blocking Wnt/Fzd signaling with the Wnt’s antagonist, Dkk-1, applied to NPCs just prior to start the co-culture with astrocytes, resulted in a significant reduction of both UAstro and TAstro- induced increase of Tuj1+/BrdU+ (Fig. 7E and P), thus suggesting that Wnt/β-catenin signaling was required for astro-induced neurogenesis from multi-potent NPCs of the MB.
We next verified the ability of exogenous Wnt1 to directly promote neurogenesis from NPCs grown in the absence of astrocytes, as above, and found that direct application of recombinant Wnt1 protein (100 ng/ml) significantly increased the number of Tuj1+ cells and the proportion of BrdU+ out of the total number of Tuj1+ cells in NPCs (Fig. 7O). In midbrain aNPCs treated with Wnt1, staining with β-catenin revealed increased β-catenin-IR signal (N) both at perinuclear (6–16 h, inset in N) and at membrane/cytoplasmic regions (72 h, N).
These findings, therefore, indicated the ability of VM astrocyte-derived factors, especially Wnt/β-catenin activation, and exogenously applied Wnt1, to promote neurogenesis from MB NPCs. We next assessed the ability VM astrocyte-derived factors to promote DA neurogenesis. A number of different experimental settings as compared to direct co-culture with VM UAstro and TAstro, or direct application of Wnt1, were investigated. Neither growing NPCs from either SVZ or MB alone in PDL, both in the absence or the presence of serum (2–20%), with or without different DAergic growth factors (BDNF, GDNF), nor direct application of different chemokines or Wnt1 to NPC cultures did produce any TH+ neuron after 7 DIV. In addition, UAstro or TAstro co-cultures with aNPC derived from SVZ failed to induce DAergic phenotype. On the other hand, when NPCs isolated from the MB were co-cultured with UAstro/TAstro for 7 DIV, a certain number of TH+ cells out of the total number of Tuj1+ cells could be detected (Fig. 7P), albeit co-culture with TAstro (in particular CXCL11) produced 3–4 times more TH+ cells out of the total number of Tuj1+, as compared to u-astro (<3%). In TAstro (CXCL11)-NPCs co-cultures, Tuj1+TH+ neurons extended long and branched TH+ processes with numerous DAergic varicosities (Fig. 7Q–S). In sharp contrast, Dkk1 treatment induced a significant inhibition in the number of TH+ cells out of the total number of Tuj1 (Fig. 7P).
Therefore, factors derived from astrocytes and chemokine activated astrocytes, including Wnt/β-catenin signaling, are likely contributing to the neuronal and dopaminergic differentiation of adult midbrain progenitors, in vitro.
Dysregulation of Wnt signaling in middle-aged mice that fail to recover from MPTP: reversal by exogenous activation of Wnt/β-catenin signaling
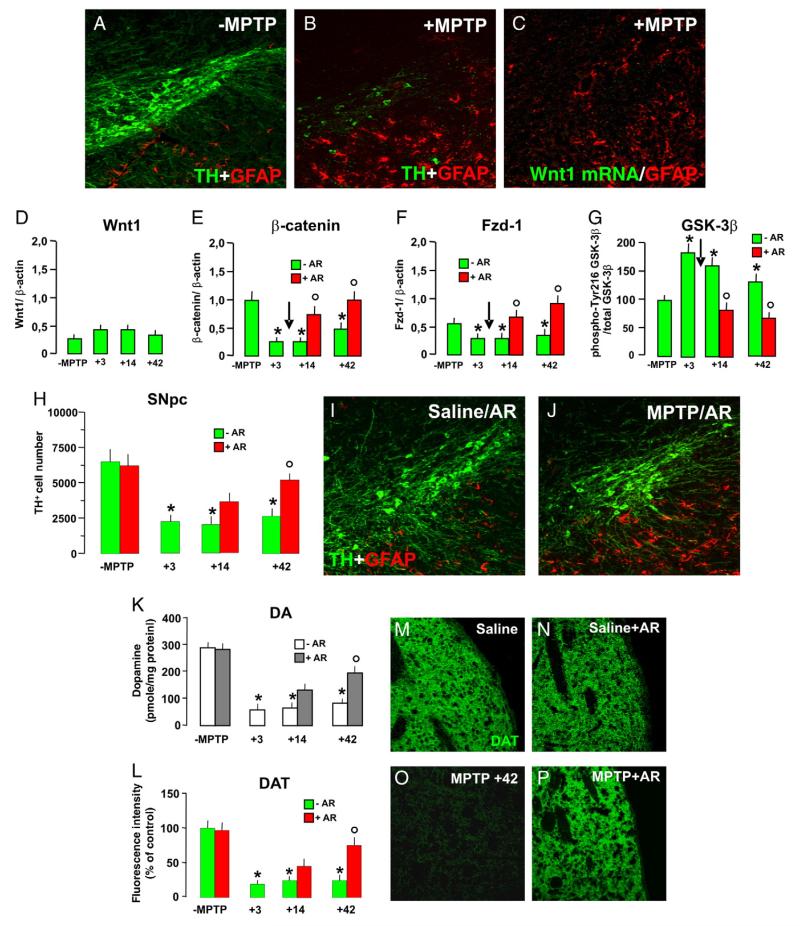
To address the relevance of Wnt/β-catenin signaling pathway to the recovery after MPTP injury and establish a functional link between Wnt signaling and DAergic neurorescue, in vivo, we investigated Wnt1/Fzd/β-catenin signaling in middle-aged mice that fail to recover after MPTP insult (Ricaurte et al., 1987; Ho and Blum, 1998). MPTP administration in 9- to 10 month-old mice induced the recognized long-lasting loss of TH+ neurons associated to the sharp increase of reactive astrocytes within the injured SNpc (Fig. 8A and B). However, despite a strong and persistent astrogliosis, the temporal analysis of Wnt1 transcript revealed lack of MPTP-induced Wnt1 mRNA upregulation in VM and VM astrocytes both by in situ hybridization (Fig. 8C) and RT-PCR (D), indicating astrocyte impairment of Wnt1 response to MPTP with age. Dysregulation of Wnt signaling in middle-aged mice exposed to MPTP was further supported by the long-lasting downregulation of β-catenin and Fzd-1 transcripts in the VM of MPTP mice thereby corroborating impaired Wnt/Fzd/β-catenin signaling in response to MPTP in middle-aged mice. Since pharmacological inhibition of GSK-3β enzyme activity results in activation of β-catenin signaling, this enabled us to examine the functional importance of this pathway in post-injury DAergic neurorescue in middle-aged mice. To this end, the specific GSK-3β inhibitor, AR, was injected i.p. (10 mg/kg twice a day) starting 72 h post-MPTP for 3 weeks (i.e., after the peak of SNpc degeneration and during the temporal window of Wnt1 expression in younger mice, Fig. 4), and mice were sacrificed 14 and 42 days after MPTP. We first verified the ability of systemic AR treatment to reduce active GSK-3β protein levels in the MPTP-injured VM. Consistently, treatment with AR reversed the MPTP-induced p216GSK-3β upregulation (Fig. 8G), as well as the concomitant downregulation of both β-catenin (Fig. 8F) and Fzd-1 (Fig. 8F) levels in the injured VM, as opposed to levels measured in middle-aged mice exposed to MPTP.
