Abstract
Aims
Caregiver distress can affect mood and cognition. Meditation can be used to reduce stress. This pilot study explored whether yogic meditation could change regional cerebral metabolism in distressed caregivers.
Methods
Nine dementia caregivers were randomized to undergo meditation training compared with relaxation for 12 min per day for 8 weeks. Caregivers received neuropsychiatric assessments and brain FDG-PET scans at baseline and postintervention.
Results
The groups did not differ on measures of mood, mental and physical health, and burden at baseline and follow-up. When comparing the regional cerebral metabolism between groups, significant differences over time were found in the bilateral cerebellum (p < 0.0005), right inferior lateral anterior temporal (p < 0.0005), right inferior frontal (p = 0.001), left superior frontal (p = 0.001), left associative visual (p = 0.002) and right posterior cingulate (p = 0.002) cortices.
Conclusion
Meditation practice in distressed caregivers resulted in different patterns of regional cerebral metabolism from relaxation. These pilot results should be replicated in a larger study.
Keywords: dementia caregiver, depression, Kirtan kriya, PET, regional brain metabolism, stress, yogic meditation
With the US population aging, the prevalence of dementia and the number of family caregivers will increase dramatically in the next two decades, adding to the cost of clinical care. Over 5 million Americans provide unpaid care for their loved ones with Alzheimer’s disease and dementia [1]. The detrimental impact of informal dementia caregiving on mental health with high prevalence and incidence of depression has been well documented in recent years [2–4]. Chronic and severe caregiver stress can lead to cognitive impairment [5–10]. Yogic meditation practice can provide stress reduction for caregivers, resulting in improvement in mental and cognitive function [11]. In this pilot study we explore the effects of meditation on brain metabolism in distressed aging dementia caregivers, which is very much in line with the journal’s interest in health-promoting practices for stress reduction that could prevent morbidity and mortality in a high-risk group of informal dementia caregivers.
Our recently published pilot study of Kirtan Kriya (KK) meditation found that brief daily practice by family dementia caregivers led to improved mental and cognitive function and lower levels of depressive symptoms, accompanied by an increase in telomerase activity [12]. KK is an ancient Kundalini yoga meditation, involving chanting, visualization of light and specific finger tapping or mudras, which has been studied in older adults with memory complaints and reported regional brain metabolic, cognitive and mood changes [13,14]. Newberg et al. compared regional cerebral blood flow (CBF) in 12 advanced meditators, who practiced KK daily for 15 years or more, to the CBF in 14 nonmeditators at rest [15]. The long-term meditators had significantly higher regional CBF in the prefrontal cortex, parietal cortex, thalamus, putamen, caudate and midbrain. Other recent neuroimaging studies have also suggested neuroprotective and neuroplastic effects of meditation [16,17], with changes in the dorsolateral prefrontal and the anterior cingulate function [18,19].
The present pilot study examined whether daily yogic meditation can reduce caregiver distress and change patterns of regional cerebral metabolism in an 8-week study of KK meditation in family dementia caregivers. We hypothesized that meditation practice would be associated with a different pattern of changes in regional metabolism reflecting the neuroplastic effect of active meditation compared with the relaxation control. Based on our review of the brain activation studies during meditation [18–20] and changes in resting brain function in the experienced meditators compared with nonmeditators [14,21,22], we anticipated that meditation practice would be associated with increased metabolism in prefrontal, and anterior and posterior cingulate cortices compared with the relaxation control condition. We expected those participating in meditation to have greater improvement in behavioral measures of depression and mental health compared with the control group.
Methods
Over a period of 6 months, we recruited ten caregivers into the PET study (mean age = 55.8 years, standard deviation = 8.7) who were identified by the family member with dementia or the physician as the primary source of support, taking care of their relatives with dementia. After providing a full description of the study to the subjects, written informed consent was obtained in accordance with the procedures set by the University of California Los Angeles Institutional Review Board. Nine subjects completed the follow-up PET scans after the 8-week intervention and were included in the final analyses.
