Abstract
The presence of pancreatic stem cells (PnSCs) has not been firmly demonstrated in the human or animal pancreas. Previous reports have suggested that ductal and acinar structures in the exocrine pancreas can be a potential source of progenitor cells. More recently, immature insulin precursors in the periphery of human islets have been found to self-replicate and differentiate to endocrine cells in vitro. Transplantation of these cells under the kidney capsule improves the diabetic state in mice. The controversy surrounding where PnSCs reside could be resolved if a specific marker were to be found that allowed their identification, purification, and directed differentiation to endocrine cells. We have identified in human pancreas cells positive for the stage-specific embryonic antigen 4 (SSEA4), a stem cell marker. These cells also express ductal, pancreatic progenitor, and stem cell protein markers. Interestingly, some of the SSEA4+ cells scattered in the ducts do not show a ductal cell phenotype. SSEA4+-sorted cells formed aggregate-like spheres in culture and robustly differentiated to pancreatic hormone-expressing cells in conditions of high glucose concentration and B27 supplementation. We hypothesize that SSEA4+ cells or a subpopulation of those cells residing in the pancreatic ducts may be the elusive PnSCs, and in this case, SSEA4 may represent a potential surface antigen marker for human PnSCs. The discovery of specific markers for the identification and purification of human PnSCs would greatly facilitate studies aimed at the expansion of these cells and the development of targeting tools for their potential induction to insulin-producing cells.
Key words: cellular biology, pancreas, regeneration, SSEA4
Introduction
Type 1-diabetes is a disease that affects millions of people throughout the world. Current cell replacement therapies involve the transplantation of pancreatic islets, which requires many islets and several cell transplant procedures. Human islets obtained from cadavers are not sufficient for the vast number of patients in need of this therapy. Human embryonic stem cells (hESCs) have been considered to be a potential source for cell replacement, but progenitors derived from these cells often generate teratomas in experimental cell transplants in mice.1 Further work will be needed to derive mature β-cells from hESCs. Transplantation of induced pluripotent stem cells or the induction in situ of pancreatic stem cells (PnSCs) to β-cells may also become possible for diabetic patients. So far, the presence of PnSCs in the pancreas has not been firmly demonstrated in humans or animals. Previous published reports suggest that ductal structures in the exocrine pancreas are a potential site where endocrine pancreatic stem/progenitors may reside until they receive inductive signals for differentiation.2–11 Acinar tissue is another potential source of progenitor cells through a process of acinar transdifferentiation to ductal and endocrine cells.12–14 Dor et al. provided evidence that mouse β-cells can regenerate through self-replication in vivo.15 In a more recent investigation, Smukler et al. showed that immature insulin cells, unique stem cells, situated in the periphery of the human islets, can self-replicate and differentiate to hormone-producing endocrine cells in vitro and in vivo.16 Xu et al. demonstrated the appearance of β-cells generated from endogenous progenitors located in the ductal lining of injured adult mouse pancreas.9 These progenitors differentiated to all islet cell types in an NGN3-dependent mode.9
The controversy of where PnSCs reside could be solved if a specific marker is found that will allow for their identification and purification. Previously, CD133 and MET ductal-positive cells were purified from mouse pancreas by flow cytometry and differentiated toward an endocrine and exocrine lineage.17–20 CD133 and MET are, however, not appropriate markers for purification of a specific population of stem/progenitors cells in the human pancreas, because CD133 is broadly expressed in stem and progenitor cells from different tissues, including hematopoietic cells, while MET is expressed in the islet.
Our preliminary data demonstrate that stage-specific embryonic antigen 4 (SSEA4) could be such a marker. SSEA4+ cells are present in the exocrine portion of the human pancreas, some surrounding the islets, but not inside islets. Progenitor markers such as Sox9 and CD133, and ductal markers such as c-Met, CK19, and carbonic anhydrase II (CA2) were all highly expressed in SSEA4+-sorted cells in comparison to SSEA4− cells (purity close to 99%). Stem cells markers such as Wnt3a and Oct4 were also significantly upregulated in SSEA4+ cells, although their expression was low. More importantly, in the human fetal pancreas, most of the SSEA4 cells were also NGN3+, a recognized marker of endocrine progenitors.