Fig. 8.
Dysregulation of Wnt1/β-catenin signaling and lack of nigrostriatal recovery of middle-aged mice are reversed by exogenous activation of Wnt/β-catenin signaling. (A–B) Representative confocal images of midbrain sections at the level of the SNpc in middle-aged (9–11 months of age) mice showing dual labeling with TH− and GFAP-Ab 42 days after saline (A) or MPTP (B) administration. (C) In situ hybridization with FITC antisense RNA probe for Wnt1 (carried out as described in the legend for Fig. 5) and immunolocalization of GFAP in midbrain sections of middle-aged mice 2 days after MPTP (n=3/group). Note the lack of Wnt1 expression after MPTP in aging mice. (D–F) Real-time PCR for Wnt1 (D), Fzd-1 (E), and β-catenin (F) mRNA transcripts in VM samples of saline and MPTP mice in the absence or the presence of a systemic treatment with saline or the specific GSK-3β inhibitor (AR, 10 mg/kg twice a day) starting 3 days after MPTP (arrow). Transcript levels were measured at different time intervals after MPTP/saline and MPTP/AR (n=4/time point), as described. (G) Active GSK-3β (GSK-3β phospho-Tyr216) protein levels as determined by wb (n=4/time point). Data from the experimental bands were normalized to beta-actin, as above, values expressed as percent (%) of saline-injected controls. *p<0.05 vs. -MPTP; °p<0.05 vs. -AR. (H) Loss of TH+ neurons within the SNpc at different time intervals in MPTP/saline and MPTP/AR (n=6/time point). *p<0.05 vs. -MPTP; °p<0.05 vs. -AR. (I–J) Representative confocal images showing dual localization of TH+ neurons (green) and GFAP+ astrocytes (red) in saline/AR (I) and MPTP/AR 42 days post-MPTP (J). (K) HPLC analysis of dopamine in the striatum (Str) of saline and MPTP mice (n=6/time point). Differences were analyzed by ANOVA followed by Newman–Keuls test, and considered significant when p<0.05. *vs. saline; °p<0.05 vs. MPTP-AR. (L) DAT-IR in striatum (Str) assessed by immunofluorescent staining as indicated in legend for Fig. 1 (n=6/time point). Fluorescence intensity values (FI, means ± SEM) are expressed as percent (%) of saline. *p<0.05 vs. ct, °p<0.05 vs. -AR. (M–P) Representative confocal images showing DAT-IF (revealed by FITC, green) in Str of saline (M), saline/AR (N), MPTP/saline (O), and MPTP/AR (P).
Immunohistochemistry revealed that MPTP/AR mice exhibited a significant recovery from MPTP insult as indicated by the significant increase in TH+ neurons by 42 dpt (Fig. 8H and J), as opposed to failure to recover of MPTP/saline counterparts. AR-induced DAergic neurorescue was not due to a difference in striatal MPTP/MPP+ metabolism, since no significant changes were observed in striatal MPP+ levels between MPTP/AR and MPTP/saline mice (not shown). At striatal level, dopamine (Fig. 7K) and DAT-IR fiber density (Fig. 7L and P) were significantly increased by 42 dpt in middle-aged MPTP mice treated with AR, as compared to age-matched MPTP/saline counterparts (Fig. 7K, L, O), thereby corroborating a significant degree of nigrostriatal recovery induced by activation of Wnt/β-catenin signaling pathway in middle-aged mice. Together these informations established a causative link between nigrostriatal self-repair and Wnt1 signaling activation in the injured midbrain (Fig. 9).
Fig. 9.
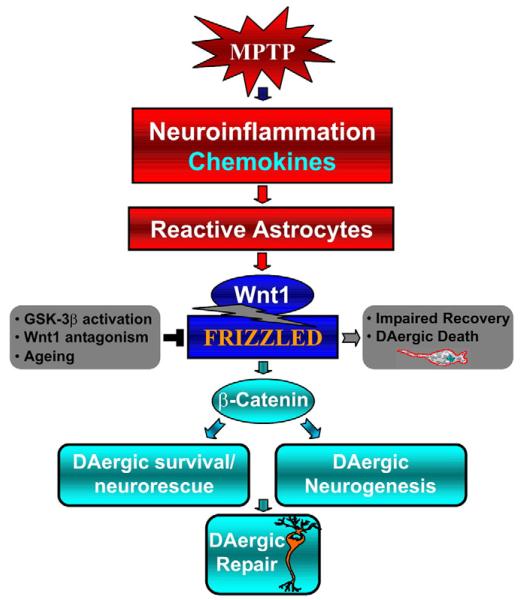
MPTP-reactive astrocytes and Wnt/β-catenin signaling link nigrostriatal injury to repair. A simplified scheme illustrating MPTP-dependent neuroinflammation and astrocyte activation of Wnt1/Fzd-1/β-catenin neurorescue signaling cascade directing towards DAergic neuron survival and/or promoting DAergic neurogenesis in the adult injured midbrain. Dysregulation of astrocyte-dependent Wnt1/Fzd signaling as a result of GSK-3β activation, Wnt antagonism or aging, may limit DAergic survival/recovery upon MPTP/MPP+ and/or direct towards DAergic degeneration, while exogenous activation of Wnt/β-catenin signaling promote neurorescue.
