Abstract
A ▵11-desaturase gene was cloned from the sex pheromone gland of the obliquebanded leafroller moth, Choristoneura rosaceana. The desaturase cDNA sequence spans 1300 nucleotides with an open reading frame encoding a 335 amino-acid protein, which has 81% identity to a Z/E11-desaturase of the redbanded leafroller moth, Argyrotaenia velutinana. A functional assay with a pYES2 yeast expression system demonstrated that the ▵11-desaturase exhibits unusual substrate and stereospecificities in producing a Z/E11 mixture (7:1) of only C14 acids. A metabolic Z9-desaturase also was cloned from fat body of this species, and proved to be in the class that produces more Z9-16:Acid than Z9-18:Acid.
Keywords: Z9-desaturase, Z/E11-desaturase, Choristoneura rosaceana, RT-PCR, RACE PCR, Functional assay, YEpOLEX, pYES2, Sex pheromone biosynthesis
Introduction
Many moth sex pheromones have been found to be produced by a unique combination of desaturation and chain-shortening or elongation reactions. The membrane-bound acyl-CoA desaturases found in the female sex-pheromone glands catalyze the introduction of double bonds into aliphatic chains of fatty acids with regio- and stereo-specificities that have not been found in other animals. Thus, the characterization of the genes for these unusual desaturases provides a database of information that is needed to define amino acid changes in the catalytic sites responsible for these diverse specificities.
The database of moth desaturases already includes ▵9, ▵10, ▵11, and ▵14 desaturases (Knipple et al., 1998; Rosenfield et al., 2001; Hao et al., 2002; Liu et al., 1999; 2002a; 2002b; Roelofs et al., 2002). These include a desaturase that makes only the E11-isomer in the light brown apple moth, Epiphyas postvittana, and a desaturase that produces a mixture of Z/E11-14:Acids in the redbanded leafroller moth, Agryrotaenia velutinana, and the European corn borer, Ostrinia nubilalis, as well as one in the European corn borer that produces a Z/E mixture of ▵14-16:Acids.
The regio- and stereo-specificity of desaturases that make a Z/E mixture specifically with a C14 acid is quite unusual, and so we have initiated research on several leafroller species that also produce one or both of these unsaturated C14 acids. In this paper, we report on the characterization of a desaturase in the obliquebanded leafroller moth (OBLR), Choristoneura rosaceana, that produces a Z/E pheromone mixture of ▵11 isomers from a C14 acid precursor.
Materials and Methods
Collection of Insect Tissue and Isolation of Poly (A)+ RNA
Female OBLR were obtained from a lab colony reared on a semisynthetic medium (Shorey, 1965) in a 16:8 h (L: D) at 25°C, 50% relative humidity. Fat bodies and pheromone glands were carefully dissected from 2-3-day old female moths and stored at −80°C. Poly A+ RNA (mRNA) was isolated and purified from fat bodies and pheromone glands by using a mRNA Isolation Kit (Ambion) according to the procedures recommended by the manufacturer.
Construction of cDNA Library
Using a GeneRacer™ Kit (Invitrogen), 1 µg of mRNA from different tissues was dephosphorylated with calf intestinal phosphatase and then decapped with tobacco acid pyrophosphatase. It was then ligated with GeneRacer™ RNA oligo and reverse-transcribed with GeneRacer™ Oligo dT Primer by reverse transcriptase. Two cDNA libraries, Cro-pheromone-gland cDNA library (Cro-PG-cDNA) and Cro fat-body cDNA library (Cro-FB-cDNA) were constructed. The abbreviation Cro uses the first letter of the species (Choristoneura) and first 2 letters of the species name (rosaceana).
Cloning of desaturase cDNAs from OBLR
Two degenerate primers, PR1 and PR2, were described previously (Liu et al., 1999; Liu et al., 2001, Hao et al., 2002 and Liu et al., 2002). These two primers were used to amplify the central region of the desaturase gene from the Cro-FB-cDNA. The PCR products were ligated to PCR2.1 TOPO vector for sequencing. Two fragments were obtained from this cDNA library: Cro-FB1-CR and Cro-FB2-CR. Two pairs of degenerate primers, PR3 plus PR4 and PR5 plus PR6 (Table 1) were designed to amplify the central region of desaturase gene from Cro-PG-cDNA. One fragment (Cro-PG-CR) was amplified from Cro-PG-cDNA. The central regions were compared with other central regions of known desaturase genes to evaluate the possibility that they were desaturase genes.
