Abstract
Previously, we have shown that systemic insults in single injury models produced immunosuppressive effects in burn, and a strong acute phase response in sepsis through hepatic gene expression. In order to investigate the implications of these effects on a combined injury, a double hit model was explored to mimic the progression of clinical burn-sepsis. Rodents were subjected to a 20% total body surface area (TSA) full-thickness burn injury, and 48 hours later underwent cecal ligation and puncture (CLP) to induce sepsis. Pathways related to innate immune signaling through cytokines and NF-KB were co regulated with xenobiotic metabolism genes and acute phase protein genes, and that these genes were suppressed early, and then activated. Furthermore, we were able to identify that, in addition to amino acid metabolism, pyruvate metabolism, fatty acid metabolism and NRF-2 mediated oxidative stress genes were down regulated over the time course. Overall, these observed trends within the double hit burn-sepsis model represent unique immune and metabolic pathways and dynamics not found in either injury, including an early suppression followed by overreaction of pro inflammatory mediators, and an increase in amino acid metabolism at the expense of central carbon pathways.
Keywords: Sepsis, burns, microarray analysis, RT-PCR analysis, liver
Introduction
After significant systemic injury, such as burn trauma, an immune response is triggered with an initial pro-inflammatory and subsequent anti-inflammatory phase. The innate immune system is critical to these mammalian immune responses and is tasked both with clearing foreign pathogens/debris and promoting wound healing. Interestingly, evidence further suggests that a series of multiple injuries, in succession, can produce an exaggerated and more pathologic immune response than after individual insults [1]. Experimental burn injury, followed by sepsis induced by cecal ligation and puncture, is a common model used to mimic this “double hit phenomenon,” as burn trauma is known to induce profound inflammation with subsequent immunosuppression and therefore increased susceptibility to polymicrobial sepsis [2,3]. Mechanistically, it is believed that burns induce an inflammatory response, followed by an anti inflammatory wound healing response that dominates the recovery phase [4]. This anti inflammatory state is believed to increase the severity of subsequent septic challenges by impeding the immune response [5,6] , which results in either overwhelming infection or a hyperinflammatory response [7]. In either setting, the end result is often multiple organ failure and, ultimately, death.
In the setting of inflammation and sepsis, the liver is at the crossroads of innate immune responses and systemic metabolic shifts [8], and therefore, examining hepatic metabolism is useful to characterize the systemic response of an immunocompromised host. With a rodent model of burn inflammation and subsequent cecal ligation and puncture (CLP) [9], the effects of burn injury on the host response to infection can be studied longitudinally. Previously, we have used a similar methodology to conduct studies of the longitudinal responses of both burn and infection independently, with an emphasis on both the first 24 hours of the acute phase injury, and the long term recovery response out to 8 days [10-12]. We observed that although both burn and CLP induce an inflammatory response, burn appears to promote an anti inflammatory state after 24 hours [12] with toll-like receptor production for recognizing infection [13], while CLP creates strong, persistent acute pro inflammatory response. By combining these injuries into a two-hit model, we aim to characterize the net response of a host that is in an anti inflammatory state due to a prior burn injury [12] when it is subject to the pro inflammatory stimulus that is CLP [11]. Furthermore, we aim to reconcile the differences in metabolic changes that were observed in both the single burn and CLP injuries by characterizing the mechanisms by which they interact in order to create the hyper metabolic conditions that plague the clinical occurrences of burn-sepsis that this animal model aims to mimic.
The complexity and diversity of the hepatic metabolic response to injury, necessitates a comprehensive and efficient analysis which can be accomplished using functional genomics. These techniques allow a wide subset of transcriptional responses and metabolic pathways to be characterized ranging from fatty acid metabolism to innate immune protein synthesis. Because the liver simultaneously manages both metabolic changes and proinflammatory signaling it is critical to characterize and interpret these responses in parallel. Significant Analysis of Microarrays (SAM) can be utilized [14] to determine significantly varying genes following injury by using multiple gene-specific t-tests to verify the significance of the observed fold changes. Subsequent Ingenuity Pathway Analysis (IPA) can then be used to identify relevant pathways that are different between groups. By integrating these techniques, as shown in Figure 1, the present study aims to characterize the long term functional and dynamic differences that are created when burn injury serves as a priming mechanism for a subsequent septic insult.