Discussion
We herein unveiled Wnt/β-catenin signaling and MPTP-reactive astrocytes “in situ” as candidate components of neurorescue/repair pathways in MPTP-induced nigrostriatal DAergic plasticity. First, wide gene expression analysis of 92 mRNA identified a major upregulation of pro-inflammatory chemokines and Wnt1 during MPTP-induced DAergic degeneration and self-recovery. Next, spatio-temporal analyses within the injured VM showed a dynamic interplay between prototypical proteins of canonical Wnt signaling pathway (e.g., Fzd-1 receptor, β-catenin, and GSK-3β), associated to DAergic degeneration and glia activation. In situ hybridization histochemistry next revealed MPTP-reactive astrocytes as candidate source of Wnt1 and in vitro experiments further suggested activated VM astrocytes as likely components of Wnt signaling pathway critically contributing to DAergic neuroprotection against MPP+ toxicity. By contrast, blocking Wnt/Fzd signaling with Dkk1 counteracted DAergic neuroprotection. Moreover, factors derived from VM-activated astrocytes, especially Wnt1, promoted neurogenesis and DAergic neurogenesis from stem/progenitor cells derived from the adult midbrain. By contrast, lack of Wnt1 expression in older as opposed to younger mice and loss of β-catenin correlated with failure to recover in response to acute MPTP injury. The fact that nigrostriatal recovery was mimicked by post-injury activation of Wnt signaling via downregulation of GSK-3β next established a functional link between Wnt signaling and DAergic plasticity (Fig. 9).
Among multiple mechanisms at play Wnt1 is a candidate signal in MPTP-induced nigrostriatal plasticity
The ability of nigrostriatal DAergic neurons to respond to injury by triggering a panel of neurochemical, molecular, and morphological compensatory mechanisms is well documented (Hornykiewicz, 1993; Blanchard et al., 1996; Bezard and Gross, 1998; Finkelstein et al., 2000; Stanic et al., 2003; Jacowec et al., 2004; Zigmond et al., 2009). Astrocyte and microglia activation, including the expression of proinflammatory cytokines and neurotrophic factors during DAergic nigrostriatal recovery upon injury have been previously underlined (Ho and Blum, 1998; Batchelor et al., 1999; Parish et al., 2002; Stanic et al., 2004). In addition, spontaneous improvement in behavioral functioning and motor recovery has also been reported after nigrostriatal injury, both in rodents and non-human primates (seePetzinger et al., 2006).
Activation of endogenous compensatory mechanisms is thought to mask the appearance of PD before the appearance of the first clinical symptoms (Hornykiewicz, 1993; Bezard and Gross, 1998), which raises the possibility that some individuals with PD suffer from a reduction of these neuroprotective mechanisms and that treatments that boost these mechanisms may provide therapeutic benefit (seeZigmond et al., 2009). Aging, the most critical risk factor for PD, is associated to a gradual decline in the intrinsic capacity of DAergic neurons to recover, at least in part via increased SNpc neuron vulnerability and dysfunctional glia–neuron crosstalk (Ho and Blum, 1998; Marchetti et al., 2005a,b,c; Boger et al., 2010; L’Episcopo et al., 2010).
Indeed, after injury, many adaptive changes occurring within the astroglial cell compartment may serve to increase the defense against oxidative stress, to reduce inflammation, to improve mitochondrial performance, to increase neurotrophic support, and/or to activate adult neurogenesis (Horner and Palmer, 2003; Barkho et al., 2006;Sandhu et al., 2009; Chen et al., 2009; Voskuhl et al., 2009; Sofroniew and Vinters, 2010). By contrast, age-related events within the SN microenviroment, including increased oxidative stress and neuroinflammatory cascades (Miller and Streit, 2007), may reduce astrocyte neuroprotective functions thereby limiting DAergic self-repair abilities, and/or reducing adult neurogenesis. The persistent expression of Wnts in the adult brain, together with their role in neuroprotection, the modulation of neurogenesis, and synaptic plasticity, indicates that Wnt signaling may play a critical role in maintaining and protecting neuronal functions, including nigrostriatal DAergic neurons (Toledo et al., 2008; Inestrosa and Arenas, 2009; He and Shen, 2009). In addition, astrocyte dysfunction and interruption of Wnt/β-catenin signaling with the aging process may contribute to increase DAergic neuron vulnerability and/or reduce neurogenic potential.
Differential regulation of Wnt/β-catenin signaling in the adult injured midbrain may direct towards degeneration/neuroprotection
Emerging evidence clearly indicates a crucial role for Wnt signaling pathway in neurodegenerative diseases, especially, Alzheimer’s disease (De Ferrari et al., 2003; Toledo et al., 2008; Chacón et al., 2008; Inestrosa and Arenas, 2009), and in particular, Wnt1 signaling has been closely tied to the control of apoptotic cell injury (Chong and Maiese, 2007; Li et al., 2007; Maiese et al., 2008). Of special interest, the Wnt-Fzd signaling pathway forms a critical component for midbrain DAergic development (Castelo-Branco et al., 2003; 2004;Prakash and Wurst, 2006; Rawal et al., 2006; Joksimovic et al., 2009). Here, the temporal analysis of Fzd1, which provides the best discrimination of the Wnt/β-catenin pathway (Gordon and Nusse, 2006), indicated a dynamic modulation after MPTP injury in younger mice as opposed to a long-lasting downregulation observed in middle-aged mice. While further studies are presently in progress to identify which specific cell type (neurons, glia, DA neuroprogenitors) harbors Fzd-1, its dynamic down- and up-modulation may be linked to different plastic responses in the injured midbrain of younger mice, including axon remodeling, death/survival responses (Chacón et al., 2008; Inestrosa and Arenas, 2009). The observed upregulation of Fzd-1 within the temporal window of Wnt1 expression prompted us to investigate two pivotal proteins of Wnt canonical signaling, i.e., β-catenin and GSK-3β. Recently GSK-3β has emerged as a critical step in MPTP/MPP+, 6-OHDA-, and rotenone-mediated neuronal cell death (Chen et al., 2004; Wang et al., 2007; Petit-Paitel et al., 2009; Duka et al., 2009). In addition, GSK-3β activation has been linked to inflammation and microglia activation (Chong and Maiese, 2007; Jope et al., 2007). In Wnt canonical pathway, GSK-3β holds a pivotal position, where it phosphorylates β-catenin in concert with APC, Fzd, and axin, causing β-catenin degradation via the ubiquitin-proteasome pathway (Aberle et al., 1997). On the other hand, activation of canonical Wnt signaling leads to GSK-3β downregulation, and un-phosphorylated/activated β-catenin accumulates in the cytoplasm and translocate in the nucleus where it interacts with cofactors to regulate the transcription of target genes that are involved in cell cycle, survival, and differentiation (Maiese et al., 2008). GSK-3β is activated by phophorylation of the tyrosine 216 residue (Tyr216) located in the kinase domain and inactivated by phosphorylation of the amino-terminal serine 9 residue (Ser9). Here, the upregulation of phosphoTyr216 GSK-3β (i.e., the active GSK-3) occurred in vivo in this acute mouse model of PD, with kinetics and topography that preceded and paralleled the degeneration of SNpc neurons, as evidenced by FJC staining and by reciprocal loss of TH+ neurons in SNpc, and the activation of microglia. These findings are in agreement with the recent study of Petit-Paitel et al. (2009) showing the involvement of cytosolic and mitochondrial pTyr216 GSK-3β in MPTP/MPP-induced DAergic cell death and further show a dramatic loss of β-catenin after GSK-3β activation and within the temporal window of active SNpc degeneration. GSK-3β is known to have a critical role in neuronal apoptosis, and PD mimetics such as 6-OHDA, rotenone, MPTP/MPP+ induce neuronal apoptosis in a GSK-dependent manner in SH-SY5Y cells, PC12 cells, cerebellar granule neurons, and DAergic neurons (Chen et al., 2004; Wang et al., 2007; Duka et al., 2009). Importantly, two functional single nucleotide polymorphisms have been reported in PD brains (see Duka et al., 2009). In addition, post-mortem striata from patients diagnosed with PD revealed increased p-GSK-3β as compared to age-matched controls (Duka et al., 2009).