Table 1.
Primers used for desaturase gene characterization

Based on the sequence information of Cro-FB1-CR and Cro-FB2-CR, gene-specific primers (Table 1) were designed for rapid amplification of cDNA ends (RACE). With Cro-FB-cDNA as template, A1 and GeneRacer 3′-P were used for the first-round PCR and A2 plus GeneRacer 3′-NP were used for the second-round PCR to amplify the 3′-end; A3 and GeneRacer 5′-P were used for the first-round PCR and A4 and GeneRacer 5′-NP were used for the second-round PCR to amplify the 5′-end. With the same cDNA library, B1 and GeneRacer 3′-P were used for the first-round PCR and B2 plus GeneRacer 3′-NP were used for the second-round PCR to amplify the 3′-end; B3 and GeneRacer 5′-P were used for the first-round PCR and B4 plus GeneRacer 5′-NP were used for the second-round PCR to amplify the 5′-end. Similarly, with Cro-PG-cDNA as template, C1 and C2 were used for the 3′-RACE PCR and C3 and C4 were used for 5′-RACE PCR. All the RACE PCR products were cloned to PCR2.1 TOPO vector for sequencing. With the sequencing results of the 5′-end, central region and 3′-end, two full-length cDNA sequences (Cro-FB1 and Cro-FB2) were generated from the Cro-FB-cDNA library and one full-length sequence (Cro-PG) was obtained from Cro-PG-cDNA library.
Functional Assay in YEpOLEX System
Gene-specific primers, A5 plus A6, B5 plus B6 and C5 plus C6 (Table 1) were designed to amplify the open reading frames (ORF) of the Cro-FB1, Cro-FB2 and Cro-PG genes. The PCR products of ORFs were digested and ligated with linearized YEpOLEX, as described previously (Knipple et al., 1998; Liu et al., 1999; Liu et al., 2001, Hao et al., 2002 and Liu et al., 2002a, 2002b). The consensus clone of the recombinant plasmids (YEpOLEX-Cro-FB1, YEpOLEX-Cro-FB2 and YEpOLEX-Cro-PG) were obtained and transformed to mutant yeast cells (strain L8-14C) (Stukey et al., 1990) for functional expression. The yeast cells were inoculated into 50 ml YPD medium and grown at 30°C overnight with shaking (300 rpm). If the cells did not grow overnight, then either Z11-18:Acid (0.5 mM) or Z9-14:Acid (0.5 mM) was added to the medium to promote growth. The induced cells were transferred to a 50-ml sterile centrifuge tube and spun at 1,500 × g for 5 min. The cell pellet was washed two times with 0.2% BSA, transferred to a 1.5-ml tube, and spun briefly to remove as much liquid as possible. The washed yeast cells were lysed with 1 ml of Y-PER (Yeast Protein Extraction Reagent, PIERCE) with brief vortex and 20 min agitation at room temperature. The cell debris was collected by centrifugation at 13,000 × g for 10 min and extracted with 0.5 ml of chloroform/methanol (2:1) at room temperature for 1 hr. The solvent was decanted from the debris and evaporated under nitrogen. The oily residue was extracted twice with 0.5 ml 10% boron trichloride/methanol, and the combined extracts heated at 100°C for 30 min. The resulting fatty acid methyl esters were extracted with 1 ml hexane and the solution concentrated under nitrogen for analyses by GC/MS, using a Hewlett Packard 5890 gas chromatograph (splitless mode) coupled to an HP 5970 B Mass Selective Detector (DB-1MS capillary column, 30 × 0.25 mm ID, 0.25 mm film thickness, J & W Scientific, Folsum, CA). The oven temperature was held at 100°C for 2 min., raised at 10°C/min to 200°C, held for 10 min, and raised at 3°C/min to 300°C. The double bond position in the products was confirmed by mass spectral analysis of the DMDS adducts (Buser et al., 1983) under the same conditions as for the methyl esters.
