Abstract
Recent studies have suggested that autophagy is a key mechanism in maintaining the integrity of podocytes. The mammalian homologue of yeast vacuolar protein sorting defective 34 (mVps34) has been implicated in the regulation of autophagy, but its role in podocytes is unknown. We generated a line of podocyte-specific mVps34-knockout (mVps34pdKO) mice, which were born at Mendelian ratios. These mice appeared grossly normal at 2 weeks of age but exhibited growth retardation and were significantly smaller than control mice by 6 weeks of age, with no difference in ratios of kidney to body weight. mVps34pdKO mice developed significant proteinuria by 3 weeks of age, developed severe kidney lesions by 5–6 weeks of age, and died before 9 weeks of age. There was striking podocyte vacuolization and proteinaceous casts, with marked glomerulosclerosis and interstitial fibrosis by 6 weeks of age. Electron microscopy revealed numerous enlarged vacuoles and increased autophagosomes in the podocytes, with complete foot process effacement and irregular and thickened glomerular basement membranes. Immunoblotting of isolated glomerular lysates revealed markedly elevated markers specific for lysosomes (LAMP1 and LAMP2) and autophagosomes (LC3-II/I). Immunofluorescence staining confirmed that the enlarged vacuoles originated from lysosomes. In conclusion, these results demonstrate an indispensable role for mVps34 in the trafficking of intracellular vesicles to protect the normal cellular metabolism, structure, and function of podocytes.
The podocyte plays an essential role in establishment of the size- and charge-selective permeability of the glomerular filtration barrier and in maintenance of the glomerular structural integrity. Although alterations in structural proteins of the podocyte are now recognized to contribute to kidney disease, much of the podocyte’s functions remain incompletely understood.1,2
Autophagy is a tightly regulated intracellular process in which portions of cytoplasm, including proteins and organelles, are sequestered within double-membrane vesicles termed autophagosomes and are delivered to lysosomes for degradation and recycling of cellular components.3,4 Mammalian cells are postulated to use autophagy as a mechanism for turnover of long-lived proteins and removal of protein aggregates and damaged organelles, and as a survival strategy under metabolic stress, including conditions of nutrient deprivation.3,5 Recent studies suggest that autophagy is a key mechanism maintaining the homeostasis and integrity of podocytes.6–9
The vacuolar protein sorting defective 34 (Vps34) was originally cloned from yeast and found to be essential for the sorting of hydrolases to the yeast vacuole.10 It was subsequently identified as the only phosphatidylinositol 3-kinase (PI3K) in yeast.11 The mammalian homologue of yeast Vps34 (mVps34) is also known as class III PI3K. Unlike the class I and class II PI3Ks, the class III PI3K, mVps34, can use only phosphatidylinositol as a substrate to generate a single product, phosphatidylinositol-3-phosphate, by specifically phosphorylating the D-3 position on the inositol ring of phosphatidylinositol.11,12 Of interest, mVps34 has been implicated in the regulation of autophagy,13–15 but the role of mVps34 in podocytes has not previously been explored.
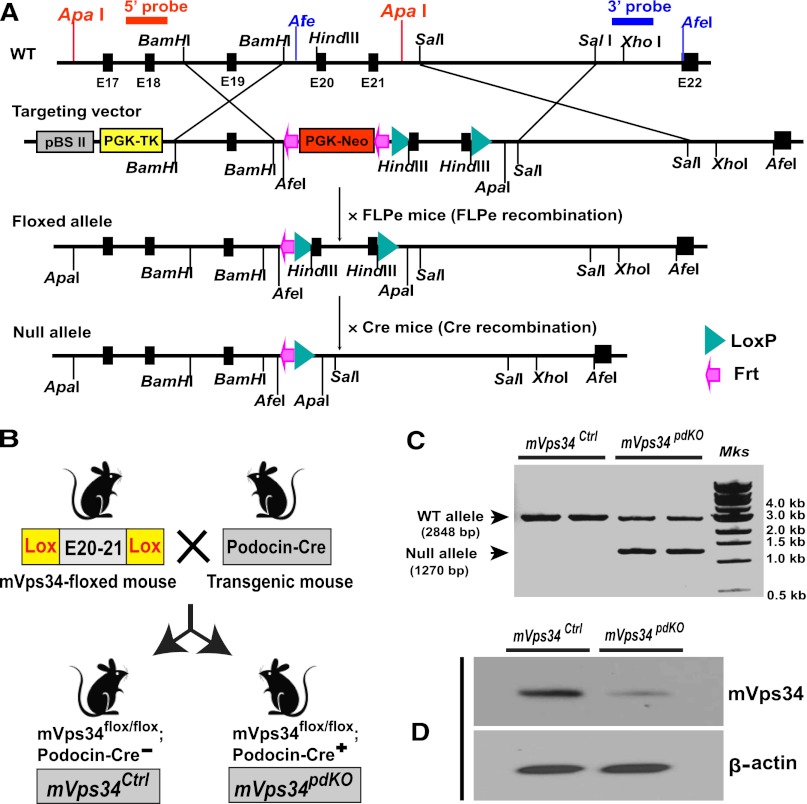
To determine the potential roles of mVps34 in podocytes, we inactivated the mouse mVps34 gene, Pik3c3, by creating a Pik3c3/mVps34-floxed mouse (mVps34flox/flox). Figure 1A depicts the structure of the mVps34 gene–targeting vector and the Cre-LoxP strategy for generating a conditional mVps34 knockout mouse. The LoxP sites were inserted to delete Pik3c3 exons 20–21, which code for the entire catalytic core and the key AsparDic acid-Fenylalanine-Glycine (DFG) motif,16 and the targeting vector was designed to render all the distal exons out of reading frame so the catalytic domain and the ATP binding domain of this kinase were deleted. As shown in Figure 1B, to delete mVps34 in podocytes, we crossed our mVps34flox/flox mice with a podocin-Cre mouse (Pod-Cre), which expresses Cre-recombinase exclusively in podocytes starting from the capillary loop stage during glomerular development,17 and generated Pik3c3/mVps34flox/flox;Pod-Cre(+) mice (subsequently called mVps34pdKO), which were compared with their sex-matched littermates with a genotype of Pik3c3/mVps34flox/flox;podocin-Cre(−) as control mice (hereafter called mVps34Ctrl). PCR of the genomic DNA from isolated glomeruli verified expected homologous recombination of mVps34 gene by podocin-Cre in mVps34pdKO, but not in mVps34Ctrl (Figure 1C). Immunoblotting confirmed significant deletion of mVps34 protein in the glomeruli of mVps34pdKO mice (Figure 1D).