SSEA4 is a known marker of hESCs, a glycolipid antigen with a globoseries carbohydrate core structure.21,22 The protein was also found in multipotent progenitors from human fetal liver23 and in human neural progenitor cells,24 as well as in mesenchymal cells of human dermis.25
Hypothesis
Accumulated evidence supports the concept that pancreatic stem/progenitor cells may originate in the pancreatic duct, where they reside in a quiescent stage.2,3,26 Nonterminally differentiated stem/progenitors persist in the adult organism and may serve as a source of terminally differentiated cells once induced. They differ from ESCs by being multipotent and are also called adult stem cells. As a progeny of ESCs, they might express markers identified in their predecessor such as stage-specific antigens. They also might coexpress progenitor cell markers, common stem cell markers, like CD133, or markers of the tissue where they reside, such as CK19 or CA2 ductal markers. Based on these assumptions and our data, we hypothesize that SSEA4 may represent a potential surface antigen marker for human pancreatic stem/progenitors. Interestingly, we found that some SSEA4+ cells did not co-localize with the CA2 ductal marker,4 although they resided in between SSEA4+CA2+ ductal cells. Based on this observation and the low number of spheres that originated from a relatively large number of SSEA4+ cells, we also hypothesize that the SSEA4+ cells that have not assumed ductal fate could be the elusive PnSCs.
We have identified SSEA4+ cells in the human exocrine pancreas by immunohistochemistry. We obtained gene expression profiles of sorted SSEA4+ and SSEA4− cells and also established conditions for their differentiation to pancreatic hormone-expressing cells. Thus, we are first to identify SSEA4+ cells in the adult human pancreas with characteristics of pancreatic progenitors. Further clonal analysis would confirm their stemness. The identification and purification of human PnSCs will greatly facilitate studies aimed at the expansion of those cells as well as development of targeting tools for their induction in situ.
Materials and Methods
Tissue culture, differentiation, and real-time quantitative polymerase chain reaction
Human exocrine pancreatic tissue was kept in a Roswell Park Memorial Institute (RPMI)/10% fetal bovine serum (FBS) medium in suspension culture. Cells were dissociated with 0.05% Trypsin/Versene (Invitrogen) and sorted by fluorescence-activated cell sorting (FACS) or by using magnetic beads as described below. Sorted cells were grown for 10 days in Dulbecco's modified Eagle's medium (DMEM)/F12 medium (Invitrogen) supplemented with 10% FBS (Invitrogen), epidermal growth factor, and basic fibroblast growth factor, 20 ng/mL each (R&D Systems). Cells were seeded in low-attachment plates at a density 0.5×106 cells/well (9.2 cm2), and the medium was changed every 3–4 days. Differentiation to pancreatic hormone–expressing cells was performed for 4 days in the presence of DMEM high glucose, 1% B27 supplement, both from Invitrogen, and PP2 (10 mM; EMD Biosciences). mRNA purification, cDNA synthesis, and real-time quantitative polymerase chain reaction (RT-qPCR) analysis of differentiated tissue were performed as previously described.27 Briefly, 20 μL PCR reactions were run using 3 μL of cDNA, combined with the TaqMan Universal PCR Master Mix (4324018; Applied Biosystems) and unlabeled PCR primers and a TaqMan FAM™ dye-labeled probe listed in Supplementary Table S1. RT-qPCR was performed using an Applied Biosystems StepOnePlus real time PCR machine. The results were analyzed by the standard curve method as previously described.27 Quantitative values for each gene of interest were normalized to cyclophilin A.
