Abstract
The identification and characterization of potential pharmacological targets in neurology and psychiatry is a fundamental problem at the intersection between medicinal chemistry and the neurosciences. Exciting new techniques in proteomics and genomics have fostered rapid progress, opening numerous questions as to the functional consequences of ligand binding at the systems level. Psycho- and neuro-active drugs typically work in nerve cells by affecting one or more aspects of electrophysiological activity. Thus, an integrated understanding of neuropharmacological agents requires bridging the gap between their molecular mechanisms and the biophysical determinants of neuronal function. Computational neuroscience and bioinformatics can play a major role in this functional connection. Robust quantitative models exist describing all major active membrane properties under endogenous and exogenous chemical control. These include voltage-dependent ionic channels (sodium, potassium, calcium, etc.), synaptic receptor channels (e.g. glutamatergic, GABAergic, cholinergic), and G protein coupled signaling pathways (protein kinases, phosphatases, and other enzymatic cascades). This brief review of neuromolecular medicine from the computational perspective provides compelling examples of how simulations can elucidate, explain, and predict the effect of chemical agonists, antagonists, and modulators in the nervous system.
BRAIN CONDITIONS AND NEURONAL DRUG TARGETS
Brain disorders affect some 16 million American adults [1], impairing important cognitive and behavioral abilities such as memory retrieval and motor control. The incidence of most neuropathologies increases with age; therefore, the considerable increase in life expectancy might imply the beginning of an epidemic with huge social and economic consequences. Pharmacological intervention is by far the most prescribed therapy for neuropsychiatric patients. However, neurological and mental diseases often involve specific biological mechanisms localized in particular regions of the nervous system and even identifiable cellular types. Epilepsy, Alzheimer, and stroke, for instance, heavily affect the hippocampus, which plays a key role in long term memory consolidation and spatial representation. Since area CA1 constitutes the hippocampal output, it is crucial to characterize the physiopathological integrative properties of its principal neurons, the pyramidal cells. Parkinson’s disease, Huntington’s disease and addiction, on the other hand, are often associated with an alteration in the basal ganglia, which are crucial neural substrates of movement planning, procedural memory, and reward learning. In particular, dopamine neurotransmission in neostriatal medium spiny projection neurons lately has attracted attention as a possible treatment target. As yet a third set of examples, anoxia and tumors in the cerebellum may lead to ataxia or damaged sensory integration. The role of intracellular calcium concentration in regulating the excitability of the cerebellar principal neurons, the Purkinje cells, has been examined recently with respect to the development of new therapies. The treatment of these conditions requires the development of highly specific therapeutic agents (sometimes for restricted subclasses of patients [2]), to target selected brain areas while sparing others in order to maximize efficacy and minimize side effects [3].
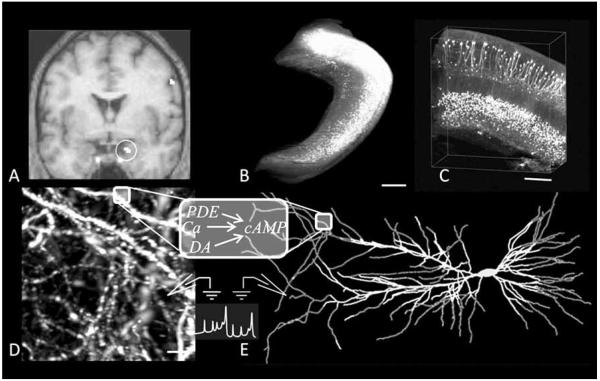
Neuroimaging techniques and cellular level experiments (Fig. 1) can inter-relate structure, activity, and function. Non-invasive imaging such as functional magnetic resonance, or fMRI (Fig. 1A), helps map the structures participating in specific brain functions by measuring the hemodynamic response to neuronal activity. To understand and control neuropharmacological specificity, however, it is important to understand the underlying molecular mechanisms, which typically involve an array of complex subcellular events, as may be illustrated considering again the case of epilepsy. Seizures, which are highly synchronized waves of electric activity, are a symptom of epilepsy regardless of the affected cortical lobes (temporal, frontal, occipital, or parietal). Triggers for epileptic seizures include excessive excitatory or defective inhibitory input (mediated by the neurotransmitters glutamate and γ-amino-butyric acid (GABA), respectively), and hyperexcitability due to an ionic channel mutation. Antiepileptic drugs can target each of these mechanisms, or any combination thereof. With techniques such as ultramicroscopy (Fig. 1B, C and D), it is possible to capture three dimensional images of single neurons at the level of dendritic processes and spines. At the same time, molecular and electrophysiological experiments investigate cell biochemical dynamics and electrical activity, respectively. Leveraging these dramatic advances in neuroscience and the ever-increasing hardware and software performance, realistic computer simulations may offer a powerful new perspective in neuropharmacology. This approach applies mathematical methods to “reverse-engineer” the neuron and test new structural or functional hypotheses (Fig. 1E).
Fig. (1).
From neuroimaging experiments to cellular and sub-cellular electrophysiological and enzymatic models. (A) Functional Magnetic Resonance Imaging represents neural activity measured through hemodynamic responses (circle indicates the position of the hippocampus), connecting function/activity to brain structures (adapted from [232], with permission granted by “Nature Publishing Group”). (B-D) Ultramicroscopy is a new neuroimaging tool allowing visualization of biological neural networks at different resolution level (adapted from [233], with permission granted by “Nature Publishing Group”) (B) Whole hippocampus imaged from multiple optical sections (scale bar: 500 μm). (C) 3D visualization of the CA1 region of the hippocampus (scale bar: 200 μm), with observable cell bodies and main branches of the dendritic trees. (D) Spines and dendritic arborization of CA1 pyramidal neurons (scale bar: 5 μm). (E) Computational/mathematical models of both electrophysiological and enzymatic activity are implemented to predict, reproduce, interpret, and explain experimental results.
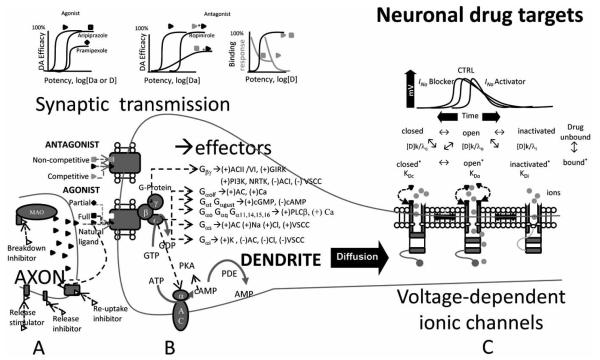
Here we overview a restricted class of interrelated biological mechanisms which can be suitable for quantitative analysis by computational modeling. These include neurotransmitter-receptor interactions, signal transduction pathways, and ionic channels, as illustrated in Fig. (2). Communication between neurons in the brain is mainly determined by activity dependent release of neurotransmitters at the synapses. The release, breakdown, and reuptake of these chemical messengers (Fig. 2, left side) are all possible neuropharmacological targets, through the action of activators, inhibitors, and modulators. Once released in the synaptic cleft, excitatory and inhibitory neurotransmitters bind to specific receptors, with opposite effects on the electrogenic activity of the receiving (post-synaptic) cell. At the synaptic receptor level, a drug can relate to the receptor as an agonist or an antagonist. An agonist triggers a response that mimics the effect of the endogenous neurotransmitter. A full agonist has the same efficacy as the natural ligand, while a partial agonist shows a weaker effect (Fig. 2, upper graphs). In contrast, an antagonist does not produce any physiological effect on its own upon binding to a receptor, but rather it reduces the effect of the neurotransmitter. A competitive antagonist binds to the receptor at the same site as the endogenous ligand. Therefore, its efficacy will be determined by the concentration ratio between the two. A non-competitive antagonist, on the contrary, binds to the receptor at a different (allosteric) site. Thus, its effect is not influenced by the concentration of the neurotransmitter.
Fig. (2).
Neuronal drug targets. (A) A drug can target the presynaptic neuron acting at many levels. It can stimulate or inhibit neurotransmitter release, reuptake or breakdown. Once released, the natural ligand diffuses in the synaptic cleft and binds to specific receptors. (B) The pharmacological effects of postsynaptic receptor-drug interactions (full/partial, agonist/antagonist) are characterized in terms of potency, efficacy and binding, or response. These interactions can be competitive or non-competitive depending on whether or not the natural ligand and drug share the same binding site. Metabotropic receptors are associated with a GTP-binding protein complex composed of three subunits, which detach upon activation and target various ion channels or enzymes. (C) A drug can also interact directly with voltage-dependent ionic channels. Depending on the membrane potential and on their kinetic properties, ionic channels can be described by at least two (open and closed) and up to four (same plus inactivated and deinactivated) different conformational and functional states. A specific drug (D) can bind to each of these states with specific association (k) and dissociation (γ) constants and affinities (K).
Ionotropic receptors at the synapses are a major target of neuroactive and psychoactive substances. These protein complexes are neurotransmitter-gated ionic channels whose opening and closing alters the membrane voltage by directly gating flow of electric current. Metabotropic receptors are coupled to guanosine 5′-triphosphate (GTP)-binding proteins (G-proteins) and are another prominent synaptic drug target. Neurotransmitter binding to these G-protein coupled receptors (GPCRs) on the outer part of the postsynaptic cell membrane initiates a biochemical reaction cascade on the intracellular side (Fig. 1, central part). The G protein consists of three associated subunits, called α, β, and γ. Upon receptor activation, the GTP bound α subunit dissociates and activates a specific target (typically an ionic channel or an enzyme), the identity of which depends on the neurotransmitter receptor type, G protein subtype, and cell type. One target of the Gαs subunit is adenylyl cyclase (AC), an enzyme that converts adenosine 5′-triphosphate (ATP) into cyclic adenosine monophosphate (cAMP). In this example, cAMP is the second messenger (the first being the neurotransmitter), which then activates ion channels or protein kinases.
Voltage-gated ionic channels are different, non-synaptic sites of possible neuropharmacological action (Fig. 2, right side). When open, these transmembrane proteins selectively allow ions such as Na+, K+, Ca++, and Cl− to move across the neuronal membrane. Ion channel dynamics are fundamental for the neuronal electrogenic activity. The conformational state of a channel and therefore its function can be influenced by a drug directly (switching from open to close or vice versa) or through a modulation of its voltage dependency. The specific binding affinity (K) of a drug for a channel, determined by its association and dissociation constants, affects the strength and duration of the interaction, and can strongly influence the generation and propagation of action potentials.
COMPUTATIONAL NEUROSCIENCE
Computational models are widely used in basic neuroscience [4,5], and medicinal chemistry could benefit greatly from this progress. In particular, the identification and characterization of potential pharmacological targets in neurology and psychiatry will become more efficient and effective as knowledge of the molecular mechanisms is integrated with a deeper understanding of the biophysical determinants of neuronal function. Computational neuroscience and bioinformatics are poised to play a major role in this functional connection. It is important to correct the false perception that computational simulations rely on ad hoc assumptions that the modeler cannot prove. In contrast, most computational models merely make explicit the implicit assumptions of the conceptual models used by all scientists. The creation of a new computational model is often an exciting scientific quest to find possible explanations for interesting experimental results that are otherwise difficult to interpret. An initial version of a model can formulate hypotheses that essentially predict responses to various conditions (i.e. drug exposure) that may not have been experimentally tested. Once the experimental results for these conditions become available, the model can be verified, or adjusted using the additional data, and then another scientific hypothesis can be formulated and tested. Given the complexity of neuronal mechanisms, and the continuous discovery of new mechanisms, a fully predictive model seems out of hand at the moment, but often the process of developing the model predicts the existence of novel mechanisms. It is important to stress that models become increasingly useful for neuropharmacology as their predictions are validated by experimental findings.
Several types of computational models have been developed in order to simulate brain dynamical activity at different levels, ranging from intracellular enzymatic and signal transduction pathways [6], to single neurons and small circuit electrophysiology [7,8], to large-scale neural networks [9]. Neuropharmacological research has only begun to take advantage of these approaches [10]; with several promising attempts to apply computational dynamical modeling to study antiepileptics [11], anxiolytics [12], and alcoholism [13]. Considering the importance of ionic channels, neurotransmitter receptors, and biochemical pathways as drugs targets, the increasing use of computational models to investigate these biophysical mechanisms in basic neuroscience provides an outstanding opportunity for discovery in medicinal chemistry. This work overviews the state of knowledge about prescribable and experimental drugs related to these molecular and biophysical mechanisms, and provides several examples to illustrate how computational models can simulate the effects of drugs at the cellular and sub-cellular levels.