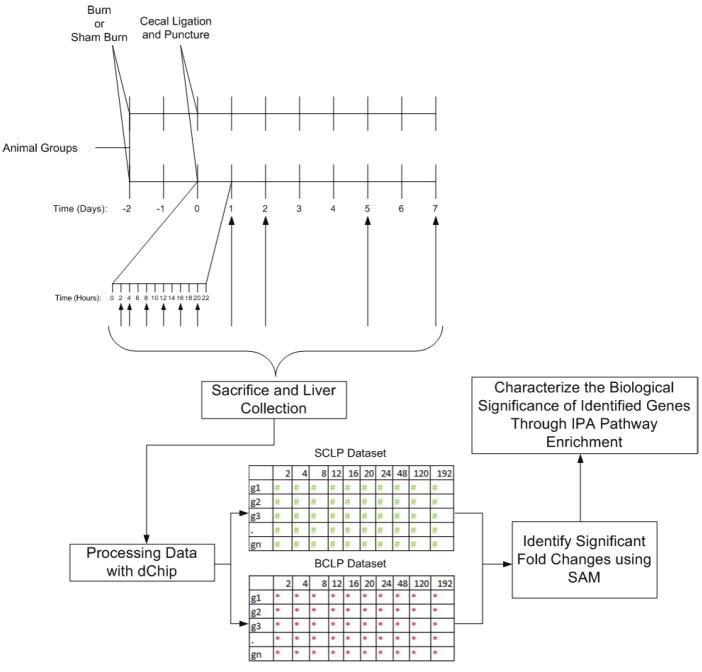
Figure 1.
Schematic Overview of the Experimental Design. Animals were first subjected to burn or sham burn treatments, and then 48 hours later, were subjected to cecal ligation and puncture. Sacrifice occurred at 2, 4, 8, 12, 16, 20, 24, 48, 120, and 192 hours post CLP. Microarray data from the liver was preprocessed using dChip software, and then compared using SAM (Statistical Analysis of Microarrays) to determine genes with significantly altered expression. This subset of genes was then processed using Ingenuity Pathway Analysis enrichment software in order to characterize the biological differences between the two injury models.
In order to analyze the maximum number of genes possible in the hepatic response, microarray chips were used to simultaneously measure mRNA levels of approximately 31,000 genes. In order to confirm that the trends we measure from the microarray samples are real experimentally, a small subset of genes was identified for further RT-PCR analysis, in order to confirm trends found in the microarray probe sets. Genes were selected that showed significant differences between injury conditions, and which were as representative as possible of the wide array of genes we aimed to observe. Therefore, genes that were selected include metabolite transporters, metabolic enzymes, pro inflammatory transcription factors, acute phase proteins, and signaling molecules. Furthermore, genes were selected at both short and long term time points, in order to increase confidence in the accuracy of the microarray results throughout the experimental period.
Materials and methods
Animal model
Male Sprague-Dawley rats (Charles River Labs, Wilmington, MA) weighing 150-200g were utilized. The animals were housed in a temperature-controlled environment (25°C) with a 12-hour light-dark cycle and provided water and standard chow ad libitum. All experimental procedures were carried out in accordance with National Research Council guidelines and approved by the Rutgers University Animal Care and Facilities Committee.
A systemic hypermetabolic response was induced by applying a full-thickness burn on an area of the dorsal skin corresponding to 20% of the total body surface area (TBSA) with 100% survival, no evidence of systemic hypoperfusion and no significant alterations on feeding patterns as performed previously [12]. Briefly, animals were shaved in the dorsal abdominal area and immersed in water at 100°C for 10 seconds to produce a full-thickness 20% TBSA scald injury.
The septic insult was induced by applying CLP treatment 48 hours post burn treatment, using the same methods previously employed by our lab for single injury CLP animals [10,15]. Briefly, the cecum of each rat was exposed and ligated just below the ileocecal valve, then punctured 4 times with a 20-gauge needle and replaced in the peritoneum once extrusions were observed.
Animals were sacrificed (starting at 9am) at different time points (2, 4, 8, 12, 16, 20, 24, 48, 120 and 192 hr post-treatment) as depicted in Figure 1. Liver tissues were collected and frozen for microarray analysis (n=3 per time point per group). The tissues were lysed and homogenized using Trizol and the RNA was further purified and treated with DNase using RNeasy columns (Qiagen). Then cRNA prepared from the RNA of liver tissues using protocols provided by Affymetrix were utilized to hybridize Rat Genome 230 2.0 Array (GeneChip, Affymetrix) comprised of more than 31,000 probe sets.
