Summary
Background
Several studies have shown that multidrug transporters, such as P-glycoprotein (PGP), are involved in cell resistance to chemotherapy and refractory epilepsy. The p38 mitogen-activated protein kinase (MAPK) signaling pathway may increase PGP activity. However, p38-mediated drug resistance associated with PGP is unclear. Here, we investigated p38-mediated doxorubicin-induced drug resistance in human leukemia K562 cells.
Material/Methods
The expression of PGP was detected by RT-PCR, Western blot, and immunocytochemistry. Cell viability and half-inhibitory concentrations (IC50) were determined by CCK-8 assay. The intracellular concentration of drugs was measured by HPLC.
Results
A doxorubicin-induced PGP overexpression cell line, K562/Dox, was generated. The p38 inhibitor SB202190 significantly decreased MDR1 mRNA expression, as well as PGP, in K562/Dox cells. The IC50 of phenytoin sodium and doxorubicin in K562/Dox cells was significantly higher than that in wild-type K562 cells, indicating the drug resistance of K562/Dox cells. During the blocking of p38 activity in the presence of SB202190, cell number was significantly reduced after the phenytoin sodium and doxorubicin treatment, and the IC50 of phenytoin sodium and doxorubicin was decreased in K562/Dox cells. HPLC showed that the intracellular levels of phenytoin sodium and doxorubicin were significantly lower in K562/Dox cells than those in K562 cells. The decrease of the intracellular level of these drugs was significantly abolished in the presence of SB202190.
Conclusions
Our study demonstrated that p38 is, at least in part, involved in doxorubicin-induced drug resistance. The mechanistic study of MAPK-mediated PGP and the action of SB202190 need further investigation.
Keywords: p38 MAPK, drug resistance, P-glycoprotein, doxorubicin, cancer
Background
The p38 mitogen-activated protein kinase (MAPK) signaling pathway mediates multiple cellular events, including proliferation, differentiation, migration, adhesion and apoptosis, in response to various extracellular stimuli, such as growth factors, hormones, ligands for G protein-coupled receptors, inflammatory cytokines, and stresses [1]. It has been shown that p38 MAPK signaling is associated with cancers in humans and mice [2] and regulates gene expression through the activation of transcription factors.
Long-term exposure of tumor cells to certain types of chemotherapy drugs causes resistance. The best example is doxorubicin, an anti-cancer drug that often leads to drug resistance [3,4]. Recent studies on cell resistance to chemotherapy and refractory epilepsy drugs showed that multidrug resistance (MDR) transporters, especially P-glycoprotein (PGP) encoded by MDR1, play an important role in multidrug resistance [5–7]. PGP is a membrane-associated protein with 6 transmembrane domains and an adenosine triphosphate (ATP) binding site. This energy-dependent structure provides the characteristics of a drug efflux transporter that can pump drugs and other hydrophobic compounds out of cells, reducing the intracellular drug concentration, thus leading to drug resistance [8–10].
PGP expression can be induced by several factors, including cytotoxic drugs, irradiation, heat shock, and other stresses [11–13]. These factors also activate the p38 MAPK signaling pathway [14–16], suggesting that the p38 MAPK signaling pathway may be involved in the regulation of PGP expression. In this study we investigated the effect of a highly selective, potent, cell-permeable inhibitor of p38 MAPK (SB202190) on doxorubicin-induced drug resistance associated with PGP in a leukemia cell line. We demonstrated that p38 MAPK is involved in doxorubicin-induced PGP expression, cell resistance to the antiepileptic drug phenytoin sodium, and the chemotherapy drug, doxorubicin, in leukemia cells.
Material and Methods
Material
Human leukemia cell line K562 was obtained from the Blood Institute of the Chinese Academy of Sciences (Tianjin, China). Doxorubicin hydrochloride (Dox), phenytoin sodium (PHT), verapamil hydrochloride (Ver), rabbit anti-human PGP antibody), SB202190 (4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole), U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene), Cell Counting Kit-8 (CCK-8), and DAB (3,3′-Diaminobenzidine) were purchased from Sigma (St. Louis, MO). RPMI-1640, penicillin and streptomycin were from Gibco (Invitrogen, NY). Anti-p38 antibodies (total and phosphor T180 - Y182) were from Santa Cruz Technologies.