β-Catenin has recently emerged as critical element regulating neuronal survival. Li et al. (2007) showed that upregulation of β-catenin prevents the cells from going into apoptosis, whereas knockdown of β-catenin produces an increase in the number of apoptotic cells (Li et al., 2007). Interestingly, in hippocampal cell cultures, in vitro, Fzd1 receptor was shown to mediate Wnt3a-induced neuroprotection against the toxicity of Alzheimer’s amyloid-β-peptide (Chacón et al., 2008). Although correlative, the present results indicated that MPTP-induced loss of β-catenin might direct towards DAergic degeneration, whereas increased endogenous Wnt/β-catenin signaling might represent a compensatory neurorescue response. Based on these observations, we postulated that Wnt1 might represent a candidate signal involved in directing the neurorepair/neurorescue (Fig. 9).
Astroglial Wnt1 as a compensatory rescue signal for mesencephalic DAergic neurons
The temporal correlation of Wnt1 mRNA expression with Wnt1 protein appeared of interest in the light of recent pieces of evidence indicating that Wnt signaling system may be reinduced in the adult CNS after injury (Osakada et al., 2007) and the studies indicating that Wnt/β-catenin activation reduces neurodegeneration in mouse models of Alzheimer’s disease (Toledo et al., 2008; Chacón et al., 2008). While little is known on the source of Wnts in adult brain, recent findings indicate that Wnt3a is expressed in adult hippocampal astrocytes (Lie et al., 2005) and expression of Wnt/β-catenin signaling components was reported in adult astrocytes acutely isolated and purified ex vivo (Cahoy et al., 2008). Here, in situ hybridization revealed MPTP-reactive astrocytes as candidate source of Wnt1 within the injured VM. In addition, increased transcription of Wnt1 mRNA was observed in astrocytes derived ex vivo from MPTP-injured VM, raising the possibility that astrocyte-derived Wnt signals might directly and/or indirectly participate to DAergic neuroprotection/rescue. These results prompted us to investigate, in vitro, the implication of Wnt/β-catenin pathway in astrocyte-induced DAergic neuroprotection against MPP+ toxicity. Accordingly, inhibition of DAergic neuron survival was obtained by directly blocking Wnt/Fzd-1/β-catenin signaling with the prototypic antagonist of Wnt canonical pathway, Dkk1. Conversely, exogenous activation of Wnt signaling in MPP+-treated astroglial–neuron co-cultures, with a specific GSK-3β antagonist, sharply magnified astrocyte-induced DAergic neuroprotection. Moreover, addition of glial inserts or direct application of Wnt1 to purified DAergic neurons just prior MPP+ insult afforded a significant degree of neuroprotection, an effect counteracted by addition of Wnt1 blocking antibody, or the Wnt antagonist, Frizzled-1-cysteine-rich domain, thus supporting the critical role of Wnt1 in DAergic neuron survival. This suggestion is consistent with recent findings showing the ability of preventive treatments with GSK-3β inhibitors, starting before MPTP administration, to exert a significant degree of DAergic neuroprotection at both Str and SNpc levels (Youdim and Arraf, 2004; Wang et al., 2007). Together, these informations raised the possibility that targeting Wnt signaling might offer therapeutic benefit by enhancing endogenous neurorescue/regeneration mechanisms and prompted us to address the functional implications of this pathway, in vivo.
Pharmacologic activation of Wnt/β-catenin signaling in middle-aged mice promotes nigrostriatal recovery
Neurochemical, morphological, and behavioral changes clearly indicate that with the aging process, the nigrostriatal DAergic system loses its ability to recover from MPTP injury (Ho and Blum, 1998). Old age is also recognized to dramatically modify the glial compartment. In particular, morphological, structural, and functional deterioration of microglia may contribute to aging-related neurodegeneration (Miller and Streit, 2007; Njie et al., 2010; Streit, 2010), including DAergic degeneration (Boger et al., 2010). On the other hand, within the midbrain microenvironment, astrocytes are recognized to create a more favorable milieu for nigrostriatal DAergic neuron survival in neurodegenerative conditions (Di Monte and Langston, 1995; Benner et al., 2004; Sandhu et al., 2009; Chen et al., 2009; Ourednik et al., 2009); however, age-dependent reduction/loss of astrocyte neurotrophic factors may contribute to the poor DAergic neurorecovery observed with age (L’Episcopo et al., 2010). This aspect appears of special importance in view of the strong decrease of adult neurogenesis with increasing age (Kronenberg et al., 2006; Sharpless, 2010) and neurodegenerative pathologies (He and Shen, 2009). Here, among the multiple mechanism(s) at play, we identified a dysregulation of Wnt/β-catenin signaling in response to MPTP in middle age, the period that precedes senescence. Interestingly enough, among other potential factors that may be dysfunctional/disrupted within the midbrain of aging mice, we identified lack of Wnt1 expression in VM and VM astrocytes, and further studies, actually in progress, are needed to clarify the mechanisms involved in this effect. Hence, a long-lasting decrease of β-catenin and Fzd-1 receptor accompanying upregulation of active GSK-3β supported a dysfunction of Wnt signaling in old injured VM. Importantly, we could mimic nigrostriatal recovery observed in young mice, by post-injury activation of Wnt/β-catenin signaling, with the same GSK-3β inhibitor used in vitro. Hence, systemic administration of AR for 3 weeks, corresponding to the temporal window of Wnt1/Fzd-1/β-catenin activation in younger mice, reversed the downregulation of β-catenin and Fzd-1 receptor and by 42 dpt, a remarkable recovery both at the SNpc and striatal levels was observed in middle-aged mice as opposed to their age-matched untreated counterparts that failed to recover. While further results are required to establish the cause for Wnt signaling dysregulation in middle age, including the potential role of altered astrocyte–microglial crosstalk, alteration in neuroinflammatory markers and/or in the innate control mechanisms regulating inflammation and repair (see Hauwel et al., 2005), the present findings implicate a causative role for Wnt signaling activation in post-injury recovery (seeFig. 9).
