Abstract
Liver granuloma are recognized as specific histological features in primary biliary cirrhosis but their significance remains enigmatic. Similarly, there are limited data on the impact of hyper-IgM on granulomas while in both cases a role of B cells has been hypothesized. The present study investigates a significant number of tissue samples from PBC and control livers as well as other granulomatous diseases to investigate the representation of dendritic cells and the role of IgM in PBC-associated granulomas. We demonstrate that the classical cellular marker for dendritc cells CD11c is highly expressed in hepatic granulomas from PBC liver samples, along with markers of immature dendritic cells, i.e. CD11b, low MHC II, IL-23 and CCR7 and CD83 expression, and C1q high expression. PBC patients with granulomas had significantly higher serum IgM levels and PBC granulomas were surrounded by B and plasma cells. Using ad in vitro approach we further demonstrate that IgM inhibits LPS-induced maturation of myeloid dendritic cells, as well as LPS-induced activation of NF-κB and the expression of proinflammatory cytokines such as TNF-α, IL-12, and IFN-β. In conclusion, we propose that immature dendritic cells are key factors in liver granulomas and that the commonly observed hyper-IgM may contribute to their appearance in patients with PBC.
Introduction
Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease characterized by immune mediated destruction of cholangiocytes lining the interlobular bile ducts [1]. The classic presentation of patients with PBC is a persistent elevation of cholestatic serum liver tests and confirmed either with a positive serum antimitochondrial antibody (AMA) or a liver biopsy demonstrating non-suppurative cholangitis [2]. Most patients with PBC have high levels of polyclonal serum IgM which are secondary to memory B cell activation [3] but the pathogenic significance remains to be determined. When histology is performed, PBC includes a portal tract inflammatory infiltrate composed of plasma cells, mononuclear cells, and polymorphonuclear cells[4]. It is also not uncommon to observe noncaseating epithelioid granulomas, particularly in early-stage disease [5].
Granulomas reflect a localized inflammatory reaction to foreign material (e.g., microorganisms, parasites) characterized by the nodular appearance of epithelioid cells (commonly representing activated macrophages) surrounded by lymphocytes. There is very limited data on the mechanisms of granuloma formation in PBC. It is believed that granulomas are formed under the influence of antigenic stimulation to a variety of professional antigen presenting cells and including such antigenic presentation to T cells [6]. Interestingly, in PBC epithelioid granulomas include the expression of CD1d [7] and often the presence of Propionibacterium acnes [8].
To extend our understanding of the immunopathology of granulomas in PBC, we have performed an extensive immunohistochemical analysis of a large series of patients with PBC, autoimmune hepatitis and chronic hepatitis B. In addition, as further controls, tissues from patients with granulomas from either sarcoidosis, granulomatous gastritis, infection and Crohn's disease, were studied. We report herein a role for not only immature dendritic cells, but also IgM in the onset and perpetuation of granulomas.
Materials and Methods
Subjects
Liver tissues were obtained from 51 patients with PBC, 37 patients with autoimmune hepatitis (AIH) [9] and 30 patients with chronic hepatitis B (CHB). In all cases, the diagnosis was confirmed by internationally accepted criteria and confirmed by histology. All tissues were obtained from excess material from liver biopsies. Essentially, the liver biopies were fixed in 10% neutral buffered formalin, embedded in paraffin, and 4 µm thick sections were cut from each paraffin block. Slides were stained with hematoxylin-eosin, and the inflammatory degree and fibrotic stages determined according to the Scheuer score system. The liver biopsy staging of PBC was based on Ludwig’s criteria [10]. As additional controls, we included gastric granulomas (n=2; this included one patient with sarcoidosis and one with granulomatous gastritis). We also included granulomas found in skin (n=2) and granulomas secondary to tuberculosis (n=5; one each for esophagus, colon, and synovium, and two from skin). Finally, intestinal granulomas from eight patients with Crohn's disease were also included (n=8). All participants gave written informed consent for the study that was approved by the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University.
Immunohistochemistry
Formalin-fixed, paraffin-embedded liver tissues were stained using antibodies for CD11c, CD11b, class II MHC, CD83, CCR7, C1q, IL-23p19 (all from Abcam, Cambridge, United Kingdom), PU.1 (Santa Cruz Biotechnology, Santa Cruz, CA), CD68, CD19 (Gene Tech, Shanghai, China), CD163 (Leica, Wetzlar, Germany); IgM (DAKO, Glostrup, Denmark). Briefly, slides were incubated with 3%H2O2 for 10min, then washed in phosphate buffer saline (PBS). After antigen retrieval, sections were incubated with 10% non-immune goat serum for 30 min and then incubated with primary antibodies overnight at 4°C. After rinsing with phosphate saline (PBS), sections were incubated with an HRP-conjugated secondy antibody (Changdao, Shanghai, China) for 30 min at room temperature, then stained with 3,3’-diaminobenzidine (DAB, Maixin-Bio, Guangzhou, China). Hematoxylin was applied for nuclear staining. Five fields were randomly selected for observation in every sample, the area of CD11c, CD19, IgM deposition was scored on a 0–4 point scale.
Immunohistochemistry double staining and confocal microscopy
Double immunohistochemical staining was performed using the DouSP™ kit (Maixin-Bio). Confocal laser scanning microscopy using Leica TCS SP5II (Wetzlar, Germany) was used for costaining detection of two primary antibodies among CD11c and MHC II, or CD11c and CD68 which were incubated together for 60min at 37°C. After washing 3 times with PBS for 5min, samples were incubated with different fluorochrome-conjugated secondary antibodies (1:500, Jackson ImmunoResearch, USA) separately for 30min at 37°C. The nucleus was stained by DAPI (Cell Signaling Technology, Boston, USA).
Murine dendritic cell culture
Bone marrow-derived DC were generated as described with some modifications [11, 12]. Briefly, bone marrow progenitors were obtained from femurs/tibias of 6–8-week-old C57BL/6 mice and cultured in a 100 mm Petri dishes (Corning, NY, USA) in RPMI1640 containing recombinant mouse GM-CSF (10ng/ml) and IL-4 (10ng/ml) (R&D Systems, Minneapolis, MN). On day 3 and 5, nonadherent cells were gently washed out and then collected and cultured at a density of 1×106 cells/ml in new 60 mm Petri dishes. On day 7, nonadherent cells were recovered and used as immature dendritic cells (iDCs), 90% of which showed the surface phenotype and morphology of iDCs. Maturation of iDCs was induced with LPS (1ug/ml, Sigma, St. Louis, MO) for another 48 hours. For stimulation studies, replicate cultures were challenged with 20µg/ml IgM (Rockland, code 010-0107,USA).