FACS and magnetic bead purification
After dissociation, the cells were stained with 20 mg/mL SSEA4 mouse monoclonal antibody in a FACS buffer (phosphate-buffered saline [PBS]/0.5% bovine serum albumin/0.025% NaN2) for 40–60 min at room temperature. After several washes with the FACS buffer, cells were stained with the secondary antibody goat-anti mouse Alexa 488 for 15–30 min atroom temperature. Cells were washed and FACS-sorted on a Becton Dickinson FACSAria. Magnetic bead purification was performed as recommended by the manufacturer (Miltenyi Biotec, Inc.). Dissociated cells were labeled first with an SSEA4-PE antibody in a concentration recommended by the manufacturer and secondly with anti-PE Beads (Miltenyi Biotec, order # 130-048-801). Cells were sorted by double purification by using LS columns, also obtained from Miltenyi Biotec.
Immunostaining
Human fetal pancreases were provided by the Birth Defects Research Laboratory, University of Washington (Seattle, WA), and approved by the University of California–San Diego (San Diego, CA) Human Research Protection Program (Protocol #081237XT). Dr. Jose Oberholzer generously provided the human adult tissue, processed at the Islet Processing Facility at the Chicago Medical Center (University of Illinois, Chicago, IL).
Tissue from fetal and adult pancreas was fixed in 4% paraformaldehyde in PBS. The human adult tissue was treated overnight with 15% sucrose in PBS. Subsequently, the tissue was cryopreserved in O.C.T. (Tissue-Tek Sakura Finetek), and 5-μm sections were cut for staining. For double immunofluorescence of SSEA4 was used 0.05% Triton X-100 in PBS for permeabilization, except for SOX9 staining where was used 0.01% Tween 20. Primary and secondary antibodies descriptions and dilutions are listed in Supplementary Tables S2 and S3, respectively. The stained sections were viewed using a Nikon Eclipse E800 and TS100 Fluorescent Microscope, and the images were captured using SPOT software (version 4.6).
Results
Characterization of SSEA4+ cells in human pancreas
We identified and purified SSEA4+ cells in the human adult pancreas by immunohistochemistry and flow cytometry. These cells appeared scattered in the exocrine pancreas and around the islets (Fig. 1B, C). The percent for SSEA4+ cells varied from 17% to 30% as estimated by FACS analysis (Supplementary Figs. S1 and S2B), and morphologically, they are small and epithelial looking (Fig. 2A).
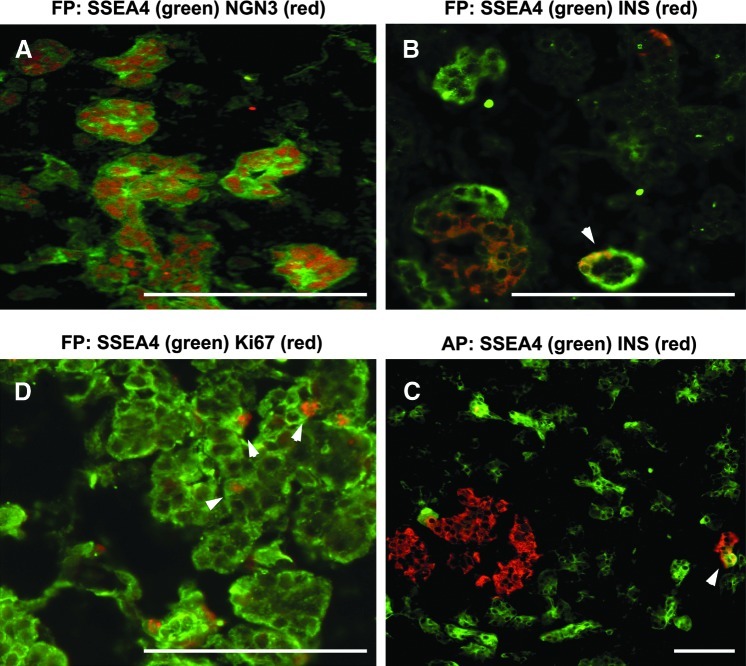
FIG. 1.