Activated astrocytes of the ventral midbrain and Wnt/β-catenin signaling are key players in promoting adult midbrain neurogenesis, in vitro
Reactive astrocytes express receptors for growth factors, cytokines, chemokines, and hormones and produce a wide array of neurotrophic and neuroprotective mediators in cooperation with those produced by microglia (Morale et al., 2006; Sofroniew and Vinters, 2010; L’Episcopo et al., 2010). In addition, astrocytes are known to release various region-specific signaling molecules, such as Shh and Wnts, which may interact with each others to dictate the neurogenic behavior in the adult CNS (Alvarez-Builla et al., 2001; Song et al., 2002; Lie et al., 2005; Barkho et al., 2006; Jiao and Chen, 2008). Indeed, canonical β-catenin regulatory mechanisms are known to be required for activation of adult neurogenesis both in vitro and in vivo (Lie et al., 2005; Yu et al., 2006; Adachi et al., 2007). Astrocytes have pivotal roles in glia–neuron interactions and for defining the stem cell niche. Hence, E13.5 VM astrocytes, but not cortex (Cx) astrocytes, express Wnt1 and Wnt5a and different DA-specific transcription factors such as Pax-2, En-1, and Otx-2 and increase the differentiation of VM embryonic precursors into TH+ neurons, in vitro, suggesting that VM astrocytes constitute part of the neurogenic niche that play a key role in VM DA neurogenesis (Wagner et al., 1999; Castelo-Branco et al., 2006). Here, ex vivo and in vitro studies suggest that MPTP injury and certain pro-inflammatory chemokines induce the expression of Wnt1 in astrocytes of the VM, indicating not only region specificity but also a defined inflammatory milieu in the modulation of Wnt1 induction in astrocytes. Recent findings indicate that interleukins 1β and 6 may be involved in astroglial modulation of adult neurogenesis (Barkho et al., 2006). In addition, certain specifically activated microglial cells can induce neural cell renewal in the adult CNS (Butovsky et al., 2006). Hence, microglia pretreated with IL-4 or IFN-γ induced neurogenesis and oligodendrogenesis in NPCs derived from the SVZ, whereas LPS-pretreated microglial cells blocked both processes in aNPCs (Butovsky et al., 2006), in line with reports that inflammation associated with LPS block adult neurogenesis (Ekdahl et al., 2009; Schwartz et al., 2009; Butovsky et al., 2006), and supporting a critical role for a specific inflammatory milieu in dictating promotion or inhibition of adult neurogenesis (Butovsky et al., 2006; Schwartz et al., 2009).
Our results together with the data of the literature suggest a model in which adult VM astrocytes may under specific controlled conditions, re-express region-specific factors, including Wnt1, contributing to the regulation of diverse aspects of DAergic neuron homeostasis in the injured VM, including amelioration of the impaired nigral milieu, mitigating DAergic neuron death, and/or enhancing survival, expansion, differentiation of DA progenitors. While the presence of DA neurogenesis within the SN, either under physiological conditions or in PD animal models, is currently debated, the presence of multipotent clonogenic neural stem cells in the adult mouse midbrain/hindbrain with functional neurogenic and DAergic potential, in vitro, was recently reported (see Hermann et al., 2006, 2009; Hermann and Storch, 2008, for review). Here, among different experimental settings, only the direct co-culture paradigm between VM astrocytes and midbrain-derived NPCs induced the TH phenotype, an effect prevented by Dkk1, suggesting that astrocyte-derived factors and endogenous Wnt/β-catenin signaling may contribute to the induction of the DAergic neuronal phenotype. Further studies, actually in progress, are clearly needed to verify whether the in vitro results may correlate with the in vivo condition, to ascertain the presence of DAergic progenitors and define the conditions that may promote adult neurogenesis within the MPTP-injured midbrain.
In conclusion, we herein highlight reactive astrocytes and Wnt/β-catenin signaling as important contributors of MPTP-induced DAergic plasticity (Fig. 9). Understanding the complex interactions required for successful DAergic recovery and how these mechanisms may be dysregulated in PD may lead to novel therapeutic approaches in the field of endogenous regeneration and PD. Enhancing neuron survival/protection and improving the efficiency of neurogenesis and/or functional integration of newly produced neurons, as well as targeting astrocytes to promote in situ nigrostriatal recovery, may represent novel avenues to be explored in PD experimental models.
Supplementary Material
Acknowledgments and Funding
The authors wish to thank the Italian Ministry of Health (Con. no. 82; Ps-CARDIO ex 56, PS-NEURO ex 56 to B.M.; Young Investigator Award 2009 to S.P.), Italian Ministry of Research (Cur. Res. 2008–2010 to B.M.), the Italian Multiple Sclerosis Foundation (FISM, grants 2004/R/15 to S.P. and 2002/R/37 to G.M.), the National Multiple Sclerosis Society (NMSS, partial grants RG-4001-A1 to S.P.; RG 3591-A-1 to G.M.; and RG 3762-A-1), the Italian Ministry of Research and University (MIUR, to B.M.), the European Research Council (Starting Independent Researcher Grant to S.P. and Established Investigator Grant to M.S.), Wings for Life (SE-013/09 to S.P.) and Banca Agricola Popolare di Ragusa (BAPR, unrestricted grant to S.P.), and the OASI (IRCCS) Institution for Research and Care on Mental Retardation and Brain Aging Troina (EN) Italy.