RT-PCR analysis
qPCR gene expression studies were performed using TaqMan gene expression assays. A detailed description of the methods can be found in the supplementary materials. The expression level of the housekeeping gene GADPH [16] was used as an internal reference, and all fold changes are displayed as comparisons between BCLP and SCLP levels of the gene at each time point.
Data analysis
Genome expression data analysis was accomplished through pairwise comparisons between the burn+CLP (BCLP) and sham burn+CLP SCLP groups at each time point. DNA chip analyzer (dChip) software [17] was used with invariant-set normalization and perfect match (PM) model to generate expression values. In order to characterize the probesets which show significant fold change at each time point, the method of statistical analysis of microarrays (SAM) was used [14]. This method compares the gene expression of the two response variables, BCLP and SCLP. Briefly, by computing a statistic gi for each gene i, the strength of the relationship between the response variable (BCLP) and the standard (SCLP) is measured. False discovery is controlled via the random permutation of the gene response sets, in order to calculate the probability that the observed response is statistically significant. Following this, we characterize the biological relevance of the genes found to be statistically significant through SAM by evaluating the enrichment of the corresponding subsets in circadian rhythm specific pathways using the pathway enrichment function (p<0.05) in Ingenuity Pathway Analysis (IPA) tools (Ingenuity Systems, Mountain View, CA) as well as analyzing the functions of individual genes extensively. For the analysis of the RT-PCR results, averaged normalized data for each experimental gene was compared between BCLP groups and SCLP groups using the 2-∆∆CT method [18].
Results
Identification of BCLP related patterns and characterization of per day changes
Hepatic gene expression levels were measured 2, 4, 8, 12, 16, 20, 24, 48, 120 and 168 hours following BCLP and SCLP treatment. Differences in gene expression in BCLP, when compared to SCLP, were identified at each time point. The complete list of genes identified can be found in Supplementary Table 2 described as up regulated or down regulated categories, relative to SCLP. Interestingly, for time points 16, 20, 48, and 168 hours post-injury, no significant gene expression differences were identified indicating identical gene expression between the BCLP and SCLP conditions for those time points. The full list of genes which passed IPA pathway analysis with p values less than .05 are shown in Supplementary Table 1 along with their specific IPA identified pathways. The most relevant to innate immunity and hepatic metabolism following injury are summarized below, and in Table 1.
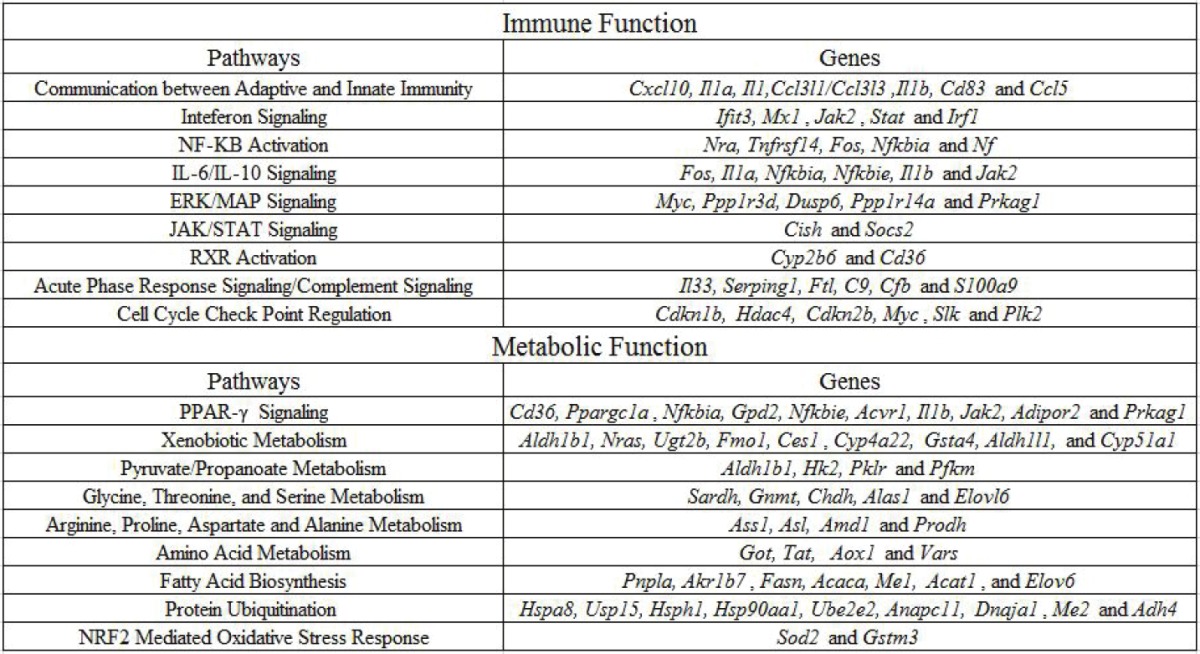
Table 1.