Figure 1.
Generation of an mVps34 gene (Pik3c3)-floxed mouse and deletion of mVps34 selectively in podocytes. (A) Strategy for making a conditional mVps34 knockout mouse. (B) Generation of renal glomerular podocyte-specific mVps34 knockout (mVps34pdKO) mice. (C) Podocin-Cre–mediated homologous recombination of mVps34 gene verified by PCR using isolated glomerular genomic DNA as template. (D) Effective deletion of mVps34 protein by podocin-Cre recombinase confirmed by immunoblotting analysis of isolated glomerular homogenates. FLPe, enhanced FLP; WT, wild type.
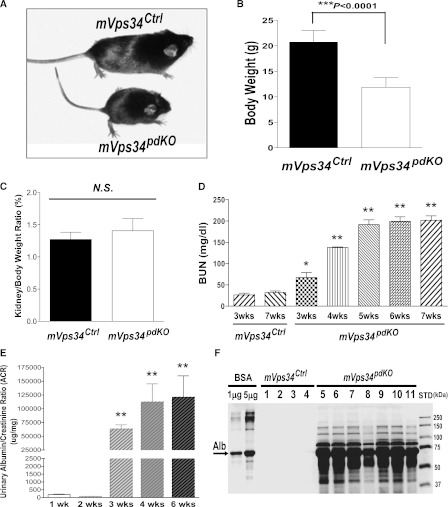
Homozygous mVps34pdKO pups were born at expected Mendelian ratios (data not shown) and were indistinguishable from their mVps34Ctrl littermates at birth. No apparent phenotype was seen by 2 weeks of age. However, mVps34pdKO mice exhibited growth retardation and were significantly smaller by 6 weeks of age (Figure 2A), with a lower body weight (Figure 2B); however, there was no difference in the ratio of kidney to body weight (Figure 2C). The BUN levels of mVps34pdKO mice were statistically higher by 3 weeks of age, and all mVps34pdKO mice developed renal failure (Figure 2D) and died before 9 weeks of age. Furthermore, mVps34pdKO mice developed proteinuria by 3 weeks of age (Figure 2E). SDS-PAGE assays revealed that albumin is the major protein species in the urine, although other plasma proteins also contributed to proteinuria (Figure 2F).
Figure 2.
Deletion of mVps34 in podocytes causing significant phenotypes after 2 weeks of age. (A–C) mVps34pdKO mice exhibited growth retardation and were significantly smaller by 6 weeks of age (A), compared with same-sex mVps34Ctrl littermates; body weight was significantly decreased (B) but ratio of kidney to body weight was similar (C). NS, not significant. (D) By 3 weeks of age, mVps34pdKO mice developed statistically higher BUN levels (knockout, 67.29±11.83 mg/dl; control, 31.80±3.91 mg/dl), which continued to increase further; values are means ± SEM (n=4 for each time point group; *P<0.05 and **P<0.0001 versus mVps34Ctrl groups at 3–7 weeks of age; no significant difference was seen among the different age groups between 3-week-old and 7-week-old mVps34Ctrl mice). (E and F) Deletion of mVps34 specifically in podocytes caused massive proteinuria by 3 weeks of age. (E) Urinary protein-to-creatinine ratios were significantly increased by 3 weeks of age in mVps34pdKO mice (values are means ± SEM [n=4 for each group; **P<0.0001 versus 1- or 2-weeks-of-age groups]). (F) SDS-PAGE assay (with Coomassie blue staining) confirmed that albumin is the major protein species in the urine, although other plasma proteins also contributed to the proteinuria. One microliter of urine from an individual 6-week-old mouse in each genotype group was loaded into each lane of the SDS-PAGE.
mVps34pdKO mice showed normal glomeruli at 9 days of age, similar to mVps34Ctrl littermates (Figure 3A), suggesting that mVps34 is not essential for early podocyte development. However, mVps34pdKO mice developed severe kidney lesions by 5–6 weeks of age. Their glomerular podocytes showed striking vacuolization (Figure 3, B–D), with focal segmental (Figure 3, E and F) and global (Figure 3G) glomerulosclerosis. mVps34pdKO mice also had renal tubular dilation, numerous proteinaceous casts, and mild to moderate interstitial inflammation and fibrosis (Figure 3, B and C), which were confirmed by Masson trichrome staining (Figure 4A). Immunohistochemistry revealed massive fibronectin deposition along the Bowman capsule but moderate increases in the glomeruli and some periglomerular areas (Figure 4B).