DRUG DATABASES
The continuous growth of neuropharmacology makes it increasingly difficult for a single medicinal chemist to acquire the available data regarding all important molecules in a particular domain of interest. New drugs aim to be specific, tackling distinct mechanisms in precise brain areas; thus, crucial knowledge on neuropharmacological agents must include the identification of the systems they interact with and their mechanisms of action. Formalizing this information will benefit both theoretical and experimental scientists involved in the process of drug development, discovery, or interaction. Several relevant resources are listed in the Neuroscience Database Gateway [14], including databases of synapse proteins [15], GPCRs [16] and numerous disease specific neuroinformatics archives and tools (e.g. [17]).
Readily available data through the medicinal chemistry and biomedical literature is typically organized by drug (molecular structure) or disease (pathological condition). From the computational neuropharmacology perspective, it would be most productive to organize this information by the un derlying sub-cellular mechanism. Such design would enable searches for drug actions at the level of key molecular constituents, cell type and brain region. References to quantitative results in the literature would then allow the implementation of new models and simulations. For example, the BrainPharm database, currently under development to support research on drugs for the treatment of different neurological disorders [18], reports on agents that act on neuronal receptors and signal transduction pathways in the normal brain and in nervous disorders [19].
Using several public sources [20, 21, 22], we have created an annotated list of 73 prescribable and experimental drugs that act on ionic channels and neurotransmitter receptors. In particular, Table 1 includes 38 drugs modulating neurotransmitter receptors, while Table 2 contains 35 drugs interacting with voltage gated ionic channels. Both tables are organized by pharmacological targets (e.g., nicotinic receptor or delayed rectifier channel), grouped in families by neurotransmitter (acetylcholine, GABA, etc.) or ionic current (potassium, calcium, etc.). The seven represented families of neurotransmitter receptors (Table 1) include both ionotropic (e.g. glutamate NMDA, AMPA and kainate) and metabotropic (e.g. serotonin 5-HT1-2 and 5-HT5-6) mechanisms. For metabotropic receptors, the G-coupled protein is specified as well (an abridged list of molecular targets of various G-protein metabotropic pathways is illustrated in Fig. (2B) above). In both tables, each entry (row) corresponds to an individual drug (e.g. diazepam), and provides the brand name (e.g. Valium) if the compound is prescribable, the action mechanism (i.e. agonist or antagonist), targeted brain region (i.e. cortex) and medical condition (i.e. anxiety), and a bibliographic citation.
Table 1.
Drug Interacting with Neurotransmitter Receptors#
| Family (G-protein) |
Drug (brand name)4 | Drug action(s) | Brain region | Condition | Ref. | |
|---|---|---|---|---|---|---|
| 5-HT | 5-HT1A,B,D (Gi/o) | Eletriptan (Relpax) | Agonist | Trigeminal sensory nerve-endings, intracra- nial blood vessels |
Migraine | [52, 53] |
| Vilazodone | Partial Agonist (SSRI) | Cortex, hippocampus | Depression | [54, 55] | ||
| Fluoxetine (Prozac) | Reuptake inhibitor | Dentate gyrus | Depression, obsessive- compulsive disorder |
[56, 57] | ||
| 5-HT2A,B,C (Gq/11) | Quetiapine (Seroquel) | Antagonist | Pre-frontal cortex, stria tum, limbic system, anterior pituitary gland |
Bipolar disorder, schizo- phrenia |
[58, 59, 60] |
|
| TGBA01AD | Reuptake inhibitor (5HT2/1A agonist) |
N/A | Depression | [61] | ||
| 5-HT5A (Gi/o) | SGS-518 | Antagonist | N/A | Schizophrenia | [62] | |
| 5-HT4A,B (Gs) | Benzoate | Antagonist (also for 5HT3) |
Frontal cortex, striatum, hippocampus |
Neurodegenerative deseases | [63, 64, 65, 66] |
|
| 5-HT6 (Gq/s) | Olanzapine (Zyprexa) | Antagonist (also for 5HT2, DA) |
Frontal cortex, hippo- campus |
Schizophrenia | [67, 68] | |
| 5-HT3*1 | Mirtazapine (Remeron) | Antagonist | Central amygdala, ante- rior insula, septum |
Depression | [69, 70, 71] |
|
| Glu | mGluR1,5 (Gq) mGluR2,3,4,6,7,8 (Gi/o) |
LY354740 | Agonist | Striatum, hippocampus, cortex, cerebellum |
Anxiety | [72, 73, 74] |
| MPEP | Antagonist (also for AMPA) |
Striatum, thalamus, cor- tex, amygdala |
Epilepsy, pain, neurode- generative diseases, anxiety |
[75, 76, 77] |
||
| Lamotrigine (Lamictal) | Release inhibitor (Ca2+ and Ih blocker) |
Striatum, thalamus, cor- tex, hippocampus, amygdala |
Epilepsy, bipolar disor- der |
[11, 67, 78, 79] |
||
| NMDA *2 | TAN-950A | Agonist | Hippocampus/CA1 | Neurodegenerative dis- ease |
[80, 81] | |
| Aptiganel | Antagonist | Hippocampus, cortex | Neurodegeneration, Parkinson, brain injury, cardiovascular ischemia |
[82, 83, 84] |
||
| Ifenprodil | Non-competitive antago- nist (Ca2+ blocker) |
Hippocampus/CA3, piriform cortex, amygdala, striatum |
Schizophrenia | [85, 86] | ||
| AMPA*1 | Talampanel | Non-competitive antago nist |
Amygdala | Epilepsy, Parkinson | [87, 88, 89, 90] |
|
| S-18986 | Modulator | Frontal cortex, dorsal hippocampus |
Neurodegeneration, cardiovascular ischemia, cognitive disorder |
[91, 92, 93, 94] |
||
| Kainate*1 | LY293558 | Antagonist (also for AMPA) |
Hippocampus, lateral and medial habenulae, supe- rior and inferior colliculi |
Post-operative pain, anxiety |
[95, 96, 97] |
|
| DA | d1/5 (Gs) | Adrogolide | Agonist | Ventral tegmental area, limbic forebrain, nucleus acubens |
Cocaine addiction | [98, 99] |
| Reserpine | Ruptake inhibitor (also for NE and 5HT) |
Striatum, frontal cortex | Antipsychotic | [100, 101, 102] |
||
| Methylphenidate (Ritalin) | Reuptake inhibitor | Prefrontal cortex, hippo- campus, striatum, hypo- thalamus |
ADD, ADHD | [103, 104, 105, 106] |
||
| Haloperidol (Haldol) | Antagonist (also for d2) | Mesocotex, limbic sys- tem, nigrostriatal path- way |
Antipsychotic | [107, 108, 109, 110] |
||
| Bupropion (Zyban, WELLBUTRIN) |
Reuptake inhibitor | Frontal cortex, hippo- campus, striatum, thala- mus |
Smoking cessation, depression |
[111, 112] | ||
| d2/3/4 (Gi) | Pramipexole (Mirapex) | Agonist | Hippocampus, premotor and posterior cingulate cortex, superior temporal gyrus |
Restless legs syndrome | [113, 114, 115, 116, 117] |
|
| Aripiprazole (Abilify) | Partial agonist | Putamen, caudate, thala- mus, superior temporal cort |
Bipolar disorder | [118, 119] | ||
| Ropinirole (Requip, Mirapex) |
Antagonist | Mesencephalus | Restless legs syndrome | [120, 121, 122] |
||
| GABA | GABAB (Gi) | Baclofen (Lioresal) | Agonist | Hypothalamus, hippo- campus/DG , frontal cortex |
Antispastic agent for multiple sclerosis |
[123, 124, 125] |
| GABAA/C*3 | Diazepam (Valium, Dias- tat) |
Agonist | Cortex, telencephalon, hypothalamus |
Anxiety, epilepsy | [30, 126, 127] |
|
| Methohexital (Brevital Sod) |
Thalamus, hippocampus | Anestetic | [128, 129] | |||
| Topiramate (Topamax) | Agonist (Na+ blocker) | Prefrontal and occipital cortex |
Epilepsy, SMEI (severe myoclonic epilepsy of infancy) |
[130, 131, 132,133] |
||
| Sodium valproate (De- pakote) |
Breakdown inhibitor (also NE, DA and 5-HT modu- lator) |
Occipital, parietal and frontal cortex, accum- bens, substantia nigra |
Mania, migraine, epi- lepsy, GEFS+ (general- ized epilepsy with feb- rile seizures plus) |
[134, 135, 136, 137] |
||
| Muscarinic1/5 (Gq) |
Donepezil (Aricept, Dyna- bac) |
Breakdown inhibitor | Cortex, hippocampus | Alzheimer | [138, 139, 140] |
|
| ACh | Nicotinic*2 | Philanthotoxin | Non-competitive antago- nist |
Muscles cells, cortex | Neurodegenerative disease, cognitive disorders |
[141, 142] |
| Nicotine (Nicotrol) | Agonist | Thalamus, straitum, anterior cingulate cortex |
Smoking cessation | [143, 144] | ||
| HP-184 | Release stimulator (also for 5-HT) |
Hippocampus | Spinal cord injury | [145, 146] | ||
| NE | α1A,B,D (Gq) α2A,B,C(Gs) β1,2,3(Gs) |
DOV 21947 | Reuptake inhibitor (also for DA and 5-HT) |
Striatum, mesocorti- colimbic, cerebrospinal fluid |
Depression | [147, 148] |
| Dextroamphetamine (Dexedrine) |
Insular cortex | ADHD | [149, 150] | |||
| Atomoxetine (Strattera) | Release inhibitor | Caudate putamen, cor- tico (Prefrontal Ventral and Orbital)-baso tha- lamic loop |
ADHD | [151, 152] |
All receptors are G-coupled unless noted with* (ionotropic);
Monovalent cations;
Mono/divalent cations;
Chloride;
Drugs without brand name are not prescribable.
Table 2.