Potential subjects were required to be adult or elderly caregivers of patients with dementia being evaluated by the geriatric psychiatry and memory clinics. In addition, the subjects were identified by the patient or clinical staff as the primary source of assistance and/or support, and were in contact with the dementia patient at least 3 days per week. First, subjects were screened using the Structured Clinical Interview for the DSM-IVR (SCID) and Hamilton Rating Scale for Depression (HAM-D-24 item) to rule out major depression that would require other treatment modalities [23]. Those who had scores between 5 and 17 signifying mild-to-moderate levels of depressive symptoms were invited to participate in the trial. Lastly, a score of 26 or higher on the Folstein Mini-Mental State Examination (MMSE) was required for inclusion [24]. Exclusion criteria were:
A history of any other psychiatric illness;
Alcohol and/or substance abuse or dependence;
Severe or acute medical illness;
Acute suicidal or violent behavior;
Any other CNS disease or dementia.
Procedures
All subjects were randomized, using a computer-generated randomization table, to participate in either KK practice or a relaxation practice for 12 min per day for 8 weeks. The protocol for KK is standard for the Kundalini Yoga practice as taught by Yogi Bhajan that was utilized in the previous studies in older adults [14,25]. We assessed the severity of depressive symptoms, mental and physical functioning and cognition at baseline, and at the end of the 8-week study, or upon early termination. In addition to the treatment protocol, all caregivers also received psychoeducation about the course and prognosis of dementia and about caregiver health, which was intended to address identified shortcomings in the care of their family members.
Intervention protocol
We provided samples of both the meditation and relaxation CDs during screening visits to assess if subjects were amenable to the interventions. Only subjects who were comfortable with both interventions and agreed to participate were recruited. The interventions were introduced and taught by a single instructor (Helen Lavretsky), a certified Kundalini Yoga teacher. All behavioral and PET ratings were done by raters, who were blinded to the group assignment (Nora Nazarian and Natalie M St Cyr – behavioral; and Kelsey Pomykala, Dan Silverman and Cheri Geist – PET ratings).
Meditation group procedures
Meditation was introduced to all caregivers during their baseline visit. A brief 12 min yogic practice included an ancient chanting meditation (KK), which was performed every day at the same time of day for a total of 8 weeks and utilized a previously tested protocol with a recorded CD [25]. The meditation involved repetitive finger movements, or mudras, as well as chanting of the mantra, ‘saa, taa, naa, maa,’ meaning ‘birth, life, death and rebirth’ [26]. Compliance with either intervention was monitored at each visit by direct interview and a review of daily diaries documenting the length of and adherence to the practice.
Relaxation group procedures
Participants in the relaxation group were asked to relax in a quiet place with their eyes closed while listening to the instrumental music on the relaxation CD for 12 min every day at the same time for 8 weeks. The music was provided to the subjects on a CD, along with instructions to find a quiet place without distractions, to close their eyes and try to relax. The choice of control was motivated by the intention to test whether relaxation response was solely responsible for the effect of meditation. Because KK involved melodic chanting, we chose to control with relaxation while listening to instrumental music.
Assessment instruments
Several instruments were used to assess psychological distress and coping. Mental Health Composite Score was assessed using the Medical Outcomes Study Short Form 36-Item Health Survey (SF-36) [27], an instrument that measures health-related quality of life, mental, physical and social functioning. The Hamilton Rating Scale for Depression (HRSD-24 item) [23] was used to quantify mood symptoms. Burden interview was used to assess perceived caregiver burden [28,29]. The remaining instruments were used to compare groups at baseline. Cognitive performance was measured by the MMSE [24]. Baseline, as well as changes in Mental Health Composite Scores, MMSE, depression and burden scores were compared between groups using nonparametric Wilcoxon tests.
PET protocol
All subjects underwent [18F]-fluorodeoxyglucose (FDG)-PET scans to measure resting metabolism at baseline and 8 weeks later. Subjects received a 5mCi intravenous injection of FDG with their eyes open and ears unplugged in a dimmed room exposed only to ambient room sound. Following a 30-min uptake period after tracer injection, the positron emission scan was acquired in a 3D mode for 15 min with a Siemens PET/CT scanner. A 3D CT scan of the brain was acquired for use in reconstruction with attenuation correction.
Image analysis
Brain images from each subject were spatially coaligned to a common reference orientation using a linear (rigid) coregistration algorithm to correct for head motion and differences in positioning between the two blocks. All images were then further spatially transformed to standardized stereotactic space using a nonlinear (elastic) transformation.
