Abstract
In addition to their antiestrogenic effects, soy isoflavones may protect against cancer through alternate biologic actions including antioxidant properties. This randomized, crossover study explored the relation between dietary isoflavone intake through common soy foods and oxidative stress quantified by urinary isoprostane levels. Eighty-two women aged 39.2±6.1 years were randomized to a high soy diet of 2 soy food servings per day and a low soy diet of <3 servings per week for 6 months each, separated by a 1 month washout period. Urine samples were collected at baseline and at the end of each dietary period. Urinary isoprostane levels were measured using enzyme-linked immunosorbent assays (ELISA) and adjusted for creatinine levels. Mixed models using log-transformed values were applied to evaluate the effect of the high soy diet. Unadjusted isoprostane excretion levels were lower during the high than the low soy diet, but this effect was not statistically significant (p=0.81). After adjustment for urinary creatinine, isoprostane excretion was slightly higher during the high soy diet (p=0.02), an observation that was confirmed in a regression analysis between urinary isoflavones and isoprostanes during the high soy diet. The original association remained significant when restricted to adherent participants; however, this effect disappeared after exclusion of three extreme values. In agreement with several previous reports, these findings do not support the hypothesis that soy exerts antioxidant effects as measured by urinary isoprostane excretions, but additional markers of oxidative stress need to be investigated in future studies.
Keywords: soy foods, isoflavones, oxidative stress, isoprostane, randomized crossover trial
INTRODUCTION
Soy foods have been associated with a lower risk for cancer. There is increasing evidence for such an association with breast cancer, in particular among Asian women1 and women exposed early in life.2–6 Various explanations for this finding have been proposed, including modulation of topoisomerase, cell signaling pathways, apoptosis, and angiogenesis.7 However, a hormonal mechanism, such as the selective estrogen receptor modulatory activity of isoflavones, is the most commonly researched mechanism of action.8–10 At the same time, antioxidant properties continue to be investigated as a possible contributor to the ability of soy isoflavones to protect against cancer.11,12 As shown in experimental studies, the isoflavones genistein, daidzein, and equol have antioxidant properties both in vitro and in vivo through their ability to directly reduce free radicals.13 Oxidative stress, particularly stress associated with nitric oxide production (NO), has been linked to the development and progression of cancer at different sites including breast cancer.14,15 Isoprostane formation is directly associated with oxidative stress caused by NO synthesis in vivo.16,17 Among the many markers of oxidative stress,18 F2 isoprostanes are a relatively good measure because they are stable and specific and are formed directly by chemical oxidation.19 This complex family of prostaglandin-like compounds is produced specifically by free radical-induced peroxidation of arachidonic acid and released into the circulation before excretion in the urine.13 Isoprostanes are not subject to auto-oxidation ex vivo in urine,20 their urinary concentration is stable in storage at −20°C,13,21 and levels are not modified by the lipid content of the diet.13 Older age, physical activity, smoking, alcohol intake, and inflammatory diseases are associated with higher urinary 15-F2t-Isoprostane (15-F2t-IsoP) levels in one report13 and postmenopausal status in another.22
Previous studies on soy intake and urinary isoprostanes have shown conflicting results. Using gas chromatography-mass spectrometry (GC-MS) to measure 15-F2t-IsoP, soy consumption reduced lipid peroxidation in vivo,23 whereas two studies reported no significant effect of isoflavone intake on urinary isoprostanes.24,25 Another study that observed no significant difference during high and low isoflavone diets only described changes associated with age.26 The current study aims to determine the effects of traditional soy food consumption on one marker of oxidative stress in premenopausal women by evaluating the relation between isoflavone intake and urinary isoprostane concentration.
MATERIALS AND METHODS
Study Design and Procedures
This study was a randomized, crossover soy intervention consisting of two 6-month diet periods (high soy and low soy) separated by a 1-month washout period. The participants were recruited through multiple sources, as described elsewhere.27 Of the 16,306 invitations sent out, 825 (5.1%) interested women replied and 310 women were identified as eligible after a telephone prescreening interview. Women were excluded from the study due to pregnancy or breast-feeding, consumption of estrogen-containing oral contraceptives or supplements containing isoflavones, cancer diagnosis, breast implants, hysterectomy, lack of a regular menstrual period, or intake of >5 soy servings per week. During the screening visit, participants completed demographic and soy questionnaires, weight and height measurements, a 24-hour dietary recall, and a nipple aspirate fluid collection. After screening and randomization, 96 participants attended 5 follow-up visits at months 3 and 6 of the first diet, after the washout period (month 7), and at months 10 and 13 of the second diet period. Overall, 14 women (15%) dropped out. The participants provided informed consent and written permission to use frozen samples for future analyses. The study protocol was approved by the Committee on Human Studies at the University of Hawaii and by the Institutional Review Boards of the participating clinics. The study was registered at clinicaltrials.gov as NCT00513916 and a Data Safety Monitoring Committee annually reviewed study progress, reasons for drop-outs, and any reported adverse health effects.
