Abstract
The objective of this study was to determine whether the tubes in which Chironomus larvae dwell protect them against chemical toxicants. A laboratory culture of an Israeli benthic midge, Chironomus luridus, was exposed to copper sulfate. Two conditions were tested in bioassay experiments: larvae within silt tubes and larvae without tubes. The non toxic, anionic, fluorescent dye, fluorescein, was used to examine the effect of sub-lethal copper sulfate concentrations on the permeability of cuticular, gill and gut epithelia of the chironomids. Increased cell permeability, which is the cause of cell damage, was reflected by an increase in fluorescence intensity. Following exposure to copper sulfate, higher fluorescence was found in different body compartments: midgut, hindgut, tracheal gills, fat body and muscles, and the Malpighian tubules. The effect was significantly higher in tube-free larvae when compared to silt tube dwelling larvae. We conclude that in addition to its other functions in feeding, respiration, and anti-predation shelter, the Chironomus luridus tube protects its inhabitant from toxins such as copper sulfate.
Keywords: Chironomus luridus, copper, chironomid tubes, fluorescein
Introduction
The floor in freshwater habitats acts as a sink for naturally occurring wastes as well as for different anthropogenic toxic substances. Winner et al. (1980) found that chironomids made up ca. 80% of total fauna in a stream heavily polluted by Cu, Cr, and Zn, whereas they constituted less than 10% in an unpolluted section of the same stream. Watanabe et al. (2000) explored the effects of drainage on a benthic macroinvertebrate community in the Ichi-kawa River, Japan. They found that benthic communities were severely damaged at sites just below the drainage where arsenic, copper and zinc concentrations in the water were distinctly higher. The impacted sites were predominated by Chironomidae. In another study, experimental flumes receiving water and aquatic invertebrates from an undisturbed, forested stream were used to determine the impact of metals in a low-conductivity stream. Chironomidae made up 80% of the benthos and showed no significant decrease in densities with increasing metal (Cu, Zn, Mn, and Pb) dose (Richardson & Kiffney 2000).
The larvae of Chironomus spp. live in tubes in the benthos of freshwater habitats. They have recently been found as pestiferous midges in covered tanks in Israel (Broza et al., 1998). Copper sulfate, which is used in water purification, was examined as a possible means to control the Chironomus population (Halpern et al. 1999). The tube-dwelling behaviors of chironomids were originally coupled with feeding and respiration (Walshe 1951). Hershey (1987) suggested that the tubes evolved as anti-predator adaptations but were later modified in some chironomid groups to facilitate feeding and respiration. Halpern et al. (2002) suggested that chironomids' tubes play a role in protecting their dwellers against toxicants. They found that larvae without substrate (i.e. naked) were significantly more sensitive to copper and chloramine than larvae within sand or silt tubes. These experiments demonstrated the protective nature of the tubes for the whole organism.
Specific fluorescent probes, fluorescent microscopy and microfluorometery are among the best ways to examine specific functions and viability of various animals (Thaer & Sernetz 1973, Bresler et al. 1990). One of the most sensitive and simple tests used to study the viability of living organisms and the alterations produced thereon by toxicants, is the examination of the permeability of plasma membranes and epithelial layers (Bresler and Yanko 1995). The plasma membrane of intact living cells is practically impermeable to water-soluble organic compounds, especially anions. Epithelial cells, joined by tight junctions, also form a layer impermeable to these compounds. Injured or dead cells can be detected by their increasing permeability to anion compounds. The fluorescent non-toxic anionic dye, fluorescein, is used to detect injured and dead cells (Bresler & Yanko 1995).
The microfluorometric technique enables identification of non-lethal stress in various larval body compartments exposed to copper. In the present study we confirm the hypothesis that the C. luridus tube protects its inhabitant against toxicants in the organ and cellular level.
Materials and Methods
Experimental conditions and chemicals:
All bioassay experiments were conducted on a laboratory culture of Chironomus luridus Strenzke that was collected from covered storage tanks of the water supply system at Shefar'am near Haifa, Israel. The laboratory culturing procedure was described in Halpern et al. 1999. Dechlorinated tap water was used in all experiments. A stock solution of 1000 mg/liter copper was prepared daily using CuSO45H20. Test concentrations were prepared by diluting appropriate aliquots of the stock solution with test water. Silt that was used for each larvae's tube substrate was collected from the same storage tank's floor as was the midge. The silt was washed, diluted (20:1 weight:weight, water to silt), sterilized, and stored at 4°C. In order to assure the addition of a homogenous amount of silt to each test cup, the silt was centrifuged (10 min at 1,000 rpm) before use, and a measured quantity of 1 ml silt was added to the appropriate test cup. The organic matter of silt was determined following a 6 h incubation at a temperature of 550°C, and was found to be 22.5%. Particle size was determined by the hydrometric method (Black 1986), and was as follows: 54.5% 200–2000 µm, 28.9% 20–200µm and 16.7% 2–20 µm.