Phagocytosis assay
Isolated thymocytes were labeled with CFSE (Molecular Probes; Invitrogen, USA), using a modified manufacturer’s protocol at a final concentration of 5µM. Subsequently, thymocytes were induced to undergo apoptosis by incubation with etoposide (Sigma) at 10µM in RPMI1640 for 15–18h. Apoptosis was confirmed based on annexin V staining by flow cytometry (Calibur, Becton Dickinson) with >90% annexin V+. To analysis DC endocytosis, equal numbers of DCs and ACs (each at 5 × 10 5) were incubated at 37°C for 2h. Cells were washed and stained with PE-cy7-anti-CD11c antibody, then analyzed with flow cytometry.
DC phenotyping
For flow cytometry purposes, DC were pretreated with IgM (20 µg/ml) for 1 hour, then stimulated with LPS (1µg/ml) for another 48 hours. To assess DC phenotype, cells were co-stained with PE-Cy7-anti-CD11C, FITC-anti-MHC-II, PE-anti-CD80, APC-anti-CD86 (BD Pharmingen, San Diego, CA) and then analyzed with flow cytometry using Cell Quest software (BD Pharmingen).
For RT-PCR cytokine expression DC were pretreated with IgM (20 µg/ml) for 1 hour, then using LPS stimulated for 6 hours. Total RNA were extracted using Trizol reagent (Invitrogen,USA) according to the manufacturer’s instructions. After quantification, 1µg RNA was reverse transcribed into cDNA, the amplification of cDNA was performed using SYBR Premix Ex Taq TM (Takara, Shiga, Japan). The primers sequence for IL-12P40, IL-6, IL-23p19, TNF-α, IFN-β, C1q and β-actin was described in the Table 1. For each sample, mRNA expression level was normalized to the level of β-actin housekeeping genes using the ΔΔCt algorithm.
Table 1.
The primers sequences used for the RT-PCR expression of DC-related genes.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| IL-12P40 | GGAAGCACGGCAGCAGAATA | AACTTGAGGGAGAAGTAGGAAT |
| IL-23P19 | TGCTGGATTGCAGAGCAGTAA | ATGCAGAGATTCCGAGAGA |
| IL-6 | GTTCTCTGGGAAATCGTGGA | CTCTGAAGGACTCTGGCTTTG |
| C1q | CCAACGCGAACGAGAACTAT | GTGGTCACCTGGAAGGTGTT |
| TNF-α | ACGTGGAACTGGCAGAAGAG | CTCCTCCACTTGGTGGTTTG |
| IFN-β | CTCCAGCTCCAAGAAAGGACG | GAAGTTTCTGGTAAGTCTTCG |
| β-actin | CTAAGGCCAACCGTGAAAAG | GGTACGACCAGAGGCATACA |
Prior to Western blotting, DC were pretreated with IgM (20 µg/ml) for an hour, then total protein was extracted from DCs after stimulation with LPS (1µg/ml) for 30 min. Cells were lysed in RIPA Lysis Buffer (Beyotime, Jiangsu, China) containing 1 mM Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktail (Kangchen Biotech, Shanghai, China). Total proteins were separated on Ready Gels (Bio-Rad, Hercules, CA) and electrotransferred to pure nitrocellulose membranes (0.45 µm, Bio-Rad). Immunoblots were reacted with primary antibodies to phosphorylated or total ERK1/2, p38, JNK, IKBα, IRF3 (Cell Signaling Technology, Boston, MA), followed by detection with horseradish peroxidase (HRP)-conjugated secondary antibodies (MR Biotech, Shanghai, China) and visualization with the Pierce ECL Plus Western Blotting Substrate (Thermo Scientific, Canada).We utilized α-Tubulin expression as loading control.
Statistical analysis
All continuous variables are expressed as mean ± standard deviation (SD). Correlations were determined using the Spearman’s correlation coefficient. Group means were compared by t-test or Mann-whitney U. Analyses were two-tailed and performed using SPSS 16.0 (SPSS, Chicago, IL); P values < 0.05 were considered statistically significant.
Results
Prevalence and clinical significance of granulomas in PBC
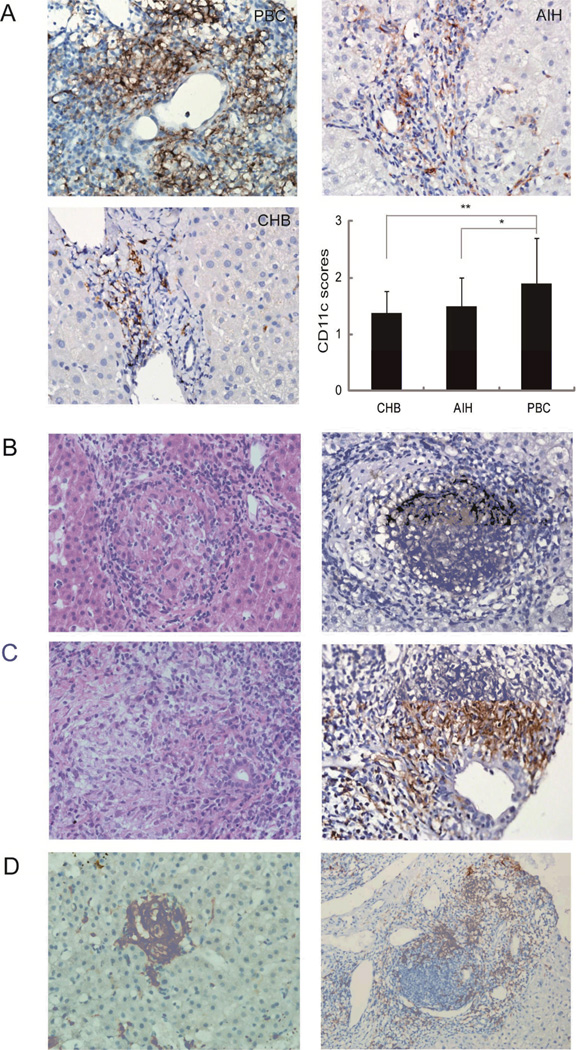
PBC granulomas were typically located within portal tracts (Fig. 1B), usually surrounding or near to, damaged bile ducts (Fig. 1B). PBC livers manifested a significantly stronger portal tract staining for CD11c compared to AIH (p<0.05) and CHB (p<0.01) controls (Figure 1A) and CD11c staining was more sensitive compared to HE for the detection of granulomas (Figure 1B) with 29/51 (57%) and 8/51 (16%) of PBC cases presenting granulomas with either staining method, respectively. Each PBC slide had a median of 2 granulomas (ranging between 0 and 8). Occasionally, small granulomas were also observed in the hepatic lobules or in proximity of the portal tract germinal centers in a minority of PBC cases (Figure 1C). No granuloma was observed in the AIH or CHB liver slides.