The 96 eligible participants were randomized into two groups, one starting on the high soy diet (Group A) and the other starting on the low soy diet (Group B) as detailed previously.27 During the high soy diet period, women were instructed to consume 2 servings of soy foods a day. One serving was equivalent to ¾ cup of soy milk, ½ cup of tofu or ¼ cup of soy nuts, providing approximately 25 mg of isoflavone aglycone equivalents per serving. During the low soy diet period, participants were instructed to maintain their usual diet and consume less than 3 servings of soy per week and no soy-containing supplements. Adherence as assessed by unannounced 24-hour dietary recalls and urinary isoflavone excretion was excellent.27 During the high soy diet, intake of >40 mg of isoflavones per day was considered compliant, while the intake limit was <10 mg of isoflavones per day during the low soy diet.
Urine Collection and Analysis
This study used existing urine samples that were stored in −80°C freezers.27 Three overnight urine samples from each participant, one at baseline, month 6, and month 13, were used for analysis. Of the 82 women who completed the study, one woman from Group B was missing a urine sample for month 6. Levels of urinary 15-F2t-IsoP were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Oxford Biomedical Research, Rochester Hills, MI). A total of 6 batches were run over the course of 6 days according to the manufacturer’s instructions, with 38 samples for 13 women on Plate 1, 39 samples for 13 women on Plate 2, and 42 samples for 14 women each on Plates 4–6. All urine samples were thawed and treated with β-glucuronidase from a glucuronidase sample prep kit (Oxford Biomedical Research, Rochester Hills, MI) before undergoing ELISA. This step increases the accuracy of the test since isoprostanes excreted in human urine are often conjugated to glucuronic acid. Batches 1–2 used 8 µl of glucuronidase for 200 µl of urine while batches 3–6 used 8 µl of glucuronidase for 100 µl of urine. We adjusted for this discrepancy during the statistical analysis. A standard curve was generated using a 4-parameter fit from the plot of concentration and absorbance and the resulting isoprostane concentrations were calculated from this curve. The minimum limit of detection was 0.1 ng/ml. With the exception of Batch 3, each plate contained at least one quality control sample from a pool of urine. The intra-assay and inter-assay coefficient of variations were 7.9% and 22.2%, respectively. Creatinine levels were analyzed with a Roche-Cobas MiraPlus chemistry analyzer using a kit from Randox Laboratories (Crumlin, UK) that is based on a kinetic modification of the Jaffe reaction. Isoprostane excretion was expressed as nmol/mg creatinine to adjust for urine volume.
Urinary isoflavonoid concentration is an excellent biomarker for soy intake because it is excreted in urine within 24–36 hours of soy food consumption. Liquid chromatography tandem mass spectrometry (LCMS) was used to measure the isoflavonoids in urine (daidzein, genistein, equol) after enzymatic hydrolysis and liquid-liquid extraction.28 Since equol producers are thought to experience more protective effects of isoflavones than non-producers,29 equol producer status was determined based on two criteria: urinary daidzein excretion ≥2 nmol/mg creatinine and the urinary equol to daidzein ratio ≥0.018. Participants who meet both criteria at least once during the study were considered equol producers.30,31
Statistical Analysis
Data were analyzed using the SAS statistical software package version 9.2 (SAS Institute, Inc., Cary, NC). Normality of distribution was checked, and non-normal values were log transformed. We performed Student’s t or χ2 tests to evaluate the differences in baseline characteristics between the two randomization groups. Medians and upper and lower quartiles were plotted for urinary isoprostane levels (adjusted and unadjusted for creatinine levels). To examine the relation between dietary isoflavone intake and urinary isoprostanes, while taking account of the repeated measures, we used mixed models (Proc Mixed) with log-transformed values of urinary isoprostane and creatinine as dependent variables.32 The models included the diet (low vs. high soy), the randomization group assignment, and time. To examine the relation between urinary isoflavone and isoprostane excretion during the high soy diet, additional linear models were performed using log-transformed values.