Condition-based and covariate analyses of cognitive performance versus brain metabolism measured with FDG-PET, were also examined with statistical parametric mapping software by methods previously described [30]. Images were coregistered and reoriented into a standardized coordinate system [31] using the nonlinear spatial transformation package in SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK) [32], smoothed three-dimensionally using a full-width half maximum kernel of 8 × 8 × 8 mm, and normalized to mean global activity. Pooled data were then statistically assessed to identify the voxels that significantly differed between treatment groups (relaxation or meditation), or within groups before and after the 8-week interventions. Results were reported in terms of locations of the largest and/or most significant effects (regionally and/or in x,y,z Talairach style millimeter coordinates; t- and p-values).
In standard volume of interest (sVOI) analyses, each of the standard regions of interest were automatically transferred to the PET images following the transformation that coregisters each individual subject’s PET images to a normal template. The 240 standard regions of interest were clustered into 47 sVOIs defined according to anatomical, magnetic resonance and PET atlases. These included (on left and right) anterior and posterior cingulate, primary sensorimotor, superior and inferior parietal, parietotemporal in the area of the angular and marginal gyri, three temporal, five frontal, primary and associative visual occipital and cerebellar cortices, as well as left and right thalamus, putamen, caudate nucleus head and brainstem at the level of midbrain and pons. sVOI activity values were normalized to the average pixel activity value across all regions.
The meditation and relaxation groups were compared regarding changes in cerebral metabolism, as measured by PET scans over the study period. Changes in FDG uptake from baseline to the end of the treatment period were quantified for each subject and mean values were compared between groups for each region assessed. Data were evaluated for significance region by region and voxel by voxel using the student t-test. Based upon previous literature, a priori hypotheses for involved regions included bilateral prefrontal, anterior cingulate and posterior cingulate cortices. For regions not covered by a priori hypotheses, they were considered significant if p < 0.0005, in order to control for multiple comparisons with Bonferroni correction.
Results
Of the nine subjects who completed the intervention, four were randomized to the meditation group (mean age = 56.0 years, standard deviation = 10.1, all four females), and five to the relaxation group (mean age = 49.8, standard deviation = 3.9, four females and one male). The groups did not differ in the time spent in support or the psychoeducation activities. The groups did not differ on any demographic and clinical measures at baseline or follow-up except for the trend for great improvement on the SF-36 Mental Health Composite Score in the meditation group (Table 1).
Table 1.
Participant characteristics and psychosocial variables at baseline and postintervention.
| Demographic & clinical varibales | Baseline† | Post-treatment† | ||
|---|---|---|---|---|
|
| ||||
| Meditation (n = 4) | Relaxation (n = 5) | Meditation (n = 4) | Relaxation (n = 5) | |
| Age | 56.0 (10.1) | 49.8 (3.9) | – | – |
|
| ||||
| Sex | ||||
| – Male | 4 (100%) | 4 (80%) | – | – |
| – Female | – | 1 (10%) | – | – |
|
| ||||
| Years of education | 16.3 (1.7) | 16.8 (2.3) | – | – |
|
| ||||
| Years of caregiving | 7.8 (2.9) | 4.2 (3.6) | – | – |
|
| ||||
| Depression score (HAM-D) | 13.5 (6.0) | 13.2 (4.0) | 5.3 (6.0) | 6.6 (5.4) |
|
| ||||
| SF-36 | ||||
| – MCS | 31.4 (5.7) | 35.7 (10.2) | 48.5 (8.9) | 36.9 (7.5) |
| – PCS | 60.0 (5.8) | 55.6 (7.7) | 51.9 (7.3) | 50.9 (12.8) |
|
| ||||
| Burden | 47.0 (20.1) | 50.8 (14.5) | 37.8 (11.5) | 38.2 (19.6) |
Frequencies (%) for categorical variables and standard deviation for continuous variables.
None of the comparisons were significant at p < 0.05 level using nonparametric analyses.
HAM-D: Hamilton Rating Scale for Depression; MCS: Mental Health Composite Score; PCS: Physical Health Composite Score; SF-36: Medical Outcomes Study Short Form 36-Item Health Survey.