Functional Assay in pYES2 System
Since Cro-FB2 was not expressed, and Cro-PG not expressed well, in the YEpOLEX system, a pYES2 expression system (Invitrogen) (Liu et al., 2002a; 2002b) was used for the functional assay. Gene-specific primers, B7 plus B8, C7 plus C8 (Table 1) were designed to amplify the ORFs of Cro-FB2 and Cro-PG for pYES2 vector construction. The pYES2 plasmid containing consensus ORF was transformed to Saccharomyces cerevisiae strain elo1 competent cells (Toke and Martin, 1996) with the methods described previously (Liu et al., 2002a; 2002b). A single elo1 colony transformed with pYES2-Cro-FB2 or pYES2-Cro-PG, which grew on an SC-U plate (0.67% YNB, 0.19% SC-U amino-acid mixture, 2% glucose and 2% agar) (Bio 101), was inoculated into 30 ml SC-U liquid medium (0.67% YNB, 0.19% SC-U amino-acid mixture, 2% glucose) in a 250-ml flask. The yeast cells were grown at 30°C overnight with shaking (300 rpm). After 16–24 hrs, the cells were collected by spinning at 500 × g for 5 min. After discarding the supernatant, the cell pellet was washed one time with induction medium (0.67% YNB, 0.19% SC-U amino-acid mixture, 1% raffinose and 1% galactose) and suspended with induction medium containing 1% tergitol with a cell density of 5 × 107/ml. Thirty ml of the cell suspension was added to each 250-ml flask with or without addition of 0.5 mM myristic acid methyl ester (MAME) for desaturase induction at 18°C with shaking (300 rpm) for three days. The induced cells were harvested and extracted with 2:1 chloroform/methanol for GC/MS analysis with the same methods described above.
Results
Cloning and functional assay of Cro-FB1 [Cro-Z9(16)]
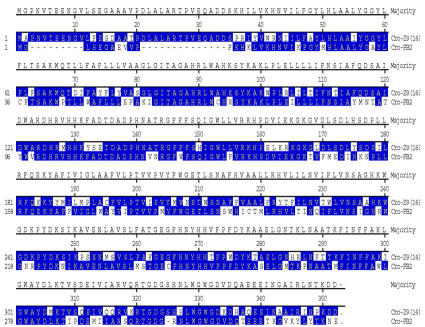
RT-PCR including RACE PCR with Cro-FB-cDNA library produced a full-length cDNA sequence (Cro-FB1) that spans 2171 nt encoding a protein with 352 amino acids (aa), The deduced aa sequence has high identity to other known moth Z9-desaturases from Tricoplusia ni, Epiphyas postvittana, Argyrotaenia velutinana, Heliothis zea, and Planotortrix octo (Liu et al., 1999; 2002a; 2002b; Rosenfield et al., 2001; and Hao et al., 2002) (Fig. 1).
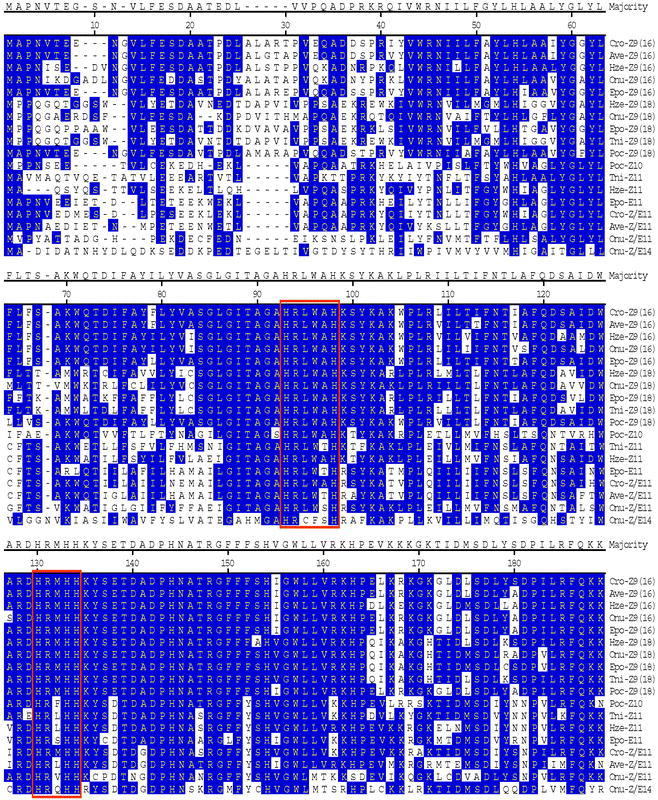
(Figure 1 continued)
Mutant ole1 yeast cells (L8-14C) were successfully used for the functional assay of Cro-FB1. After transformation with YEpOLEX-Cro-FB1 the yeast cells grew well in YPD medium without addition of UFAs. GC/MS analysis of the unsaturated fatty acid methyl esters generated by transmethylation of acyl compounds extracted from the yeast cells showed that this desaturase produced Z9-16:Acid and Z9-18:Acid in a 2:1 ratio (Fig. 2). It was labeled Cro-Z9(16) to differentiate it from the Z9-desaturases that produce 18:Acid>16:Acid.