RESULTS
Of the 82 women who completed the study, 40 women were in Group A and 42 women in group B. The two randomization groups did not differ by ethnicity, BMI, equol producer status, dietary isoflavone intake, or urinary isoprostane levels at baseline (Table 1). However, Group B was younger by 4.1 years (p<0.01) and excreted lower levels of urinary isoflavonoids (p=0.03) and creatinine (p=0.03) than Group A.
Table 1.
Baseline characteristics of study participants by randomization groupa
| Characteristic | All | Group Ab | Group Bb | P value | |
|---|---|---|---|---|---|
| N | 82 | 40 | 42 | ||
| Ethnicity | White | 42 (51%) | 20 (50%) | 22 (52%) | 0.98 |
| Asian | 22 (27%) | 11 (27%) | 11 (26%) | ||
| Other | 18 (22%) | 9 (23%) | 9 (22%) | ||
| Age at screening, y | 39.2, 6.1 | 41.3, 5.6 | 37.3, 6.0 | <0.01 | |
| Body mass index, kg/m2 | 25.8, 5.6 | 25.8, 5.2 | 25.9, 6.0 | 0.91 | |
| Equol producer statusc | 43 (52%) | 23 (58%) | 20 (48%) | 0.37 | |
| Dietary isoflavone intake, mg/d | 21.2, 39.7 | 16.3, 38.8 | 25.8, 40.5 | 0.28 | |
| Urinary isoflavonoids, pg/mL | 5.0, 9.2 | 7.3, 11.3 | 2.8, 6.1 | 0.03 | |
| Urinary isoprostaned, ng/mL | 14.6, 5.3 | 15.7, 6.3 | 13.5, 3.8 | 0.08 | |
| Urinary creatinined, mg/L | 917, 513 | 1022, 512 | 817, 499 | 0.03 | |
| Urinary isoprostane/creatinined, ng/mg creatinine | 19.7, 9.9 | 17.4, 6.2 | 21.8, 12.2 | 0.11 | |
Data are n (%) or mean and standard deviation (separated by a comma).
Group A: high-soy diet, then low-soy diet, Group B: high-soy diet, then low-soy diet.
Equol producer status is defined as urinary daidzein excretion ≥2 nmol/mg creatinine and urinary equol to daidzein ≥0.018; equol producer status is missing for one woman in Group A and one woman in Group B.
Used log transformed values for the calculation of p values
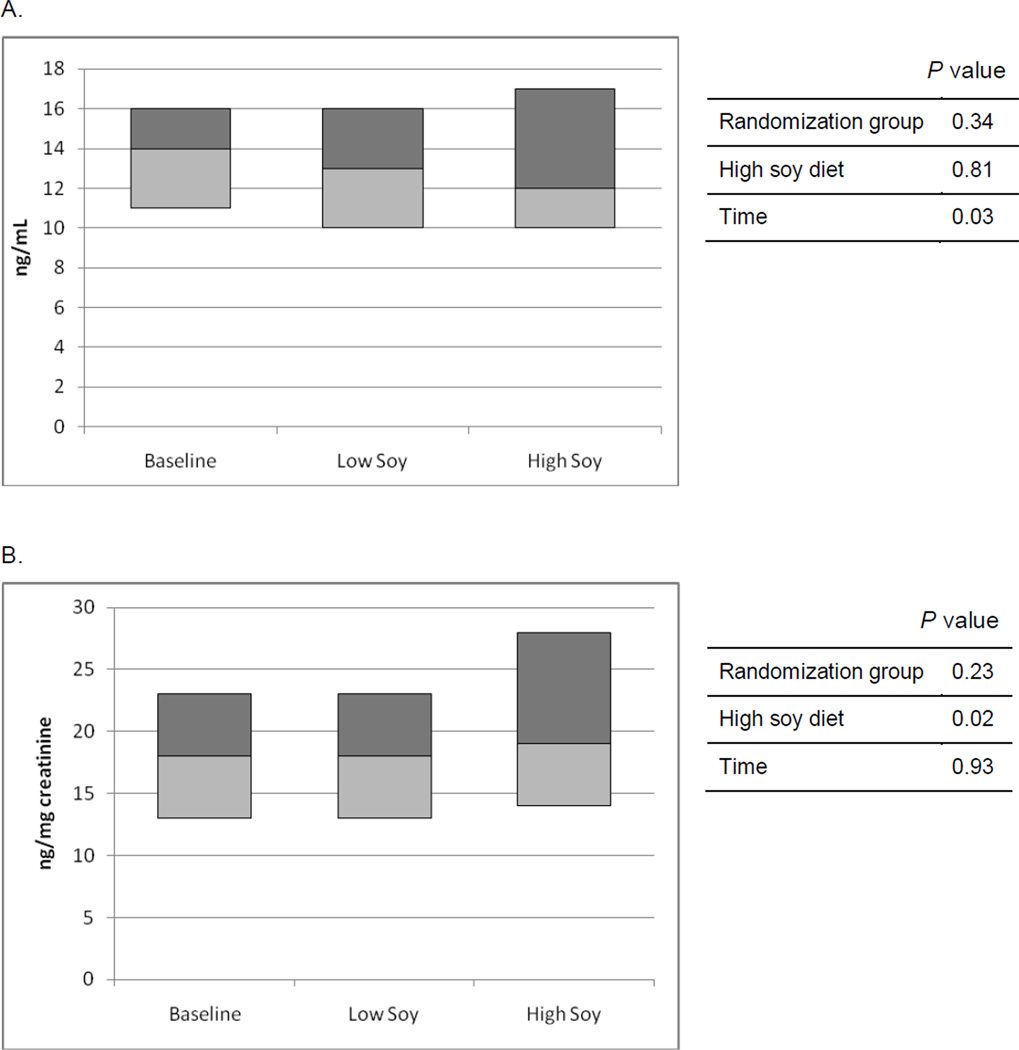
Unadjusted median isoprostane levels were 14, 13, and 12 ng/ml at baseline, during the low and high soy diet, respectively (Figure 1A), but the difference between the low and high soy diet was not significant (p=0.81). There was no difference in isoprostane level by randomization group, but as the significant effect for time (p=0.03) indicated, isoprostane levels decreased over time. When creatinine-adjusted excretion levels of isoprostanes were compared, this trend was reversed (Figure 1B). The median creatinine-adjusted isoprostane