Figure 1.
Panel A: CD11c immunostain clearly define hepatic granulomas in PBC patients. (A) Representative immunohistochemical staining for CD11c in liver sections from PBC (n=51), AIH (n=37) and CHB (n= 30) (magnification 400×). Five fields were randomly selected and the area of CD11c deposition scored on a 0-4 scale to be compared between PBC, AIH and CHB patients (*p<0.05, ** p<0.01). Panel B: Representative hematoxylin-eosin staining and CD11c immunostaining of hepatic granulomas in portal tracts illustrating the vicinity with injured bile ducts (magnification 400×). Panel C: Representative CD11c immunostaining of small granulomas in hepatic lobules (Left) (magnification 400×), which in some cases are located in the vicinity of the portal tracts germinal center (Right) (magnification 200×).
When patients were subdivided according to the presence of granulomas (Table 2), we observed significantly higher levels of serum IgM and lower bile acids in the 29 patients with this histological finding; moreover, these patients had significantly earlier histological stages.
Table 2.
Clinical and biochemical features of patients with PBC according to the presence of granulomas with CD11c staining. Variables are expressed as mean±SD (median, range). Not significant, NS.
| With granulomas (n=29) | Without granulomas (n=22) | P value | |
|---|---|---|---|
| Female sex | 28/1 | 21/1 | NS |
| Age | 51.3±9.1(53, 34–70) | 51.9±12.1(52, 29–85) | NS |
| ALT | 88.4±48.8(79, 24–250) | 108.4±85.1(81.2, 19–348) | NS |
| AST | 75.2±39.5(68, 30–215) | 95±61.6(79, 20–266) | NS |
| ALP | 333±282(228.5,77–1377) | 275±184.2(243, 67–847) | NS |
| GGT | 324.6±199.9(305, 27–739) | 426.9±503.4(230.5, 24–1654) | NS |
| TBil | 23.4±13.2(20, 9.9–72.7) | 43.2±40.5(24.2, 3.4–139.3) | NS |
| DBil | 8.8±8.0(6.3, 1.5–39.3) | 23.7±25.7(9.4, 1.1–79.2) | NS |
| Bile acids | 28.09±31.48(11.1,2.1–109.7) | 90.4±97.2(29.75, 3.7–291.7) | 0.023 |
| IgG | 16.4±3.8(15.7, 9.4–29.2) | 17.3±5.5(16.9, 9.3–31.1) | NS |
| IgM | 4.7±2.8(4.18, 1.48–13.5) | 3.0±1.1(2.72, 1.0–5.11) | 0.045 |
| IgA | 2.8±1.0(2.88, 0.74–4.77) | 3.4±1.7(2.52, 1.78–8.74) | NS |