Generation of K562/Dox cell line and treatment
To generate a resistant cell line K562/Dox, K562 cells were cultured in RPMI-1640 medium supplemented with 15% fetal calf serum (FCS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C overnight, followed by treatment with 10 μg/mL doxorubicin at 37°C for 2 hours. Cells were then centrifuged and recovered with fresh medium. After recovery, cells were retreated with doxorubicin at the same dose. These processes were repeated several times until drug resistance was acquired. All K562 cells were kept in the logarithmic growth phase during doxorubicin treatment. The established cell line was then maintained in fresh complete medium supplemented with 0.1 μg/mL doxorubicin.
All K562/Dox cells were cultured in the absence of Dox for 10 days prior to compound treatment. The cells were then plated and treated with 10 μM phenytoin sodium, 10 μM doxorubicin, 10 μM SB202190, 10 μM U0126, or 10 μM verapamil in DMSO for a period of time as indicated below. Equal amount DMSO was used for a negative control.
Immunocytochemical staining
K562 and K562/Dox cells were seeded on 0.1% poly-lysine coated slides (Sigma) and fixed in ice-cold acetone for 10 min. After washing 3 times with PBS, the cells were permeated with 0.25% Triton X-100 plus 5% DMSO in PBS for 10 min. After washing 3 times with PBS, the cells were treated with 1.5% and 3% hydrogen peroxide for 15 min each to block endogenous peroxidase and peroxidase-like activity. Following block with 10% goat serum in PBS for 1 hour, the cells were incubated with specific antibody against human PGP (1: 200 dilution) at 4°C overnight. After incubation of horseradish peroxidase-labeled goat anti-rabbit secondary antibody (1:1000 dilution) at 37°C for 1 hour, the cells were washed with 0.1 mol/L Tris-HCl buffer for 5 min. The cells were then incubated with 0.05% DAB substrate in 0.05 mol/L Tris-HCl buffer, followed by incubation of 2 drops of 3% hydrogen peroxide for 5~15 min until cells were coloured light brown. The reaction was stopped by putting the slides into 0.05 mol/L Tris-HCl buffer and air-drying. After mounting, the cells were observed under the microscope and photos were taken.
RNA extraction and RT-PCR
Total RNA was extracted from cells using Trizol reagent (Invitrogen). One microgram of total RNA was reversely transcribed using a reverse transcription kit (MBI Fermentas, Burlington, Canada). The PCR amplification was carried out in a volume of 25 μl using the Fermentas kit. The primers of MDR1 and β-actin were synthesized by Shanghai Saibaisheng Company (Shanghai, China). The primer sequences were 5′-TTTTCATGCTATAATGCGAC-3′ (forward) and 5′-TCCAAGAACAGGACTGATGG-3′ (reverse) for MDR1 (226 bp) and 5′-CCTCGCCTTTGCCGATCC-3′ (forward) and 5′-GGATCTTCATGAGGTAGTCAGTC-3′ (reverse) for β-actin (620 bp). PCR amplification was performed at 94°C for 45 sec, 54°C for 45 sec, and 72°C for 1 min for 30 cycles. An initial step to denature RNA at 95°C for 2 min and a final extension of 5 min at 72°C were also performed. PCR products were then separated on 1.5% agarose gel and analyzed using a gel imaging system (GeneGenius, USA).
Western blotting
Total protein was extracted from 1×109 cells. Equal amount protein samples were run on SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 1% skim milk in TBS-T at room temperature for 1 hour, the membrane was probed with mouse anti-human primary antibody (1:500 dilution) at 4°C overnight and subsequently incubated with goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:2000 dilution) at room temperature for 2 hours. Signals were detected using ECL-Plus (Santa Clara, CA) and quantified using the Bio-Rad2000 gel imaging system with QUANTITY ONE software (Bio-Rad Laboratories, Hercules, CA).