values were equal at baseline and during the low soy diet (18 ng/mg creatinine) and marginally higher during the high soy diet (19 ng/mg creatinine). This difference was statistically significant at first (p=0.02) but not after excluding 3 women with extremely low creatinine values during the high soy diet (p=0.17). When the analysis was restricted to women who were adherent to the dietary intervention (195 observations instead of 245), the increase in creatinine-adjusted excretion of isoprostanes was smaller but still statistically significant (p=0.04). In a regression model based on observations during the high soy diet, a significant positive association (p=0.02) between urinary isoflavone and isoprostane excretion was observed; including BMI, age, or ethnicity into the model did not change this finding. Stratification by equol producer status showed a significant effect for the 43 equol producers (p=0.03) but not for the 39 subjects who did not produce equol (p=0.32).
Figure 1.
Median and upper and lower quartiles of urinary isoprostane concentration ([A] unadjusted and [B] adjusted for urinary creatinine concentration) at baseline and at the end of each diet phase in a soy trial; p values are derived from mixed models comparing mean values using log transformed data
DISCUSSION
Contrary to our hypothesis, the current analysis observed no protective effect of soy food consumption against oxidative stress as assessed by urinary isoprostane excretion. After adjustment for urinary creatinine levels, the isoprostane levels were slightly higher at the end of the high soy than the low soy diet. This observation was confirmed in a regression analysis between urinary isoflavones and isoprostanes during the high soy diet. Of some interest was the reduction in urinary creatinine for a few women during the high soy diet. The abnormally high isoprostane levels in these women may indicate an unknown effect of soy on creatinine formation. Since creatinine is generated from arginine, which is also the precursor of NO, the mediator of isoprostane formation, the use of creatinine as an adjustment factor for urinary isoprostane excretion should be reconsidered in future studies. As an alternative, careful measurement of urine volume and time during which urine was collected may be advisable in order to be able to express urinary excretion rates accurately.