Drugs Interacting with Ionic Channels#
| Family | Drug (Brand Name)4 | Drug Action(s) | Brain Region | Condition | Ref. | |
|---|---|---|---|---|---|---|
| Na+ | Navα1.1/1.9 | AM-36 | Blocker | Middle cerebral artery | Cerebrovascular ischemia, Alzheimer, spinal chord injury |
[153, 154, 155] |
| BIII-890-CL | Cortical and subcortical | Pain, cerebrovascular ische- mia |
[156, 157,158] |
|||
| RS-100642-198 | Cerebellar neurons | Neurodegenerative disease, cerebrovascular ischemia |
[159, 160] | |||
| SUN-N8075 | Blocker (also Ca2+) | Middle cerebral artery | Cerebral infarction | [161, 162] | ||
| ADCI | Blocker (NMDA antago- nist) |
Superior cervical gan- glion (SCG) and hippocampus |
Neurodegenerative disease, anticonvulsant |
[163, 164] | ||
| Lithium (Eskalith) | Alters nerve Na+ trans- port |
Anterior cingulate cortex, striatum, caudal midbrain, preoptic area, hypothalamus |
Bipolar disorder, mood stabilizer |
[165, 166,167] |
||
| Nap (slowly inactivating) |
Topiramate (Topamax) | Blocker (GABAA ago- nist, AMPA/Kainate antagonist) |
Hippocampus, hypo- thalamus |
Migraine prevention, epi- lepsy |
[130, 131, 132,133] |
|
| Phenytoin (Dilantin) | Blocker | Motor cortex, brainstem | Epilepsy | [168, 169, 170] |
||
| Mixed | Hyperpolarization- activated (NA+,K+) |
Lamotrigine (Lamictal) | Shifts the inactivation curve |
Striatum, thalamus, cor- tex, hippocampus, amygdala |
Epilepsy, bipolar disorder | [11, 67, 78, 79] |
| K+ | Kdr (non- inactivating) |
Flindokalner | Activator | N/A | Cerebrovascular ischemia | [31, 32, 33, 34, 35, 36] |
| KM (muscarinic dependent) |
Phenobarbital (Pb) | Blocker | Cerebral cortex, hippocampus, hypothalamus, striatum, medulla oblongata |
BNFC (benign familial neonatal convulsions) |
[171, 172] | |
| DMP-543 | Blocker (also Ach re- lease enhancer) |
Hippocampus | Alzheimers, epilepsy | [173, 174] | ||
| Kir (inward- rectifying) |
Spironolactone (Aldac- tone) |
K+-sparing diuretic (glu- cocorticoid receptor antagonist) |
Lateral septum, hippo- campus |
Andersen-Tawil syndrome | [175, 176, 177, 178, 179] |
|
| KATP | BAY-X-9227 | Activator | CNS neurons | Neurodegenerative disease | [180, 181, 182] |
|
| KAHP, BK, SK, IK*1 |
NS-1608 | BK activator | dorsal root ganglia | Cerebrovascular ischemia | [183, 184, 185, 186] |
|
| BMS-204352 | Modulator; Kdr, maxi (K) and KCNQ4 activator |
Hippocampus, thalamus and adjacent cortex |
Stroke, cerebrovascular ischemia, brain injury |
[187, 188, 189] |
||
| Ca2+ | L-type | MEM-1003 | Blocker | Medial prefrontal cortex, hippocampus |
Dementia, Alzheimer, Cog- nitive disorder |
[190, 191, 192] |
| E-2051 | N/A | Cerebrovascular ischemia | [75] | |||
| S-312-d | Forebrain neurons and astrocytes, hippocampus, cerebral cortex |
Neurodegenerative disease, cerebrovascular ischemia, antiepileptic |
[193, 194, 195, 196] |
|||
| NS-649 | Cerebellar granule cells | Alzheimer, neurodegenera- tive disease, cognitive disor- der |
[197, 198] | |||
| BayK 8644 | Activator | Cerebellar granule cells, striatum |
Mood regulator | [199, 200, 201] |
||
| N-type | NS-638 | Blocker (also for L-type) | Dorsal root ganglion cells | Neurodegenerative disease, cerebrovascular ischemia |
[202, 203] | |
| R-type | CNS-2103 | Blocker (also for L-type) | N/A | Neurodegenerative disease | [204, 205] | |
| SNX-482 | Blocker (also for P/Q- type, delays Na+ inacti- vation) |
Entorhinal and motor cortex |
Neurodegenerative disease | [206, 207] | ||
| P/Q-type | 4-aminopyridine | Blocker (also for K+) | Entorhinal cortex, cere- bellar Purkinje cells |
Epidodic ataxia type 2 | [208, 209, 210] |
|
| Safinamide | Blocker (also for K+, MAOB inhibitor, Ach modulator) |
Muscles | Parkinson, epilepsy | [191, 211, 212, 213] |
||
| Pregabalin (Lyrica) | Blocker | Entorhinal cortex, cere- bellum, hippocampus |
Neuropathic pain, Post her- petic neuralgia, Fibromyal- gia, ataxia type 6 |
[44, 214, 215] |
||
| CPC-304 | Hippocampus/CA1 | Neurodegenerative disease, Alzheimer, cerebrovascular ischemia |
[205] | |||
| Diperdipine | Blood vessels | Neurodegenerative disease, cerebral infarction, cere- brovascular disease, cere- brovascular ischemia |
[216, 217] | |||
| AR-R18565 | N/A | Ischemia | [205] | |||
| T-type | Ethosuximide (Zarontin) |
Blocker | Thalamic relay neurons, cerebellar Purkinje cells |
Epilepsy | [218, 219, 220, 221] |
|
| IP3R1/3*2 | Heparin (Heparin) | Release inhibitor | Cerebral cortex, midbrain | Stroke | [222, 223] | |
| Ryanodine 1/3*3 | Dantrolene (Dantrium) | Release inhibitor | Hippocampus/CA1, parietal cortex, cerebel- lum |
Malignant hyperthermia | [224, 225, 226, 227] |
|
| Caffeine (Cafcit, Caf- feine and Sodium Benzoate Inj) |
Agonist | Cerebellum, forebrain | Headache, migraine | [228, 229, 230, 231] |
Voltage-gated transmembrane unless noted with;
(ligand gated and/or intracellular);
Calcium dependent;
Inositol triphosphate dependent, endoplasmic reticulum;
Calcium dependent endoplasmic reticulum;
Drugs without brand name are not prescribable.
This initial overview of drugs acting on various neural targets with different mechanisms in several brain regions and conditions provides the seed of a reference tool that could aid the development of computational neuroscience models relevant to medicinal chemistry. To illustrate this potential, we report the results of the application of this information onto two types of dynamical models. The first one consists of a single neuron, multi-compartmental model of membrane biophysics and electrophysiology. The second is a computational model of enzymatic pathways in a single subcellular compartment of a neuron.
COMPARTMENTAL SIMULATIONS OF NEURONAL ELECTROPHYSIOLOGY
Neurons integrate and generate electrical signals through intricate dendritic arborizations that possess ion channels. Without arborizations or ion channels, the change in dendritic membrane potential in space and time can be calculated by solving a partial differential equation. Realistically, this analytic task is prohibited by the complex branching, variable diameter, and numerous non-linear ion channels of dendrites [23]. To calculate membrane potential computationally, the neuron is described as a set of small, interconnected isopotential, cylindrical compartments. Each of these “units” is numerically represented as an electric circuit, with longitudinal (internal) resistance or conductivity, and transverse (membrane) resistance and capacitance. Using this representation, accurate neuronal morphologies are imported from digital reconstructions of microscopically imaged real cells [24] into mature software environments, such as NEURON [7] and Genesis [8], for neuron modeling. Membrane and synaptic properties, including the dynamics of voltageand ligand-gated ionic channels, are distributed through the neuronal arborization to match experimental data or test new hypotheses. Then, it is possible to simulate the effect of drugs on neuronal activity via modulation of neurotransmitter receptors and electric currents.
The examples presented here are based on a realistic model of a CA1 pyramidal neuron of the hippocampus [25]. This model was previously validated against a number of experimental findings [26] and includes digitally reconstructed dendritic morphology (see NeuroMorpho.Org [27]), a sodium current (INa), delayed rectifying and A-type potassium currents (IKDR and IKA, respectively), and a mixed sodium/potassium current activated by membrane hyperpolarization (Ih). The distributions of these ionic channels, all of which are represented in Table 2, were consistent with the experimental data of CA1 pyramidal neurons [4]. We simulated the electrophysiological behavior of this cell (using NEURON v6.1) under control conditions and in the presence of antiepileptic drugs interacting with different biological targets, with a particular emphasis on the effect of drug administration on the spiking output. Five excitatory synapses with large conductances (2 nS in the control condition) were randomly distributed on the proximal dendrites (50-400 μm from the soma), and stimulated at a constant frequency (100 Hz) to represent synchronized populations of incoming synaptic inputs. Five inhibitory synapses (0.2 nS) were randomly distributed in the perisomatic region of the dendritic tree (0-20 μm from the soma), and subjected to stochastic activation with the same average frequency (100 Hz).
The effects of three antiepileptic agents were simulated based on their distinct mechanism of action. Lamotrigine (Fig. 3A) is a phenyltriazine that is well absorbed orally (>95% bioavailability) and ~55% protein bound, reducing the probability of clinical interaction with other proteinbound drugs. Lamotrigine has a fairly broad spectrum of activity against multiple types of seizures, and does not impair cognition. It inhibits voltage-dependent Na+ channels, thereby making the neuron less excitable, but the exact mechanism of action is not fully understood; Lamotrigine also reduces glutamate release [28], inhibits postsynaptic AMPA receptor [29], and shifts the inactivation voltage dependence of the Ih current [11]. In the lamotrigine simulations, the strength of excitatory synapses was decreased from 2 nS to 1.5 nS to model the combined glutamate antagonist effect, while shifting the Ih inactivation curve by 10 mV, consistent with experimental data [26]. The second drug, diazepam (Fig. 3B), is one of the simplest and most prescribed benzodiazepines (e.g. Valium), a powerful and popular class of GABAA agonists. Diazepam allosterically locks the GABAA receptor into a conformation with higher affinity for the neurotransmitter, therefore increasing the frequency of opening of the associated chloride channel and hyperpolarizing the neuronal membrane. This potentiation of the effect of GABA was modeled in our simulations with a 10-fold increase in the conductance of the inhibitory synapses (from 0.2 nS to 2 nS) [30]. Finally, flindokalner (Fig. 3C) is a chiral candidate drug for post stroke neural protection [31], and blocks the calcium-dependent potassium current [32]. Given its possible interaction with other K+ channels [33,34,35,36], we explored its potential as an antiepileptic agent by increasing the IKDR conductance (from 0.001 pS/μm2 to 0.002 pS/μm2). Moreover, we also tested the putative combined effect of lamotrigine and flindokalner by simultaneously changing both sets of simulation parameters. Of course, increasing or decreasing an ionic conductance and modifying a kinetic scheme is hardly enough to understand how a drug can affect synaptic excitation of a neuron. Details on neurotransmitter diffusion and short-term plasticity can be critical for predicting the effects of a drug. Likewise, the input patterns need to be selected appropriately. Thus, the examples shown here should be understood for illustrative purposes only rather than interpreted literally.
Fig. (3).
Molecular structures and functions of drugs interacting with the simulated mechanisms. (A) Lamotrigine (C9H7Cl2N5) is an anticonvulsant and mood stabilizer used to treat epilepsy and bipolar disorder. (B) Diazepam (C16H13ClN2O) is a benzodiazepine commonly prescribed in the treatment of anxiety, insomnia, seizures, alcohol withdrawal, and muscle spasms. (C) Flindokalner (C16H10ClF4NO2) is a drug candidate for post-stroke neuroprotection. It is an activator of large conductance calcium dependent K+ conductances, but the therapeutic mechanism of action is still under investigation. (D) Reserpine (C33H40N2O9) is a bulky molecule used as an antipsychotic. It acts by blocking the norepinephrine, serotonin, and dopamine transporters. (E) Methylphenidate (C14H19NO2) is a stimulant used to treat attention-deficit hyperactivity disorder, chronic fatigue syndrome, and daytime drowsiness due to narcolepsy. It works by binding the dopamine transporter and inhibiting its reuptake. (F) Bupropion (C13H18ClNO) is an atypical antidepressant acting mainly as a dopamine reuptake inhibitor. It is also a weak nicotinic antagonist and a norepinephrine reuptake inhibitor. (G) Ifenprodil (C21H27NO2), a candidate drug in schizophrenia, is a selective non-competitive NMDA antagonist, which reduces cell excitability by blocking calcium influx. (H) LY354740 (C8H11NO4), also known as eglumegad, is a drug candidate for the treatment of anxiety and drug addiction. It is a highly selective agonist of metabotropic glutamate receptors (mGluR2/3). (I) Pregabalin (C8H17NO2) is effective in treating neuropathic pain, generalized anxiety disorder and as an adjunct therapy for partial seizures. It inhibits P/Q type Ca++ channels by binding to their α2δ subunit. (J) Bayk 8644 (C16H15F3N2O4), is an experimental drug candidate known to act as a mood regulator. It is an L-type Ca++ channel activator. (K) MPEP (C14H11N) is an extensively studied drug candidate to treat epilepsy, pain, neurodegenerative diseases, and anxiety. It is a highly selective and potent mGluR and AMPA antagonist.
The spiking activity of the CA1 pyramidal cell was monitored for 1 second in response to the above described synaptic stimulation. In particular, the ability to modulate spike timing is crucial in pathologies such as epilepsy, where cell excitability and network synchrony play fundamental roles. The results shown in Fig. (4) correspond to five simulations: the control condition (in gray), the three individual drug treatments (in black), and the combination of lamotrigine and flindokalner (case 4, also in black). The model suggests that different drugs (each with a separate mechanism of action) can variably affect signal integration and generation, and thus spike timing (Fig. 4A). Flindokalner produced a lower overall spike number compared to lamotrigine (50 vs. 53). This relatively minor difference must be interpreted in the context of the underlying mechanisms: by modulating IKDR, flindokalner heavily affects the interspike interval, whereas lamotrigine reduces the excitatory strength without affecting signal integration. Thus, both the overall number of spikes generated during a given time period (as a measure of cell excitability) and the ability to modulate individual spike timing (Fig. 4A) are important. Interestingly, the combined effect of the two drugs results in an interaction comparable to the results obtained from diazepam (spike number = 44). Another quantitative metric is the drug’s ability to change the shape of the interspike interval histogram of the neuron. In all cases, the main effect of these antiepileptic drugs is to abate the instantaneous output frequency compared to control conditions (Fig. 4B). However, compared with the individual treatment condition, the cumulative action of lamotrigine and flindokalner may more effectively decrease the overall cell clusters of synchronized activity which are crucial in epilepsy.
Fig. (4).
Computational neuropharmacology and electrophysiology. (A) Raster plot describing how various antiepileptic drugs targeting different neuronal mechanisms can affect spike patterns and sequences (signal generation/integration). (B) All these simulated treatment conditions (black lines) significantly abate the instantaneous frequencies. (C) Compared to control (gray histograms), individual drugs can preferentially favor certain frequency ranges, cutting off others. (D) Even if they have equal average output frequencies (top bars), two drugs (3 vs. 4) can differentially modulate membrane voltage (bottom bars). (E) Drug effects on the timing of the first and second spike.