Figure 3.
Podocyte-specific loss of mVps34 causing severe glomerulosclerosis. mVps34pdKO mice had normal renal histologic findings at 4 days of age (data not shown) and 9 days of age (A) but developed severe kidney lesions by 6 weeks of age, including striking glomerular podocyte vacuolization (B–D), focal segmental (E and F) and global (G) glomerulosclerosis, renal tubular dilation, proteinaceous casts, and mild to moderate interstitial inflammation and fibrosis (B and C), as indicated by hematoxylin and eosin (H-E) (A, B, and D) and periodic acid-Schiff (PAS) staining (C, E, F, and G). Original magnification, ×100 in A, B, and Upper panel C; ×200 in lower panel C; ×400 in D–G.
Figure 4.
Confirmation of renal fibrosis using Masson trichrome staining and immunohistochemical staining for fibronectin. (A) Masson trichrome staining highlighted tubulointerstitial fibrosis and glomerulosclerosis in mVps34pdKO mice at 6 weeks of age. (B) Immunohistochemical staining with an antibody specific for fibronectin revealed massive fibronectin deposition along the Bowman capsule, with a moderate increase in the glomeruli and some periglomerular areas of mVps34pdKO mice, compared with mVps34Ctrl mice.
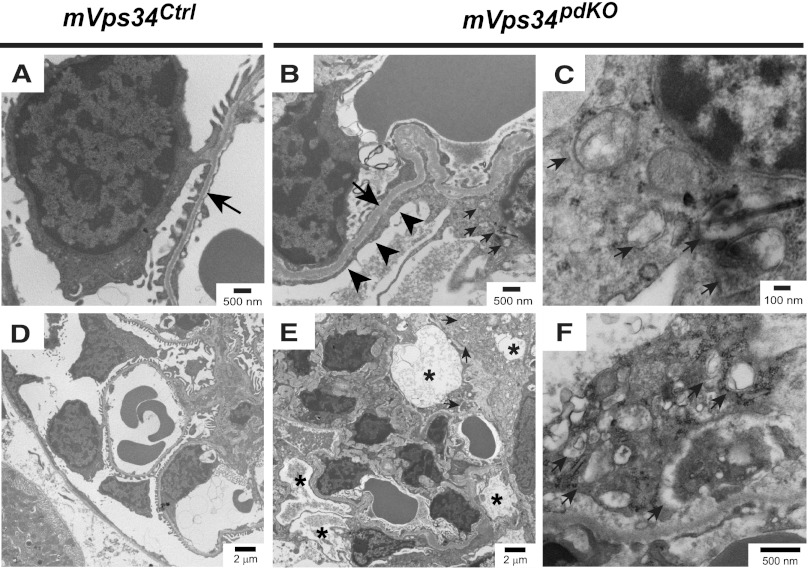
Electron microscopy revealed complete podocyte foot process effacement, with irregular and thickened glomerular basement membrane (Figure 5, A and B). The podocytes of mVps34pdKO mice contained numerous enlarged vacuoles (Figure 5, D and E) and increased number of double-membraned cytoplasmic vesicles characteristic of autophagosomes, which, however, had irregular inner membranes (Figure 5, C and F). Accumulation of these aberrant autophagosomes in mVps34pdKO mice is consistent with the fact that the enzymatic product of mVps34, phosphatidylinositol-3-phosphate, is enriched on the inner membranes of autophagosomes.18–20
Figure 5.
Micrographs of electron microscopy from mVps34Ctrl and mVps34pdKO glomeruli at 6 weeks of age. (A and B) A representative micrograph revealing complete podocyte foot process effacement (black arrowheads), with irregular and thickened glomerular basement membrane in mVps34pdKO mice compared with mVps34Ctrl littermates (black arrows). (D and E) Lower-magnification image reveals numerous enlarged vacuoles (indicated by black asterisks) in the podocytes of mVps34pdKO mice, but not in the podocytes of mVps34Ctrl littermates. (C and F) Higher-magnification image shows the accumulation of aberrant double-membraned autophagosomes with irregular inner membranes in the podocytes of mVps34pdKO mice (small arrows). Magnifications are indicated by the scale bars in the corresponding micrographs.