Cell viability and half maximal inhibitory concentration
Cell viability and drug resistance was determined by cell counting method using CCK-8 assay according to the protocol of the manufacturer. Briefly, cells were pre-treated with 10 μM SB202190 or 10 μM verapamil for 1 hour and treated with phenytoin or doxorubicin for 48 hours. After adding 10 μl of the CCK-8 solution to each well of the plate, cells were incubated for 2 hours in the incubator. The absorbance was measured at 450 nm using a microplate reader. Cell viability was calculated using the data obtained from the wells that contain known numbers of viable cells. The 50% inhibitory concentration (IC50) of each drug was calculated using a weighted regression of the plot. Reversal index (RI) was calculated as RI=IC50 without inhibitor/IC50 with inhibitor.
Measurement of intracellular concentration of PHT and Dox
The concentration of intracellular phenytoin or doxorubicin was measured by HPLC. Briefly, cells were pretreated with 10 μM SB202190 or 10 μM verapamil for 1 hour and treated with phenytoin sodium or doxorubicin for 36 hours at a final concentration of 10 μM. Cells (2×106 in 2 ml culture medium) were then collected and re-suspended in 0.3 mol/L HCl/50% ethanol. After centrifugation at 10,000 rpm for 10 min, the supernatant (20 μl) was loaded into the column of HPLC for the measurement of the intracellular concentration of drug according to the protocol of the manufacturer.
Statistical analysis
All statistical analyses were carried out using SigmaStat (Chicago, IL). Comparisons between groups were performed using either a paired Student t-tests or one-way ANOVA, where indicated. Data are presented as mean ±SD or SEM. Differences were considered significant at values of P<0.05.
Results
Generation of K562/Dox cells by doxorubicin and responsiveness to p38 inhibitor
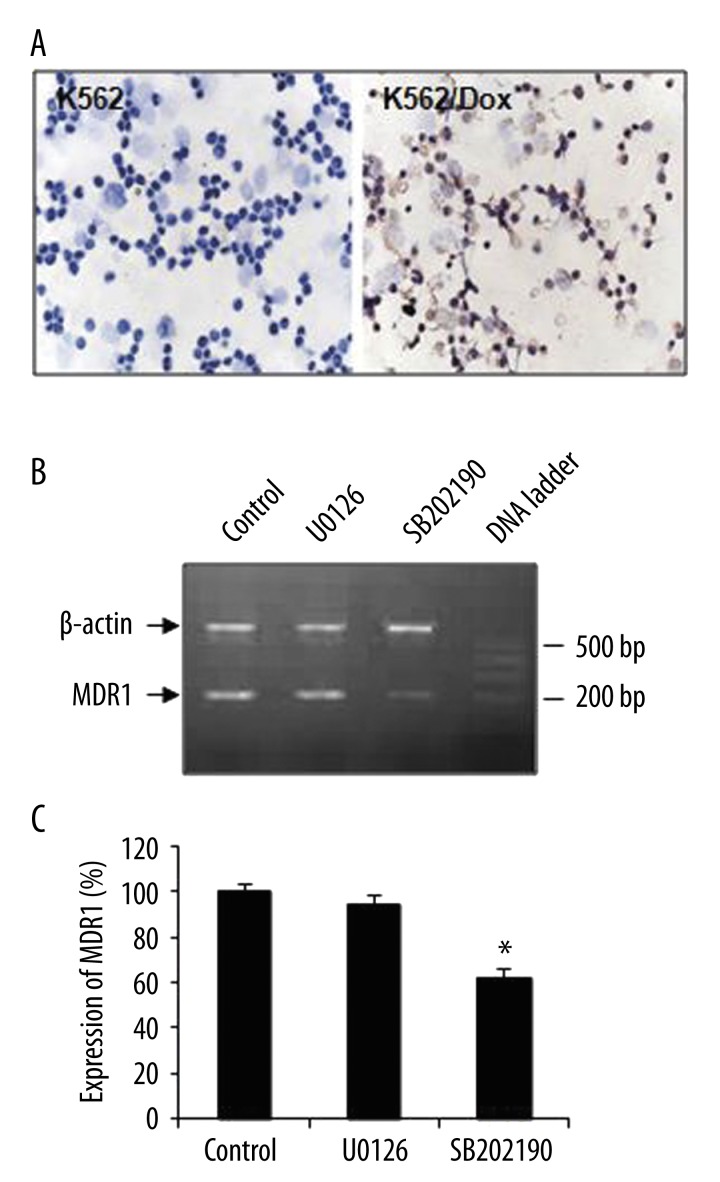
K562/Dox cells were generated by repeating treatments of doxorubicin and confirmed by the induction of PGP expression. Immunocytochemistry showed that wild-type K562 cells had an undetectable level of PGP expression (Figure 1A, left panel), whereas most K562/Dox cells were PGP-positive and appeared light-brown (Figure 1A, right panel), indicating the induction of PGP expression by doxorubicin. This drug-resistant cell line was further confirmed by the detection of multi-drug resistance 1 gene, MDR1, in the absence or presence of a p38 inhibitor, SB202190 (Figure 1B). After quantitative analysis of RT-PCR, we found that SB202190 treatment significantly decreased MDR1 expression in K562/Dox cells (Figure 1C; P<0.001; n=10). However, the treatment of U0126, a highly selective inhibitor of both MEK1 and MEK2, had no effect on MDR1 expression.