Genes that show significant fold change following burn priming when compared with CLP within key metabolic and immune pathways. All listed genes were identified by SAM to be significantly different between both conditions, and in agglomerate found by IPA to be significant in their associated pathways
|
Innate immunity related pathways
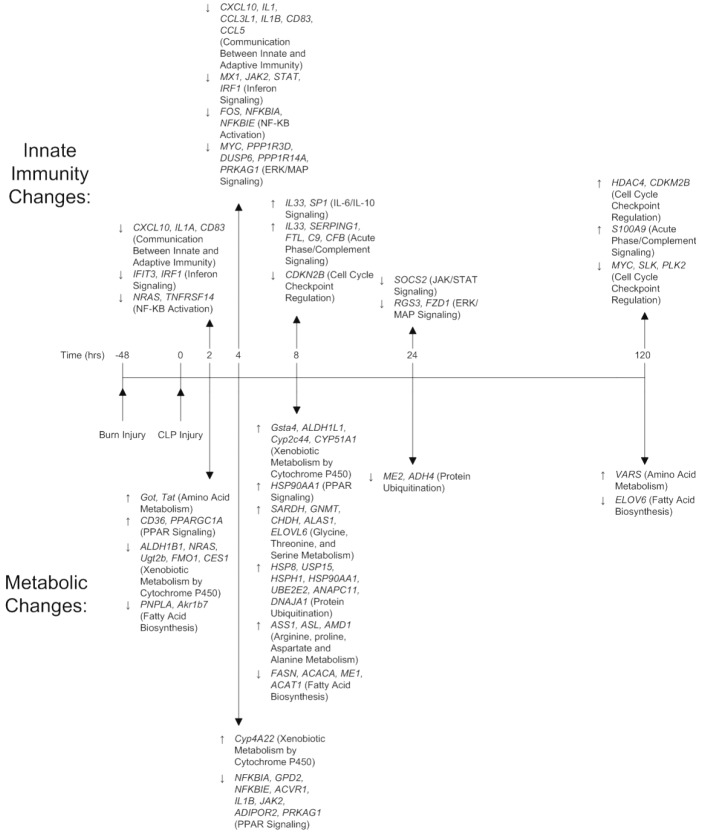
Over the 7 days post injury, 9 total pathways related to innate immunity showed significant changes in expression between BCLP and SCLP, indicating a change in transcriptional output between the two injuries. Specific genes associated with these pathways can be found in Table 1. Pathways which were down regulated in the first four hours include Communication between Adaptive and Innate Immune Systems, Interferon Signaling and NF-κB activation, which all correspond to key cytokines and intracellular proteins that recognize and propagate the inflammatory response. The IL-6/IL-10 Signaling pathway, related to the activity of key pro inflammatory cytokines, had similar dynamics, but remained suppressed 4 hours longer than previous pathways. The ERK/MAP signaling pathway, associated with G protein activation of the immune response, was similarly suppressed early, but also showed suppression at one day post injury. The JAK/STAT signaling pathway, associated in this case with suppression of cytokine signaling, does not follow a similar regulatory pattern, but is instead suppressed halfway through the first day. In addition, the chemokine receptor related pathway called RXR Activation was elevated midway through the first day as well. The acute phase response signaling/complement signaling pathway, associated with anti bacterial acute phase protein production, was activated both early in the time course, but also very late, at 5 days post injury. The final pathway is associated with Cell Cycle Check Point Regulation, which controls cell proliferation both during the immune response, and shows similar patterns to the complement signaling pathway, with early suppression of anti proliferators in the first day of injury, and late activation of anti proliferators on the fifth day of injury. Overall, significant activity is observed very early in the time course, as well as at the first day, and the fifth day marks.