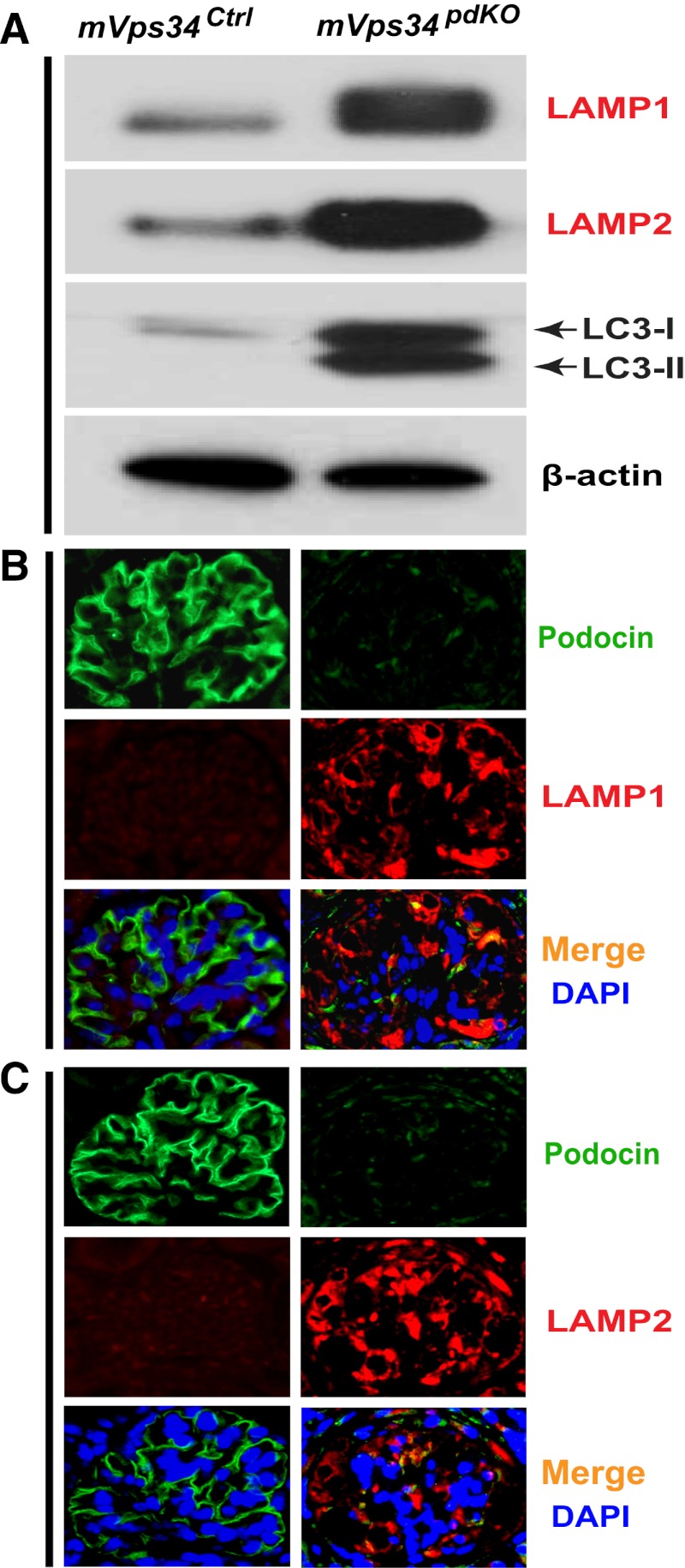
In an attempt to determine the origin of these enlarged vacuoles (Figure 5E), we determined that the lysosome-associated membrane proteins 1 and 2 (LAMP1 and LAMP2) were both markedly elevated (Figure 6A). Immunofluorescence staining revealed that the enlarged vacuoles were positive for both LAMP1 (Figure 6B) and LAMP2 (Figure 6C). In contrast, podocin levels were decreased markedly (Figure 6, B and C). Thus, the enlarged vacuoles may have originated from lysosomes. During autophagy, the cytosolic form of microtubule-associated protein 1 light chain 3 (LC3)-I forms LC3-phosphatidylethanolamine conjugate (LC3-II), which is recruited to autophagosomal membranes and plays a critical role in autophagy and thus has become a reliable marker for autophagosomes.21,22 We found LC3-II and even the cytosolic LC3-I were both markedly increased in the mVps34pdKO mice (Figure 6A).
Figure 6.
Increased protein levels of LAMP1 and LAMP2 localized to the membranes of the enlarged vacuoles in the podocytes of mVps34pdKO mice. (A) Immunoblotting of isolated glomerular homogenates revealed markedly increased protein levels of the lysosome markers, LAMP1 and LAMP2, as well as the autophagosome marker, LC3-II and the cytosolic LC3-I. (B and C) Immunofluorescence staining localized the increased protein levels of LAMP1 (B) and LAMP2 (C) to the enlarged vacuoles in the podocytes of mVps34pdKO mice; in contrast, podocin levels were markedly diminished compared with mVps34Ctrl mice (B and C). DAPI, 4′,6-diamidino-2-phenylindole. Original magnification, ×400 in B and C.