Sections of human FP and AP were immunostained for co-localization of SSEA4+ cells with markers as noted. White arrowheads represent Ki67 or insulin+SSEA4+ cells as noted. Scale bars: 115-μm FP (A, B, D), 60-μm AP (C). FP, fetal pancreas; AP, adult pancreas; SSEA, stage-specific embryonic antigen 4.
FIG. 2.
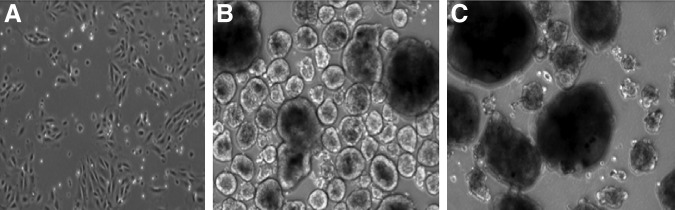
SSEA4+ and SSEA4− cell culture. (A) SSEA4+ cells expanded in monolayer for 1 week in RPMI/10% FBS on an HTB9 matrix. (B) SSEA4+ cell-derived spheres after 10 days in a suspension culture. (C) SSEA4− cell-derived spheres after 10 days in a suspension culture. FBS, fetal bovine serum; RPMI, Roswell Park Memorial Institute medium.
Gene expression profiles of SSEA4+ and SSEA4− cells are shown in Figure 3. SSEA4+ cells expressed the pancreatic progenitor markers such as CD133 and Sox9 and the ductal markers c-Met, CK19, and CA2. They also expressed low amounts of Pdx1 and stem cell markers such as Oct4 and Wnt3a. Interestingly, c-Myc was highly expressed in SSEA4+ cells (Fig. 4). These cells did not express endothelial or endocrine markers as shown by PECAM-1 or insulin, respectively. They also did not express the endocrine progenitor marker Ngn3. Nestin expression was lower in the SSEA4+ than in the SSEA4− cells.
FIG. 3.
Representative study showing an RT-qPCR profile of SSEA4− (gray bars) and SSEA4+ cells (green bars). Adult human exocrine pancreases were dissociated and labeled with an SSEA4 antibody. SSEA4− and SSEA4+ cells were purified closed to 99% purity by flow cytometry cell sorting. Cells were lyzed, and total RNA was purified for cDNA synthesis and RT-qPCR analysis. Numbers on the right scale in graphs represent relative mRNA message. l (dashed line), SSEA4− cell mRNA message; RT-qPCR, real time-quantitative polymerase chain reaction.
FIG. 4.
Two adult human exocrine pancreases were dissociated and labeled separately with SSEA4 antibody. SSEA4− and SSEA4+ cells were purified closed to 95%–98% purity by flow cytometry cell sorting. Cells were lyzed, and total RNA was purified for cDNA synthesis and RT-qPCR analysis. The numbers on the scale are relative to the expression of c-Myc in human embryonic stem cells. The RT-qPCR results shown in this study are the average values of two experiments (n=2); p<0.05.
We used immunofluorescence to demonstrate the co-localization of SSEA4+ cells with the pancreatic duct, progenitor markers, and emerging insulin cells. SOX9, CK19, and CA2 are established ductal markers. As shown in Figure 5B, staining of pancreas sections showed that a majority of SSEA4+ cells were ductal cells, but notably some SSEA4+ cells were not CA2+. By staining 7-day cultures of dissociated adult pancreases, we confirmed that the majority of SSEA4+ cells co-localize with SOX9 (Fig. 6C), and most of them co-localize with the ductal marker CK19 (Fig. 6B); however, they were not positive for carboxypeptidase A1 (Fig. 6A), a marker for cells localized in the tip domain of the duct.10 This is consistent with other report where SOX9 cells mark a population of cells at the interface of the tip and trunk domain.28 SSEA4+ cells also grew in clusters in between the duct cells (Fig. 6B, C).