| Stage | 2.1±1.14(2, 0–4) | 2.86±1.36(3, 0–4) | 0.021 |
Immature DC in granulomas
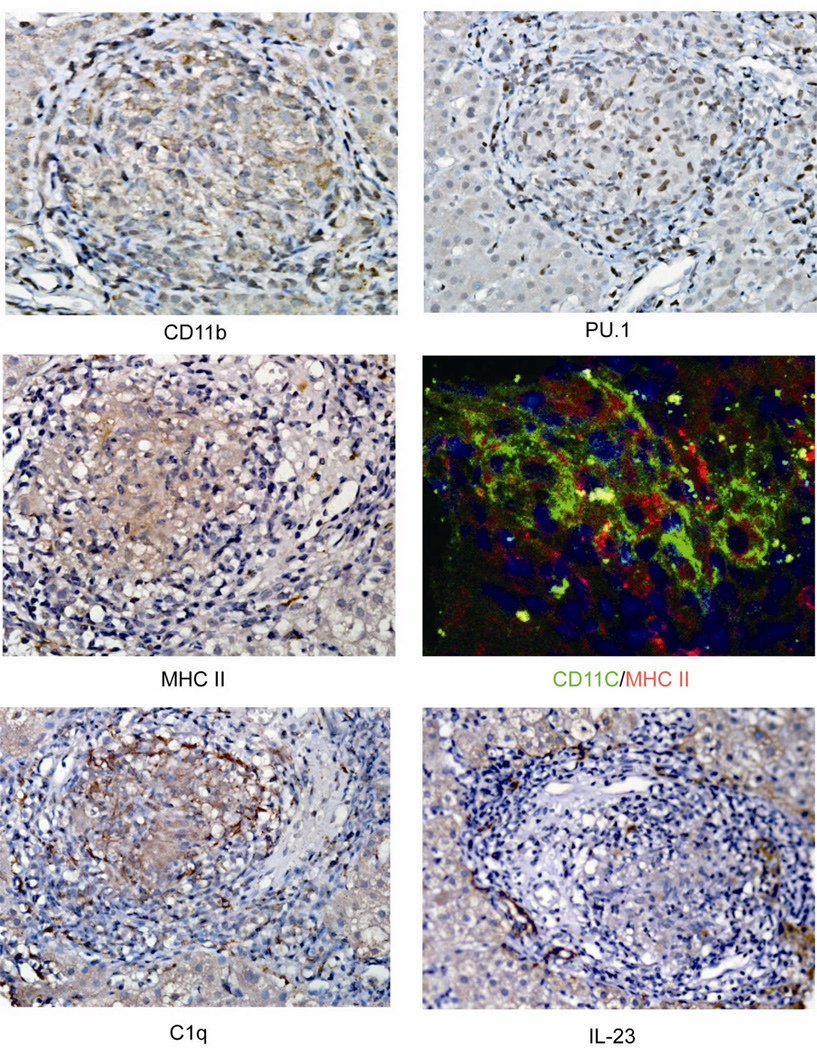
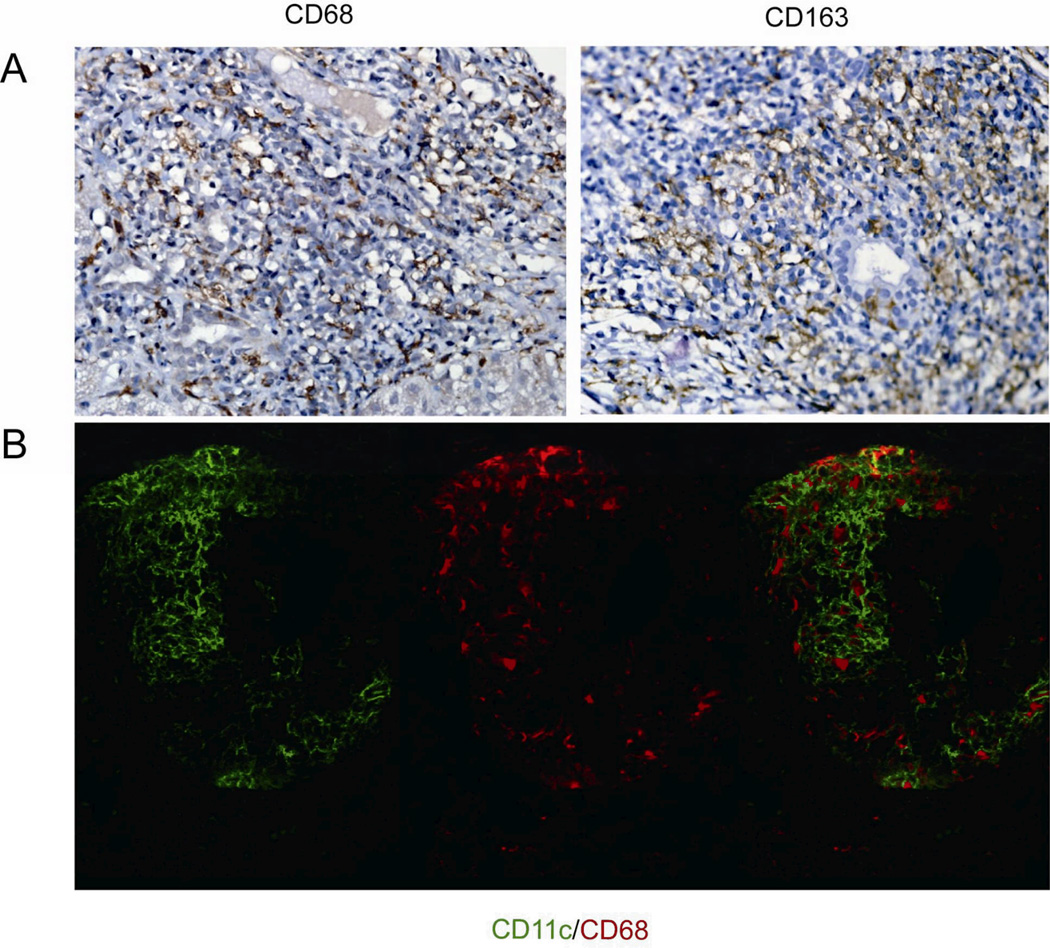
As mentioned, PBC granulomas had significant expression of CD11c and this was observed also in other control granulomas from other tissues (Supplemental Figure 1), Beside CD11c strong positivity, PBC granulomas were investigated for additional markers of DC, their maturity and of macrophages. First, PBC granulomas manifested other DC markers as represented by the positivity of CD11b, the nuclear transcription factor for both conventional and plasmacytoid DC development PU.1 [13], MHC-II, with the latter colocalizing with CD11c at confocal imaging (Figure 2). Second, granulomas were positive for C1q but not for IL-23, CD83, or CCR7 (Figure 2), supporting the DC immature features. Third, when stained with antibodies against CD68 and CD163, both classical cellular markers of macrophages, we observednumerous positive cells among the epithelial cells of PBC granulomas (Figure 3A) while confocal imaging demonstrated no overlapping with CD11c positive cells (Figure 3B).
Figure 2.
Immature dendritic cell properties in PBC granulomas. Representative immunohistochemical staining for CD11b, PU.1, MHC II, C1q and IL-23 expression (magnification 400×). Confocal staining and imaging were performed for CD11c and MHC-II.
Figure 3.
Presence of macrophages in PBC granulomas. Panel A: Representative immunohistochemical staining for CD68, CD163, as classical cellular markers of macrophages (magnification 400×). Panel B: Confocal staining analysis of CD11c and CD68 (magnification 400×).
B cells and IgM mechanisms
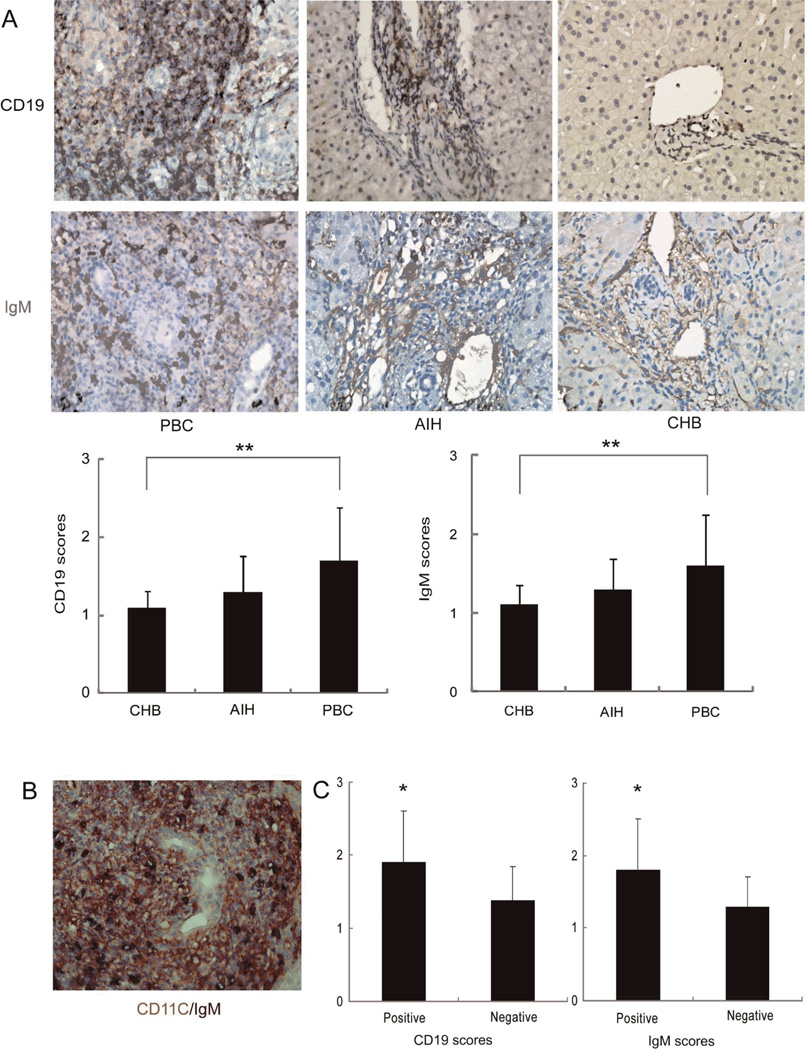
PBC liver slides demonstrated numerous CD19-positive cells (i.e. B cells) and IgM-positive cells (i.e. plasma cells) around and within hepatic granulomas (Figure 4A), in close proximity to CD11c-positive cells at confocal imaging (Figure 4B). Cells demonstrating positive CD19 and IgM expression were significantly more represented in PBC compared to CHB but similar to AIH (Figure 4A).