S.P. holds a John and Lucille van Geest University Lecturership in Brain Repair at the Cambridge Centre for Brain Repair, University of Cambridge, UK.
C.C. is receiving a fellowship (SFRH/BD/15899/2005) from the Fundação para a Ciĉncia e a Tecnologia (FCT). A.E.C.I is receiving a John and Lucille van Geest post-doctoral fellowship.
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.nbd.2010.10.023.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin–proteasome pathway. EMBO J. 1997;16(13):3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, et al. β-Catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Alvarez-Builla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2(4):2287–2293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T, Nakashima K, et al. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15(3):407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J. Neurosci. 1999;19(5):1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner EJ, Mosley RL, Destache CJ, Lewis TB, Jackson-Lewis V, Gorantla S, et al. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc. Natl Acad. Sci. USA. 2004;101:9435–9440. doi: 10.1073/pnas.0400569101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog. Neurobiol. 1998;55:93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Blackburn D, Sargsyan S, Monk PN, Shaw PJ. Astrocyte function in motor neuron disease: a future therapeutic target? Glia. 2009;57:1251–1264. doi: 10.1002/glia.20848. [DOI] [PubMed] [Google Scholar]
- Blanchard V, Anglade P, Dziewczapolski G, Savasta M, Agid Y, Raisman-Vozari R. Dopaminergic sprouting in the rat striatum after partial lesion of the substantia nigra. Brain Res. 1996;709(2):319–325. doi: 10.1016/0006-8993(95)01391-1. [DOI] [PubMed] [Google Scholar]
- Boger HA, Granholm AC, McGinty JF, Middaugh LD. A dual-hit animal model for age-related parkinsonism. Prog. Neurobiol. 2010;90:217–229. doi: 10.1016/j.pneurobio.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Cadet JL, Kent JD, Karanas AL, Jackson-Lewis V. An assessment of the validity of densitometric measures of striatal tyrosine hydroxylase-positive fibers: relationship to apomorphine-induced rotation in 6-hydroxydopamine lesioned rats. J. Neurosci. Meth. 1990;35:63–73. doi: 10.1016/0165-0270(90)90095-w. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Gennady L, Talpalar AE, Pluchino S, et al. Microglia activated by IL-4 or IFN-gamma differentiaally induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 2008;28(1):264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, Pasolli HA, Fuchs E, Kitajewski J, Arenas E. Differential regulation of midbrain dopaminergic neuron development by Wnt1, Wnt3a, and Wnt5a. Proc. Natl Acad. Sci. USA. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco G, Rawal N, Arenas E. GSK-3β inhibition/β-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J. Cell Sci. 2004;117:5731–5737. doi: 10.1242/jcs.01505. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco G, Sousa KM, Bryja V, Pinto L, Wagner J, Arenas E. Ventral midbrain glia express region-specific transcription factors and regulate dopaminergic neurogenesis through Wnt-5a secretion. Mol. Cell. Neurosci. 2006;31(2):251–262. doi: 10.1016/j.mcn.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Chacón MA, Varela-Nallar L, Inestrosa NC. Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Abeta oligomers. J. Cell. Physiol. 2008;217(1):215–227. doi: 10.1002/jcp.21497. [DOI] [PubMed] [Google Scholar]
- Chen G, Bower KA, Ma C, Ma C, Fang S, Thiele CJ, et al. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxy dopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, et al. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: critical role for the astrocyte. PNAS. 2009;106(8):2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signalling pathways. Cell. Signal. 2007;19(6):1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Conti B, Sugama S, Lucero J, Winshy-Sommer R, Wirz SA, Maher P, et al. Uncoupling protein 2 protects dopaminergic neurons from acute 1,2,3,6-methyl-phenyl-tetrahydropyridine toxicity. J. Neurochem. 2005;93:493–501. doi: 10.1111/j.1471-4159.2005.03052.x. [DOI] [PubMed] [Google Scholar]
- De Ferrari GF, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G, Reyes AE, Alvarez A, Bronfman M, Inestrosa NC. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol. Psychiatry. 2003;8:195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- Di Monte DA, Langston JW. Idiopathic and 1-methyl-4phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford University Press; 1995. pp. 997–989. Chapter 65. [Google Scholar]
- Duka T, Duka V, Joyce JN, Sidhu A. α-Synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009;23(9):2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Ekonomou A, Kazanis I, Malas S, Wood H, Alifragis P, Denaxa M, et al. Neuronal migration and ventral subtype identity in the telencephalon depend on SOX1. PLoS Biol. 2005;3:e186. doi: 10.1371/journal.pbio.0030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engele J, Bohn MC. The neurotrophic effects of fibroblast growth factors on dopaminergic neurons in vitro are mediated by mesencephalicglia. J. Neurosci. 1991;11(10):3070–3078. doi: 10.1523/JNEUROSCI.11-10-03070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press Inc.; 1997. [Google Scholar]
- Gallo F, Morale MC, Spina-Purrello V, Tirolo C, Testa N, Farinella Z, et al. Basic fibroblast growth factor (bFGF) acts on both neurons and glia to mediate the neurotrophic effects of astrocytes on LHRH neurons in culture. Synapse. 2000;36:233–253. doi: 10.1002/(SICI)1098-2396(20000615)36:4<233::AID-SYN1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglia NADPH-oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003;17:1954–1966. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennuso F, Fernetti C, Tirolo C, Testa N, L’Episcopo F, Caniglia S, et al. Bilirubin protects astrocytes from its own toxicity inducing up-regulation and translocation of multigrug resistance-associated protein 1 (Mrp 1) Proc. Natl Acad. Sci. USA. 2004;101:2470–2475. doi: 10.1073/pnas.0308452100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Griffiths MR, Gasque P, Neal JW. The multiple roles of the innate immune system in the regulation of apoptosis and inflammation in the brain. J. Neuropathol. Exp. Neurol. 2009;68:217–226. doi: 10.1097/NEN.0b013e3181996688. [DOI] [PubMed] [Google Scholar]
- Griffiths M, Neal JW, Gasque P. Innate and protective neuroinflammation: new emphasis on the role of neuroimmune regulatory proteins. Int. Rev. Neurobiol. 2007;82:29–55. doi: 10.1016/S0074-7742(07)82002-2. [DOI] [PubMed] [Google Scholar]