Figure 2.
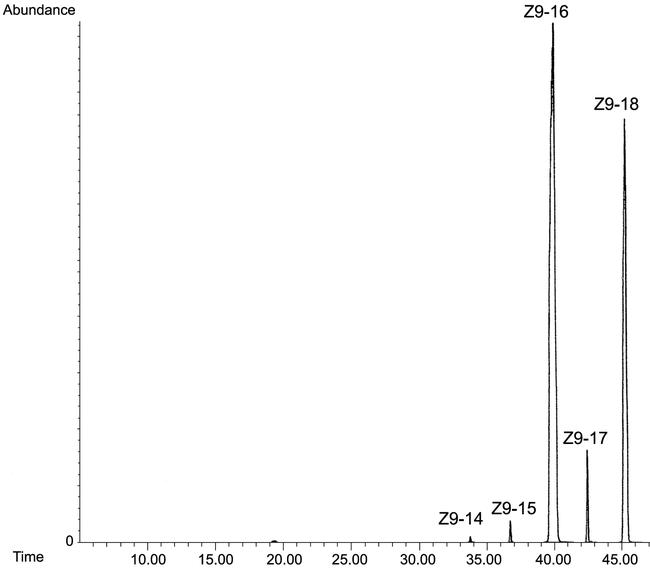
SIM (ion 217 m/z) GC/MS analysis of Z9-unsaturated fatty acid methyl esters (DMDS adducts) from L8-14C yeast cells complemented with YEpOLEX-Cro-FB1 plasmid. Desaturase is labeled Cro-Z9(16) because it produces Z9-16:Acid>Z9-18:Acid.
Cloning and functional assay of Cro-FB2
RT-PCR including RACE PCR with Cro-FB-cDNA library produced a full-length cDNA sequence (Cro-FB2) that spans 1142 bp encoding a protein with 328 amino acids (aa). The deduced aa (Fig. 3) has low identity to other known moth Z9-desaturases (44% to Cro-FB1, 41% to T. ni, 43% to A. velutinana, 46% to H. zea and 45% to P. oct) and ▵11-desaturases (48% to T. ni, 53% to H. zea, 49% to A. velutinana, and 49% to E. postvittana. This deduced aa sequence has 51% identity to that of Cro-PG.
Figure 3.
Comparison of deduced amino-acid-sequences for Cro-Z9(16) and Cro-FB2. No product was found to be produced by the latter purported desaturase.
Yeast cells L8-14C transformed with YEpOLEX-Cro-FB2 were not able to grow without addition of UFAs. With addition of Z9-14, the transformants grew in YPD, but the GC/MS assay did not reveal any new products. Yeast cells elo1 transformed with pYES2-Cro-FB2 also did not yield any new fatty-acid products. Attempts to obtain any unsaturated product from this clone with various saturated and mono-unsaturated precursors, such as 14:Acid, E9-14:Acid, Z11-14:Acid, E11-14:Acid, in the YEpOLEX and the pYES2 expression systems all failed.