Microfluorometry procedures
The fluorescent non-toxic dye, “fluorescein” (FLU; 3′,6′-dihydroxy spiro[isobenzo-furan-1(3M)9′-xanthen]-3-one, disodium salt; pKa=7.0), in a final concentration of 100 µM, was used to examine the permeability of 5 body compartments of C. luridus larvae: midgut, hindgut, tracheal gills, fat body and muscles (body), and the Malpighian tubules. A Nikon Labophot-2 fluorescent microscope was used to determine the fluorescence intensity of larval cells, using the vital contact microfluorometry technique (Thaer & Sernetz, 1973 and Bresler et al., 1990). Fluorescence intensity was measured by a microfluorometer (1uVolts-10Volts) and expressed by arbitrary units (0.1–100,000) (Thaer & Sernetz 1973).
Bioassay procedure
Bioassays were conducted with larvae in silt tubes or without tubes at 25°C. Five 4th instar larvae were placed in a 120-ml plastic cup containing 90-ml tap water, with or without silt, and were allowed to acclimate and construct tubes for 24h before experimentation. Test concentrations were prepared by diluting appropriate aliquots of the stock solution with tap water; 10 ml were added to the plastic cups, to yield a total of 100 ml. Each bioassay consisted of two copper concentrations (50 or 100 mg/liter) and a control of dechlorinated tap water. After 1h exposure to copper concentration, FLU, in a final concentration of 100µM was added to the test cups for another hour of incubation. Fluorescence intensity was measured in the 5 larval body compartments, 20 measures per compartment.
Data analysis
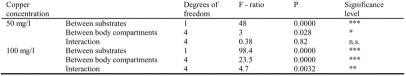
Results were subjected to Nested ANOVA (Sokal & Rohlf 1998) (Data not shown). As the variances between larvae were higher than the variances between measures in each larva (for each body compartment), an average of all 20 measures in each larval body compartment was calculated. Significant differences in fluorescence intensity were tested in 2 way ANOVA between substrates and between copper concentration in 5 different larval body compartments (Table 1), as well as between substrates and between body compartments in each concentration (Table 2).
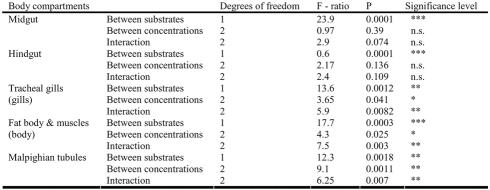
Table 1.
Significant differences in fluorescence intensity between substrates and between copper concentration in different larval body compartments of Chironomus luridus (2 way ANOVA) (see data in Figure 2).
Table 2. T.
he significant differences in fluorescence intensity (fluorescein penetration) between substrates (with silt tube or naked) and between larval body compartments of Chironomus luridus as a result of the exposure to different copper concentrations (2 way ANOVA).
Results
The midgut of a naked larva exposed to tap water with fluorescent dye is shown in Fig. 1. The fluorescent dye fills the midgut lumen and does not penetrate other body compartments. Exposure of the larvae to copper causes cell injury that results in FLU penetration into larval organs. Following the exposure to copper, significant differences were found between high FLU penetration into larval organs in larvae without tubes, as opposed to low FLU penetration in larvae within silt tubes (Table 1). Significant differences were found in all the 5 examined body compartments and in all tested concentrations (Table 2). The average FLU intensity calculated for three tissue types (the larval body, gills, and Malpighian tubules) in the 50 mg/l treatment, as compared to tap water controls, was doubled in larvae in tubes, whereas it was elevated 36-fold in naked larvae (Fig. 2 c, d, e). When the copper concentration was elevated to 100 mg/l, permeability into all body compartments of the naked larvae decreased, while permeability into the various tissue types of larvae in silt tubes increased, as compared to the 50 mg/l treatment (Fig. 2).
Figure. 1.