PET imaging
The meditation group demonstrated decreased metabolism over time in the right inferior frontal cortex (t=4.438; p=0.021), while the relaxation group demonstrated an increase in metabolism over time (t = 2.88; p = 0.046). In between-group analyses, the right inferior frontal cortex significantly differentiated the meditation and control groups (Figure 1: t = 4.74, p = 0.001, peak voxel [30, 26, -24], 160 voxels, largest region, 2.754% difference due to a 1.059% decrease in the meditation group and a 1.695% increase in the relaxation group). The right posterior cingulate cortex was the second largest area of significant difference between the meditation group and the control group (t = 3.98, p = 0.002), due to decreases in the meditation group (0.918%) and increases in the control group (1.772%). In addition, after the intervention, the left associative visual cortex (AVC) (t = 4.15; p = 0.002, peak voxel [-44, -74, -16] Figure 2) demonstrated decreased metabolism in meditators compared with the control group. Though not a region proposed a priori, this finding was seen in both sVOI (p = 0.01) and statistical parametric mapping (p = 0.002) analyses. In the direction of increases in the meditation group, the left superior frontal cortex (lGFs) demonstrated significant differences when the two groups were compared (t = 5.37; p = 0.001). In addition, a trend toward increasing activity was seen over time in the right lentiform nucleus (LN) (3.101% increase, t = 4.214; p = 0.024) within the meditation group, as well as a trend towards higher LN metabolism in direct statistical comparison to the control group (t = 2.09; p = 0.075). Lastly, bilateral cerebellar (bilaterally t ≥ 5; p<0.0005, differences due to increases in the meditation group and decreases in the relaxation group) and right anterior hippocampal metabolism significantly differed when comparing the two groups (peak voxel [28, -28, -4], t = 5.86; p < 0.0005, 1.164% difference due to a 0.344% increase in the meditation group and a larger 1.508% increase in the relaxation group) (Table 2).
Figure 1. Group differences in metabolic brain changes in the right inferior frontal cortex.
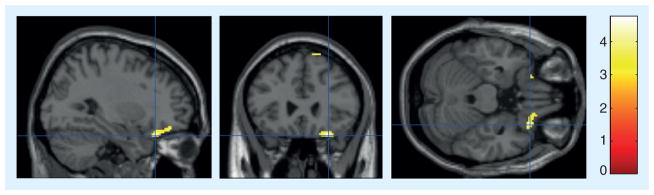
Cross-sectional view displays the crosshair intersection at (30, 26, -24 mm [x,y,z] coordinates), within the right inferior frontal area. Lighter pixels represent the regions of the brain that were significantly different between the meditation group and the relaxation group over time (t = 4.74 with p = 0.001, 160 contiguous voxels at p < 0.01).
Figure 2. Group differences in metabolic brain changes in the left associative visual cortex.
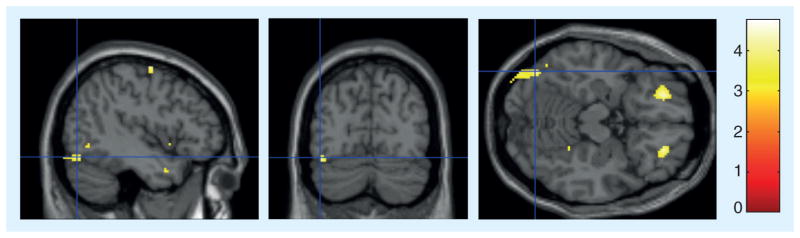
Cross-sectional view displays the crosshair intersection at (-44, -74, -16 mm [x,y,z] coordinates), within the left associative visual cortex. Lighter pixels represent the regions of the brain that had lower metabolism in the meditation group compared with the control group postintervention (t = 4.15; p = 0.002).
Table 2.
Brain regions that most significantly change over the period of the intervention when comparing the meditation and control group.
| sVOI | Significance† of difference of intervention-associated change between groups | Magnitude‡ of difference in intervention-associated change between groups (%) |
|---|---|---|
| riLAT | p < 0.0005 | 1.164 |
| rCbm | p < 0.0005 | 2.561 |
| lCbm | p < 0.0005 | 1.994 |
| rGFi | p = 0.001 | 15.287 |
| lGFs | p = 0.001 | 0.808 |
| rPCC | p = 0.002 | 2.426 |
Signficance determined by SPM analysis described in the methods section.
Magnitude determined by sVOI analysis described in the methods section.
lCbm: Left cerebellum; lGFs: Left superior frontal cortex; rCbm: Right cerebellum; rGFi: Right inferior frontal cortex; riLAT: Right inferior lateral anterior temporal cortex; rPCC: Right posterior cingulate cortex; sVOI: Standard volume of interest.