Even though the foremost aim of antiepileptic drugs is to reduce cellular excitability, in order to carry out their physiological functions, neurons still need to span different ranges of frequencies. Grouping the instantaneous frequencies in 10 Hz bins (Fig. 4C) shows how different drugs differentially skew the control distribution. Lamotrigine can reduce (without completely cutting off) the highest frequencies better than the other drugs, leaving the lowest frequencies almost untouched. Another important parameter related to cell excitability is the average membrane voltage (Fig. 4D, lower panel) which is mostly driven by the number of spikes and therefore by the average output frequency (Fig. 4D, upper panel). Interestingly, for the same average output frequency, diazepam resulted in a lower average membrane voltage (~3mV) than flindokalner. The net inhibitory action of diazepam tends to hyperpolarize the membrane more often, driving down its voltage. Somatic voltage traces can be compared directly (Fig. 4E) to examine how the four drug conditions can modulate spike properties. These results can be best appreciated by considering the recent surge of interest in the potential role of spike timing in processing and storage of information in neural circuits. In particular, the temporal order of pre- and postsynaptic spiking has been shown to affect the induction of long-term modifications of neuronal excitability and dendritic integration [37]. Such temporal specificity at the synaptic and cellular level is believed to play an important functional role from basic neuronal activity to human cognition.
SIGNALING PATHWAY MODELS
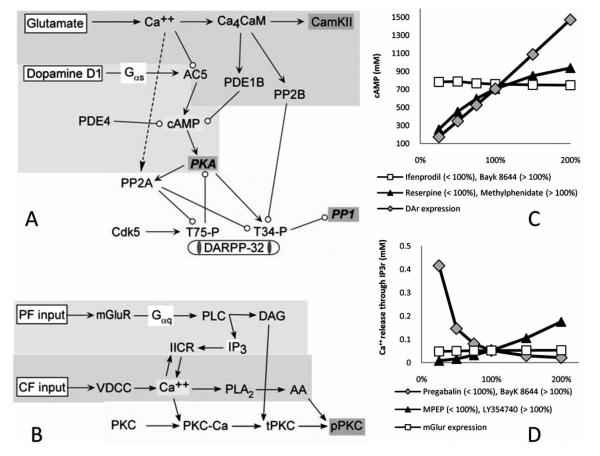
A complementary way to simulate the effect of drugs on brain activity is by modeling their influences at the level of the different components of the cellular signaling pathways. Synaptic inputs activate intracellular molecular cascades [38] which in turn modulate ionic channels. Several drugs affect neuronal excitability by activating signaling pathways which phosphorylate or dephosphorylate proteins such as channels, receptor or transporters. Mathematical models based on specific biochemical reactions allow prediction of drugdependent changes in the second messenger concentration (i.e. Ca++ and cAMP) and downstream modulation of target phosphoproteins. The software tool XPPAUT [6] was designed to solve systems of differential equations and is therefore well suited to simulate biochemical reaction kinetics. Here we illustrate this approach with two sub-cellular, single-compartment (spine-like) computational models (Fig. 5). The signaling pathways from both examples are involved in synaptic plasticity, one in a neostriatal medium spiny projection neuron and one in a cerebellar Purkinje cell. Both models have been implemented using XPPAUT and consist of a series of ordinary differential equations computing mass action kinetics of biochemical cascades over time. Complete equations, network cascades, and other details of these two models have been published in, and can be retrieved from, peer reviewed journals [5,39].
Fig. (5).
Two intracellular signaling models of molecular dynamics involved in plasticity. (A) Dopaminergic and glutamatergic activated signaling pathways of medium spiny projection neurons involved in the production of cyclic adenosine monophosphate (cAMP). Glutamate stimulation leads to calcium elevation; Ca++ binding to calmodulin (CaM) activates calcium - Calmodulin kinase 2 (CaMKII), protein phosphatase 2B (PP2B) and phosphodiesterase 1B (PDE1B), the latter of which degrades cAMP. Dopamine (D1) receptor stimulation activates the protein kinase A (PKA) pathway via adenylate cyclase type V (AC5) and cAMP. PKA activation increases phosphorylation of DARPP-32, which then inhibits protein phosphatase 1 (PP1). (B) The Purkinje cell model incorporates three separate biochemical pathways. The first pathway describes parallel fiber (PF) activation and consequent diacylglycerol (DAG), and inositol trisphosphate (IP3) production via activation of G-proteins and phospholipase C (PLC) and subsequent intracellular Ca2+ release. Another biochemical pathway depicts how Ca++ elevation due to CF stimulation activates voltage dependent calcium channels (VDCC) and leads to arachidonic acid (AA) production. The last set of reactions describes how calcium, DAG, and AA elevation influence the activity of the persistently active form of protein kinase C (pPKC). (C) Dose-dependent effects on cAMP of different drugs modulating dopamine reuptake (Reserpine and methylphenidate), dopamine receptor density (DaR expression, a possible target for genetic therapies) and neuronal calcium influx (ifenprodil and Bayk 8644). (D) Simulated effect of different dosages of drugs modulating glutamate release (MPEP and LY354740), glutamate receptor density (mGluR expression), and intracellular Ca++ concentration (pregabalin and Bayk 8644) on the IP3 receptor dependent release of intracellular calcium from the endoplasmic reticulum.
The involvement of cAMP in intracellular signal transduction is of particular importance, as this second messenger activates cAMP-dependent protein kinase (protein kinase A, or PKA). Thus, we decided to investigate its intracellular dynamics in the neostriatal spiny projection neuron (see signaling pathway schematic in Fig. 5A). In this neuron glutamate leads to calcium influx, whereas dopamine activates GPCRs couple to adenylyl cyclase. Dopamine stimulates adenylyl cyclase to increase cAMP production (Fig. 1), whereas calcium inhibits production of cAMP by adenylyl cyclase. Thus, activation of PKA by cAMP depends on the interaction between dopamine and calcium, and drugs that modify dopamine stimulation or Ca++ influx should modulate cAMP levels. For example, while reserpine (Fig. 3D), the active principle of Harmonyl, is a generic reuptake inhibitor (norepinephrine, serotonin, and dopamine) used as an antipsychotic; methylphenidate (Fig. 3E), the active principle of Ritalin, specifically inhibits the dopamine transporter and is prescribed for attention deficit disorder. Another drug with the same action mechanism is bupropion (Fig. 3F), found in Zyban and Wellbutrin, and used against depression or as an aid to smoking cessation. Dopamine reuptake inhibitors allow the neurotransmitter to stay in the synaptic cleft longer, amplifying the effect of dopamine, the deficiency of which is thought to be the main problem in these conditions. We also investigated drugs that modify calcium influx, since this, too, can modulate cAMP via an inhibitory effect on adenylyl cyclase. For instance, ifenprodil (Fig. 3G) influences the intracellular calcium concentration by blocking the NMDA receptor. Another not mutually exclusive possible etiology is that the number of neurotransmitter receptors in the pathological brain region is altered. Since at the moment there are no drugs that modulate the post synaptic receptor densities, we simulated this issue as a probe for future drug target or genetic therapies.
The proposed model (Fig. 5A) predicts the effects on the intracellular enzymatic activity and second messenger molecular concentration (i.e. cAMP) of five different neuropharmacological agents, which are or might be used to treat diseases involving dopamine and striatum dysfunction. We simulated the effect of methylphenidate, which increases the concentration of dopamine in the synaptic cleft [39] by inhibiting its reuptake, with a change in the dopamine concentration at the synapse, from 100% to 200% of the control condition. On the other hand, reserpine decreases the synaptic dopamine concentration [40]; we simulated this effect by decreasing the dopamine concentration at the synapse from 100% to 25%. The effects of ifenprodil (NMDA channel blocker) and bayk 8644 (enhances open state of calcium channels) were simulated by modulating the calcium influx in the same range [41,42,43]. Finally, the effect of a possible future genetic therapy was simulated by increasing up to two-fold or decreasing by one quarter the dopamine receptor densities relative to control. We investigated how cAMP depends on the presence of dopamine in the synaptic cleft (Fig. 5C, black triangles), the increase in the number of dopamine receptors (Fig. 5C, gray diamonds), and the modulation of Ca++ flowing in the intracellular medium (Fig. 5C, white squares). Our simulations show that when either the number of dopamine receptors or the amount of dopamine in the pre-synaptic cleft was increased, the amount of cAMP monotonically increased. When neuronal Ca++ influx increased, the amount of cAMP slightly decreased. These results suggest the experimentally testable hypothesis that decreasing either the number of receptors or the amount of dopamine can have a similar effect (Fig. 5C, <100%), while increasing them leads to very different results (Fig. 5C, >100%). The rise of cAMP due to the dopamine receptor increase was linear while that due to the increase in the amount of the endogenous ligand was sub-linear. Therefore, therapies that target dopamine release (or reuptake) and the amount of the receptor expression can differently affect the cAMP second messenger cascades in a dose dependent manner.
The second example that illustrates the computational modeling approach to medicinal chemistry and neuropharmacology at the signaling pathway level concerns synaptic convergence in the principal neurons of the cerebellum, the Purkinje cells. The dendrites of these neurons receive excitatory inputs through two axonal pathways. Parallel fibers activate GPCRs coupled to phospholipase C, which produces inositol triphosphate (IP3), whereas climbing fiber stimulation leads to calcium influx through voltage gated calcium channels. Activation of protein kinase C (PKC) and subsequent synaptic plasticity requires both of these two conditions, which are ultimately related to the control and regulation of calcium release. Fig. (5B) describes this set of biochemical reactions triggered by cerebellar Purkinje cell climbing and parallel fiber stimulation [44,45]. The model simulates these biochemical reactions including Ca++ release into the cytosol from the endoplasmatic reticulum due to IP3 receptor stimulation. Calcium release is modulated by the calcium influx through voltage dependent channels, by the amount of available glutamate in the synaptic cleft, and by the density of glutamate receptors.
The model simulates the effects of five different therapies on IP3-induced intracellular calcium release, because calcium is one of the most important intracellular second messengers in neuronal function. The new compound LY354740 (Fig. 3H) is a potent metabotropic glutamate receptor (mGluR) agonist acting also in the cerebellum. This drug is not yet marketed and requires detailed investigation of its therapeutic potential. In addition to the mGluR-activating LY354740, we examined the action of pregabalin, bayk 8644, MPEP, and mGluR expression. Pregabalin (Fig. 3I) is an anticonvulsant that regulates intracellular calcium concentration by blocking the P/Q-type voltage-gated Ca++ channels [44], making the cell less likely to fire an action potential. Bayk 8644 (Fig. 3J) as mentioned above enhances the open state of L-type voltage-gated Ca++ channels, while MPEP (Fig. 3K) is an mGlurR antagonist. The amount of glutamate receptors may be alterable in the future using genetic therapy. Model simulations of the effect of these drugs (Fig. 5D) show that an increase in glutamate release (black triangles) leads to a supra-linear increase in the amount of calcium released from the IP3 receptor. Moreover, this same measure drastically drops upon elevation of calcium influx through voltage dependent channels (gray diamonds). In contrast, when the amount of glutamate receptors is increased (white squares), the resulting effect on intracellular IP3-dependent calcium release is barely noticeable.
CONCLUSIONS
This overview aimed at building a bridge between basic, theoretical, and neuropharmacological research through dynamical computational modeling. Drugs can affect signal integration in the central nervous system by targeting a variety of mechanisms. Some chemical structures may be more effective at stopping bursts of action potentials, while others could be more suitable to modulate the mean firing frequency, or sub-threshold dynamics. Relevant molecular interactions might involve ionic channels (ligand-gated in the synapse or voltage-gated outside of it) or second messenger biochemical pathways. Understanding the effects of different types of modulation could play a crucial role in the future of medicinal neurochemistry. An increasingly accurate, complete, and quantitative characterization of the biochemical and biophysical interactions between drugs and neural activity will aid the design of new classes of drugs with enhanced selectivity and targeting specific classes of patients and pathological conditions.
Computer simulations are already used in basic and clinical research for several other similar applications, and could speed up or otherwise improve the neuropharmacological process of drug design and characterization. For one effective compound that makes it into the market there are millions of unsuccessful trials. The strength of plausible biophysical models consists of the ability to make realistic predictions, which can be used to prescreen a discrete number of compounds for bench testing in the laboratory, thus increasing the possible success ratio. Computational modeling is an intrinsically exploratory approach, because the simplifications embedded in the methodology must be ultimately verified empirically. Continuous testing against experimental results is constantly required in order to develop new hypotheses and realistically update the models. Tightly paired with experimental research, computational neuropharmacology can be both cost- and time-effective.