Metabolism related pathways
In addition to pathways associated with innate immunity, 9 pathways related to hepatic metabolic changes showed significant changes following injury in BCLP compared to SCLP over a 7 day time course. Specific genes associated with these pathways can be found in Table 1. Pathways with heavy activity early in the time course include PPAR-γ Signaling and Xenobiotic Metabolism, which both are metabolic functions that crosstalk with innate immunity to degrade foreign bodies, and are activated early, then suppressed, or suppressed and then activated, respectively. Pyruvate/Propanoate Metabolism is a pathway critical to central carbon metabolism which is suppressed over the first four hours of injury. Specific amino acid pathways related to Glycine, Threonine, and Serine Metabolism and Arginine, Proline, Aspartate and Alanine Metabolism are activated 8 hours post injury, and in the case of the latter, persist to 12 hours post injury, at a time when previous pathways are winding down in activity. More general pathways related to Amino Acid Metabolism (including branched chain amino acid activity) were consistently activated early in the time course, and again as late as 5 days post injury, while the Fatty Acid Biosynthesis pathway is suppressed at those same time points. The Protein Ubiquitination pathway, which is related to the catabolism of proteins into their component amino acids, was activated by 8 hours post injury, but then suppressed at 24 hours post injury. The final metabolic pathway observed is related to NRF2 Mediated Oxidative Stress Response, which shows suppression midway through the first day, and whose functions primarily relate to the scavenging of potentially damaging reactive oxygen species within the cell. Similar to the innate immune pathways, genes associated with metabolic function appear to show activity early in the time course, or later, at 1 day or 5 days post injury.
RT-PCR confirmation of expression levels
In order to confirm the experimental microarray results, select genes were chosen for RT-PCR analysis that had passed both the SAMS tests and the false positive tests, and were significant biologically in the context of observed pathways. The full list of genes that were selected are Slc1a4, Angptl4, Pcolce, Nfkbia, Stam2 and G6pd, which were up and down regulated at 2, 4 and 168 hours respectively. Slc1a4 and G6pd are metabolic genes that are responsible for producing proteins responsible for amino acid transport into the cell, and central carbon metabolism respectively, while Angptl4 and Pcolce are genes that encode acute phase proteins produced by the liver. Nfkbia is a component of the NF-KB transcription factor, which is well known for its activity in acute inflammation, and Stam2 is a component of a downstream cytokine receptor signaling pathway. These genes reinforce trends, for example, RT-PCR results for Nfkbia, and G6pd, which encode a sub unit of the critical immune response transcription factor NF-KB and central carbon metabolism through Glucose-6-phosphate dehydrogenase respectively, lend significant weight to the more general observations of immune suppression and lowered carbon based metabolic activity. The results are shown in Table 2, where the trends in fold change match between the microarray experimental data and the RT-PCR data in every case, although the magnitude of the fold changes sometimes differs between the two experimental methods.
Table 2.
RTPCR Confirmation of Affymetrix Gene Expression Fold Change. Above are shown the 6 genes that were selected for RT-PCR confirmation: mean fold change compares the average BCLP expression levels to those of the SCLP condition, in both the microarray and the RT-PCR
| Mean Fold Change | ||||
|---|---|---|---|---|
|
| ||||
| Time | Gene Symbol | Gene Name | Affymetrix | RTPCR |
| 2h post CLP | Slc1a4 | Solute carrier family 1 (glutamate/neutral amino acid transporter), member 4 | 2.69 | 3.47 |
| Angptl4 | Angiopoietin-like 4 | 0.33 | 0.36 | |
|
| ||||
| 4h post CLP | Pcolce | Procollagen C-endopeptidase enhancer | 3.05 | 6.43 |
| Nfkbia | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha | 0.49 | 0.77 | |
|
| ||||
| Day 7 post CLP | Stam2 | Signal transducing adaptor molecule (SH3 domain and ITAM motif) 2 | 2.49 | 1.07 |
| G6pd | Glucose-6-phosphate dehydrogenase | 0.42 | 0.54 | |
Discussion
While the innate immune system usually responds to systemic threats with both pro inflammatory and anti inflammatory phases [6], recent evidence suggests that the magnitude of the inflammatory response is highly dependent on the status of the host at the time of injury [19]. Because inflammatory outcomes differ significantly between patients [20], it is necessary to understand how prior injuries that alter the innate immune system, such as burn [9], influence the progression of the inflammatory response during sepsis. In the experimental design outlined in Figure 1, we utilized rat model of thermal injury, which we have shown in previous works to induce an immunosuppressive state at 16 hours post injury that continues to be persistent past the 24 hour mark [21] and assessed its effects on a subsequent septic insult.