The results of our analysis agree with several previous studies24–26 that detected no association of urinary isoprostane levels with a soy diet. After 16 weeks of a soy drink (706 mL/day), the concentration of urinary 8-isoprostane, as analyzed by an enzyme immunoassay method, did not differ from baseline in 52 postmenopausal women.24 A trial examining levels of oxidative damage before and during soy supplementation using Novasoy tablets detected no difference in plasma levels of total 8-isoprostane by enzyme immunoassay after dietary supplementation with 50 mg/day for women and 100 mg/day for men.25 These two studies24,25 noted that ELISA was a less reliable method of measuring isoprostane than GC-MS. Our finding also agrees with a study among 8 premenopausal women that described no difference in F2 isoprostane during a high (113–207 mg/day) as compared to a low soy diet (<5 mg/day) that lasted one month.26 On the other hand, an intervention among 19 healthy women and 5 men observed a significant decrease in plasma concentrations of 8-epi-prostaglandin after a high soy treatment with 56 mg isoflavones versus the low soy from which the isoflavones had been removed (<2 mg isoflavones).23 However, there were no baseline 8-epi-prostaglandin levels available in that report. Susceptibility to oxidation of low density lipoprotein was reduced in a soy intervention with 6 healthy volunteers33 and in the report described above.23
Strengths of the present study include a relatively large number of women with diverse ethnic backgrounds; 82 women provided urine samples for the current analysis, whereas previous studies reported sample sizes ranging from 8 to 52 women. The randomized crossover design with a 6-month intervention period, the repeated sampling over 2 × 6-month periods, and measurement of the exposure to isoflavones by common soy foods were additional strengths. Adherence to the intervention diet, as monitored by multiple unannounced dietary recalls and urinary isoflavonoid excretion, remained high throughout the trial.27
Our study also had a number of limitations. The randomization did not lead to perfectly balanced groups as indicated by the baseline differences in age. Given the excellent health status of our participants, oxidative stress levels were probably not elevated and, thus, a measurable effect caused by dietary change is unlikely. As well known in lipid research, substantial effects are more likely observed in high-risk populations. To measure urinary isoprostanes, ELISA assays rather than GCMS were used. Isoprostane levels might have been influenced by infections, sunburn, stress, and other factors13 outside our control. It is also possible that isoflavones affect other types of oxidative stress that are not fully captured by isoprostane measurements, given the relative specificity of isoprostane formation by NO generation.16,17 The application of several assays to capture different aspects of oxidative stress would have been desirable; the lack of correlation across markers observed in some reports indicates that oxidative stress cannot be defined in universal terms.18,34 In addition to isoprostanes as a marker of lipid peroxidation, assays evaluating the oxidative and reductive capacity of biological fluids and the ex vivo susceptibility of lipids to oxidation may be informative. Establishing a stable and unrelated molecular adjustment for isoprostane in urine is another important consideration for future studies.
In conclusion, this soy trial observed an unexpected modest increase in a well validated measure of oxidative stress and questions whether an NO-based antioxidative mechanism contributes to the cancer-preventive effects of soy isoflavones. Future studies with multiple markers assessing oxidative stress would allow more valid conclusions with respect to the spectrum of potential oxidative reactions than this study based on a single marker.
ACKNOWLEDGEMENTS
This research was supported by grants from the National Cancer Institute (R01 CA80843 and P30 CA71789). We thank all participants for their dedication and time to be part of this intervention.
REFERENCES
- 1.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shu XO, Jin F, Dai Q, et al. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–488. [PubMed] [Google Scholar]
- 3.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian- Americans. Carcinogenesis. 2002;23:1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 4.Thanos J, Cotterchio M, Boucher BA, Kreiger N, Thompson LU. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada) Cancer Causes Control. 2006;17:1253–1261. doi: 10.1007/s10552-006-0062-2. [DOI] [PubMed] [Google Scholar]
- 5.Korde LA, Wu AH, Fears T, et al. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18:1050–1059. doi: 10.1158/1055-9965.EPI-08-0405. [DOI] [PubMed] [Google Scholar]
- 6.Lee SA, Shu XO, Li H, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai Women's Health Study. Am J Clin Nutr. 2009;89:1920–1926. doi: 10.3945/ajcn.2008.27361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford D. Mechanistic explanations for the chemopreventive action of soyabean isoflavones: reducing the possibilities. Br J Nutr. 2002;88:439–441. doi: 10.1079/BJN2002711. [DOI] [PubMed] [Google Scholar]