Besides the important repercussions in neuropharmacological research, computational models in the future could have practical clinical applications. Since brain activity changes dynamically and individual exogenous factors acting on various biological mechanisms alter the nervous signal in a different manner, the type and dosage of drug administered in a specific moment should adapt accordingly in real time. One could envision a system in which the patient’s brain activity is monitored with a set of permanently implanted in vivo electrodes. Subsequently, the right combination of different drugs is dynamically dosed to counteract a specifically detected pathological activity pattern, and automatically injected with a drug pump [46,47,48] just when and where needed.
In the examples illustrated here, computational models already implemented and validated in basic neuroscience research, specifically of a pyramidal, a medium spiny, and a Purkinje cell, were deployed to address open neuropharmacological questions. All three neuron classes are involved in plasticity and operate in three circuits (hippocampus, striatum, and cerebellum) crucial for encoding different types of memories. Restricting these examples to principal neurons was useful in this case to illustrate the insights that models can offer on the potential action of a drug at the single cell level. Nonetheless, addressing firing properties in the principal neurons only provides a very simplistic view of the effects of the drug on the circuit as a whole. For instance, interneurons play a critical role in regulating network dynamics, and their modulation may have even more dramatic functional consequences than altering the activity of principal cells. Moreover, given their structural simplicity, the excitable properties of the interneurons are often understood at a level of precision and reliability that is higher than in principal neurons, as documented in the hippocampus [49], neocortex [50], and cerebellum [51]. Nevertheless, our preliminary findings strongly suggest that drugs with functionally similar targets (e.g. neurotransmitter release or postsynaptic receptor density) can have considerably different effects on the second messenger enzymatic cascades, and therefore cell activity. We surmise that the spread of this approach will soon generate an avalanche of strides toward the resolution of many brain conditions.
ACKNOWLEDGEMENTS
The authors are indebted to several members of the Center for Neural Informatics, Structures, and Plasticity (krasnow.gmu.edu/cn3) for critical comments on a previous version of this manuscript. This work was supported in part by R01 grant NS396000 to GAA, funded by NINDS, NIMH, and NSF; by R01 grant AG025633 to GAA, funded by NIA under the CRCNS program; and by SGER grant 747864 to GAA, funded by NSF. KTB was supported by the CRCNS program through R01 grant AA16022, and an HFSP program grant.
REFERENCES
- [1]. http://www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=4 38.
- [2].Malphrus AD, Wilfong AA. Curr. Treat. Options Neurol. 2007;9:256. doi: 10.1007/s11940-007-0012-7. [DOI] [PubMed] [Google Scholar]
- [3].Youdim MB, Buccafusco JJ. J. Neural. Transm. 2005;112:519. doi: 10.1007/s00702-004-0214-z. [DOI] [PubMed] [Google Scholar]
- [4].Migliore M, Shepherd GM. Nat. Rev. Neurosci. 2002;3:362. doi: 10.1038/nrn810. [DOI] [PubMed] [Google Scholar]
- [5].Lindskog M, Kim M, Wikström MA, Blackwell KT, Kotaleski JH. PLoS Comput. Biol. 2006;2:119. doi: 10.1371/journal.pcbi.0020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. http://www.math.pitt.edu/;bard/xpp/xpp.html.
- [7].Hines ML, Carnevale NT. Neural Comput. 1997;9:1179. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- [8].Bower JM, Beeman D. Book of Genesis: Exploring Realistic Neural Models with the General Neural Simulation System with CDROM. Springer-Verlag; New York: 1995. [Google Scholar]
- [9].Markram H. Nat. Rev. Neurosci. 2006;7:153. doi: 10.1038/nrn1848. [DOI] [PubMed] [Google Scholar]
- [10].Aradi I, Erdi P. Trends Pharmacol. Sci. 2006;27:240. doi: 10.1016/j.tips.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [11].Poolos NP, Migliore M, Johnston D. Nat. Neurosci. 2002;5:767. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- [12].Kiss T, Erdi P. Biosystems. 2006;86:46. doi: 10.1016/j.biosystems.2006.02.016. [DOI] [PubMed] [Google Scholar]
- [13].Migliore M, Cannia C, Canavier CC. J. Neurophysiol. 2008 doi: 10.1152/jn.00024.2008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [14].Gardner D, Shepherd GM. Neuroinformatics. 2004;2:271. doi: 10.1385/NI:2:3:271. [DOI] [PubMed] [Google Scholar]
- [15].Zhang W, Zhang Y, Zheng H, Zhang C, Xiong W, Olyar-chuk JG, Walker M, Xu W, Zhao M, Zhao S, Zhou Z, Wei L. Nucleic Acids Res. 2007;35:737. doi: 10.1093/nar/gkl876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Elefsinioti AL, Bagos PG, Spyropoulos IC, Hamodrakas SJ. BMC Bioinformatics. 2004;5:208. doi: 10.1186/1471-2105-5-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lam HY, Marenco L, Clark T, Gao Y, Kinoshita J, Shepherd G, Miller P, Wu E, Wong GT, Liu N, Crasto C, Morse T, Stephens S, Cheung KH. BMC Bioinformatics. 2007;8:4. doi: 10.1186/1471-2105-8-S3-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. http://senselab.med.yale.edu/BrainPharm.
- [19].Crasto CJ, Marenco LN, Liu N, Morse TM, Cheung KH, Lai PC, Bahl G, Masiar P, Lam HY, Lim E, Chen H, Nadkarni P, Migliore M, Miller PL, Shepherd GM. Brief Bioinform. 2007;8:150. doi: 10.1093/bib/bbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. http://www.ncbi.nlm.nih.gov/sites/entrez.
- [21]. http://www.drugs.com/
- [22]. http://www.rxlist.com/script/main/hp.asp.
- [23].Abbott LF, Farhi E, Gutmann S. Biol. Cybern. 1991;66:49. doi: 10.1007/BF00196452. [DOI] [PubMed] [Google Scholar]
- [24].Ascoli GA. Nat. Rev. Neurosci. 2006;4:318. doi: 10.1038/nrn1885. http://www. neuromorpho.org. [DOI] [PubMed] [Google Scholar]
- [25].Migliore M, Morse TM, Davison AP, Marenco L, Shepherd GM, Hines ML. Neuroinformatics. 2003;1:141. doi: 10.1385/NI:1:1:135. http://senselab.med.yale.edu/ModelDb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Migliore M, Messineo L, Ferrante M. J. Comput. Neurosci. 2004;16:5. doi: 10.1023/b:jcns.0000004837.81595.b0. [DOI] [PubMed] [Google Scholar]
- [27].Ascoli GA, Donohue DE, Halavi M. J. Neurosci. 2007;27:9247. doi: 10.1523/JNEUROSCI.2055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Teoh H, Fowler LJ, Bowery NG. Neuropharmacology. 1995;34:1273. doi: 10.1016/0028-3908(95)00104-e. [DOI] [PubMed] [Google Scholar]
- [29].Lee CY, Fu WM, Chen CC, Su MJ, Liou HH. Epilepsia. 2008;49:888. doi: 10.1111/j.1528-1167.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- [30].Eghbali M, Curmi JP, Birnir B, Gage PW. Nature. 1997;388:71. doi: 10.1038/40404. [DOI] [PubMed] [Google Scholar]
- [31].Young BL, Cooks RG, Madden MC, Bair M, Jia J, Aubry AF, Miller SA. J. Pharm. Biomed. Anal. 2007;43:1602. doi: 10.1016/j.jpba.2006.12.027. [DOI] [PubMed] [Google Scholar]
- [32].Jensen BS. CNS Drug Rev. 2003;8:353. doi: 10.1111/j.1527-3458.2002.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang D, Krishna R, Wang L, Zeng J, Mitroka J, Dai R, Narasimhan N, Reeves RA, Srinivas NR, Klunk LJ. Drug Metab. Dispos. 2005;33:83. doi: 10.1124/dmd.104.001412. [DOI] [PubMed] [Google Scholar]
- [34].Hewawasam P, Gribkoff VK, Pendri Y, Dworetzky SI, Meanwell NA, Martinez E, Boissard CG, Post-Munson DJ, Trojnacki JT, Yeleswaram K, Pajor LM, Knipe J, Gao Q, Perrone R, Starrett JE. Bioorg. Med. Chem. Lett. 2002;12:1023. doi: 10.1016/s0960-894x(02)00101-4. [DOI] [PubMed] [Google Scholar]
- [35].Gribkoff VK, Starrett JE, Jr., Dworetzky SI, Hewawasam P, Boissard CG, Cook DA, Frantz SW, Heman K, Hibbard JR, Huston K, Johnson G, Krishnan BS, Kinney GG, Lombardo LA, Meanwell NA, Molinoff PB, Myers RA, Moon SL, Ortiz A, Pajor L, Pieschl RL, Post-Munson DJ, Signor LJ, Srinivas N, Taber MT, Thalody G, Trojnacki JT, Wiener H, Yeleswaram K, Yeola SW. Nat. Med. 2001;7:471. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]
- [36].Hewawasam P, Erway M, Moon SL, Knipe J, Weiner H, Boissard CG, Post-Munson DJ, Gao Q, Huang S, Gribkoff VK, Meanwell NA. J. Med. Chem. 2002;45:1487. doi: 10.1021/jm0101850. [DOI] [PubMed] [Google Scholar]
- [37].Dan Y, Poo MM. Physiol. Rev. 2006;86:1033. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- [38].Bhalla US, Iyengar R. Science. 1999;283:381. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- [39].Schiffer WK, Volkow ND, Fowler JS, Alexoff DL, Logan J, Dewey SL. Synapse. 2006;59:243. doi: 10.1002/syn.20235. [DOI] [PubMed] [Google Scholar]
- [40].Verheij MM, Cools AR. J. Neurochem. 2007;100:810. doi: 10.1111/j.1471-4159.2006.04259.x. [DOI] [PubMed] [Google Scholar]
- [41].Lee CR, Tepper JM. J. Neurosci. 2007;27:6531. doi: 10.1523/JNEUROSCI.1678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang L, Liu Y, Chen X. J. Physiol. 2005;568:469. doi: 10.1113/jphysiol.2005.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bell DC, Butcher AJ, Berrow NS, Page KM, Brust PF, Nesterova A, Stauderman KA, Seabrook GR, Nürnberg B, Dolphin AC. J. Neurophysiol. 2001;85:816. doi: 10.1152/jn.2001.85.2.816. [DOI] [PubMed] [Google Scholar]
- [44].Gazulla J, Tintoré MA. Med. Hypotheses. 2007;68:131. doi: 10.1016/j.mehy.2006.06.014. [DOI] [PubMed] [Google Scholar]
- [45].Kotaleski JH, Lester D, Blackwell KT. Integr. Physiol. Behav. Sci. 2002;37:265. doi: 10.1007/BF02734249. [DOI] [PubMed] [Google Scholar]
- [46].Gauthier S, Leblanc R, Robitaille Y, Quirion R, Carlsson G, Beaulieu M, Bouchard R, Dastoor D, Ervin F, Gauthier L. Can. J. Neurol. Sci. 1986;13:394. doi: 10.1017/s0317167100036969. [DOI] [PubMed] [Google Scholar]
- [47].Cohen SP, Dragovich A. Anesthesiol. Clin. 2007;25:863. doi: 10.1016/j.anclin.2007.07.001. [DOI] [PubMed] [Google Scholar]
- [48].Baert L, Schueller L, Tardy Y, Macbride D, Klooster GV, Borghys H, Clessens E, Van Den Mooter G, Van Gyseghem E, Van Remoortere P, Wigerinck P, Rosier J. Int. J. Pharm. 2008;355:38. doi: 10.1016/j.ijpharm.2008.01.029. [DOI] [PubMed] [Google Scholar]
- [49].Saraga F, Wu CP, Zhang L, Skinner FK. J. Physiol. 2003;552:673. doi: 10.1113/jphysiol.2003.046177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Korngreen A, Kaiser KM, Zilberter Y. J. Physiol. 2005;562:421. doi: 10.1113/jphysiol.2004.077032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].D’Angelo E, Nieus T, Maffei A, Armano S, Rossi P, Taglietti V, Fontana A, Naldi G. Neuroscience. 2001;21:759. doi: 10.1523/JNEUROSCI.21-03-00759.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Silberstein SD, Cady RK, Sheftell FD, Almas M, Parsons B, Albert KS. Headache. 2007;47:673. doi: 10.1111/j.1526-4610.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- [53].Sandrini G, Perrotta A, Nappi G. Expert Rev. Neurother. 2006;6:1413. doi: 10.1586/14737175.6.10.1413. [DOI] [PubMed] [Google Scholar]
- [54].Hughes ZA, Starr KR, Langmead CJ, Hill M, Bartoszyk GD, Hagan JJ. Eur. J. Pharmacol. 2005;510:49. doi: 10.1016/j.ejphar.2005.01.018. [DOI] [PubMed] [Google Scholar]
- [55].de Paulis T. IDrugs. 2007;10:193. [PubMed] [Google Scholar]
- [56].Gourion D, Perrin E, Quintin P. Encephale. 2004;30:392. doi: 10.1016/s0013-7006(04)95453-x. [DOI] [PubMed] [Google Scholar]
- [57].Solai LK, Mulsant BH, Pollock BG. Drugs Aging. 2001;18:355. doi: 10.2165/00002512-200118050-00006. [DOI] [PubMed] [Google Scholar]
- [58].Siddiqui A, Niazi A, Shaharyar S, Wilson CA. Pharmacol. Biochem. Behav. 2007;87:386. doi: 10.1016/j.pbb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- [59].Gefvert O, Lundberg T, Wieselgren IM, Bergström M, Långström B, Wiesel F, Lindström L. Eur. Neuropsychopharmacol. 2001;11:105. doi: 10.1016/s0924-977x(00)00133-4. [DOI] [PubMed] [Google Scholar]
- [60].Li Z, Ichikawa J, Huang M, Prus AJ, Dai J, Meltzer HY. Psychopharmacol. (Berl.) 2005;183:144. doi: 10.1007/s00213-005-0170-9. [DOI] [PubMed] [Google Scholar]
- [61].Szarfman A, Tonning JM, Levine JG, Doraiswamy PM. Pharmacotherapy. 2006;26:748. doi: 10.1592/phco.26.6.748. [DOI] [PubMed] [Google Scholar]
- [62]. http://www.fabrekramer.com/TGBA01AD.html.