The effect of burn priming on immune function
In previous work [15], we identified key motifs in the short term liver response of rodents to CLP when compared to uninjured animals, including a predominantly pro inflammatory cluster, with multiple cytokines, cytokine signals, complement, and coagulation proteins consistently upregulated. Therefore, fold changes observed between Burn-CLP animals and CLP animals represent deviations from this acute pro inflammatory trend. These changes are summarized in the top half of Figure 2, which shows key genes involved in Innate Immune pathways which are altered during the time course. During the first 4 hours after the septic insult, many of the immune regulated genes were suppressed under BCLP compared to SCLP. These genes are primarily involved in cytokine production, NF-κB signaling, and innate and adaptive communication through chemokines and other motifs. The suppression appears to be significantly stronger at the 4 hour time point, with critical inflammatory genes suppression (including IL1, STAT1, and JAK2) after thermal priming. In contrast the subsequent 4 hours post injury (the 8 hour time point) show significant up regulation of the immune system including several cytokines such as IL-33, a known Th2 cell attractor [22]. Further, at this time point, proinflammatory complement proteins (anti-bacterial acute phase reactants), are elevated in contrast with the suppression of inflammatory mediators observed previously. The observed early suppression of pro inflammatory functions is likely caused by the burn priming: in single injury animals, burn injuries caused the animal to enter an immunosuppressive state during the first 24 hours, which would potentially dampen the acute response to CLP. This suppression gives way to an overshoot of pro inflammatory mediators not long after, indicating that burn does not promote pro or anti inflammatory mediators, but instead disrupts the balance between both in the healthy CLP response. In this case, this “spring back” of pro inflammatory mediators is dampened back down to a normal CLP response before 24 hours, however, in a more severe injury, the first 8 hours may represent a critical time period where an overshoot may overwhelm anti inflammatory forces that are designed to counterbalance it.
Figure 2.
Key Metabolic and Immune Genes Over Time. Genes were identified first by Stastical Analysis of Microarrays (SAM), and then by pathway analysis through IPA. Genes related to transcriptional regulation of immune function, and cytokine signaling can be found down regulated by hour 4, and up regulated by hour 8. Metabolic functions related to amino acid catabolism are up regulated early relative to carbon metabolic pathways, while xenobiotic metabolism follows similar patterns to immune signaling genes.
In previous studies, we have compared the hepatic response to single injuries that involved a surgical trauma with septic complications (CLP), to a surgical trauma that did not have septic complications (sham-CLP: a procedure involving the same incisions, but with no puncture of the cecum), and discovered that these injuries, despite their similarities, provoked huge differences in the hepatic response over the short and long term [10]. In contrast to those results, the temporal nature of the burn priming of CLP appears to affect the strength of the pro inflammatory and anti inflammatory stimuli, rather than shifting the dynamic entirely. While there is early immune suppression relative to the CLP condition, the number of genes observed as suppressed that have innate immune functions in the burn-CLP case are much smaller than the size of the pro inflammatory clusters observed in the single injury CLP results, and thus, the observed trend is more a dampening of the acute pro inflammatory CLP response, as opposed to an immune suppressive response.
Furthermore, although acute phase protein production and cytokine production have been analyzed together in innate immune function due to their common functionalities, they are traditionally produced by heterogeneous cell types in the liver, which cannot differentiate. Though it is most likely that acute phase protein production originates from hepatocytes, while cytokine production originates from Kupffer cells, further investigations are required to understand how these cells communicate between one another to produce the observed response.