- 8.Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr. 2010;140:2326S–2334S. doi: 10.3945/jn.110.124230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper L, Ryder JJ, Kurzer MS, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messina M, Caskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98:1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- 11.Patel RP, Boersma BJ, Crawford JH. Antioxidant mechanisms of isoflavones in lipid systems: paradoxical effects of peroxyl radical scavenging. Free Radic Biol Med. 2001;31:1570–1581. doi: 10.1016/s0891-5849(01)00737-7. [DOI] [PubMed] [Google Scholar]
- 12.Wei H, Wei L, Frenkel K, Bowen R, Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer. 1993;20:1–12. doi: 10.1080/01635589309514265. [DOI] [PubMed] [Google Scholar]
- 13.Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol Sci. 2002;23:360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- 14.Roberts RA, Laskin DL, Smith CV. Nitrative and oxidative stress in toxicology and disease. Toxicol Sci. 2009;112:4–16. doi: 10.1093/toxsci/kfp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pervin S, Chaudhuri G, Singh R. NO to breast: when, why and why not? Curr Pharm Des. 2010;16:451–462. doi: 10.2174/138161210790232130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marnett LJ, Wright TL, Crews BC, Tannenbaum SR, Morrow JD. Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric-oxide synthase. J Biol Chem. 2000;275:13427–13430. doi: 10.1074/jbc.275.18.13427. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Wood LA, Cooney RV. Enhancement of intracellular gamma-tocopherol levels in cytokine-stimulated C3H 10T1/2 fibroblasts: relation to NO synthesis, isoprostane formation, and tocopherol oxidation. BMC Chem Biol. 2007;7:2. doi: 10.1186/1472-6769-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magalhaes LM, Segundo MA, Reis S, Lima JL. Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta. 2008;613:1–19. doi: 10.1016/j.aca.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 19.Tsimikas S. Measures of oxidative stress. Clin Lab Med. 2006;26:571, 575. doi: 10.1016/j.cll.2006.06.004. vi. [DOI] [PubMed] [Google Scholar]
- 20.Pratico D, Barry OP, Lawson JA. IPF2alpha-I: an index of lipid peroxidation in humans. Proc Natl Acad Sci U S A. 1998;95:3449–3454. doi: 10.1073/pnas.95.7.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 22.Helmersson J, Mattsson P, Basu S. Prostaglandin F(2alpha) metabolite and F(2)-isoprostane excretion rates in migraine. Clinical science. 2002;102:39–43. [PubMed] [Google Scholar]
- 23.Wiseman H, O'Reilly JD, Adlercreutz H, et al. Isoflavone phytoestrogens consumed in soy decrease F(2)-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am J Clin Nutr. 2000;72:395–400. doi: 10.1093/ajcn/72.2.395. [DOI] [PubMed] [Google Scholar]
- 24.Ryan-Borchers TA, Park JS, Chew BP, McGuire MK, Fournier LR, Beerman KA. Soy isoflavones modulate immune function in healthy postmenopausal women. Am J Clin Nutr. 2006;83:1118–1125. doi: 10.1093/ajcn/83.5.1118. [DOI] [PubMed] [Google Scholar]
- 25.Djuric Z, Chen G, Doerge DR, Heilbrun LK, Kucuk O. Effect of soy isoflavone supplementation on markers of oxidative stress in men and women. Cancer Lett. 2001;172:1–6. doi: 10.1016/s0304-3835(01)00627-9. [DOI] [PubMed] [Google Scholar]
- 26.Nhan S, Anderson KE, Nagamani M, Grady JJ, Lu LJ. Effect of a soymilk supplement containing isoflavones on urinary F2 isoprostane levels in premenopausal women. Nutr Cancer. 2005;53:73–81. doi: 10.1207/s15327914nc5301_9. [DOI] [PubMed] [Google Scholar]
- 27.Maskarinec G, Morimoto Y, Conroy SM, Pagano IS, Franke AA. The volume of nipple aspirate fluid is not affected by 6 months of treatment with soy foods in premenopausal women. J Nutr. 2011;141:626–630. doi: 10.3945/jn.110.133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franke AA, Halm BM, Kakazu K, Li X, Custer LJ. Phytoestrogenic isoflavonoids in epidemiologic and clinical research. Drug Test Anal. 2009;1:14–21. doi: 10.1002/dta.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 30.Franke AA, Lai JF, Pagano I, Morimoto Y, Maskarinec G. Equol production changes over time in pre-menopausal women. Br J Nutr. 2011:1–6. doi: 10.1017/S0007114511004223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franke AA, Lai JF, Halm BM. Equol production changes over time in postmenopausal women. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute Inc.; 1996. [Google Scholar]
- 33.Tikkanen MJ, Wahala K, Ojala S, Vihma V, Adlercreutz H. Effect of soybean phytoestrogen intake on low density lipoprotein oxidation resistance. Proc Natl Acad Sci U S A. 1998;95:3106–3110. doi: 10.1073/pnas.95.6.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dotan Y, Lichtenberg D, Pinchuk I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog Lipid Res. 2004;43:200–227. doi: 10.1016/j.plipres.2003.10.001. [DOI] [PubMed] [Google Scholar]