- [63]. http://www.technologypartners.com/assets/Saegis.pdf.
- [64].Laramie M, Gaster, Frank D. King. Med. Res. Rev. 1998;17:163. [Google Scholar]
- [65].Reynolds GP, Mason SL, Meldrum A, De Keczer S, Parnes H, Eglen RM, Wong EH. Br. J. Pharmacol. 1995;114:993. doi: 10.1111/j.1476-5381.1995.tb13303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bai O, Zhang H, Li XM. Brain Res. 2004;1010:81. doi: 10.1016/j.brainres.2004.02.064. [DOI] [PubMed] [Google Scholar]
- [67].Green W, Patil P, Marsden CA, Bennett GW, Wigmore PM. Brain Res. 2006;1070:242. doi: 10.1016/j.brainres.2005.11.047. [DOI] [PubMed] [Google Scholar]
- [68].Montgomery SA, Baldwin DS, Blier P, Fineberg NA, Kasper S, Lader M, Lam RW, Lépine JP, Möller HJ, Nutt DJ, Rouillon F, Schatzberg AF, Thase ME. Int. Clin. Psychopharmacol. 2007;22:323. doi: 10.1097/YIC.0b013e3282eff7e0. [DOI] [PubMed] [Google Scholar]
- [69].Slattery DA, Morrow JA, Hudson AL, Hill DR, Nutt DJ, Henry B. Neuropsychopharmacology. 2005;30:1278. doi: 10.1038/sj.npp.1300717. [DOI] [PubMed] [Google Scholar]
- [70].Schüle C. J. Neuroendocrinol. 2007;19:213. doi: 10.1111/j.1365-2826.2006.01516.x. [DOI] [PubMed] [Google Scholar]
- [71].Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. Stress. 2003;6:18. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- [72].Spinelli S, Ballard T, Gatti-McArthur S, Richards GJ, Kapps M, Woltering T, Wichmann J, Stadler H, Feldon J, Pryce CR. Psychopharmacology (Berl) 2005;179:292. doi: 10.1007/s00213-004-2126-x. [DOI] [PubMed] [Google Scholar]
- [73].Nordquist RE, Steckler T, Wettstein JG, Mackie C, Spooren W. Psychopharmacol. (Berl.) 2008 doi: 10.1007/s00213-008-1096-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [74].Lea PM, Faden AI. CNS Drug Rev. (4th) 2006;12:149. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Simonyi A, Schachtman TR, Christoffersen GR. Drug News Perspect. 2005;18:353. doi: 10.1358/dnp.2005.18.6.927927. [DOI] [PubMed] [Google Scholar]
- [76].Slassi A, Isaac M, Edwards L, Minidis A, Wensbo D, Mattsson J, Nilsson K, Raboisson P, McLeod D, Stormann TM, Hammerland LG, Johnson E. Curr. Top. Med. Chem. 2005;5:897. doi: 10.2174/1568026054750236. [DOI] [PubMed] [Google Scholar]
- [77].Mutani R, Cantello R, Gianelli M, Civardi C. Ital. J. Neurol. Sci. 1995;16:217. doi: 10.1007/BF02282992. [DOI] [PubMed] [Google Scholar]
- [78].Katz I, Kim J, Gale K, Kondratyev A. J. Pharmacol. Exp. Ther. 2007;322:494. doi: 10.1124/jpet.107.123133. [DOI] [PubMed] [Google Scholar]
- [79].Kwon MO, Herrling P. Neurodegener. Dis. 2006;3:148. doi: 10.1159/000094773. [DOI] [PubMed] [Google Scholar]
- [80].Iwama T, Nagai Y, Tamura N, Harada S, Nagaoka A. Eur. J. Pharmacol. 1991;197:187. doi: 10.1016/0014-2999(91)90520-z. [DOI] [PubMed] [Google Scholar]
- [81].Hoyte L, Barber PA, Buchan AM, Hill MD. Curr. Mol. Med. 2004;4:131. doi: 10.2174/1566524043479248. [DOI] [PubMed] [Google Scholar]
- [82].Muir KW, Grosset DG, Lees KR. Clin. Neuropharmacol. 1997;20:311. doi: 10.1097/00002826-199708000-00003. [DOI] [PubMed] [Google Scholar]
- [83].Kroppenstedt SN, Schneider GH, Thomale UW, Unterberg AW. Acta Neurochir. Suppl. 1998;71:114. doi: 10.1007/978-3-7091-6475-4_34. [DOI] [PubMed] [Google Scholar]
- [84].Chapman DE, Keefe KA, Wilcox KS. J. Neurophysiol. 2003;89:69. doi: 10.1152/jn.00342.2002. [DOI] [PubMed] [Google Scholar]
- [85].Berger ML, Rebernik P. J. Pharmacol. Exp. Ther. 1999;289:1584. [PubMed] [Google Scholar]
- [86].Howes JF, Bell C. Neurotherapeutics. 2007;4:126. doi: 10.1016/j.nurt.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Denes L, Szilágyi G, Gál A, Nagy Z. Brain Res. Bull. 2006;70:260. doi: 10.1016/j.brainresbull.2006.02.024. [DOI] [PubMed] [Google Scholar]
- [88].Rogawski MA. Epilepsy Res. 2006;69:273. doi: 10.1016/j.eplepsyres.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jakus R, Graf M, Ando RD, Balogh B, Gacsalyi I, Levay G, Kantor S, Bagdy G. Brain Res. 2004;1008:236. doi: 10.1016/j.brainres.2004.01.087. [DOI] [PubMed] [Google Scholar]
- [90].Béracochéa D, Philippin JN, Meunier S, Morain P, Bernard K. Psychopharmacology (Berl.) 2007;193:63. doi: 10.1007/s00213-007-0765-4. [DOI] [PubMed] [Google Scholar]
- [91].Lockhart BP, Rodriguez M, Mourlevat S, Peron P, Catesson S, Villain N, Galizzi JP, Boutin JA, Lestage P. Eur. J. Pharmacol. 2007;561:23. doi: 10.1016/j.ejphar.2007.01.030. [DOI] [PubMed] [Google Scholar]
- [92].Rosi S, Giovannini MG, Lestage PJ, Muñoz C, Corte LD, Pepeu G. Neurosci. Lett. 2004;361:120. doi: 10.1016/j.neulet.2003.12.061. [DOI] [PubMed] [Google Scholar]
- [93].Bourasset F, Bernard K, Muñoz C, Genissel P, Scherrmann JM. Drug Metab. Dispos. 2005;33:1137. doi: 10.1124/dmd.105.004424. [DOI] [PubMed] [Google Scholar]
- [94].Lee HJ, Pogatzki-Zahn EM, Brennan TJ. J. Pain. 2006;7:768. doi: 10.1016/j.jpain.2006.03.010. [DOI] [PubMed] [Google Scholar]
- [95].Alt A, Weiss B, Ogden AM, Li X, Gleason SD, Calligaro DO, Bleakman D, Witkin JM. Psychopharmacology (Berl.) 2006;185:240. doi: 10.1007/s00213-005-0292-0. [DOI] [PubMed] [Google Scholar]
- [96].Jones N, O’Neill MJ, Tricklebank M, Libri V, Williams SC. Psychopharmacology (Berl.) 2005;180:743. doi: 10.1007/s00213-005-2254-y. [DOI] [PubMed] [Google Scholar]
- [97].Giardina WJ, Williams M. CNS Drug Rev. 2001;7:305. doi: 10.1111/j.1527-3458.2001.tb00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gorelick DA, Gardner EL, Xi ZX. Drugs. 2004;64:1547. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- [99].Foster JD, Cervinski MA, Gorentla BK, Vaughan RA. Handb. Exp. Pharmacol. 2006;175:197. doi: 10.1007/3-540-29784-7_10. [DOI] [PubMed] [Google Scholar]
- [100].O’Leary OF, Bechtholt AJ, Crowley JJ, Hill TE, Page ME, Lucki I. Psychopharmacology (Berl.) 2007;192:357. doi: 10.1007/s00213-007-0728-9. [DOI] [PubMed] [Google Scholar]
- [101].Sastre-Coll A, Esteban S, Miralles A, Zanetti R, García-Sevilla JA. Neurosci. Lett. 2001;301:29. doi: 10.1016/s0304-3940(01)01599-3. [DOI] [PubMed] [Google Scholar]
- [102].Reimann W, Schneider F. Eur. J. Pharmacol. 1998;349:199. doi: 10.1016/s0014-2999(98)00195-2. [DOI] [PubMed] [Google Scholar]
- [103].Heidbreder C. Eur. J. Pharmacol. 2005;526:101. doi: 10.1016/j.ejphar.2005.09.038. [DOI] [PubMed] [Google Scholar]
- [104].Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Makris N, Surman C, Aleardi M, Mick E, Biederman J. Arch. Gen. Psychiatry. 2008;65:102. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- [105].Reneman L, De Bruin K, Lavalaye J, Gunning WB, Booij J. Synapse. 2001;39:193. doi: 10.1002/1098-2396(20010301)39:3<193::AID-SYN1000>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- [106].Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Expert Rev. Neurother. 2007;7:1337. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Menon M, Jensen J, Vitcu I, Graff-Guerrero A, Crawley A, Smith MA, Kapur S. Biol. Psychiatry. 2007;62:765. doi: 10.1016/j.biopsych.2006.10.020. [DOI] [PubMed] [Google Scholar]
- [108].Maguire GA. Am. J. Health Syst. Pharm. 2002;59:4. doi: 10.1093/ajhp/59.suppl_5.S4. [DOI] [PubMed] [Google Scholar]
- [109].Reymann K, Pohle W, Müller-Welde P, Ott T. Biomed. Biochim. Acta. 1983;42:1247. [PubMed] [Google Scholar]
- [110].Juckel G, Schlagenhauf F, Koslowski M, Wüstenberg T, Villringer A, Knutson B, Wrase J, Heinz A. Neuroimage. 2006;29:409. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- [111].Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S. Psychopharmacology (Berl.) 2002;163:102. doi: 10.1007/s00213-002-1166-3. [DOI] [PubMed] [Google Scholar]
- [112].Vann RE, Rosecrans JA, James JR, Philibin SD, Robinson SE. Brain Res. 2006;1117:18. doi: 10.1016/j.brainres.2006.07.110. [DOI] [PubMed] [Google Scholar]
- [113].Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, Perlmutter JS. Proc. Natl. Acad. Sci. USA. 2002;99:17113. doi: 10.1073/pnas.012260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hall ED, Andrus PK, Oostveen JA, Althaus JS, VonVoigtlander PF. Brain Res. 1996;742:80. doi: 10.1016/s0006-8993(96)00968-7. [DOI] [PubMed] [Google Scholar]
- [115].Zarate CA, Jr., Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, Charney DS, Manji HK. Biol. Psychiatry. 2004;56:54. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]
- [116].Aiken CB. J. Clin. Psychiatry. 2007;68:1230. [PubMed] [Google Scholar]
- [117].Ramirez AD, Wong SK, Menniti FS. Eur. J. Pharmacol. 2003;475:29. doi: 10.1016/s0014-2999(03)02087-9. [DOI] [PubMed] [Google Scholar]
- [118].Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Am. J. Psychiatry. 2007;164:1411. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- [119].Gründer G, Boy c., Bröcheler A, Fellows C, Hiemke C, Büll U, Rösch F, Vernaleken I, Schäfer W. J. Nucl. Med. 2007;48:263. [Google Scholar]
- [120].Du F, Li R, Huang Y, Li X, Le W. Eur. J. Neurosci. 2005;22:2422. doi: 10.1111/j.1460-9568.2005.04438.x. [DOI] [PubMed] [Google Scholar]
- [121].Bogan RK. Expert Opin. Pharmacother. 2008;9:611. doi: 10.1517/14656566.9.4.611. [DOI] [PubMed] [Google Scholar]
- [122].Happe S, Sauter C, Klösch G, Saletu B, Zeitlhofer J. Neuropsychobiology. 2003;48:82. doi: 10.1159/000072882. [DOI] [PubMed] [Google Scholar]
- [123].Obrietan K, van den Pol AN. J. Neurophysiol. 1999;82:94. doi: 10.1152/jn.1999.82.1.94. [DOI] [PubMed] [Google Scholar]
- [124].Uruno K, O’Connor MJ, Masukawa LM. Brain Res. 1995;695:163. doi: 10.1016/0006-8993(95)00652-7. [DOI] [PubMed] [Google Scholar]
- [125].Hasselmo ME. Behav. Brain Res. 1995;67:1. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- [126].McLeod MC, Sundram S, Dean B. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:560. doi: 10.1016/j.pnpbp.2007.10.017. [DOI] [PubMed] [Google Scholar]
- [127].Vargas ML, Abella C, Hernandez J. Br. J. Pharmacol. 2001;133:1355. doi: 10.1038/sj.bjp.0704201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kendall TJ, Minchin MC. Br. J. Pharmacol. 1982;75:219. doi: 10.1111/j.1476-5381.1982.tb08776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zhang L, Zhang Y, Wennberg R. J. Pharmacol. Exp. Ther. 1998;286:1177. [PubMed] [Google Scholar]