Cloning and functional assay of Cro-PG (Cro-Z/E11)
Cro-PG was amplified from Cro-PG-cDNA library and the full-length cDNA sequence spans 1300 nt and contains an ORF encoding a 335-aa protein. This deduced aa has high identity to other Z11-, Z/E11- and E11-desaturases (57% to T. ni; 66% to H. zea; 81% to A. velutinana, 75% to E. postvittana; 62% to O. nubilalis) (Knipple et al., 1998; Rosenfield et al., 2001; Liu et al., 2002a; 2002b; Roelofs et al., 2002) and low identity (ca. 48%) to Z9-desaturases (Fig. 1).
The ole1 cells transformed with YEpOLEX-Cro-PG did not grow in YPD medium, and with the addition of UFAs, a trace amount of Z/E11-14:Acids was detected from the YEpOLEX system (data not shown). With the pYES2 system, GC/MS analysis of the unsaturated fatty acid methyl esters generated by transmethylation of acyl compounds extracted from yeast cells elo1 transformed with pYES2-Cro-PG showed that Cro-PG desaturase produced both Z11-14:Acid and E11-14:Acid with a ratio of 7:1 when MAME was added into the induction medium. Control elo1 cells with pYES2 plasmid only produced normal Z9-UFAs, but no Z/E11: acids were produced under the same conditions (Fig. 4). The elo1 cells are deficient in the chain-elongation enzyme and so the products were not elongated to Z/E13-16:Acids as in normal yeast cells.
Figure 4.
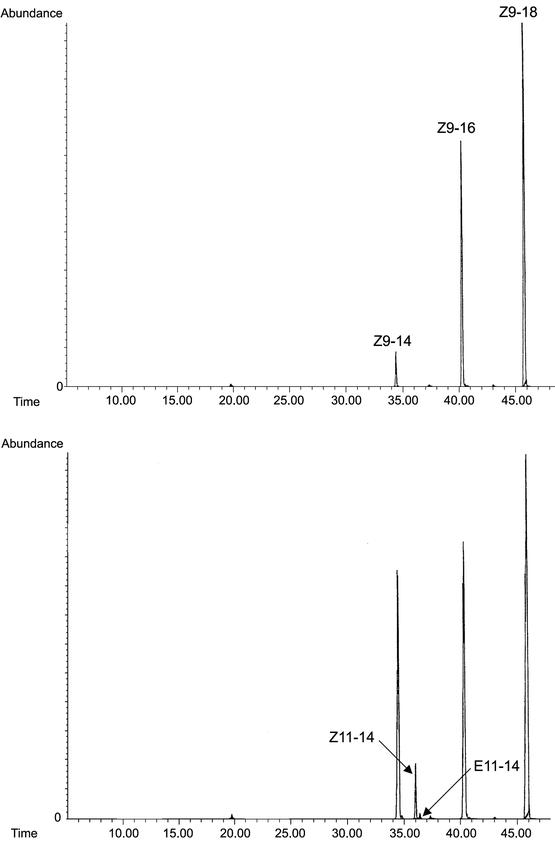
SIM GC/MS analysis of methyl esters (DMDS adducts) for ions 217 (▵9) and 245 (▵11) m/z. Top: control elo1 yeast cells transformed with pYES2 only and incubated with MAME exhibit normal Z9-unsaturated acids. Bottom: Elo1 yeast cells transformed with pYES-Cro-PG and incubated with MAME produced Z/E11-14:Acids as new products.
Discussion
cDNA libraries produced from mRNA isolated from fat-body tissue and pheromone-gland tissue of female OBLR moths yielded two desaturase clones from the fat body and one from the pheromone gland. One of the fat-body desaturases was found to be a Z9-desaturase that produces Z9-16:Acid more abundantly than Z9-18:Acid. No function could be found for the second clone (CRO-FB2) in two yeast expression systems with various precursors added. Although the clone has all the characteristics of a desaturase gene, it does not appear to produce an active enzyme. An NCBI BLAST search using the deduced aa sequence showed that it had high homology with desaturase genes, but not to any other class of enzymes. It could possibly be a pseudogene that is carried along in a species after a gene duplication event.