The effect of burn priming on metabolism
Our previous work on the short-term metabolic response following CLP indicates that the induced sepsis is associated with a shift toward increased metabolism via central carbon metabolism, fatty acid metabolism, and amino acid metabolism [15]. In contrast, the priming of the burn injury does not appear to increase net metabolism, but instead shifts the emphasis of consumed metabolites towards nitrogen based sources. This can be observed through the bottom half of Figure 2, which shows key genes related to metabolic enzymes involved in nitrogen metabolism, fatty acid metabolism, and oxidative stress. Almost immediately following CLP injury, we observed significant differences in metabolic response in burn-primed animals compared to the unprimed CLP group, particularly with respect to amino acid metabolism. Genes associated with amino acid metabolism are up regulated over the first 8 hours following injury, and then return to normal. Furthermore, the up regulation of amino acid degradation coincides with a down regulation of fatty acid energy sources, indicating that the burn injury predisposes the host to utilize an amino acid based energy source for the acute phase response. Given that amino acid degradation, through the urea cycle, is the primary mechanism by which the liver enters a negative nitrogen balance [23], this metabolic shift can be characterized as a move towards a state of hypermetabolism following burn injury, despite a suppressed immune response. Interestingly, there is no indication in our previous works that the single burn injury shifts metabolism towards nitrogen sources at the expense of carbon based energy [12]. The phenomenon of an energy shift towards amino acids is remarkably different from the single injuries, which simply increase metabolic activity through multiple channels, and bears further investigation.
In addition to catabolic changes, xenobiotic metabolism continued to increase over the first 8 hours following injury, while NRF2 mediated-oxidative responses were down regulated. A similar increase in xenobiotic metabolism is also observed halfway through the first day, corresponding to moderately increased in immune function. This implies that systemic oxidation is higher post burn at this time point than seen with CLP alone, though to what degree remains unknown. Our previous characterizations of the hepatic response following CLP as a single injury [11] have also linked xenobiotic metabolism through cytochrome P450 to inflammation, indicating that this metabolic pathway is at least partially co-regulated with innate immunity. It is not clear how interconnected these elements of xenobiotic metabolism are with other metabolic pathways within hepatocytes, but their communication may represent an important link between inflammatory signaling elements and energy regulation that has hitherto been overlooked.
The effect of burn priming on the long term response
One of the interesting observations that came out of our previous work on the long term effects of CLP in a single injury model was the emergence of significant anti inflammatory gene expression at the 5 day mark, which occurred following a return to baseline at day 2 [24]. Interestingly, while most of the long term data points show no difference following burn priming, activity at the 5-day mark does occur, indicating that there is “memory” of the burn injury that persists through into the long term recovery response for CLP. Observed gene changes in this peak following burn priming include the activation of further wound repair, the down regulation of fatty acid synthesis, and the up regulation of amino acid synthesis. The preference for amino acid synthesis over fatty acid synthesis in the long term is likely the direct result of burn injury priming: creating a preference for amino acid degradation at the expense of lipid degradation in the short term response. These findings may be compensatory for relatively larger loss of amino acids in the early phase leading to increased synthesis later. None of the observed genes indicate a suppression of the anti inflammatory wound healing response, and to the contrary, they appear to enhance it. Due to the scarcity of data around this point, further investigations centering around 5 days post injury would be required to understand whether the wound healing response observed at this time point is persistent, and whether it is a mechanistic function of burn, or just indicative of increased injury to be repaired.
Conclusion
Using high throughput functional genomic techniques, we are able to compare previous data from single injury models of both burn and sepsis with a combined double hit model of the injury in order to understand the mechanisms by which disparate injuries might combine to create a response more severe than the sum of its parts. The priming with thermal injury before CLP creates an early immune suppression characteristic of the burn injury, followed by a resurgent inflammatory response that overshoots the baseline CLP response through a “spring back” mechanism. Pathways critical to this mechanism include NF-KB activation, IL-6/IL-10 signaling, acute phase response signaling, and communication between the adaptive and innate immune systems. Further, priming with thermal injury exacerbates post-CLP hypermetabolism through significantly increased amino acid catabolism at the expense of carbon catabolism through pyruvate and fatty acid metabolism pathways, a phenomenon unique to the double hit injury, while xenobiotic metabolism appears to be closely linked with immune functions. This indicates that excess urea production associated with amino acid degradation may be a byproduct of this metabolic shift which involves pathways not observed in the clinical pathologies. Further, we identify that the 5 day long term response, previously identified as critical in single injuries, remains a hitherto unexplored point of resurgent gene expression that promotes wound healing and an immune suppressive state over the long term. Thus the immune suppressive state imposed by burn creates a more severe acute CLP response that has potentially severe metabolic and inflammatory effects, which appear to be partially co-regulated. Mitigating this immune suppression from burn may therefore be a viable clinical intervention for burn-sepsis related pathologies.