Discussion
This is the first pilot study of yogic meditation in caregivers documenting change in brain metabolism over 8 weeks of meditative practice compared with relaxation. As anticipated, we found differences in the metabolic cerebral changes between the yogic meditation and relaxation conditions, suggesting that the effects of the meditation on the brain are not solely due to relaxation response as discussed by the previous literature [33]. The meditation group demonstrated both decreases and increases in metabolism in several brain regions. The largest region to decrease in metabolism was the right inferior frontal cortex. The bilateral AVC and the right posterior cingulate also demonstrated decreases in metabolism. In the other direction, the left superior frontal cortex and the right LN demonstrated the most significant increases in metabolism. Finally, the group differences in the bilateral cerebellum and the right hippocampal were attributable to the more prominent change seen in the relaxation group. Unlike in our parent study [11], we did not find group differences in the behavioral measures of distress or cognition, most likely due to the small sample size.
The majority of the previous literature examined the acute and chronic effects of meditation on the brain and most of the studies used single-photon emission computed tomography or functional MRI to measure the CBF [34–36]. Few investigations have addressed how a chronic regimen of meditation affects resting cerebral activity and the present investigation is the first to use PET to measure the impact upon resting metabolism. Newberg et al. published two papers concerning changes in baseline CBF. The first paper examined a group of 14subjects with memory loss randomized into a KK meditation group or a music control group [14], very similar to our study. Many of the brain regions affected by meditation in their study were also found to be sites of altered metabolism in the present study. We found decreases in metabolism in the right inferior frontal cortex and right posterior cingulate cortex, while they found increases in CBF in the right inferior frontal cortex and posterior cingulate [14]. Together these findings suggest that meditation may be leading to specific regions of greater metabolic efficiency at rest, which are activated during the practice of meditation procedures. The right inferior frontal region controls both response inhibition and task switching, skills that Alzheimer’s disease caregivers need to use on a daily basis [37]. Meditation-based exercise of these regions, in addition to contributions to relief of chronic stress, may lead to greater efficiency of resting metabolism.
Interestingly, when measuring the CBF during KK meditation, Khalsa et al. found the posterior cingulate region to demonstrate increased CBF in healthy individuals who were experienced meditators compared with their resting baseline measurement [25]. Again, finding that reduced regional cerebral metabolic rates at rest may correspond to the meditators developing greater metabolic efficiency during mental relaxation. Previous studies are also consistent with this hypothesis. For example, Small et al. demonstrated that a group of 17 subjects without dementia, participants in a 14-day healthy longevity lifestyle program, increased their word fluency, while, demonstrating a decreased resting metabolism in the brain region involved with this activity [37]. Subjects may be able to improve the efficiency of their cerebral functioning, whether it is from meditation or a healthy lifestyle program, decreasing their resting cerebral metabolism.
The last significant region of decreased metabolism in the meditators was the bilateral AVC. This finding can be similarly explained. The visual cortex is more strongly activated during the KK meditation regimen because of the visualization of the L form. This stronger activation is associated with the AVC becoming more efficient at rest.
The two regions identified here with significantly increased metabolism were also previously reported [14,15,25]. In the Newberg et al. study on the subjects with memory loss, they found an increase in resting CBF in meditators’ right and left superior frontal cortices over the 8-week meditation regimen [14] and we identified an increase in the meditators’ metabolism in the left superior frontal cortex. Likewise, examining CBF during meditation and at rest, comparing the CBF of 12 advanced meditators and 14 non-meditators at rest, the advanced meditators had a higher average CBF in the right putamen [15], similar to our finding of increased metabolism in the right LN.
Our pilot study is the first to document brain metabolic effects of meditation in distressed caregivers. The limitations of the study include the small sample size limiting our ability to examine the relationships with clinical and cognitive outcomes in distressed caregivers. Although we have not observed the mood benefits in this small sample, the results of our parent study demonstrated group differences in mood and mental health probably due to a larger sample [12]. Observed differences in the metabolic brain changes between the meditation and relaxation conditions suggest different neural mechanisms of yogic meditation compared with relaxation. Another limitation was the lack of treatment-as-usual control group that would allow controlling for nonspecific time effects. These pilot results should be replicated in a larger study and examine the effects of metabolic brain changes in mediating the clinical and cognitive outcomes of meditation.