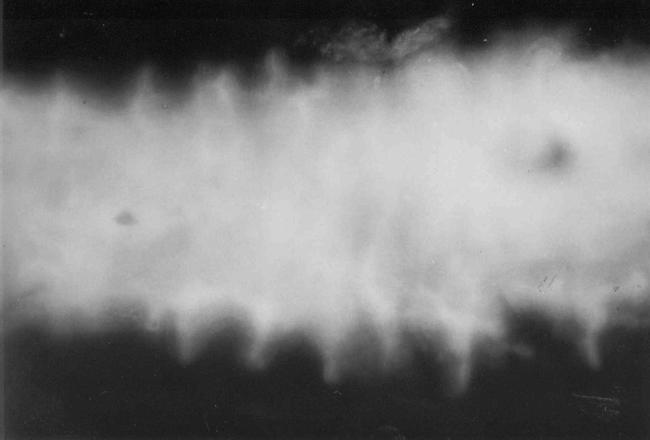
Fluorescence intensity of midgut lumens of Chironomus luridus larva (untreated and without a tube), vital stained with fluorescein. The picture was taken while in peristaltic movement. Taken with fluorescent microscope at 100×.
Figure. 2.
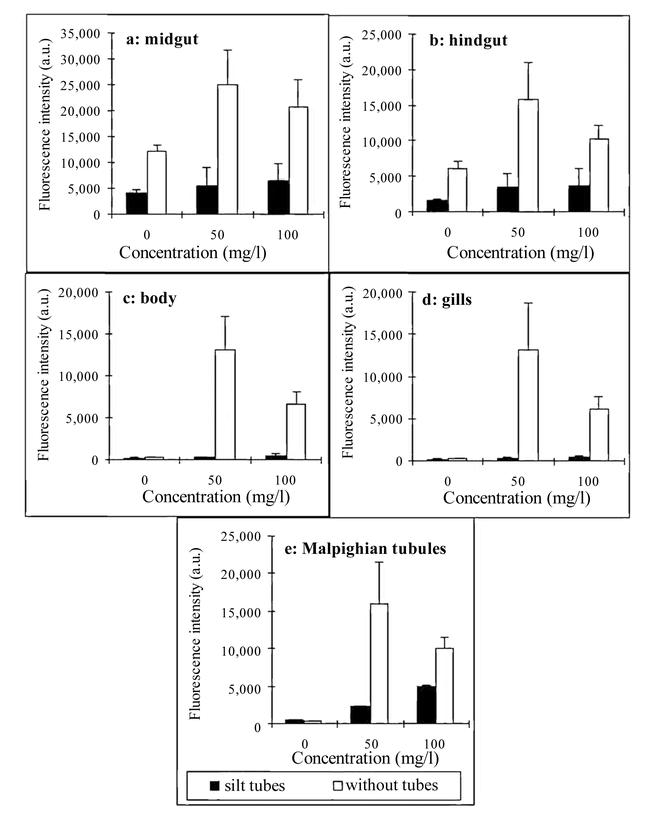
Effect of Chironomus luridus 4th instar larvae exposure (within and without tubes) to different copper concentrations, on the permeability of fluorescein into (a) midgut, (b) hindgut, (c) body, (d) gills, (e) Malpighian tubules. The data are expressed as fluorescence intensity, mean a.u.± S.D. Larvae with silt tubes were exposed to copper and FLU while within the tubes.
To examine the response to copper more carefully, the fluorescence intensity of the internal body compartments was calculated as a percentage of the midgut fluorescence intensity. As the fluorescence intensity measured in the midgut was the highest under all experimental conditions (Fig. 2), it was considered the reference level of intensity. When the larvae were not exposed to copper, fluorescence intensity in the hindgut was 45% of that in the midgut, and penetration of FLU into gills, Malpighian tubules, and body lumen was negligible (Fig. 3a). Exposure of larvae to 50-mg/l copper raised the fluorescence intensity in the hindgut to 65% (Fig. 3b). The relative fluorescence in gills, Malpighian tubules, and body lumen rose about 25 times in larvae without tubes as compared to controls, whereas the fluorescence intensity in gills and body lumen of larvae within silt tubes was only about 1.5 times higher than in the control. Penetration of the dye into Malpighian tubules was more intensive, being about 4 times higher than in the control (Fig. 3b).
Figure. 3.