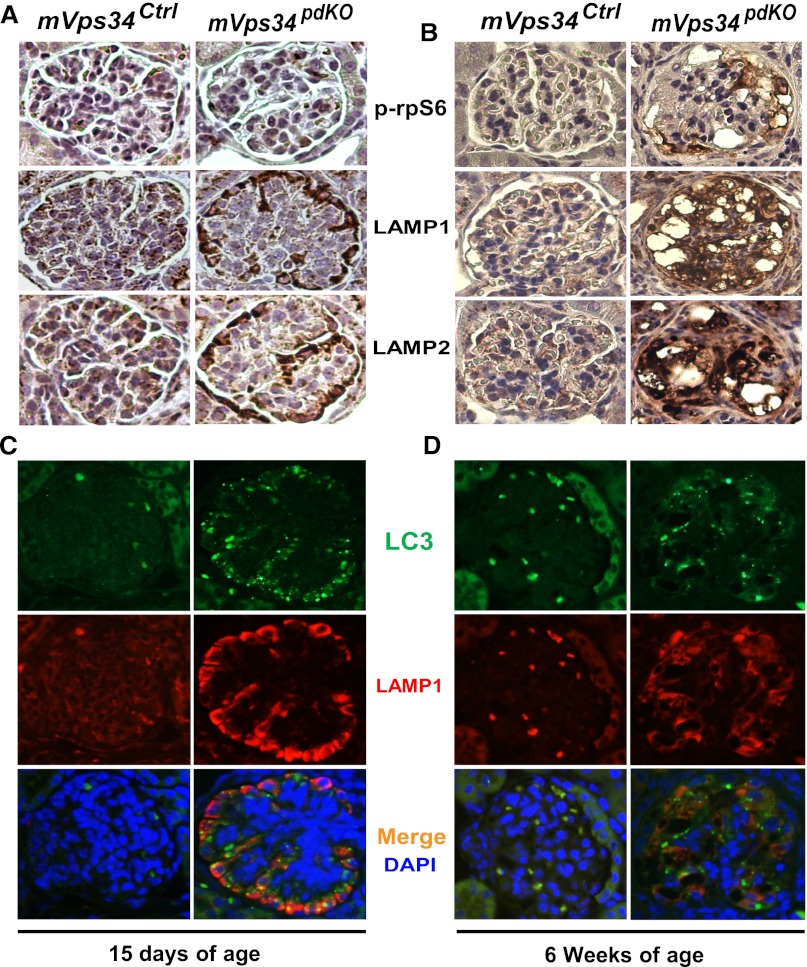
Recent studies indicate that inactivation of the mammalian target of rapamycin (mTOR) by either double knocking out Raptor (an essential protein for mTOR complex 1 activity) and Rictor (key for mTORC2 activity)23 or directly deleting the mTOR gene in podocytes24 caused a phenotype similar to what we have seen in our mVps34pdKO mice, including podocyte vacuolization, massive proteinuria, progressive glomerulosclerosis, and renal failure within 6 weeks after birth.23,24 Podocyte-specific mTOR deletion also caused an accumulation of autophagosomes and autolysosomes by disrupting autophagic flux in podocytes.24 Because mVps34 has been shown to lie upstream of the mTOR pathway,25,26 it is possible that mVps34 deletion in podocytes may result in the similar phenotype by inactivating the mTOR pathway. Surprisingly, when we examined the phosphorylation levels of the ribosomal protein S6 (rpS6), a downstream target of mTOR commonly used as an in vivo readout for mTOR activities, we found that the mTOR pathway was activated, rather than inactivated, along with increased levels of both LAMP1 and LAMP2 (Figure 7B). Proteinuria was not detected until 17 days of age (data not shown), but significant increases in both LAMP1 and LAMP2 had already occurred by 15 days of age, without increases in rpS6 phosphorylation (Figure 7A). Thus, mTOR activation, rather than inactivation, seen in 6-week-old mVps34pdKO mice (Figure 7B) might be secondary to proteinuria or dysregulated autophagy. However, our data in this study cannot rule out the possibility that mTOR inactivation by mVps34 knockout may be an early signaling event that leads to a phenotype similar to that seen in the podocyte-specific mTOR knockout mice.23,24 Future studies are required to clarify the precise interplay between mVps34 and mTOR.
Figure 7.
Deletion of mVps34 in podocytes increases the protein levels of LAMP1, LAMP2, and LC3 but does not activate the mTOR pathway before the onset of proteinuria. (A) Immunohistochemistry showed no apparent alterations in the phosphorylation levels of rpS6 in 15-day-old mVps34pdKO mice compared with their mVps34Ctrl littermates; however, these 15-day-old mVps34pdKO mice had increased levels of the lysosome-associated membrane proteins, LAMP1 and LAMP2. (B) The phosphorylation levels of rpS6 were increased selectively in the vacuolated podocytes of mVps34pdKO mice at 6 weeks of age when massive proteinuria had already developed (as shown in Figure 2, E and F) and glomerulosclerosis occurred (as shown in Figure 3, B–G). (C and D) Immunofluorescence staining revealed markedly elevated levels of both LC3-II/I and LAMP-1 in mVps34pdKO mice by 15 days of age before the onset of proteinuria and remained elevated at 6 weeks of age when detrimental podocyte vacuolization was observed and severe glomerulosclerosis occurred. DAPI, 4′,6-diamidino-2-phenylindole. Original magnification, ×400 in A–D.
Additional experiments with immunofluorescence staining revealed markedly increased levels of both LC3 and LAMP1 in mVps34pdKO mice at 15 days of age (Figure 7C) as well as 6 weeks of age (Figure 7D), consistent with the immunoblotting data shown in Figure 6A. As indicated in Figure 7C, some of the increased LC3-positive granules or punctate dots were co-localized with the increased LAMP1 (suggesting increases in autolysosomes), whereas others were not co-localized with LAMP1, indicating increases in autophagosomes. Double immunofluorescence staining confirmed that the increases in both LC3 (Figure 8A) and LAMP1 (Figure 8B) occurred only in nephrin-positive glomerular cells (the podocytes). Indeed, electron microscopy further confirmed that formation of numerous vacuoles (Figure 9, E and F) and an increase of autophagosomes (Figure 9, G and H) occurred only in the podocytes, with occasional focal foot process effacement (Figure 9, E and F), compared with mVps34Ctrl littermates (Figure 9, A–D). Thus, both marked elevations of LC3 and LAMP1/2 and the earliest ultrastructural alterations (podocyte vacuolization, focal foot process effacement, and increased autophagosome formation) had occurred before the onset of proteinuria.
Figure 8.
Podocyte-specific mVps34 knockout increases the protein levels of both LC3 and LAMP1, but not that of nephrin, by 15 days of age before the onset of proteinuria. (A) Triple immunofluorescence staining with the indicated antibodies verified that compared with their mVps34Ctrllittermates, mVps34pdKO mice showed markedly increased LC3 protein levels specifically in the nephrin-positive glomerular cells, which are podocytes. (B) Triple immunofluorescence staining also confirmed that increased LAMP1 protein levels were confined to the podocytes. In contrast, the nephrin protein level of Vps34pdKO mice was not increased, compared with that of mVps34Ctrl mice. DAPI, 49,6-diamidino-2-phenylindole. Original magnification, ×400.