Future perspective
The public health significance of dementia caregiver stress and associated cost of healthcare is rapidly growing alongside the increasing number of individuals with dementia. The available standard treatment and preventive strategies for caregiver stress and depression have only limited efficacy. At the same time, the interest in and use of complementary and alternative medicine is rising among aging individuals. The currently available evidence of the efficacy of complementary and alternative medicine interventions is limited due to the serious methodological limitations and the lack of understanding of Oriental medicine. Gaining an understanding of various approaches and application of those in clinical research, as well as the use of biomarkers of response, will likely benefit researchers, clinicians and older caregivers. The number of studies of various complementary and alternative medicine interventions and translational research is steadily rising, and will likely lead to the combined use of methodologies and approaches of the eastern and western medicine. It is important to identify biomarkers of clinical response to meditation that may help identify subgroups of individuals who are more likely to respond to such interventions. Hence, more research in this area can enrich clinical practice and help individualize and tailor behavioral interventions to individual brain metabolic profiles. This research is in the very beginning phases of development that generates a significant impetus because of increased use of mind body techniques by general public and the baby-boomers.
Executive summary.
With the US population aging, the prevalence of dementia and the number of family caregivers will increase dramatically in the next two decades, adding to the cost of clinical care. Over 5 million Americans provide unpaid care for their loved ones with Alzheimer’s disease and dementia.
The detrimental impact of informal dementia caregiving on mental health with high prevalence and incidence of depression has been well documented in recent years. Caregiver distress can affect mood and cognitive functioning.
Yogic meditation practice can provide stress reduction for caregivers, resulting in improvement in mental and cognitive function. This pilot study explored whether an ancient yogic meditation, Kirtan Kriya, could change regional cerebral metabolism in caregivers.
Nine dementia caregivers were randomized to undergo meditation training to be compared with relaxation for 12 min per day for 8 weeks. Caregivers received neuropsychiatric assessments and brain FDG-PET scans at baseline and postintervention.
The groups did not differ on measures of mood, mental and physical health, and burden at baseline and follow-up.
When comparing the regional cerebral metabolism between groups, significant differences over time were found in the bilateral cerebellum (p < 0.0005), right inferior lateral anterior temporal (p < 0.0005), right inferior frontal (p = 0.001), left superior frontal (p = 0.001), left associative visual (p = 0.002) and right posterior cingulate (p = 0.002) cortices.
Meditation practice in distressed caregivers resulted in different patterns of regional cerebral metabolism compared with relaxation. These pilot results should be replicated in a larger study.
The use of biomarkers such as PET imaging can help to understand metabolic changes in the brain with meditation use and relate those to clinical outcomes.
It is important to identify biomarkers of clinical response to meditation that may help identify subgroups of individuals who are more likely to respond to such interventions. Hence, more research in this area can enrich clinical practice and help individualize and tailor behavioral interventions to individual brain metabolic profiles. This research is in the very beginning phases of development that generates a significant impetus because of increased use of mind–body interventions.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This work was supported by the Alzheimer’s Research and Prevention Foundation grant. NIH grants MH077650, MH086481 and AT003480 supported H Lavretsky, and the National Institute on Aging grant AG016570 was awarded to the University of California Los Angeles Alzheimer’s Disease Research Center. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Biegel DE, Sales E, Schulz R. Family Caregiving in Chronic Illness: Heart Disease, Cancer, Stroke, Alzheimer’s Disease, and Chronic Mental Illness. Sage Publications; Newbury Park, CA, USA: 1991. [Google Scholar]
- 3••.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. Description of caregiver stress associated with greater mortality. [DOI] [PubMed] [Google Scholar]
- 4•.Schulz R, Martire LM. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry. 2004;12(3):240–249. Detailed description of health effects of caregiver stress. [PubMed] [Google Scholar]
- 5.Bremner JD. Does stress damage the brain? Biol Psychiatry. 1999;45(7):797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 6.Lupien SJ, Fiocco A, Wan N, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Mendl M. Performing under pressure: stress and cognitive function. Appl Anim Behav Sci. 1999;65:221–244. [Google Scholar]