FIG. 5.

Adult human pancreas sections. SSEA4+ cells co-localize with (A) SOX9 and (B) CA2 duct markers. SSEA4+ cells that do not co-localize with CA2 are noted with white arrowheads; (C) shows co-localization of SOX9 and CA2 duct markers. Some SOX9, most probably acinar cells, also do not co-localize with CA2. Scale bars: 60 μm (A, C), 115 μm (B).
FIG. 6.

Cells from adult human pancreatic tissue were cultured for 7 days in RPMI medium with 10% FBS and stained for markers as noted. (A) SSEA4+ cells were not carboxypeptidase A1+; see white arrowheads.The majority of SSEA4+ cells co-localize with (B) CK19+ and (C) SOX9+ duct cells. Scale bars: 70 μm (A–C). CPA, carboxypeptidase A1.
We reasoned that if SSEA4 cells were endocrine progenitors, they had to be positive for NGN3, a well-characterized endocrine progenitor marker during pancreatic development. Therefore, we assayed human fetal pancreases from age 9.6- to 22-week gestation for the presence of SSEA4/NGN3 double-positive cells. We found that SSEA4+ cells co-localized with NGN3 (Fig. 1A).
We also looked at SSEA4 cells' proliferative potential by co-staining with Ki67. Very few SSEA4+ cells co-stained with Ki67 (Fig. 1D), indicating that few of them might have proliferative potential in vivo.
In the adult pancreas, SSEA4+ cells did not co-localize with insulin+ cells, except for one cell detected as shown in Figure 1C, which may suggest that SSEA4 expression was lost during the endocrine progenitor transformation to a mature cell and underlines SSEA4 marker progenitor's specificity.
We thought that in the fetal pancreas, we might detect more of these rare transient events, and indeed, Figure 1B, we saw one such demonstration of SSEA4+INS+ cells. Often, we observed SSEA4 cells surrounding an emerging islet (Fig. 1B, C).
Differentiation of SSEA4+ and SSEA4− cells to pancreatic cells
To test the differentiation potential of SSEA4+ and SSEA4− cells, sorted cells were grown in low-attachment plates for 10 days in a medium described in the Material and Methods section. After 2 days, sphere formation appeared in the SSEA4+ and SSEA4− cultures. A week later, the spheres in the SSEA4+ cultures were more uniform in comparison to the SSEA4− cultures (Fig. 2B, C). Samples were taken every day, and the Ngn3 mRNA expression was analyzed by RT-qPCR. In the SSEA4+ cells, Ngn3 expression was three times higher by day 2 in comparison to day 1, and it peaked at day 5 (Fig. 7). The purity of SSEA4+ cells was 95% as estimated by FACS (Supplementary Fig. S2A). However, SSEA4− cells contained 20% low-expressing SSEA4+ cells (Supplementary Fig. S2A), which probably contributed to the sporadic low Ngn3 expression (Fig. 7) and elevated insulin expression in differentiated SSEA4− cells (Fig. 8). Despite the fact that Ngn3 was gradually and significantly upregulated in spheres derived from SSEA4+ cells in comparison to the SSEA4− cells, the expression of Ngn3 mRNA was low.
FIG. 7.
RT-qPCR analysis of spheres for neurogenin 3 and insulin expression. Samples were taken every day, as noted, from SSEA4+ and SSEA4− cell-derived sphere formations. Cells were lyzed by standard methods for mRNA purification, and generation of cDNA. RT-qPCR was performed in a TaqMan mix by using standard procedures (Applied Biosystems). This is one representative experiment. Standard deviations represent PCR replicates of the same sample.
FIG. 8.
RT-qPCR analysis of spheres, derived from magnetic-activated cell sorting–purified SSEA4+ and SSEA4− cells, after 4-day culture in high-glucose medium supplemented with B27 and PP2. Dark bars on the right represent SSEA4+ cell-derived spheres; gray bars are for the SSEA4− cell-derived spheres with controls (CNTR), spheres grown in a regular growth medium. The numbers on the scale are relative to the expression of those genes in the FP. This is one representative experiment. Standard deviations represent PCR replicates of the same sample.