Figure 9.
Lack of functional mVps34 in the glomerular podocyte disrupts the dynamics of intracellular vesicle forming and processing. Podocyte-specific mVps34 knockout resulted in striking ultrastructural podocyte vacuolization (E and F) and increased autophagosome formation (G–H) by 15 days of age before the onset of proteinuria, compared with mVps34Ctrl littermates (A–D). Focal foot process effacement was seen occasionally as indicated by a black arrowhead (F). Shown are representative micrographs of electron microscopy from three mice in each group with similar results. A scale bar is shown in each corresponding micrograph to indicate the magnification, with black asterisks representatively indicating enlarged vacuoles (F and H) and small white arrows representatively pointing to increased number of autophagosomes (F–H) in the podocytes of mVps34pdKO mice.
Of note, previous studies suggested that mVps34 plays an indispensable role at the initiation step of autophagosome formation,13–15,27 which would not be consistent with the increases in autophagosome formation (Figure 9) and a striking accumulation of autophagic vesicles in the enlarged autolysosomes of the mVps34-deleted podocytes (Figure 5, C and F). Thus, our results suggest the existence of an mVps34-independent mechanism that can initiate the formation of autophagosomes in the podocyte. Follow-up studies are required to define the precise roles of mVps34 and its interactions with many other autophagy-related genes (Atg). It is also important to determine whether endocytic pathway has also been disrupted and contributed to the enlarged vacuoles in the mVps34pdKO podocytes.
In summary, this study represents the first report of podocyte-specific mVps34 knockout. The major findings include markedly increased LC3-II/I and LAMP1/2 but decreased podocin levels, causing a lethal accumulation of aberrant autophagosomes and enlarged autolysosomes, resulting in striking podocyte vacuolization, foot process effacement, glomerulosclerosis, and interstitial fibrosis, consequently leading to massive proteinuria and renal failure. Thus, there is no compensation by related genes in the mVps34pdKO mice, which all died before 9 weeks of age. In contrast, podocyte-specific Atg5 knockout mouse caused age-dependent late-onset glomerulosclerosis.6–9 mVps34pdKO mice reported herein exhibited a much more pronounced and severe phenotype than did the Atg5 knockout mice, even when 6-week-old mVps34pdKO mice were compared with the 24-month-old Atg5pdKO mice.9 In future studies, it will be important to determine whether mVps34 inactivation contributes to the pathogenesis of proteinuria and glomerulosclerosis in some cases of glomerulosclerosis, such as FSGS and diabetic nephropathy.
Concise Methods
Reagents and Antibodies
Bacto peptone, Bacto yeast extract, and Bacto agar were obtained from Difco Laboratories (Detroit, MI). All restriction enzymes and T4 DNA ligase used in this study were purchased from New England Biolabs (Beverly, MA). Rabbit anti-Vps34 antibodies were purchased from Invitrogen (Carlsbad, CA). Antibodies against β-actin, phospho-rpS6, and LC3 were from Cell Signaling Technology (Beverly, MA). Antibodies to LAMP1, LAMP2, WT1, fibronectin, nephrin, and all secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against podocin and fibronectin, chloramphenicol, and other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Construction of the Targeting Vector
A bacterial artificial chromosome clone, SV129/195A12, containing the targeting regions of the HVPS34 genomic locus was used for the construction of the conditional targeting vector. A 1.5-kb HindIII-HindIII fragment containing exons 20 and 21 was first subcloned into pBluescript II (pBS II) plasmid vector. After identification of a clone with a correctly oriented HindIII-HindIII fragment, a short arm (2.2-kb) BamHI-BamHI containing exon 19 of mVps34 gene, Pik3c3, was then inserted and verified to be correctly oriented, followed by cloning of a long arm (5.1-kb) SalI-SalI fragment within the intron 21 of mVps34 into the vector. A first loxP site was inserted upstream of exon 20, and a second loxP site was inserted downstream of exon 21 in the same orientation as the first one. A Frt-flanked phosphoglycerol kinase-neomycin (PGK-Neo)–positive selection cassette was cloned upstream of the first loxP site, and the phosphoglycerol kinase-thymidine kinase (PGK-TK)–negative selection marker upstream and outside of the short homologue arm.
Generation of ES Cell Clones
The targeting vector was linearized with XhoI and electroporated into 129Sv mouse ES cells, which were selected with G418. G418-resistant ES clones were screened by Southern blotting analysis. Genomic DNA from the ES clones was digested with ApaI and AfeI, respectively, and Southern hybridization was performed using 5′- and 3′-external probes, correspondingly.
Generation of Chimeras and Breeding
After identification of positive clones that underwent correct homologous recombination, two independent positive ES clones were microinjected into C57BL/6J host blastocysts to generate chimeras. Ten chimeras were bred to inbred C57BL/6 mice, and F1 agouti offspring mice were genotyped by PCR and confirmed by Southern blotting analysis. Three chimeras were confirmed to have undergone germline transmission. The Frt-flanked PGK-Neo cassette was deleted by breeding the mVps34flox/+ offspring with the enhanced FLP (FLPe)-recombinase transgenic deleter mice from Jackson Laboratory.28 The Vanderbilt University Institutional Animal Use and Care Committee approved all experiments, and all experiments were conducted according to National Institutes of Health guidelines.