Figure 4.
Presence of B cells and IgM in PBC granulomas. Panel A: Representative immunohistochemical staining for CD19 (magnification 400×) and IgM (magnification 400×) in liver samples from PBC (n=51), AIH (n=37) and CHB (n=30). Five fields were randomly selected for observation in every sample and CD19 and IgM-positive areas scored on a 0-4 point scale for comparison between diseases (** p<0.01, respectively). Panel B: double immunohistochemical staining performed for CD11c (Red) and IgM (Black). Panel C: CD19 and IgM scores in the portal tracts were compared between granuloma positive (n=29) and granuloma negative (n=22) patients with PBC (* p<0.05).
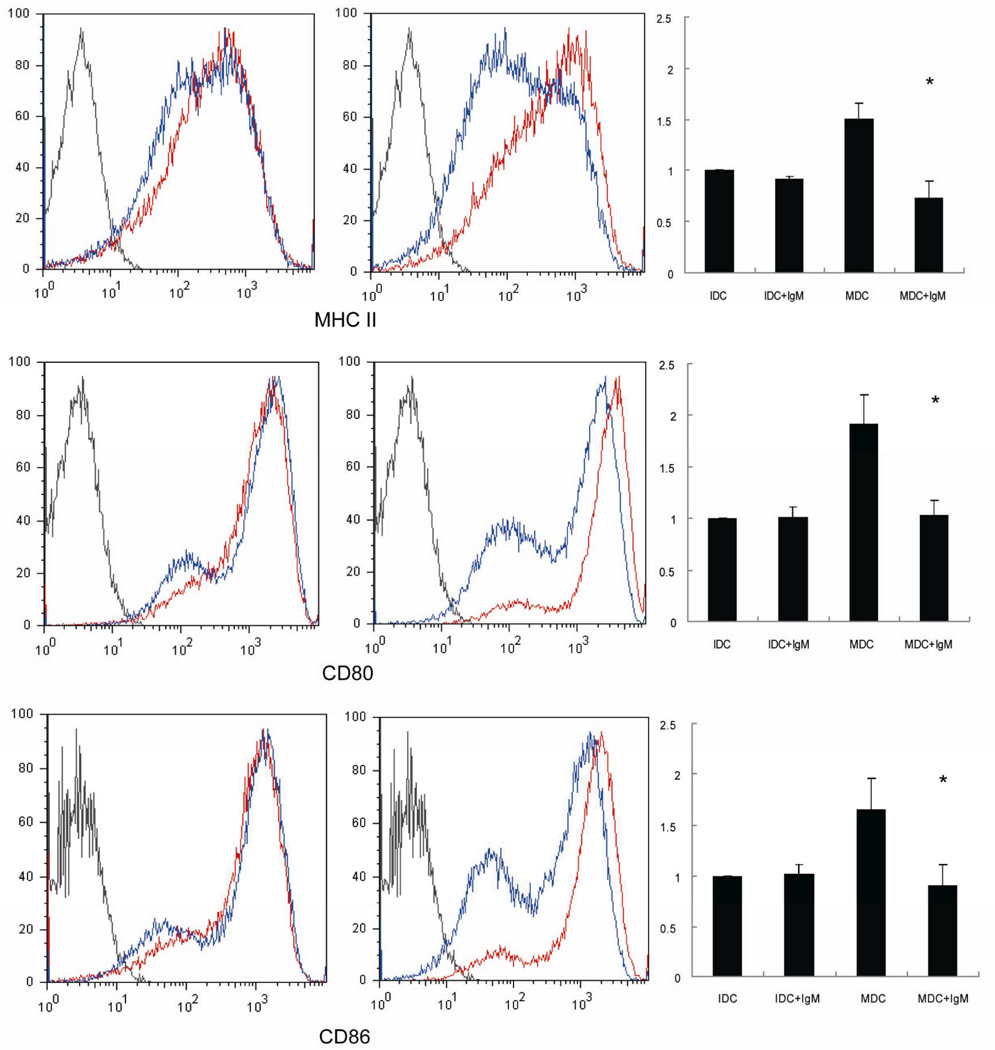
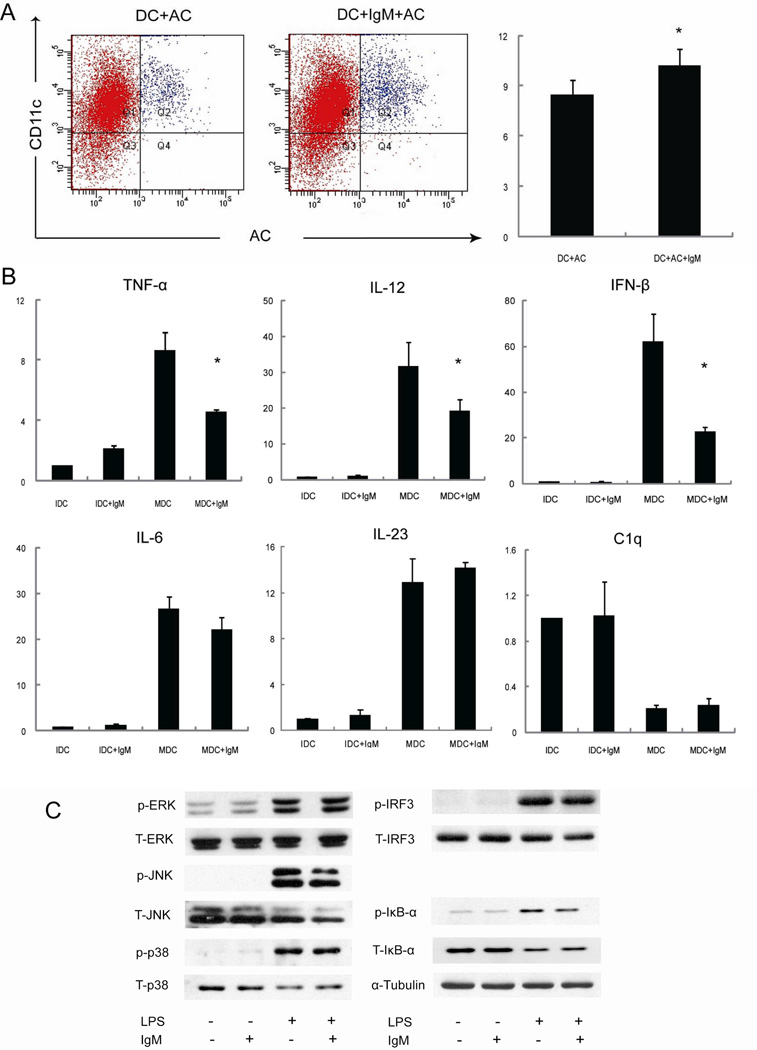
Our subsequent experiments on murine cells were designed to investigate the open issues of B cells and IgM in DC biology. IgM significantly inhibited the expression of MHC-II, CD80 and CD86 in LPS-induced mature DC (Figure 5) but not on immature DC. Western blot analysis showed that LPS induced the phosphorylation of 3 MAPK pathways (e.g. ERK, JNK and p38 MAPK), IRF3 and IκB-α [14], while IgM decreased LPS induced activation of IκB-α but not of MAPK and IRF3 pathways (Figure 6). Finally, IgM enhanced the phagocytosis of apoptotic thymocytosis by immature DC (Figure 6) as demonstrated by the significant decrease in the expression of proinflammatory cytokines such as TNF-α, IL-12 and IFN-β in LPS-treated DC.
Figure 5.
IgM inhibition of the maturation of bone marrow-derived DC. Myeloid DC were pretreated with IgM (20 µg/ml) for an hour, then stimulated with LPS (1µg/ml) for 48 hours. Flow cytometry ananlysis was performed to observe the expression of MHC II, CD80 and CD86 in immature (left) and mature (Right) myeloid DC treated with (Blue) or without (Red) IgM (* p<0.05).
Figure 6.
IgM inhibition of DC phagocytosis. Panel A: myeloid DC were pretreated with IgM (20 µg/ml) then incubated with apoptotic cells (CSFE stained). The percentages of CD11c and CSFE double positive cells were compared between groups treated with or without IgM (* p<0.05). Panel B: DC were pretreated with IgM (20 µg/ml) for 1 hour, then using LPS stimulated for 6 hours. RT-PCR was used to analyze the expression of IL-12p40, IL-6, IL-23p19, TNF-α, IFN-β, C1q, and β-actin (* p<0.05). Panel C: DC were pretreated with IgM (20 µg/ml) for an hour, then total protein was extracted from DCs after stimulated with LPS (1µg/ml) for 30 min. Immunoblots were performed with primary antibodies against phosphorylated or total ERK1/2, JNK p38, IKB-α, IRF3. α-Tubulin expression was determined as the loading control.