The desaturase clone from the pheromone gland was found to produce a mixture of Z/E11-14:Acids, similar to the desaturase characterized from the pheromone gland of the redbanded leafroller moth (Liu et al., 2002b). Although they exhibit the same unusual set of stereo- and regio-specificities, there are many amino acid differences in certain regions of the proteins (Fig. 1). Characterization of additional desaturases with similar specificities would be valuable in determining which set of changes are important in effecting a change from Z9 to Z11, or to E11, or to Z/E11, and changing from a substrate specificity of C16/C18 to one of only C14 (Fig. 1).
The Z/E11-desaturase characterized from OBLR described here is sufficient to produce all pheromone precursor acids required for the pheromone blend of this species. The pheromone components have been identified as (Z)-11-tetradecenyl acetate, (E)-11-tetradec enyl acetate, (Z)-11-tetradecen-1-ol, and Z)-11-tetradecenal (Hill and Roelofs, 1989; Vakenti et al., 1988). All four compounds can be produced by reduction of one of the products (Z11- and E11-14:Acid) from this desaturase.
GenBank Accession Numbers
AF518017 for Cro-Z9(16)
AF518018 for Cro-FB2
AF545481 for Cro-Z/E11
Figure 1.
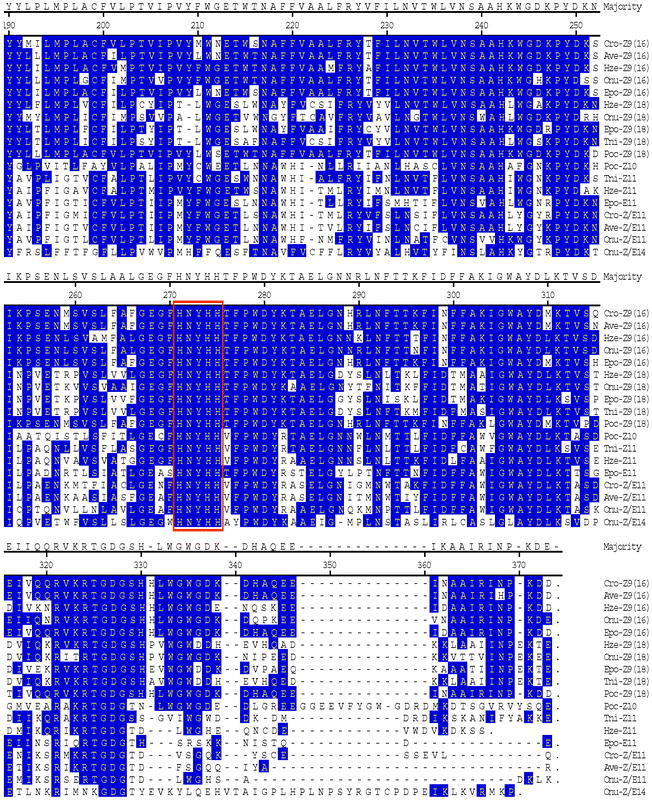
Comparison of deduced amino-acid-sequences for insect desaturases from Argyrotaenia velutinana (Ave), Choristoneura rosaceana (Cro), Epiphyas postvittana (Epo), Heliothis zea (Hze), Ostrinia nubilalis (Onu), Planotortrix octo (Poc), and Tricoplusia ni (Tni). The Z9 desaturases are classified as Z9(16) or Z9(18) to define whether they produce more Z9-16:Acid or more Z9-18:Acid. The three histidine domains are boxed in red.
Acknowledgments
We thank Kathy Poole and Callie Musto for rearing and supplying the female OBLR pupae, and Charles Martin for the gift of the ole1 and elo1 yeast strains. This work was supported by the National Science Foundation (NSF #1BN-9870669).