- [130].Bauer J, Schwalen S. Nervenarzt. 2000;71:495. doi: 10.1007/s001150050614. [DOI] [PubMed] [Google Scholar]
- [131].Coppola G, Capovilla G, Montagnini A, Romeo A, Spanò M, Tortorella G, Veggiotti P, Viri M, Pascotto A. Epilepsy Res. 2002;49:45. doi: 10.1016/s0920-1211(02)00010-4. [DOI] [PubMed] [Google Scholar]
- [132].Jansen JF, Aldenkamp AP, Marian Majoie HJ, Reijs RP, de Krom MC, Hofman PA, Eline Kooi M, Nicolay K, Backes WH. Epilepsy Behav. 2006;9:181. doi: 10.1016/j.yebeh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- [133].Petroff OA, Hyder F, Rothman DL, Mattson RH. Epilepsia. 2001;42:543. doi: 10.1046/j.1528-1157.2001.18800.x. [DOI] [PubMed] [Google Scholar]
- [134].Baf MH, Subhash MN, Lakshmana KM, Rao BS. Neurochem. Int. 1994;24:67. doi: 10.1016/0197-0186(94)90130-9. [DOI] [PubMed] [Google Scholar]
- [135].Gernert M, Fedrowitz M, Wlaz P, Löscher W. Eur. J. Neurosci. 2004;20:2377. doi: 10.1111/j.1460-9568.2004.03699.x. [DOI] [PubMed] [Google Scholar]
- [136].Audic-Gerard F, Szepetowski P, Genton P. Rev. Neurol. (Paris) 2003;159:189. [PubMed] [Google Scholar]
- [137].Reynolds MF, Sisk EC, Rasgon NL. Curr. Med. Chem. 2007;14:2799. doi: 10.2174/092986707782360088. [DOI] [PubMed] [Google Scholar]
- [138].Fujiki M, Kobayashi H, Uchida S, Inoue R, Ishii K. Brain Res. 2005;1043:236. doi: 10.1016/j.brainres.2005.02.063. [DOI] [PubMed] [Google Scholar]
- [139].Mikami A, Masuoka T, Yasuda M, Yamamoto Y, Kamei C. Eur. J. Pharmacol. 2007;575:82. doi: 10.1016/j.ejphar.2007.07.010. [DOI] [PubMed] [Google Scholar]
- [140].Lazareno S, Popham A, Birdsall NJ. J. Mol. Neurosci. 2003;20:363. doi: 10.1385/JMN:20:3:363. [DOI] [PubMed] [Google Scholar]
- [141].Brier TJ, Mellor IR, Tikhonov DB, Neagoe I, Shao Z, Brierley MJ, Strømgaard K, Jaroszewski JW, Krogsgaard-Larsen P, Usherwood PN. Mol. Pharmacol. 2003;64:954. doi: 10.1124/mol.64.4.954. [DOI] [PubMed] [Google Scholar]
- [142].Anis N, Sherby S, Goodnow R, Jr, Niwa M, Konno K, Kallimopoulos T, Bukownik R, Nakanishi K, Usherwood P, Eldefrawi A, et al. J. Pharmacol. Exp. Ther. 1990;254:764. [PubMed] [Google Scholar]
- [143].Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, Turkington TG. Neuropsychopharmacology. 2007;32:2441. doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- [144].Levin ED, McClernon FJ, Rezvani AH. Psychopharmacology (Berl.) 2006;184:523. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- [145].Tang L, Kongsamut S. Eur. J. Pharmacol. 1996;300:71. doi: 10.1016/0014-2999(96)00002-7. [DOI] [PubMed] [Google Scholar]
- [146].Smith CP, Woods-Kettelberger AT, Corbett R, Chesson SM, Bores GM, Petko WW, Roehr JE, Kongsamut S. Neurochem. Res. 1996;21:575. doi: 10.1007/BF02527756. [DOI] [PubMed] [Google Scholar]
- [147].Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. Eur. J. Pharmacol. 2003;461:99. doi: 10.1016/s0014-2999(03)01310-4. [DOI] [PubMed] [Google Scholar]
- [148].Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS. Life Sci. 2003;73:3175. doi: 10.1016/j.lfs.2003.06.007. [DOI] [PubMed] [Google Scholar]
- [149].Schwarz AJ, Gozzi A, Reese T, Heidbreder CA, Bifone A. Magn. Reson. Imaging. 2007;25:811. doi: 10.1016/j.mri.2007.02.017. [DOI] [PubMed] [Google Scholar]
- [150].Schwarz AJ, Gozzi A, Reese T, Bifone A. Neuroimage. 2007;34:1627. doi: 10.1016/j.neuroimage.2006.11.010. [DOI] [PubMed] [Google Scholar]
- [151].Easton N, Marshall F, Fone K, Marsden C. Neuropharmacology. 2007;52:812. doi: 10.1016/j.neuropharm.2006.09.024. [DOI] [PubMed] [Google Scholar]
- [152].Pattij T, Vanderschuren LJ. Trends Pharmacol. Sci. 2008;29:192. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [153].Callaway JK, Knight MJ, Watkins DJ, Beart PM, Jarrott B. Stroke. 1999;30:2704. doi: 10.1161/01.str.30.12.2704. [DOI] [PubMed] [Google Scholar]
- [154].Callaway JK, Castillo-Melendez M, Giardina SF, Krstew EK, Beart PM, Jarrott B. Neuropharmacology. 2004;47:146. doi: 10.1016/j.neuropharm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- [155].Nicolazzo JA, Nguyen TT, Katneni K, Steuten JA, Smith G, Jarrott B, Callaway JK, Charman SA. J. Pharm. Pharmacol. 2008;60:171. doi: 10.1211/jpp.60.2.0005. [DOI] [PubMed] [Google Scholar]
- [156].Carter AJ, Grauert M, Pschorn U, Bechtel WD, Bartmann-Lindholm C, Qu Y, Scheuer T, Catterall WA, Weiser T. Proc. Natl. Acad. Sci. USA. 2000;97:4944. doi: 10.1073/pnas.040577097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Meythaler J. Curr. Opin. Investig. Drugs. 2002;3:1733. [PubMed] [Google Scholar]
- [158].Dekker LV, Daniels Z, Hick C, Elsegood K, Bowden S, Szestak T, Burley JR, Southan A, Cronk D, James IF. Eur. J. Pharmacol. 2005;528:52. doi: 10.1016/j.ejphar.2005.10.035. [DOI] [PubMed] [Google Scholar]
- [159].Dave JR, Lin Y, Ved HS, Koenig ML, Clapp L, Hunter J, Tortella FC. Neurotox. Res. 2001;3:381. doi: 10.1007/BF03033199. [DOI] [PubMed] [Google Scholar]
- [160].Dave JR, Yao C, Moffett JR, Berti R, Koenig M, Tortella FC. Neurotox. Res. 2003;5:213. doi: 10.1007/BF03033141. [DOI] [PubMed] [Google Scholar]
- [161].Annoura H, Nakanishi K, Toba T, Takemoto N, Imajo S, Miyajima A, Tamura-Horikawa Y, Tamura S. J. Med. Chem. 2000;43:3372. doi: 10.1021/jm000143w. [DOI] [PubMed] [Google Scholar]
- [162].Kotani Y, Morimoto N, Oida Y, Tamura Y, Tamura S, Inoue T, Shimazawa M, Yoshimura S, Iwama T, Hara H. Neuroscience. 2007;149:779. doi: 10.1016/j.neuroscience.2007.08.029. [DOI] [PubMed] [Google Scholar]
- [163].Sun L, Lin SS. Epilepsia. 2000;41:263. doi: 10.1111/j.1528-1157.2000.tb00154.x. [DOI] [PubMed] [Google Scholar]
- [164].Danysz W, Parsons CG. Neurotox. Res. 2002;4:119. doi: 10.1080/10298420290015872. [DOI] [PubMed] [Google Scholar]
- [165].Contreras M, Ceric F, Torrealba F. Science. 2007;318:655. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- [166].Ramaprasad S, Ripp E, Pi J, Lyon M. Magn. Reson. Imaging. 2005;23:859. doi: 10.1016/j.mri.2005.07.007. [DOI] [PubMed] [Google Scholar]
- [167].Quiroz JA, Gould TD, Manji HK. Mol. Interv. 2004;4:259. doi: 10.1124/mi.4.5.6. [DOI] [PubMed] [Google Scholar]
- [168].Meshkibaf MH, Subhash MN, Lakshmana KM, Rao BS. Neurochem. Res. 1995;20:773. doi: 10.1007/BF00969688. [DOI] [PubMed] [Google Scholar]
- [169].Tarnawa I, Bölcskei H, Kocsis P. Recent Patents CNS Drug Discov. 2007;2:57. doi: 10.2174/157488907779561754. [DOI] [PubMed] [Google Scholar]
- [170].Iivanainen M. J. Intellect. Disabil. Res. 1998;42:24. [PubMed] [Google Scholar]
- [171].Soldovieri MV, Cilio MR, Miceli F, Bellini G, Miraglia del Giudice E, Castaldo P, Hernandez CC, Shapiro MS, Pascotto A, Annunziato L, Taglialatela M. J. Neurosci. 2007;27:4919. doi: 10.1523/JNEUROSCI.0580-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Marzatico F, Dagani F, Curti D, Benzi G. Neurochem. Res. 1987;12:33. doi: 10.1007/BF00971361. [DOI] [PubMed] [Google Scholar]
- [173].Chorvat RJ, Zaczek R, Brown BS. Expert Opin. Investig. Drugs. 1998;7:499. doi: 10.1517/13543784.7.4.499. [DOI] [PubMed] [Google Scholar]
- [174].Zaczek R, Chorvat RJ, Saye JA, Pierdomenico ME, Maciag CM, Logue AR, Fisher BN, Rominger DH, Earl RA. J. Pharmacol. Exp. Ther. 1998;285:724. [PubMed] [Google Scholar]
- [175].Sansone V, Tawil R. Neurotherapeutics. 2007;4:233. doi: 10.1016/j.nurt.2007.01.005. [DOI] [PubMed] [Google Scholar]
- [176].Camerino DC, Tricarico D, Desaphy JF. Neurotherapeutics. 2007;4:184. doi: 10.1016/j.nurt.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [177].Wong EY, Herbert J. Eur. J. Neurosci. 2005;22:785. doi: 10.1111/j.1460-9568.2005.04277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Zhu MY, Wang WP, Bissette G. Neuroscience. 2006;141:2019. doi: 10.1016/j.neuroscience.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Calfa G, Bussolino D, Molina VA. Behav. Brain Res. 2007;181:23. doi: 10.1016/j.bbr.2007.03.020. [DOI] [PubMed] [Google Scholar]
- [180].Gopalakrishnan M, Whiteaker KL, Molinari EJ, Davis-Taber R, Scott VE, Shieh CC, Buckner SA, Milicic I, Cain JC, Postl S, Sullivan JP, Brioni JD. J. Pharmacol. Exp. Ther. 1999;289:551. [PubMed] [Google Scholar]
- [181].Buckner SA, Milicic I, Daza A, Davis-Taber R, Scott VE, Sullivan JP, Brioni JD. Eur. J. Pharmacol. 2000;400:287. doi: 10.1016/s0014-2999(00)00388-5. [DOI] [PubMed] [Google Scholar]
- [182].Hunnicutt EJ, Jr., Davis JN, Chisholm JC. Eur. J. Pharmacol. 1994;261:R1–3. doi: 10.1016/0014-2999(94)90325-5. [DOI] [PubMed] [Google Scholar]
- [183].Shieh CC, Turner SC, Zhang XF, Milicic I, Parihar A, Jinkerson T, Wilkins J, Buckner SA, Gopalakrishnan M. Br. J. Pharmacol. 2007;151:798. doi: 10.1038/sj.bjp.0707278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Mora TC, Suarez-Kurtz G. Br. J. Pharmacol. 2005;144:636. doi: 10.1038/sj.bjp.0706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Parihar AS, Groebe DR, Scott VE, Feng J, Zhang XF, Warrior U, Gopalakrishnan M, Shieh CC. Assay Drug Dev. Technol. 2003;1:647. doi: 10.1089/154065803770381002. [DOI] [PubMed] [Google Scholar]
- [186].Kwon MO, Fischer F, Matthisson M, Herrling P. Neurodegener. Dis. 2004;1:113. doi: 10.1159/000081242. [DOI] [PubMed] [Google Scholar]
- [187].Cheney JA, Weisser JD, Bareyre FM, Laurer HL, Saatman KE, Raghupathi R, Gribkoff V, Starrett JE, McIntosh TK. J. Cereb. Blood Flow Metab. 2001;21:396. doi: 10.1097/00004647-200104000-00008. [DOI] [PubMed] [Google Scholar]
- [188].Korsgaard MP, Hartz BP, Brown WD, Ahring PK, Strøbaek D, Mirza NR. J. Pharmacol. Exp. Ther. 2005;314:282. doi: 10.1124/jpet.105.083923. [DOI] [PubMed] [Google Scholar]
- [189].Schroder RL, Jespersen T, Christophersen P, Strobaek D, Jensen BS, Olesen SP. Neuropharmacology. 2001;40:888. doi: 10.1016/s0028-3908(01)00029-6. [DOI] [PubMed] [Google Scholar]
- [190].Rose GM, Ong VS, Woodruff-Pak DS. Neurobiol. Aging. 2007;28:766. doi: 10.1016/j.neurobiolaging.2006.03.006. [DOI] [PubMed] [Google Scholar]
- [191].Bayés M, Rabasseda X, Prous JR. Methods Find Exp. Clin. Pharmacol. 2007;29:625. [PubMed] [Google Scholar]
- [192]. http://www.memorypharma.com/news/PR_061207.html.