Discussion
Two major unanswered questions remain in the pathogenesis of PBC: i.e. the presence of non-caseating granulomas in the liver and the frequenly elevated polyclonal serum IgM. We herein report for the first time that (i) the DC marker CD11c is a sensitive tool to identify liver granulomas in PBC, (ii) epithelioid cells share features of immature DC, (iii) B and plasma cells infiltrate such granulomas, and (iv) IgM inhibit LPS-induced maturation of myeloid dendritic cells. Take altogether, the data suggest that DC and IgM are crucial players in the formation and perpetuation of liver granulomas. Of major importance, the present study was performed on a large number of liver samples from PBC cases and different types of granulomatous and chronic liver disease controls and was complemented with data from an in vitro study on murine cells.
In general terms, granulomas are circumscribed and organized tissue structures characterized by a central accumulation of mononuclear cells, primarily activated macrophages, with a surrounding rim consisting of lymphocytes and fibroblasts. The reported prevalence of granulomas in unselected liver biopsy samples is approximately 4% [6] The formation of liver granulomas is usually in response to antigenic stimulation [15] triggered by noninfectious immunologic insults, infections, foreign body reactions, drugs, and tumors [16]. In a complementary fashion, PBC is commonly associated with the presence of granulomas [17, 18][19]. Nevertheless, there is limited knowledge on the mechanisms linking PBC with granulomas, similar to the largely unexplained serum hyper-IgM that accompanies the disease [3, 20]. Our study provides new intriguing insights into the mechanisms leading to granulomas in PBC and the putative role of the elevated IgM levels. One hint was provided by the observation that PBC cases in which granulomas were found had lower serum bile acids levels and were more likely at earlier disease stages, thus suggesting that the formation of granulomas may be an adaptive reaction to the chronic bile duct injury, and may be effectively reducing the inflammatory process. Nevertheless, the obvious objection that granulomas may disappear due to the onset of cirrhosis cannot be overlooked.