Acknowledgements
The authors gratefully acknowledge the financial support from NIH grant GM082974.
Funding
National Institute of Health (NIH) GM082974.
Subject category
Shock /Sepsis/Trauma/Critical Care.
Supporting Information
References
- 1.Fitzwater J, Purdue GF, Hunt JL, O’Keefe GE. The risk factors and time course of sepsis and organ dysfunction after burn trauma. J Trauma. 2003;54:959–966. doi: 10.1097/01.TA.0000029382.26295.AB. [DOI] [PubMed] [Google Scholar]
- 2.Moss NM, Gough DB, Jordan AL, Grbic JT, Wood JJ, Rodrick ML, Mannick JA. Temporal correlation of impaired immune response after thermal injury with susceptibility to infection in a murine model. Surgery. 1988;104:882–887. [PubMed] [Google Scholar]
- 3.Schwacha MG, Schneider CP, Chaudry IH. Differential expression and tissue compartmentalization of the inflammatory response following thermal injury. Cytokine. 2002;17:266–274. doi: 10.1006/cyto.2001.1003. [DOI] [PubMed] [Google Scholar]
- 4.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- 5.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Adhesion molecule and proinflammatory cytokine gene expression in hepatic sinusoidal endothelial cells following cecal ligation and puncture. World J Gastroenterol. 2001;7:128–130. doi: 10.3748/wjg.v7.i1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejager L, Pinheiro I, Dejonckheere E, Libert C. Cecal ligation and puncture: the gold standard model for polymicrobial sepsis? Trends Microbiol. 2011;19:198–208. doi: 10.1016/j.tim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Mattick JS, Yang Q, Orman MA, Ierapetritou MG, Berthiaume F, Androulakis IP. Long-term gene expression profile dynamics following cecal ligation and puncture in the rat. J Surg Res. 2012;178:431–442. doi: 10.1016/j.jss.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Mattick JS, Orman MA, Nguyen TT, Ierapetritou MG, Berthiaume F, Androulakis IP. Dynamics of Hepatic Gene Expression Profile in a Rat Cecal Ligation and Puncture Model. J Surg Res. 2012 Aug;176:583–600. doi: 10.1016/j.jss.2011.11.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Orman MA, Berthiaume F, Ierapetritou MG, Androulakis IP. Dynamics of short-term gene expression profiling in liver following thermal injury. J Surg Res. 2011;176:549–558. doi: 10.1016/j.jss.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 14.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001 Apr 24;98:5116–21. doi: 10.1073/pnas.091062498. Epub 2001 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Q, Mattick JS, Orman MA, Nguyen TT, Ierapetritou MG, Berthiaume F, Androulakis IP. Dynamics of hepatic gene expression profile in a rat cecal ligation and puncture model. J Surg Res. 2012;176:583–600. doi: 10.1016/j.jss.2011.11.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005 May 11;21:389–95. doi: 10.1152/physiolgenomics.00025.2005. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of Inflammatory Responses by Chronic Circadian Disruption. The Journal of Immunology. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Critical Crit Care Med. 2003 Sep;31:2316–23. doi: 10.1097/01.CCM.0000085178.80226.0B. [DOI] [PubMed] [Google Scholar]
- 21.Yang Q, Orman MA, Berthiaume F, Ierapetritou MG, Androulakis IP. Dynamics of short-term gene expression profiling in liver following thermal injury. J Surg Res. 2012;176:549–558. doi: 10.1016/j.jss.2011.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komai-Koma M, Xu D, Li Y, McKenzie ANJ, Mc-Innes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007 Oct;37:2779–86. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 23.Kamin H, Handler P. The metabolism of parenterally administered amino acids. II. Urea synthesis. J Biol Chem. 1951;188:193–205. [PubMed] [Google Scholar]
- 24.Mattick JS, Yang Q, Orman MA, Ierapetritou MG, Berthiaume F, Androulakis IP. Long-term gene expression profile dynamics following cecal ligation and puncture in the rat. J Surg Res. 2012 Nov;178:431–42. doi: 10.1016/j.jss.2012.03.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.