- Hauwel M, Furon E, Canova C, Griffiths M, Neal J, Gasquue P. Brain Res. Exp. Brain Res. Rev. 2005;48:220–233. doi: 10.1016/j.brainresrev.2004.12.012. [DOI] [PubMed] [Google Scholar]
- He P, Shen Y. Interruption of β-catenin signaling reduces neurogenesis in Alzheimer’s disease. J. Neurosci. 2009;29:6545–6557. doi: 10.1523/JNEUROSCI.0421-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Maisel M, Wegner F, Liebau S, Kim DW, Gerlach M. Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells. 2006;24:949–964. doi: 10.1634/stemcells.2005-0192. [DOI] [PubMed] [Google Scholar]
- Hermann A, Storch A. Endogenous regeneration in Parkinson’s disease: do we need orthotopic dopaminergic neurogenesis? Stem Cells. 2008;26:2749–2752. doi: 10.1634/stemcells.2008-0567. [DOI] [PubMed] [Google Scholar]
- Hermann A, Suess C, Fauser M, Kanzler S, Witt M, Fabel K, et al. Rostrocaudal loss of cellular diversity within the periventricular regions of the ventricular system. Stem Cells. 2009;27:928–941. doi: 10.1002/stem.21. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Ho A, Blum M. Induction of interleukin-1 associated with compensatory dopaminergic sprouting in the denervated striatum of young mice: model of aging and neurodegenerative disease. J. Neurosci. 1998;18(15):5614–5629. doi: 10.1523/JNEUROSCI.18-15-05614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Choi DK, Nagai M, Wu DC, Nagata T, Prou D, et al. Neuronal NOS and cyclooxygenase-2 contribute to DNA damage in a mouse model of Parkinson disease. Free Radic. Biol. Med. 2009;47:1049–1056. doi: 10.1016/j.freeradbiomed.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Palmer TD. New roles for astrocytes : the night life of an “astrocyte”. La vida loca! Trends Neurosci. 2003;26:597–603. doi: 10.1016/j.tins.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Parkinson’s disease and the adaptive capacity of the nigrostriatal dopamine system: possible neurochemical mechanisms. Advances in neurology. 1993;vol. 60 [PubMed] [Google Scholar]
- Hu X, Zhang D, Pang H, Caudle WM, Li Y, Gao H, et al. Macrophage antigen complex-1 mediates reactive microgliosis and progressive dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J. Immunol. 2008;181(10):7194–7204. doi: 10.4049/jimmunol.181.10.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging role of Wnts in the adult nervous system. Nat. Rev. Neurosci. 2009;11(2):77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1, 2, 3, 6 tetrahydropyridine. Neurodegeneration. 1995;4(3):257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP model of Parkinson’s disease. Nat. Protoc. 2007;2(1):141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Jacowec MW, Nixon K, Hogg E, McNeill T, Petzinger G. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4-phenyl-1,2,3,6-tetra-hydroppyridine-induced degeneration of the mouse nigrostriatal pathway. J. Neurosci. Res. 2004:76539–76550. doi: 10.1002/jnr.20114. [DOI] [PubMed] [Google Scholar]
- Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26:1221–1230. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic M, Yun BA, Kittappa R, Anderegg AM, Chang WW, Taketo MM, et al. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat. Neurosci. 2009;12(2):125–131. doi: 10.1038/nn.2243. [DOI] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem. Res. 2007;32(4–5):577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996 Aug.19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercice prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol. Aging. 2006;27(10):1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Marchetti B. Glia as a turning point in the therapeutic strategy of Parkinson’s disease. CNS Neurol. Disord. 2010;9:349–372. doi: 10.2174/187152710791292639. [DOI] [PubMed] [Google Scholar]
- Li HL, Wang LL, Liu SJ, Deng YQ, Zhang YJ, Tian YJ, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing β-catenin, a mechanism involved in Alzheimers’s degeneration. Proc. Natl Acad. Sci. USA. 2007;104:3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HG, Désiré L, Mira H, Consiglio A, et al. Wnt signaling regulates adult hippocampal neurogenesis. Nature. 2005;473:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc. Natl Acad. Sci. USA. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Faqi L, Chong ZZ, Chen SY. The Wnt signalling pathway: aging gracefully as a protectionist? Pharmacol. Ther. 2008;118(1):58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti B, Abbracchio MP. To be or not to be (inflammed) is that the question in anti-inflammatory drug therapy of neurodegenerative diseases? Trends Pharmacol. Sci. 2005;26:517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Serra PA, Tirolo C, L’Episcopo F, Caniglia S, Gennuso F, et al. Glucocorticoid receptor-nitric oxide crosstalk and vulnerability to experimental Parkinsonism: pivotal role for glia–neuron interactions. Brain Res. Rev. 2005a;48(2):302–321. doi: 10.1016/j.brainresrev.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Serra PA, L’Episcopo F, Tirolo C, Caniglia S, Testa N, et al. Hormones are key actors in gene × environment interactions programming the vulnerability to Parkinson’s disease: glia as a common final pathway. Ann. NY Acad. Sci. 2005b;1057:296–318. doi: 10.1196/annals.1356.023. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Kettenmann H, Streit WJ. Glia–neuron crosstalk in neuroinflammation, neurodegeneration and neuroprotection. Brain Res. Rev. 2005c;482(2):129–489. Special Issue. [Google Scholar]
- McGeer P, McGeer EG. Glial reactions in Parkinson’s disease. Mov. Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- McNaught KSP, Jenner P. Altered glial function causes neuronal death and increases neuronal susceptibility to 1-methyl-4-phenylpyridinium-and 6-hydro-xydopamine-induced toxicity in astrocytic/ventral mesencephalic co-cultures. J. Neurochem. 1999;73:2469. doi: 10.1046/j.1471-4159.1999.0732469.x. [DOI] [PubMed] [Google Scholar]
- Miller KR, Streit WJ. The effect of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3(3):245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- Morale MC, Serra P, Delogu MR, Migheli R, Rocchitta G, Tirolo C, et al. Glucocorticoid receptor deficiency increases vulnerability of the nigrostriatal dopaminergic system: critical role of glial nitric oxide. FASEB J. 2004;18:164–166. doi: 10.1096/fj.03-0501fje. [DOI] [PubMed] [Google Scholar]