First, we report that staining with the DC marker CD11c is more powerful than HE to detect granulomas, and may be useful in routine evaluations. DC are a complex lineage of APC that orchestrate a variety of immune responses [21]; in particular, immature DC are very efficient at capturing and processing antigen, while fully mature DC are equipped to display these antigens on their surface via major MHC molecules and thus deliver signals to T cells [22]. The role of DC had been previously reported in other granulomatous diseases. A unique CD11c+ DC-like cell subset with phenotypic features of immature myeloid DC was recently described in intestinal granulomas; these cells express macrophage markers, produce IL-23, and induce granulomas under a Th1-predominant intestinal inflammatory condition [23]. Similarly, a CD11c-positive inflammatory DC subset was found in granulomas induced by Mycobacteria bovis in a murine model. with lower expression of MHC II and co-stimulatory molecules CD40, CD80 and CD86, and higher expression of inhibitory molecules PD-L1 and PD-L2 [24]. Similarly, we demonstrated that PBC granulomas include CD11chighCD11bmoderate cells with low expression of MHC-II, IL-23, CD83, and CCR7, to suggest that DC are immature and tolerogenic. This may well be in agreement with the proposed protective effect suggested by the less advanced disease stages of PBC when granulomas are present. Further, immature DC with tolerogenic properties produce C1q [25] and we observed that high levels of C1q produced by immature bone-marrow derived DC were downregulated by LPS-induced maturation [26] while granulomas were strongly C1q-positive.
Second, we investigated the putative role of IgM in the proposed scenario, based on the commonly increased serum levels in PBC [27] secondary to B1 cell production [28] and their prominent role in the maintenance of immune tolerance and in clearance of cell debris [29]. Similarly, B cells and plasma cells may play a critical role in the immune-mediated injury of intrahepatic bile ducts [30] although B cell depletion exacerbated cholangitis in a murine PBC model [31] and data were conflicting in small clinical trials [32]. We demonstrated that IgM inhibit the process of DC maturation and increase the phagocytosis of cells undergoing apoptosis, a key phenomenon in PBC etiopathogenesis [33–35]. Further, the uptake of apoptotic DC converts immature viable DC into tolerogenic dendritic cells resistant to LPS-induced maturation [36]. Finally, IgM also inhibit the LPS-induced activation of NF-κB and the expression of proinflammatory cytokines such as TNF-α, IL-12 and IFN-β. Ultimately, we may surmise that B cells play an immune regulatory role in the PBC liver through regulating the maturation of DC through IgM secretion.
In summary, we provide histological evidence that immature DC are important to the mechanisms leading to granuloma formation in the PBC liver. Further, our data suggest that IgM may be the link for B cells to modulate this process. We are particularly intrigued by these observation which may hold a promising potential to new therapeutic approaches and we warrant that further studies may continue this effort to address the role of female sex [37, 38], past infectious agents [39–41], or new molecular mediators such as microRNA [42] in the pathogenesis of PBC granulomas.
Supplementary Material
Acknowledgments
This work was supported by the Awards from National Natural Science Foundation of China (#30770963, 30972751, XM), Shanghai Pujiang program: Innovative Research Team of Shanghai Municipal Education Commission (XM) and by National Institutes of Health grant DK39588.
References
- 1.Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010;52:745–758. doi: 10.1016/j.jhep.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 2.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 3.Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, Miyakawa H, Coppel RL, Gershwin ME. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–312. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 5.Drebber U, Mueller JJ, Klein E, Kasper HU, Schulze F, Schardt K, Quasdorff M, Schulte S, Odenthal M, Dienes HP. Liver biopsy in primary biliary cirrhosis: clinicopathological data and stage. Pathol Int. 2009;59:546–554. doi: 10.1111/j.1440-1827.2009.02405.x. [DOI] [PubMed] [Google Scholar]
- 6.Lagana SM, Moreira RK, Lefkowitch JH. Hepatic granulomas: pathogenesis and differential diagnosis. Clin Liver Dis. 2010;14:605–617. doi: 10.1016/j.cld.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Tsuneyama K, Yasoshima M, Harada K, Hiramatsu K, Gershwin ME, Nakanuma Y. Increased CD1d expression on small bile duct epithelium and epithelioid granuloma in livers in primary biliary cirrhosis. Hepatology. 1998;28:620–623. doi: 10.1002/hep.510280303. [DOI] [PubMed] [Google Scholar]
- 8.Harada K, Tsuneyama K, Sudo Y, Masuda S, Nakanuma Y. Molecular identification of bacterial 16S ribosomal RNA gene in liver tissue of primary biliary cirrhosis: is Propionibacterium acnes involved in granuloma formation? Hepatology. 2001;33:530–536. doi: 10.1053/jhep.2001.22653. [DOI] [PubMed] [Google Scholar]
- 9.Vergani D, Mieli-Vergani G. Cutting Edge Issues in Autoimmune Hepatitis. Clin Rev Allergy Immunol. 2011 doi: 10.1007/s12016-010-8236-9. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis) Virchows Arch A Pathol Anat Histol. 1978;379:103–112. doi: 10.1007/BF00432479. [DOI] [PubMed] [Google Scholar]
- 11.Xu S, Han Y, Xu X, Bao Y, Zhang M, Cao X. IL-17A-producing gammadeltaT cells promote CTL responses against Listeria monocytogenes infection by enhancing dendritic cell cross-presentation. J Immunol. 2010;185:5879–5887. doi: 10.4049/jimmunol.1001763. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, Guo J, Huang X, Chen T, Wang J, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 13.Carotta S, Dakic A, D'Amico A, Pang SH, Greig KT, Nutt SL, Wu L. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 2010;32:628–641. doi: 10.1016/j.immuni.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez PM, Lopez-Bravo M, Kalinke U, Ardavin C. Statins inhibit iNOS-mediated microbicidal potential of activated monocyte-derived dendritic cells by an IFN-beta-dependent mechanism. Eur J Immunol. 2011;41:3330–3339. doi: 10.1002/eji.201141674. [DOI] [PubMed] [Google Scholar]
- 15.Almadi MA, Aljebreen AM, Sanai FM, Marcus V, Almeghaiseeb ES, Ghosh S. New insights into gastrointestinal and hepatic granulomatous disorders. Nat Rev Gastroenterol Hepatol. 2011;8:455–466. doi: 10.1038/nrgastro.2011.115. [DOI] [PubMed] [Google Scholar]