- [193].Yagami T, Ueda K, Sakaeda T, Itoh N, Sakaguchi G, Okamura N, Hori Y, Fujimoto M. Biochem. Pharmacol. 2004;67:1153. doi: 10.1016/j.bcp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- [194].Amano T, Aihua Z, Matsubayashi H, Seki T, Serikawa T, Sasa M, Sakai N. J. Pharmacol. Sci. 2004;95:355. doi: 10.1254/jphs.fp0040233. [DOI] [PubMed] [Google Scholar]
- [195].Eigyo M, Shiomi T, Inoue Y, Sakaguchi I, Ishibashi C, Murata S, Koyabu K, Matsushita A, Adachi I, Ueda M. Jpn. J. Pharmacol. 1994;65:175. doi: 10.1254/jjp.65.175. [DOI] [PubMed] [Google Scholar]
- [196].Yasui M, Kawasaki K. Brain Res. 1994;642:146. doi: 10.1016/0006-8993(94)90916-4. [DOI] [PubMed] [Google Scholar]
- [197].Varming T, Christophersen P, MOller A, Peters D, Axelsson O, Nielsen E. Bioorg. Med. Chem. Lett. 1996;6:245. [Google Scholar]
- [198].O’Neill MJ, Bath CP, Dell CP, Hicks CA, Gilmore J, Ambler SJ, Ward MA, Bleakman D. Eur. J. Pharmacol. 1997;332:121. doi: 10.1016/s0014-2999(97)01074-1. [DOI] [PubMed] [Google Scholar]
- [199].Amico C, Marchetti C, Nobile M, Usai C. J. Neurosci. 1995;15:2839. doi: 10.1523/JNEUROSCI.15-04-02839.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Kasim S, Blake BL, Fan X, Chartoff E, Egami K, Breese GR, Hess EJ, Jinnah HA. Dev. Neurosci. 2006;28:505. doi: 10.1159/000095113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Striessnig J, Koschak A, Sinnegger-Brauns MJ, Hetzenauer A, Nguyen NK, Busquet P, Pelster G, Singewald N. Biochem. Soc. Trans. 2006;34:903. doi: 10.1042/BST0340903. [DOI] [PubMed] [Google Scholar]
- [202].Møller A, Christophersen P, Drejer J, Axelsson O, Peters D, Jensen LH, Nielsen EO. Neurol. Res. 1995;17:353. [PubMed] [Google Scholar]
- [203].Mordvintcev PI, Busse R, Mülsch A. Acta Neurol. Scand. 1997;95:219. doi: 10.1111/j.1600-0404.1997.tb00102.x. [DOI] [PubMed] [Google Scholar]
- [204].McBurney RN, Daly D, Fischer JB, Hu LY, Subbarao K, Knapp AG, Kobayashi K, Margolin L, Reddy NL, Goldin SM. J. Neurotrauma. 1992;9:531. [PubMed] [Google Scholar]
- [205].Fischer F, Matthisson M, Herrling P. Neurodegener. Dis. 2004;1:50. doi: 10.1159/000077879. [DOI] [PubMed] [Google Scholar]
- [206].Shan H, Messi ML, Zheng Z, Wang ZM, Delbono O. J. Physiol. 2003;553:49. doi: 10.1113/jphysiol.2003.047746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Castelli L, Magistretti J. Brain Res. 2006;1090:76. doi: 10.1016/j.brainres.2006.03.037. [DOI] [PubMed] [Google Scholar]
- [208].Giovannini F, Sher E, Webster R, Boot J, Lang B. Br. J. Pharmacol. 2002;136:1135. doi: 10.1038/sj.bjp.0704818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Strupp M, Zwergal A, Brandt T. Neurotherapeutics. 2007;4:267. doi: 10.1016/j.nurt.2007.01.014. [DOI] [PubMed] [Google Scholar]
- [210].Egorov AV, Heinemann U, Müller W. Eur. J. Neurosci. 2002;16:1305. doi: 10.1046/j.1460-9568.2002.02197.x. [DOI] [PubMed] [Google Scholar]
- [211].Fernandez HH, Chen JJ. Pharmacotherapy. 2007;27:174. doi: 10.1592/phco.27.12part2.174S. [DOI] [PubMed] [Google Scholar]
- [212].Adis International Limited Drugs R D. 2004;5:355. [Google Scholar]
- [213].Chazot PL. Curr. Opin. Investig. Drugs. 2001;2:809. [PubMed] [Google Scholar]
- [214].Cunningham MO, Woodhall GL, Thompson SE, Dooley DJ, Jones RS. Eur. J. Neurosci. 2004;20:1566. doi: 10.1111/j.1460-9568.2004.03625.x. [DOI] [PubMed] [Google Scholar]
- [215].Micheva KD, Taylor CP, Smith SJ. Mol. Pharmacol. 2006;70:467. doi: 10.1124/mol.106.023309. [DOI] [PubMed] [Google Scholar]
- [216].Di Donato M, Fantini F, Marchionni N. Cardiovasc. Drug Rev. 1992;10:404. [Google Scholar]
- [217].Di Donato M, Maioli M, Venturi F, Bürgisser C, Fantini F, Biamino G, Marchionni N. Am. Heart J. 1991;121:776. doi: 10.1016/0002-8703(91)90188-n. [DOI] [PubMed] [Google Scholar]
- [218].Gören MZ, Onat F. CNS Drug Rev. 2007;13:224. doi: 10.1111/j.1527-3458.2007.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Posner EB, Mohamed K, Marson AG. Seizure. 2005;14:117. doi: 10.1016/j.seizure.2004.12.003. [DOI] [PubMed] [Google Scholar]
- [220].Chung JM, Huguenard JR, Prince DA. J. Neurophysiol. 1993;70:20. doi: 10.1152/jn.1993.70.1.20. [DOI] [PubMed] [Google Scholar]
- [221].Huguenard JR. Annu. Rev. Physiol. 1996;58:329. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- [222].Nasledov GA, Skvortsova IV, Skorobovichuk NF. J. Evol. Biochem. Physiol. 2001;37:95. [PubMed] [Google Scholar]
- [223].Cui G, Bernier BE, Harnett MT, Morikawa H. J. Neurosci. 2007;27:4776. doi: 10.1523/JNEUROSCI.0139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Kondashevskaya MV, Kudrin VS, Malikova LA, Klodt PM, Makarova OV. Bull. Exp. Biol. Med. 2006;141:599. doi: 10.1007/s10517-006-0231-z. [DOI] [PubMed] [Google Scholar]
- [225].Rosenberg H, Davis M, James D, Pollock N, Stowell K. Orphanet. J. Rare Dis. 2007;24:2. doi: 10.1186/1750-1172-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Zhang L, Andou Y, Masuda S, Mitani A, Kataoka K. Neurosci. Lett. 1993;158:105. doi: 10.1016/0304-3940(93)90623-s. [DOI] [PubMed] [Google Scholar]
- [227].Popescu BO, Oprica M, Sajin M, Stanciu CL, Bajenaru O, Predescu A, Vidulescu C, Popescu LM. J. Cell. Mol. Med. 2002;6:555. doi: 10.1111/j.1582-4934.2002.tb00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Bezprozvanny I, Bezprozvannaya S, Ehrlich BE. Mol. Biol. Cell. 1994;5:97. doi: 10.1091/mbc.5.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Shapiro RE. Neurol. Sci. 2007;28:179. doi: 10.1007/s10072-007-0773-5. [DOI] [PubMed] [Google Scholar]
- [230].Goldstein J, Silberstein SD, Saper JR, Ryan RE, Jr, Lipton RB. Headache. 2006;46:444. doi: 10.1111/j.1526-4610.2006.00376.x. [DOI] [PubMed] [Google Scholar]
- [231].Boulenger JP, Marangos PJ. J. Neural Transm. Gen. Sect. 1989;78:9. doi: 10.1007/BF01247109. [DOI] [PubMed] [Google Scholar]
- [232].Winston JS, Strange BA, O’Doherty J, Dolan R. J. Nat. Neurosci. 2002;3:192. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- [233].Dodt HU, Leischner U, Schierloh A, Jährling N, Mauch CP, Deininger K, Deussing JM, Eder M, Zieglgänsberger W, Becker K. Nat. Methods. 2007;4:307. doi: 10.1038/nmeth1036. [DOI] [PubMed] [Google Scholar]



 Author Profile: Dr. Giorgio Ascoli received a Chemistry Ph.D. from Scuola Normale Superiore (Pisa, Italy), continued investigating neuronal protein binding at NIH, and moved to George Mason in 1997. He founded and directs the Neural Informatics, Structure, and Plasticity Center, a multidisciplinary team of psychologists, biologists, physicists, and engineers. He is Professor of Molecular Neuroscience and founding Editor-in-Chief of Neuroinformatics.
Author Profile: Dr. Giorgio Ascoli received a Chemistry Ph.D. from Scuola Normale Superiore (Pisa, Italy), continued investigating neuronal protein binding at NIH, and moved to George Mason in 1997. He founded and directs the Neural Informatics, Structure, and Plasticity Center, a multidisciplinary team of psychologists, biologists, physicists, and engineers. He is Professor of Molecular Neuroscience and founding Editor-in-Chief of Neuroinformatics.