PCR Primers and Genotyping
Genomic DNA was isolated from mouse tail biopsy samples, and mice carrying wild-type and mVps34-floxed alleles were genotyped using PCR and confirmed by Southern blotting according to standard protocols. PCR primers used for genotyping are as follows: 5′-CTGGACGTAAACTCCTCTTCAGACC-3′ and 5′-CTAGCTTTCGGAGTCTCAGTGCAGC-3′. PCR reactions for genotyping were carried out under the following conditions: 94°C for 2 minutes, 30 cycles at 94°C, and 60°C each for 30 seconds, followed by an extension at 72°C for 1 minute, and a final extension at 72°C for 7 minute.
Generation of HVPS34podKO Mice
The F1 offspring carrying the mVps34-targeted allele (mVps34flox/WT) was bred to a high-efficiency deleter mouse carrying the FLPe recombinase transgene to remove the neomycin selection cassette, FRT-PGK-neo-FRT.28 We then deleted mVps34 selectively in glomerular podocytes using the Cre-Lox strategy. Specifically, to generate mVps34flox/flox;Pod-Cre(+) (mVps34pdKO), we bred the HVPS34-floxed, neo-cassette–removed mice to a transgenic mouse line carrying Cre recombinase under the control of the podocin promoter (Pod-Cre).17 Sex-matched mVps34flox/flox littermates lacking the pod-Cre transgene, mVps34flox/flox;Pod-Cre(-), were used as control mice (mVps34Ctrl).
Histologic Analysis and Immunohistochemistry
Immunohistochemistry was performed as we have previously described.29 Briefly, kidneys from both mVps34pdKO and wild-type control mice were assessed by light and electron microscopy. Kidneys were dissected and fixed in 4% paraformaldehyde. The fixed kidneys were dehydrated through a graded series of ethanol, embedded in paraffin, sectioned (5 µm), and mounted on glass slides. After deparaffinization and rehydration, sections were stained with periodic acid-Schiff or hematoxylin and eosin. For immunohistochemistry, antigen retrieval was performed using Antigen Unmasking Solution (Vector, Burlingame, CA) followed by blocking with 2.5% normal goat serum; the sections were incubated with primary antibodies against fibronectin overnight at 4°C. The sections were washed three times in PBS followed by immunostaining using VECTASTAIN ABC kits (Vector). The sections were counterstained with hematoxylin. Images were captured by using an AxioCam HRc digital camera (Carl Zeiss).
Immunofluorescence Analysis
Immunofluorescence staining was performed as previously described.29 Briefly, 5-μm kidney sections were deparaffinized, rehydrated, and subjected to antigen retrieval followed by blocking with 2.5% BSA in PBS; the sections were incubated with primary antibodies against WT1 (1:200), LAMP1 (1:200), LAMP2 (1:200), podocin (1:200), or nephrin (1:200) overnight at 4°C, and then incubated with Alexa Fluor 594 or 488-conjugated secondary antibodies for 1 hour. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Images were captured by using an Axioplan2 fluorescence microscope and AxioCam HRc digital camera (Carl Zeiss).
Isolation of Glomeruli
Mouse glomeruli are isolated as previously described,30 with some modifications. Briefly, renal cortical tissues were dissected from both mVps34podKO and mVps34Ctrl mice; they were minced and digested for 45 minutes with 0.03% collagenase in the presence of 0.01% soybean trypsin inhibitor prepared in an isotonic buffer containing (in mM) 105 NaCl, 24 NaHCO3, 5 KCl, 1.5 CaCl2, 1 MgSO4, 2.0 NaH2PO4, 10 HEPES, 8.3 glucose, and 1 alanine, as well as 0.2% bovine serum albumin, while being gassed with 95% O2-5% CO2 at 37°C. The cortical suspension was strained through a 250-µm-pore sieve, washed three times with the isotonic buffer, resuspended in 30 ml of a 44% Percoll solution in the isotonic buffer described above, and centrifuged at 12,200 g for 30 minutes at 4°C. After centrifugation, the tissue was separated into four distinct bands. Glomeruli were enriched in the top-most band, and 5 ml of this band was collected and 10 ml of ice-cold isotonic buffer was added, followed by sitting on ice for 5 minutes. The glomeruli were precipitated and transferred into a clean tube, which contains approximately 95% purity of glomeruli.
Renal Function Measurement
Blood samples were collected from different ages of mice, and BUN levels were measured as previously described.31,32
Measurement of Urine Creatinine and Albumin
To determine the absolute amount of albumin excretion, we collected 24-hour urine samples by housing mVps34pdKO and mVps34Ctrl mice in metabolic cages and used an albumin-specific monoclonal antibody-based murine microalbuminuria ELISA kit, AlbuwellM (Exocell, Philadelphia, PA), to specifically measure albumin concentration in the urine. Urinary creatinine was measured by using the microplate assay kit, Creatinine Companion (Exocell). All measurements were performed in triplicate, and the ratio of urinary albumin (µg/ml) to creatinine (mg/ml) (ratio expressed as μg/mg) was calculated. Results are expressed as the means ± SEM.
Immunoblotting
These procedures were performed as we previously described.33,34 Isolated glomeruli were homogenized in radioimmunoassay precipitation buffer for 45 minutes, followed by centrifugation at 10,000 g for 5 minutes at 4°C, and protein concentrations were measured. Equal amounts of protein were loaded directly onto 7%–15% SDS-PAGE, transferred onto Immobilon-P transfer membranes (Millipore, Bedford, MA) and probed with the indicated primary antibody. The primary antibodies were detected with peroxidase-labeled goat antirabbit IgG or goat antimouse IgG and exposed on film by using enhanced chemiluminescence (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom).