- 16.Lefkowitch JH. Hepatic granulomas. J Hepatol. 1999;30(Suppl 1):40–45. [PubMed] [Google Scholar]
- 17.Drebber U, Kasper HU, Ratering J, Wedemeyer I, Schirmacher P, Dienes HP, Odenthal M. Hepatic granulomas: histological and molecular pathological approach to differential diagnosis--a study of 442 cases. Liver Int. 2008;28:828–834. doi: 10.1111/j.1478-3231.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- 18.Dourakis SP, Saramadou R, Alexopoulou A, Kafiri G, Deutsch M, Koskinas J, Archimandritis AJ. Hepatic granulomas: a 6-year experience in a single center in Greece. Eur J Gastroenterol Hepatol. 2007;19:101–104. doi: 10.1097/01.meg.0000243882.09820.d2. [DOI] [PubMed] [Google Scholar]
- 19.Turhan N, Kurt M, Ozderin YO, Kurt OK. Hepatic granulomas: a clinicopathologic analysis of 86 cases. Pathol Res Pract. 2011;207:359–365. doi: 10.1016/j.prp.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Lleo A, Liao J, Invernizzi P, Zhao M, Bernuzzi F, Ma L, Lanzi G, Ansari AA, Coppel RL, Zhang P, et al. Immunoglobulin M levels inversely correlate with CD40 ligand promoter methylation in patients with primary biliary cirrhosis. Hepatology. 2012;55:153–160. doi: 10.1002/hep.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumpter TL, Abe M, Tokita D, Thomson AW. Dendritic cells, the liver, and transplantation. Hepatology. 2007;46:2021–2031. doi: 10.1002/hep.21974. [DOI] [PubMed] [Google Scholar]
- 22.Hosszu KK, Santiago-Schwarz F, Peerschke EI, Ghebrehiwet B. Evidence that a C1q/C1qR system regulates monocyte-derived dendritic cell differentiation at the interface of innate and acquired immunity. Innate Immun. 2010;16:115–127. doi: 10.1177/1753425909339815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizoguchi A, Ogawa A, Takedatsu H, Sugimoto K, Shimomura Y, Shirane K, Nagahama K, Nagaishi T, Mizoguchi E, Blumberg RS, et al. Dependence of intestinal granuloma formation on unique myeloid DC-like cells. J Clin Invest. 2007;117:605–615. doi: 10.1172/JCI30150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreiber HA, Hulseberg PD, Lee J, Prechl J, Barta P, Szlavik N, Harding JS, Fabry Z, Sandor M. Dendritic cells in chronic mycobacterial granulomas restrict local anti-bacterial T cell response in a murine model. PLoS One. 5:e11453. doi: 10.1371/journal.pone.0011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellano G, Woltman AM, Nauta AJ, Roos A, Trouw LA, Seelen MA, Schena FP, Daha MR, van Kooten C. Maturation of dendritic cells abrogates C1q production in vivo and in vitro. Blood. 2004;103:3813–3820. doi: 10.1182/blood-2003-09-3046. [DOI] [PubMed] [Google Scholar]
- 26.Castellano G, Trouw LA, Fiore N, Daha MR, Schena FP, van Kooten C. Infiltrating dendritic cells contribute to local synthesis of C1q in murine and human lupus nephritis. Mol Immunol. 2010;47:2129–2137. doi: 10.1016/j.molimm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Duarte-Rey C, Bogdanos DP, Leung PS, Anaya JM, Gershwin ME. IgM predominance in autoimmune disease: Genetics and gender. Autoimmun Rev. 2011 doi: 10.1016/j.autrev.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 29.Klimovich VB. IgM and its receptors: structural and functional aspects. Biochemistry (Mosc) 2011;76:534–549. doi: 10.1134/S0006297911050038. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T, Miura T, Nakamura J, Yamada S, Miura T, Yanagi M, Matsuda Y, Usuda H, Emura I, Tsuneyama K, et al. Plasma cells and the chronic nonsuppurative destructive cholangitis of primary biliary cirrhosis. Hepatology. 2012;55:846–855. doi: 10.1002/hep.24757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhirapong A, Lleo A, Yang GX, Tsuneyama K, Dunn R, Kehry M, Packard TA, Cambier JC, Liu FT, Lindor K, et al. B cell depletion therapy exacerbates murine primary biliary cirrhosis. Hepatology. 2011;53:527–535. doi: 10.1002/hep.24044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuda M, Moritoki Y, Lian ZX, Zhang W, Yoshida K, Wakabayashi K, Yang GX, Nakatani T, Vierling J, Lindor K, et al. Biochemical and immunologic effects of rituximab in patients with primary biliary cirrhosis and an incomplete response to ursodeoxycholic acid. Hepatology. 2012;55:512–521. doi: 10.1002/hep.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lleo A, Shimoda S, Ishibashi H, Gershwin ME. Primary biliary cirrhosis and autoimmune hepatitis: apotopes and epitopes. J Gastroenterol. 2011;46(Suppl 1):29–38. doi: 10.1007/s00535-010-0303-8. [DOI] [PubMed] [Google Scholar]
- 34.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, Coppel RL, Worman HJ, Gores GJ, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, Ansari AA, Van de Water J, Gershwin ME. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kushwah R, Wu J, Oliver JR, Jiang G, Zhang J, Siminovitch KA, Hu J. Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg. Eur J Immunol. 2010;40:1022–1035. doi: 10.1002/eji.200939782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya JM. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun. 2012;38:J109–J119. doi: 10.1016/j.jaut.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Shoenfeld Y, Tincani A, Gershwin ME. Sex gender and autoimmunity. J Autoimmun. 2012;38:J71–J73. doi: 10.1016/j.jaut.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Grossman C, Dovrish Z, Shoenfeld Y, Amital H. Do infections facilitate the emergence of systemic sclerosis? Autoimmunity reviews. 2011;10:244–247. doi: 10.1016/j.autrev.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Shapira Y, Poratkatz BS, Gilburd B, Barzilai O, Ram M, Blank M, Lindeberg S, Frostegard J, Anaya JM, Bizzaro N, et al. Geographical differences in autoantibodies and anti-infectious agents antibodies among healthy adults. Clin Rev Allergy Immunol. 2012;42:154–163. doi: 10.1007/s12016-010-8241-z. [DOI] [PubMed] [Google Scholar]
- 41.Meron MK, Amital H, Shepshelovich D, Barzilai O, Ram M, Anaya JM, Gerli R, Nicola B, Shoenfeld Y. Infectious aspects and the etiopathogenesis of rheumatoid arthritis. Clin Rev Allergy Immunol. 2010;38:287–291. doi: 10.1007/s12016-009-8158-6. [DOI] [PubMed] [Google Scholar]
- 42.Tian L, De Hertogh G, Fedeli M, Staats KA, Schonefeldt S, Humblet-Baron S, Van Den Bosch L, Dellabona P, Dooley J, Liston A. Loss of T cell microRNA provides systemic protection against autoimmune pathology in mice. J Autoimmun. 2012;38:39–48. doi: 10.1016/j.jaut.2011.12.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.