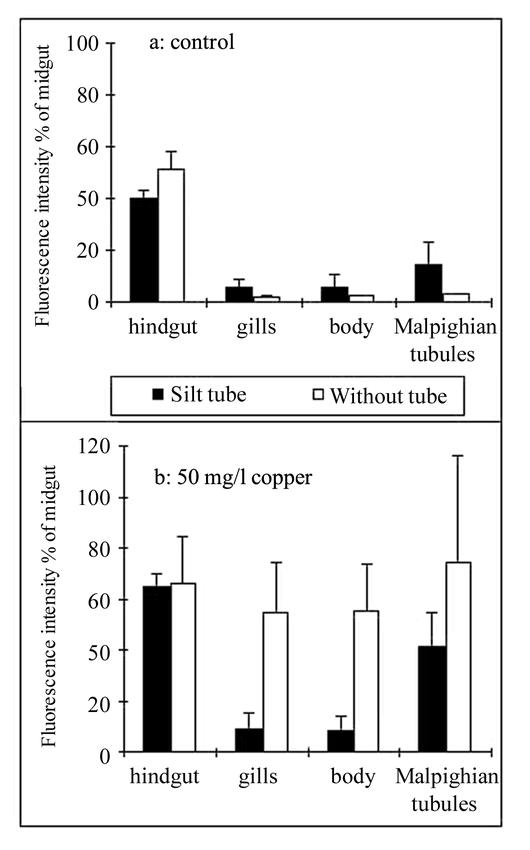
The effect of increased copper concentrations: (a) tap water control, (b) 50 mg/l copper as CuSO4, on the fluorescence intensity of hindgut, body, gills and Malphigian tubules of Chironomus luridus 4th instar larvae with and without tubes. Data are expressed as a mean percentage (± S.D.) relative to the fluorescence intensity of the midgut in each concentration.
Discussion
The vital contact microfluorometry technique enables the detection of larval cell injury after exposure to copper sulfate. Copper sulfate increases cytoplasmatic membrane permeability. Cells in untreated larvae showed a selective permeability, which did not enable penetration of FLU. Fluid in the lumen of the gut has free access to the fluid outside the insect; thus, FLU remained in the lumen of the gut (mid- and hindgut) in both, treated and untreated larvae. However, FLU penetration of the tracheal gills, fat body and muscles, and the Malpighian tubules in naked larvae exposed to 50 mg/l copper was about 20 times higher than in larvae protected in silt tubes. Exposure of naked larvae to 100 mg/l copper caused a decrease in permeability in all body compartments. The decrease can be explained by the poor physiological condition of those larvae, which may have affected their water filtration function. In comparison, only minor damages were caused by the exposure of larvae in silt tubes to both 50 and 100 mg/l copper.
Halpren (2002) found that larvae without tubes are more sensitive to copper and chloramines than are those living in sand or silt tubes. Silt had a higher protective value for C. luridus larvae than sand tubes, especially when they were exposed to copper for a short period of time. The differences found between larvae's sensitivity to copper (in the 3 substrate situations) were due to the protection that the tube gave to its resident and not because silt or sand by themselves reduce the effective concentration of the toxicant (Halpern et al. 2002). Use of the microfluorometric technique enabled us to identify non-lethal stress caused by copper. The naked larvae showed a “dose-response” reaction, with a gradual increase in permeability. On the other hand, the larvae within the silt tube were almost indifferent to the lower concentration of copper, as a result of tube protection. They responded only to the dose that was higher than LC50 /24h (80.5 mg/liter). This assay supports our hypothesis (Halpern et al. 2002) that Chironomus tubes serve as a barrier against introduction of toxicants.
In all the experiments, both with and without tubes, the fluorescence intensity in the midgut was always the highest. Elevating copper concentrations resulted in an increase in fluorescence intensity relative to midgut, most notable in Malpighian tubules. This can be explained by the active transport of the toxicant (Cu+2) into the Malpighian tubule lumen. The toxicant is transported from the Malpighian tubules into the hindgut, which was second to the midgut in FLU intensity. These results are consistent with previous studies. Bresler et al. (1990) studied the transport of FLU into isolated Malpighian tubules of Periplaneta americana, Locusta migratoria, and the larva of the wax moth, Galleria mellonella. They found active transport of FLU in all 3 species against gradient concentrations. Snyder et al. (1994) reported a process of nicotine detoxification starting in the midgut of Manduca sexta larvae, followed by an active transport into the lumen of Malpighian tubules, and then excretion through the hindgut.