Glossary
| Abbreviation: | |
|---|---|
| aa | amino acid |
| Cro | initial letters from C. rosaceana |
| DMDS | dimethyl disulfide |
| MAME | myristic acid methyl ester |
| OBLR | oblique banded leaf roller |
| ORF | open reading frame. |
| nt | nucleotide |
| RACE | rapid amplification of cDNA ends |
| SC-U | amino acid mixture without uracil |
| UFA | unsaturated fatty acid |
| YPD | yeast extract/peptone/dextrose |
| YNB | Yeast Nitrogen Base |
References
- Buser HR, Arn H, Guerin P, Rauscher S. Determination of double bond position in mono unsaturated acetates by mass spectrometry of dimethyl disulfide adducts. Analytical Chemistry. 1983;55:818–822. [Google Scholar]
- Hao G, Liu W, O'Connor M, Roelofs WL. Acyl-CoA Z9 and Z10-desaturase genes from a New Zealand leafroller moth species, Planotortrix octo. Insect Biochemistry and Molecular Biology. 2002;32:961–966. doi: 10.1016/s0965-1748(01)00176-x. [DOI] [PubMed] [Google Scholar]
- Knipple DC, Miller SJ, Rosenfield CL, Liu W, Tang J, Ma PWK, Roelofs WL. Cloning and characterization of a cDNA encoding a pheromone gland-specific acyl-CoA ▵11-desaturase of the cabbage looper moth, Trichoplusia ni. Proceedings of the National Academy of Sciences USA. 1998;95:15287–15292. doi: 10.1073/pnas.95.26.15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Roelofs WL. Sex pheromone components of the obliquebanded leafroller moth, Choristoneura rosaceana. Journal of Chemical Ecology. 1989;5:3–11. [Google Scholar]
- Liu W, Ma PWK, Marsella-Herrick P, Rosenfield CL, Knipple DC, Roelofs WL. Cloning and functional expression of a cDNA encoding a metabolic acyl-CoA ▵9-desaturase of the cabbage looper moth, Trichoplusia ni. Insect Biochemistry and Molecular Biology. 1999;29:435–443. doi: 10.1016/s0965-1748(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Liu W, Jiao H, Murray NC, O'Connor M, Roelofs WL. Gene characterized for membrane desaturase that produces (E)-11 isomers of mono-and diunsaturated fatty acids. Proceedings of the National Academy of Sciences USA. 2002a;99:620–624. doi: 10.1073/pnas.221601498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Jiao H, O'Connor M, Roelofs WL. Moth desaturase characterized that produces both Z and E isomers of ▵11-tetradecenoic acids. Insect Biochemistry and Molecular Biology. 2002b;32:1489–1495. doi: 10.1016/s0965-1748(02)00069-3. [DOI] [PubMed] [Google Scholar]
- Roelofs WL, Liu W, Hao G, Jiao H, Rooney AP, Linn CE Jr. Evolution of moth sex pheromones via ancestral genes. Proceedings of the National Academy of Sciences. 2002;99:13621–13626. doi: 10.1073/pnas.152445399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield CL, You KM, Marsella-Herrick P, Roelofs WL, Knipple DC. Structural and functional conservation and divergence among acyl-CoA desaturases of two noctuid species, the corn earworm, Helicoverpa zea and the cabbage looper, Trichoplusia ni. Insect Biochemistry and Molecular Biology. 2001;31:949–964. doi: 10.1016/s0965-1748(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Shorey HH, Hale RL. Mass-rearing of the larvae of nine noctuid species on a simple artificial medium. Journal of Economic Entomology. 1965;58:522–524. [Google Scholar]
- Stukey JE, McDonough VM, Martin CE. The OLE1 gene of Saccharomyces cerevisiae encodes the delta-9 fatty acid desaturase and can be functionally replaced by the fat stearoyl coenzyme A desaturase gene. Journal of Biological Chemistry. 1990;265:20144–20149. [PubMed] [Google Scholar]
- Toke DA, Martin CE. Isolation and characterization of a gene affecting fatty acid elongation in Saccharomyces cerevisiae. Journal of Biological Chemistry. 1996;271:18413–18422. doi: 10.1074/jbc.271.31.18413. [DOI] [PubMed] [Google Scholar]
- Vakenti JM, Gaunce AP, Slessor KN, King GGS, Allan SA, Madsen HF, Borden JH. Sex pheromone components of the oblique-banded leafroller, Choristoneura rosaceana in the Okanagan Valley of British Columbia. Journal of Chemical Ecology. 1988;14:605–621. doi: 10.1007/BF01013910. [DOI] [PubMed] [Google Scholar]