Ten-day SSEA4+ and SSEA4− spheres were each divided in two plates. Half of the spheres were transferred to one plate and kept in a growth medium (control), and the other half were kept in a differentiation medium, which contained DMEM high glucose, B27 supplement and PP2, a Src family kinase inhibitor, since we recently showed that Src family kinase inhibition is involved in endocrine specification.27 After 4 days, the spheres were harvested and analyzed for pancreatic markers of progenitor and hormone-expressing cells. We found robust expression of Pdx1, Nkx6.1, insulin, glucagon, and somatostatin in SSEA4+ in comparison to the controls and to SSEA4− cell-derived spheres (Fig. 8).
Interestingly, Sox9 ductal expression was not observed in the control population of SSEA4+ cell-derived spheres, but glucagon and somatostatin were upregulated in the SSEA4+ control in comparison to the SSEA4− control population (Fig. 8). As mentioned before, we observed some SSEA4 cells that were not positive for ductal markers in the adult pancreas (Fig. 5B, white arrow heads). Clonal analysis of SSEA4+ and SSEA4− control cells will be necessary to test whether the SSEA4+ nonductal cells are the ones that robustly differentiate to pancreatic cells in high-glucose medium. We believe that the cells that give rise to endocrine cells are derived from the SSEA4+ cells, and they are not a result of contamination with replicating INS+SSEA4− cells, because the expression of insulin in the SSEA4+ control spheres was close to zero (Ct38) in comparison to the SSEA4− spheres (Ct25) (Figs. 7 and 8, insulin). In addition, we saw Ngn3 expression predominantly in SSEA4+ cells, which might explain the consequent elevated expression of somatostatin and glucagon in the control SSEA4+ spheres. Sustained Hes1 expression in SSEA4− spheres might explain their inefficient differentiation (Fig. 8). The absence of Hes1 expression in SSEA4+ cells is consistent with their endocrine progenitor phenotype acquired during the culture, as manifested by Ngn3 upregulation.29
Hypothesis evaluation and discussion
We have identified and purified SSEA4+ cells from human adult pancreases. Based on genetic profiling and immunostaining, most of these cells appeared to have ductal origin, although some did not show expression of the CA2 ductal marker (Fig. 5B). SSEA4 cells appeared to express low levels of Oct4 and Wnt3a markers, related to pluripotency, but they express high levels of CD133 and c-Myc, which is characteristic of multipotent progenitors.30,31 Pdx1 low expression in the SSEA4+ cell pool most probably is due to a small contamination with insulin cells from the SSEA4− cell pool during sorting. It could also reflect SSEA4+ cells that are on their path to become INS+ cells. SSEA4 is an established marker for multipotent progenitors in human fetal liver and in neural progenitor cells.23,24
We hypothesize that a subpopulation of the SSEA4 cells in the human pancreas has arrested multipotent stem cells, which in conditions of high-glucose media and B27 can be induced to become pancreatic hormone-expressing cells. Confirmation of these experiments would require that SSEA4+ and SSEA4− (control) cells be clonally analyzed for self-renewal and differentiation to pancreatic cells. The genetic profiles of expanded clonally derived SSEA4+ cells need a more complete analysis for markers related to self-renewal, ductal, acinar, and pancreatic endoderm. We have not examined what other marker SSEA4+ nonductal cells express.
A current belief is that the pancreatic duct and acinar cells may serve as a source of potential new cells in the pancreas by undergoing a process of dedifferentiation and redifferentiation.14,32 We hypothesize that SSEA4+ cells could directly differentiate to insulin cells under circumstances that promote tissue regeneration in the pancreas. Our observation that SSEA4+, but not SSEA4−, cells expressed Ngn3, although low levels, in vitro, supports this hypothesis (Fig. 7). In addition, Xu et al. recently showed that INS+ cells appeared from NGN3+ cells in the mouse postnatal pancreas9 after an injury.