Statistical Analyses
Data are presented as means ± SEM for at least three separate experiments (each in triplicate). An unpaired t test was used for statistical analyses; for multiple group comparisons, ANOVA and Bonferroni t tests were used. A P value <0.05 compared with control was considered to represent a statistically significant difference.
Disclosures
None.
Acknowledgments
We thank Dr. Ronald Emeson, Jennifer Skelton, and other members at Vanderbilt Transgenic Mouse/ES Cell Shared Resource Core for their helpful advice and technical support during the generation of this floxed mouse. Some of the data in this paper were presented as an oral presentation during a free communication session at the 2011 American Society of Nephrology meeting in Philadelphia, PA. This work was supported by funds from National American Heart Association Scientist Development grant 0630274N, Vanderbilt Diabetes Research and Training Center Pilot & Feasibility grant 2P60DK020593, Academic Program Support fund from the Department of Medicine at Vanderbilt University, Startup fund from Georgia Regents University Augusta, and National Institutes of Health R01 grant DK83575 (to J.K.C.) as well as a Veterans Affairs Merit Award and National Institutes of Health PPG DK38226 (to R.C.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Asanuma K, Mundel P: The role of podocytes in glomerular pathobiology. Clin Exp Nephrol 7: 255–259, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Patrakka J, Tryggvason K: New insights into the role of podocytes in proteinuria. Nat Rev Nephrol 5: 463–468, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Klionsky DJ, Emr SD: Autophagy as a regulated pathway of cellular degradation. Science 290: 1717–1721, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizushima N: Autophagy: Process and function. Genes Dev 21: 2861–2873, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Dunn WA, Jr: Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol 4: 139–143, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Asanuma K, Tanida I, Shirato I, Ueno T, Takahara H, Nishitani T, Kominami E, Tomino Y: MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J 17: 1165–1167, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Kitamura H, Adachi A, Sasaki Y, Ghazizadeh M: Two types of autophagy in the podocytes in renal biopsy specimens: Ultrastructural study. J Submicrosc Cytol Pathol 38: 167–174, 2006 [PubMed] [Google Scholar]
- 8.Yadav A, Vallabu S, Arora S, Tandon P, Slahan D, Teichberg S, Singhal PC: ANG II promotes autophagy in podocytes. Am J Physiol Cell Physiol 299: C488–C496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB: Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120: 1084–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman PK, Emr SD: Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol 10: 6742–6754, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD: Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 260: 88–91, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Volinia S, Dhand R, Vanhaesebroeck B, MacDougall LK, Stein R, Zvelebil MJ, Domin J, Panaretou C, Waterfield MD: A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J 14: 3339–3348, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itakura E, Kishi C, Inoue K, Mizushima N: Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19: 5360–5372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT, Heintz N, Yue Z: Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1-phosphatidylinositol-3-kinase complex. Nat Cell Biol 11: 468–476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, Maejima I, Shirahama-Noda K, Ichimura T, Isobe T, Akira S, Noda T, Yoshimori T: Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol 11: 385–396, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Walker EH, Perisic O, Ried C, Stephens L, Williams RL: Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature 402: 313–320, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Obara K, Ohsumi Y: PtdIns 3-kinase orchestrates autophagosome formation in yeast. J Lipids 2011: 498768, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baba M, Takeshige K, Baba N, Ohsumi Y: Ultrastructural analysis of the autophagic process in yeast: Detection of autophagosomes and their characterization. J Cell Biol 124: 903–913, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T: Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol 152: 657–668, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giménez-Xavier P, Francisco R, Platini F, Pérez R, Ambrosio S: LC3-I conversion to LC3-II does not necessarily result in complete autophagy. Int J Mol Med 22: 781–785, 2008 [PubMed] [Google Scholar]
- 22.Tanida I, Ueno T, Kominami E: LC3 and autophagy. Methods Mol Biol 445: 77–88, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB: Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cinà DP, Onay T, Paltoo A, Li C, Maezawa Y, De Arteaga J, Jurisicova A, Quaggin SE: Inhibition of MTOR disrupts autophagic flux in podocytes. J Am Soc Nephrol 23: 412–420, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, Zwartkruis FJ, Thomas G: Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A 102: 14238–14243, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byfield MP, Murray JT, Backer JM: hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem 280: 33076–33082, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Engelman JA, Luo J, Cantley LC: The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7: 606–619, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM: High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet 25: 139–140, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC: EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinay P, Gougoux A, Lemieux G: Isolation of a pure suspension of rat proximal tubules. Am J Physiol 241: F403–F411, 1981 [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Chen JK, Wang SW, Moeckel G, Harris RC: Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J Am Soc Nephrol 14: 3147–3154, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Chen JK, Chen J, Thomas G, Kozma SC, Harris RC: S6 kinase 1 knockout inhibits uninephrectomy- or diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol 297: F585–F593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JK, Capdevila J, Harris RC: Heparin-binding EGF-like growth factor mediates the biological effects of P450 arachidonate epoxygenase metabolites in epithelial cells. Proc Natl Acad Sci U S A 99: 6029–6034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen JK, Chen J, Neilson EG, Harris RC: Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J Am Soc Nephrol 16: 1384–1391, 2005 [DOI] [PubMed] [Google Scholar]