Sediment provides a habitat for various aquatic organisms and at the same time is a major repository for many of the more persistent chemicals that are introduced into surface water. To such habitats Chironomus species are well adapted. Mattingly et al. (2001) isolated cDNA that encodes a protein, designated Chironomus tentans alpha-tubulin1 (CTTUB1). The RNA and protein abundances were increased in larvae exposed to copper or cadmium. The pattern of cellular distribution of CTTUB1 protein in the midgut epithelial cells was radically affected by cadmium. In the midgut cells of unexposed larvae, CTTUB1 was found evenly distributed throughout the cytoplasm, while in cadmium-exposed larvae, CTTUB1 was mostly concentrated along the basolateral plasma membrane.
Our data suggest that the chironomids' defense against copper is comprised of two barriers. The first barrier is the tube, which consists of foreign particles embedded within and around the larva's own protein secretion. The second barrier is composed of proteins that play a role in protecting the larvae against copper and other metals or toxicants. The approach presented in the current study may provide a better understanding of the tube-dwelling chironomids' adaptation to the benthos.
Glossary
| Abbreviation: | |
|---|---|
| FLU | fluorescein: 3′,6′-dihydroxy spiro[isobenzo-furan-1(3M)9′-xanthen]-3-one, disodium salt |
References
- Black WC. 1986 Methods of soil analysis 1. American Society of Agronomy, Madison, WI. [Google Scholar]
- Bresler V, Belyaeva EA, Mozahaeva MG. A comparative study on the system of active transport of organic acids in Malpighian tubules of insects. Journal of Insect Physiology. 1990;36:259–270. [Google Scholar]
- Bresler V, Yanko V. Acute toxicity of heavy metals for benthic epiphytic Foraminifera Pararotalia spinigera (Le calvez) and influence of seaweed-derived DOC. Environmental Toxicology & Chemistry. 1995;14:1687–1695. [Google Scholar]
- Broza M, Halpern M, Teltsch B, Porat R, Gasith A. Shock chloramination: Potential treatment for Chironomidae (Diptera) larvae nuisance abatement in water supply systems. Journal of Economic Entomology. 1998;91:834–840. doi: 10.1093/jee/91.4.834. [DOI] [PubMed] [Google Scholar]
- Halpern M, Gasith A, Porat R, Telsch B, Broza M. Synergistic effect of chloramine and copper sulfate as control agents of planktonic midge larvae (Diptera: Chironomidae) in drinking water supply systems. Journal of American Mosquito Control Association. 1999;15:453–457. [PubMed] [Google Scholar]
- Halpern M, Gasith A, Broza M. Does the tube of a benthic chironomid larva play a role in protecting its dweller against chemical toxicants? Hydrobiologia. 2002;470:49–55. [Google Scholar]
- Hershey AE. Tubes and foraging behavior in larval Chironomidae: Implication for predator avoidance. Oecologia. 1987;73:236–241. doi: 10.1007/BF00377513. [DOI] [PubMed] [Google Scholar]
- Mattingly KS, Beaty BJ, Mackie RS, McGaw M, Carlson JO, Rayms-Keller A. Molecular cloning and characterization of a metal responsive Chironomus tentans alpha-tubulin cDNA. Aquatic Toxicology. 2001;54:249–60. doi: 10.1016/s0166-445x(00)00181-8. [DOI] [PubMed] [Google Scholar]
- Richardson JS, Kiffney PM. Responses of a macroinvertebrate community from a pristine, southern British Columbia, Canada, stream to metals in experimental mesocosms. Environmental Toxicology and Chemistry. 2000;19:736–743. [Google Scholar]
- Snyder NJ, Walding JK, Feyereisen R. Metabolic Fate of the allelochemical nicotine in the tabaco hornworm, Manduca sexta. Insect Biochemistry and Molecular Biology. 1994;24:837–46. [Google Scholar]
- Sokal RR, Rohlf FJ. 1998 Biometry, 4th edition. W. H. Freeman & Company, NY. [Google Scholar]
- Thaer AA, Sernetz M. 1973 Fluorescense Techniques in Cell Biology. Springer - Verlage, Berlin. [Google Scholar]
- Watanabe NC, Harada S, Komai Y. Long-term recovery from mine drainage disturbance of a macroinvertebrate community in the Ichi-kawa River, Japan. Hydrobiologia. 2000;429:171–180. [Google Scholar]
- Walshe BM. The feeding habits of certain Chironomid larvae (subfamily Tendipedinae) Proceedings of the Zoological Society of London. 1951;121:63–79. [Google Scholar]
- Winner RW, Bossel MW, Farrel MP. Insect community structure as an index of heavy-metal pollution in loic ecosystems. Canadian Journal of Fisheries & Aquatic Sciences. 1980;37:647–655. [Google Scholar]