- 8.Mackenzie CS, Smith MC, Hasher L, Leach L, Behl P. Cognitive functioning under stress: evidence from informal caregivers of palliative patients. J Palliat Med. 2007;10(3):749–758. doi: 10.1089/jpm.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahoney AM, Dalby JT, King MC. Cognitive failures and stress. Psychol Rep. 1998;82(3 Pt 2):1432–1434. doi: 10.2466/pr0.1998.82.3c.1432. [DOI] [PubMed] [Google Scholar]
- 10.Sandstrom A, Rhodin IN, Lundberg M, Olsson T, Nyberg L. Impaired cognitive performance in patients with chronic burnout syndrome. Biol Psychol. 2005;69(3):271–279. doi: 10.1016/j.biopsycho.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 11•.Black DS, Cole SW, Irwin MR, et al. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.06.011. (Epub ahead of print). Parent study description. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2012 doi: 10.1002/gps.3790. (Epub ahead of print). Parent study description. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moss AS, Wintering N, Roggenkamp H, et al. Effects of an 8-week meditation program on mood and anxiety in patients with memory loss. J Altern Complement Med. 2012;18(1):48–53. doi: 10.1089/acm.2011.0051. [DOI] [PubMed] [Google Scholar]
- 14.Newberg AB, Wintering N, Khalsa DS, Roggenkamp H, Waldman MR. Meditation effects on cognitive function and cerebral blood flow in subjects with memory loss: a preliminary study. J Alzheimers Dis. 2010;20(2):517–526. doi: 10.3233/JAD-2010-1391. [DOI] [PubMed] [Google Scholar]
- 15•.Newberg AB, Wintering N, Waldman MR, Amen D, Khalsa DS, Alavi A. Cerebral blood flow differences between long-term meditators and non-meditators. Conscious Cogn. 2010;19(4):899–905. doi: 10.1016/j.concog.2010.05.003. Describes the use of Kirtan Kriya in experienced meditators. [DOI] [PubMed] [Google Scholar]
- 16.Hölzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45(3):672–678. doi: 10.1016/j.neuroimage.2008.12.061. Describes brain changes associated with prolonged meditation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132(2):180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 19.Holzel BK, Ott U, Hempel H, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421(1):16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 20•.Newberg A, Pourdehnad M, Alavi A, D’Aquili EG. Cerebral blood flow during meditative prayer: preliminary findings and methodological issues. Percept Mot Skills. 2003;97(2):625–630. doi: 10.2466/pms.2003.97.2.625. Another study of Kirtan Kirya in older adults. [DOI] [PubMed] [Google Scholar]
- 21.Davidson RJ, Kabat-Zinn J, Schumacher J, et al. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65(4):564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- 22.Orme-Johnson D. Evidence that the Transcendental Meditation program prevents or decreases diseases of the nervous system and is specifically beneficial for epilepsy. Med Hypotheses. 2006;67(2):240–246. doi: 10.1016/j.mehy.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, Mchugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Khalsa DS, Amen D, Hanks C, Money N, Newberg A. Cerebral blood flow changes during chanting meditation. Nucl Med Commun. 2009;30(12):956–961. doi: 10.1097/MNM.0b013e32832fa26c. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) I Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 27.Zarit SH, Zarit JM. Cognitive impairment. In: Lewinsohn PM, Teri L, editors. Clinical Geropsychology. Pergamon Press; NY, USA: 1983. pp. 38–81. [Google Scholar]
- 28.Zarit SH. Diagnosis and management of caregiver burden in dementia. Handb Clin Neurol. 2008;89:101–106. doi: 10.1016/S0072-9752(07)01209-2. [DOI] [PubMed] [Google Scholar]
- 29.Silverman DH, Geist CL, Kenna HA, et al. Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology. 2011;36(4):502–513. doi: 10.1016/j.psyneuen.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; NY, USA: 1998. [Google Scholar]
- 31.Ashburner J, Barnes G, Chen C, et al. SPM8 Manual. Wellcome Trust Centre for Neuroimaging; London, UK: 2012. pp. 1–475. [Google Scholar]
- 32.Benson H, Beary JF, Carol MP. The relaxation response. Psychiatry. 1974;37(1):37–46. doi: 10.1080/00332747.1974.11023785. [DOI] [PubMed] [Google Scholar]
- 33.Goldin P, Ziv M, Jazaieri H, Hahn K, Gross JJ. MBSR vs. aerobic exercise in social anxiety: fMRI of emotion regulation of negative self-beliefs. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holzel BK, Carmody J, Evans KC, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2011;5(1):11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilpatrick LA, Suyenobu BY, Smith SR, et al. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. Neuroimage. 2011;56(1):290–298. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127(Pt 7):1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- 37.Small GW, Silverman DH, Siddarth P, et al. Effects of a 14-day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry. 2006;14(6):538–545. doi: 10.1097/01.JGP.0000219279.72210.ca. [DOI] [PubMed] [Google Scholar]
Website
- 101.Foundation ASRP: Kirtan Kriya. 2011 www.alzheimersprevention.org/kirtan_kriya.htm.