Maritxell et al. demonstrated that centroacinar/terminal ductal progenitors that express ALDH1a1 might contribute to the maintenance of tissue homeostasis in the adult mouse pancreas.13 We examined whether SSEA4+ and SSEA4− cells express aldh1a1 and found that although they both do expressed it, aldh1a1 expression was higher in SSEA4+ then SSEA4− cells in some pancreatic tissue preparations (data not shown).
Seaberg et al. and later Smukler et al. have demonstrated the existence of PMP (pancreas-derived multipotent precursor) cells in the mouse and human pancreas.7,16 PMPs expressed low levels of insulin and Glut2, propagated in vitro, and differentiated to neuronal and pancreatic cells. It is possible that the detected proliferating cells are early derivatives of SSEA4+ cells that had lost SSEA4 expression. We have demonstrated that rare SSEA4+ cells co-localize with insulin, but the majority of them co-localize with NGN3. We hypothesize that once SSEA4 cells transition to insulin-expressing cells, they lose SSEA4 expression. Another interesting observation was that although SSEA4 cells were located throughout the adult exocrine pancreas, some of them were found around the islet (Fig. 1B, C). Smukler et al. reported that the INS+ PMPs were also often observed around the islets.16
The spheres in our cultures could potentially be the result of cell-to-cell aggregation and expansion rather than clonal single-cell expansion. Further clonal analysis will prove helpful in resolving this problem.
This is the first description of SSEA4+ endocrine progenitor cells in the human fetal and adult pancreas. Marker identification for the putative pancreatic stem/progenitor cells is of great importance for their purification and the initiation of studies for the generation of β-cells in vitro and in vivo.
Supplementary Material
Acknowledgments
We are grateful to Dr. James F. Markmann and colleagues at the Pancreas Transplantation Program, Massachusetts General Hospital (Boston, MA), for providing human exocrine pancreatic tissue. Funds were provided by the Larry Hillblom Foundation, grant 2006/1An, to A.H.
Author Disclosure Statement
We declare no competing financial interests exist.
References
- 1.Matveyenko AV. Georgia S. Bhushan A, et al. Inconsistent formation and nonfunction of insulin-positive cells from pancreatic endoderm derived from human embryonic stem cells in athymic nude mice. Am J Physiol Endocrinol Metab. 2010;299:713–720. doi: 10.1152/ajpendo.00279.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner-Weir S. Toschi E. Inada A, et al. The pancreatic ductal epithelium serves as a potential pool of progenitors cells. Pediatr Diabetes. 2004;5:16–22. doi: 10.1111/j.1399-543X.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 3.Bonner-Weir S. Sharma A. Pancreatic stem cells. J Pathol. 2002;197:519–526. doi: 10.1002/path.1158. [DOI] [PubMed] [Google Scholar]
- 4.Inada A. Nienaber C. Katsuta H, et al. Carbonic anhydrase II-positive cells are progenitors for both endocrine and exocrine pancreas after birth. PNAS. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YQ. Kritzik M. Sarvetnick N. Identification and expansion of pancreatic stem/progenitor cells. J Cell Mol Med. 2005;9:331–344. doi: 10.1111/j.1582-4934.2005.tb00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YQ. Sarvetnick N. Development of cell markers for identification and expansion of islet progenitor cells. Diabetes Metab Res Rev. 2003;19:363–374. doi: 10.1002/dmrr.406. [DOI] [PubMed] [Google Scholar]
- 7.Seaberg RM. Smukler SR. Kieffer TJ, et al. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol. 2004;22:1115–1124. doi: 10.1038/nbt1004. [DOI] [PubMed] [Google Scholar]