- Morale MC, Serra PA, L’Episcopo F, Tirolo C, Caniglia S, Testa N, et al. Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience. 2006;138:869–878. doi: 10.1016/j.neuroscience.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and agent rodent brain reveal age-related changes in microglial function. Neurobiol. Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signalling promotes regeneration in the retina of adult mammals. J. Neurosci. 2007;27(15):4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourednik VC, Ourednik J, Xu Y, Zhang Y, Lynch WP, Snyder EY, et al. Cross-talk between stem cells and the dysfunctional brain is facilitated by manipulating the niche: evidence from an adhesion molecule. Stem Cells. 2009;27:2846–2856. doi: 10.1002/stem.227. [DOI] [PubMed] [Google Scholar]
- Parish CL, Finkelstein DI, Tripanichkul W, Satoskar AR, Drago J, Horne MK. The role of interleukin-1, interleukin-6, and glia in inducing growth of neuronal terminal arbors in mice. J. Neurosci. 2002;22:8034–8041. doi: 10.1523/JNEUROSCI.22-18-08034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr. Opin. Neurobiol. 2000;10(3):392–399. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Paitel A, Brau F, Cazareth J, Chabry J. Involment of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/Mpp+-treated neurons. PLoS ONE. 2009;4(5):e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Fisher B, Hogg E, Abernathy A, Arevalo P, Nixon K, Jacowec MW. Behavioral motor recovery in the 1-methyl-4-phenyl-1,2,3,6-tetrahdropyridine-lesioned squirrel monkey: changes in striatal dopamine and expression of tyrosine hydroxylase transporter proteins. J. Neurosci. Res. 2006;83:332–347. doi: 10.1002/jnr.20730. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422(6933):688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Prakash N, Wurst W. Genetic networks controlling the development of midbrain dopaminergic neurons. J. Physiol. 2006;575:403–410. doi: 10.1113/jphysiol.2006.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal N, Castelo-Branco G, Souse KM, Kele J, Kobayashy K, Okano H, et al. Dynamic temporal and cell type-specific expression of Wnt signaling components in the developing midbrain. Exp. Cell Res. 2006;312:1626–1636. doi: 10.1016/j.yexcr.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Irwin I, Forno LS, DeLanney LE, Langston E, Langston JW. Aging and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced degeneration of dopaminergic neurons in the substantia nigra. Brain Res. 1987;403(1):43–51. doi: 10.1016/0006-8993(87)90120-x. [DOI] [PubMed] [Google Scholar]
- Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, et al. Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol. Dis. 2009;33:405–414. doi: 10.1016/j.nbd.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertston C, Slikker W., Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Schwartz M, London A, Shecter R. Boosting T-cell immunity as a therapeutic approach for neurodegenerative conditions: the role of innate immunity. Neuroscience. 2009;158:1133–1142. doi: 10.1016/j.neuroscience.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Schwartz JP, Wilson DJ. Preparation and characterization of type 2 astrocytes cultured from adult rat cortex, cerebellum, and striatum. Glia. 1992;5:75–80. doi: 10.1002/glia.440050111. [DOI] [PubMed] [Google Scholar]
- Serra PA, Sciola L, DeLogu MR, Spano A, Monaco G, Miele E, et al. The neurotoxin MPTP induces apoptosis in mouse nigrostriatal glia. Relevance to nigral neuronal death and striatal neurochemical changes. J. Biol. Chem. 2002;277:34451–34461. doi: 10.1074/jbc.M202099200. [DOI] [PubMed] [Google Scholar]
- Sharpless NE. Hot topics in stem cells and self-renewal: 2010. Aging Cell. 2010 doi: 10.1111/j.1474-9726.2010.00592.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon HH, Saueressig H, Wurst W, Goulding MD, O’Leary DD. Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J. Neurosci. 2001;21:3126–3134. doi: 10.1523/JNEUROSCI.21-09-03126.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew M, Vinters HB. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Song W, Su H, Song S, Paudel HK, Schipper HM. Over-expression of heme oxygenase-1 promotes oxidative mitochondrial damage in rat astroglia. J. Cell. Physiol. 2006;206:655–663. doi: 10.1002/jcp.20509. [DOI] [PubMed] [Google Scholar]
- Stanic D, Finkelstein DI, Bourke DW, Drago J, Horne MK. Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. Eur. J. Neurosci. 2003;18(5):1175–1188. doi: 10.1046/j.1460-9568.2003.02800.x. [DOI] [PubMed] [Google Scholar]
- Stanic D, Tripanichkul W, Drago J, Finkelstein DI, Horne MK. Glial responses associated with dopaminergic striatal reinnervation following lesions of the rat substantia nigra. Brain Res. 2004;1023(1):83–91. doi: 10.1016/j.brainres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglial activation and neuroinflammation in Alzheimer’s disease: a critical examination of recent history. Front. Aging Neurosci. 2010;3:2–22. doi: 10.3389/fnagi.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima T, Johnston JM, Commissiong JW. Mesencephalic type 1 astrocytes rescue dopaminergic neurons from death induced by serum deprivation. J. Neurosci. 1994;14:4769–4779. doi: 10.1523/JNEUROSCI.14-08-04769.1994. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo EM, Clombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog. Neurobiol. 2008;88:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Guegan C, Wu D, Teismann P, Choi DK, et al. The role of glial cells in Parkinson’s disease. Curr. Opin. Neurol. 2001;14:483–489. doi: 10.1097/00019052-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson R, Song B, Ao Y, Morales LBJ, Tiwari-Woodruff S, et al. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J. Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Akerud DS, Castro PC, Holm JM, Canals EY, Snyder T, Perlmann T, Arenas E. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat. Biotechnol. 1999;17:653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, et al. Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22(5):1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Arraf Z. Prevention of MPTP (n-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium involvements of Bcl-2 and bax. Neuropharmacology. 2004;46:1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Yu JM, Kim JH, Song GS, Jung JS. Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol. Cell. Biochem. 2006;288:17–28. doi: 10.1007/s11010-005-9113-3. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Leak K, Smejne RJ, Smith AD. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat. Disord. 2009;15S3:S42–S45. doi: 10.1016/S1353-8020(09)70778-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.