- 8.Ramiya VK. Maraist M. Arfors KE, et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 9.Xu X. D'Hoker J. Stange G, et al. β-Cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q. Law AC. Rajagopal J, et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Bonner-Weir S. Taneja M. Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue. PNAS. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeyens L. Bouwens L. Can β-cell be derived from exocrine pancreas? Diabetes Obes Metab. 2008;4:170–178. doi: 10.1111/j.1463-1326.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- 13.Maritxell R. Sherri-Gae S. Andrew S, et al. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. PNAS. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippsett MA. Castellarin ML. Rosenberg L. Development of a novel in vitro model to study human acinar-to-duct-to-islet differentiation. Pancreas. 2007;34:452–457. doi: 10.1097/MPA.0b013e3180335c80. [DOI] [PubMed] [Google Scholar]
- 15.Dor Y. Brown J. Martinez OL, et al. Adult pancreas b-cells are formed by cell duplication rather then stem cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 16.Smukler SR. Arntfield ME. Razavi R, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;3:281–293. doi: 10.1016/j.stem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Oshima Y. Suzuki A. Kawashimo K, et al. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132:720–732. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Sugiyama T. Rodriguez RT. McLean GW, et al. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. PNAS. 2007;104:175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Immervoll H. Hoem D. Sakariassen PO, et al. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kania G. Corbeil D. Fuchs J, et al. Somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem-cell-derived progenitors. Stem Cells. 2005;23:791–804. doi: 10.1634/stemcells.2004-0232. [DOI] [PubMed] [Google Scholar]
- 21.Kannagi R. Cochran NA. Ishigami F, et al. Stage-specific embryonic antigen (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;12:2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson JK. Draper JS. Baillie HS, et al. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells. 2002;20:329–337. doi: 10.1634/stemcells.20-4-329. [DOI] [PubMed] [Google Scholar]
- 23.Dan YY. Riehle KJ. Lazaro C, et al. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. PNAS. 2006;103:9912–9917. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barraud P. Stott S. Mollqard K, et al. In vitro characterization of a human neural progenitor cell co-expressing SSEA4 and CD133. J Neurosci Res. 2007;85:250–259. doi: 10.1002/jnr.21116. [DOI] [PubMed] [Google Scholar]
- 25.Vaculik C. Schuster C. Bauer W, et al. Human dermis harbors distict mesenchymal stromal cell subsets. J Investig Dermatol. 2012;132:536–574. doi: 10.1038/jid.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland AM. Gonez L. Harrison LC. Progenitor cells in the adult pancreas. Diabetes Metab Res Rev. 2004;20:13–27. doi: 10.1002/dmrr.430. [DOI] [PubMed] [Google Scholar]
- 27.Afrikanova I. Yerba M. Simpkinson M, et al. Inhibition of Src-family kinase and focal adhesion kinase (FAK) activity promotes the expression of early endocrine specification factors required for the derivation of b-cells from human pluripotent stem cells. J Biol Chem. 2011;286:36042–36052. doi: 10.1074/jbc.M111.290825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopp JL. Dubois CL. Hao E, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Dev Stem Cells. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JC. Smith SB. Watada H, et al. Regulation of the pancreatic pro-endocrine gene neurogenin 3. Diabetes. 2001;50:928–936. doi: 10.2337/diabetes.50.5.928. [DOI] [PubMed] [Google Scholar]
- 30.Honneycutt KA. Roop DR. C-Myc and epidermal stem cells determination. J Dermatol. 2004;31:368–375. doi: 10.1111/j.1346-8138.2004.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 31.Meregalli M. Farini A. Belicchi M, et al. CD133 (+) cells isolated from various sources and their role in future clinical perspectives. Expert Opin Biol Ther. 2010;10:1521–1528. doi: 10.1517/14712598.2010.528386. [DOI] [PubMed] [Google Scholar]
- 32.Li WC. Rukstalis JM. Nishimura W, et al. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci. 2010;123:2792–2802. doi: 10.1242/jcs.065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.