Figure 1.
Generation of K562/Dox cell line and effect of p38 inhibitor in K562/Dox cells. (A) Expression of PGP in K562 and K562/Dox cells. Cells were treated with Dox and stained with anti-PGP antibody using immunohistochemistry. PGP-positive cells showed light brown color. Left panel, K562 cells; right panel, K562/Dox cells (magnification ×400). (B) Expression of MDR1 in K562/Dox cells. MDR1 was detected using RT-PCR in the absence (Control) or presence of U0126 or SB202190. bp, base pair. (C) Quantitative analysis of RT-PCR. The relative level of the expression of MDR1 was normalized to β-actin and compared. Data were presented as mean ±SEM. *, p<0.01 as compared to control and U0126 (n=10).
Inhibition of p38 leading to a decrease of PGP expression in K562/Dox cells
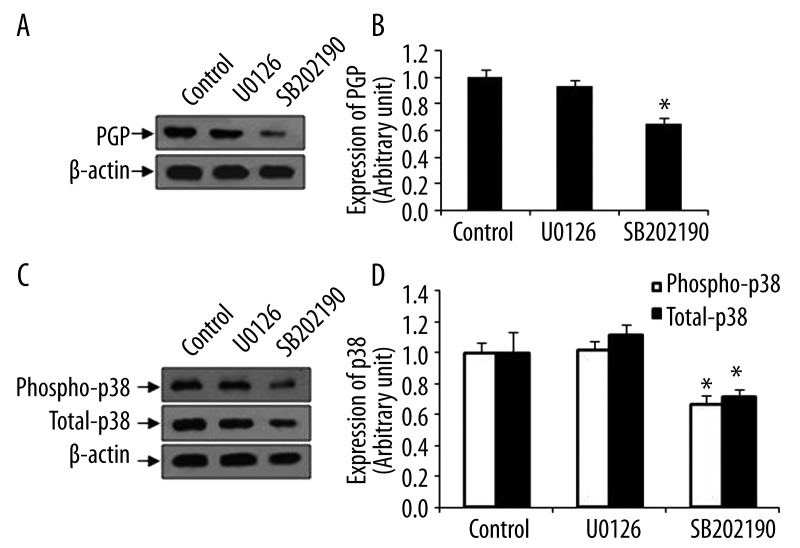
The expression of PGP in K562/Dox cells was further detected by Western blot. SB202190 treatment for 48 hours decreased PGP expression, whereas U0126 treatment had no effect (Figure 2A). After quantitative analysis, we found that p38 inhibitor significantly reduced the expression of PGP (Figure 2B). Next, we confirmed that SB202190 indeed significantly suppressed phopho-p38, an active form of p38, as well as total-p38 in K562/Dox cells (Figure 2C, D).
Figure 2.
Effect of p38 inhibitor SB202190 in K562/Dox cells. Total proteins were extracted from cells treated with SB202190 or U0126. Untreated cells ware used as a control. Equal amount protein was subjected to Western blot using specific antibody against PGP. β-actin was used as for equal loading control. (A) Expression of PGP in K562/Dox cells. Representative images were shown. (B) Quantitative analysis of Western blot for A. (C) Expression of phospho-p38 and total-p38 in K562/Dox cells. Representative images were shown. (D) Quantitative analysis of Western blot for C. Data were presented as mean ±SEM. *, p<0.001 as compared to control and U0126 (n=8).
Reducing drug resistance by p38 inhibitor in K562/Dox cells
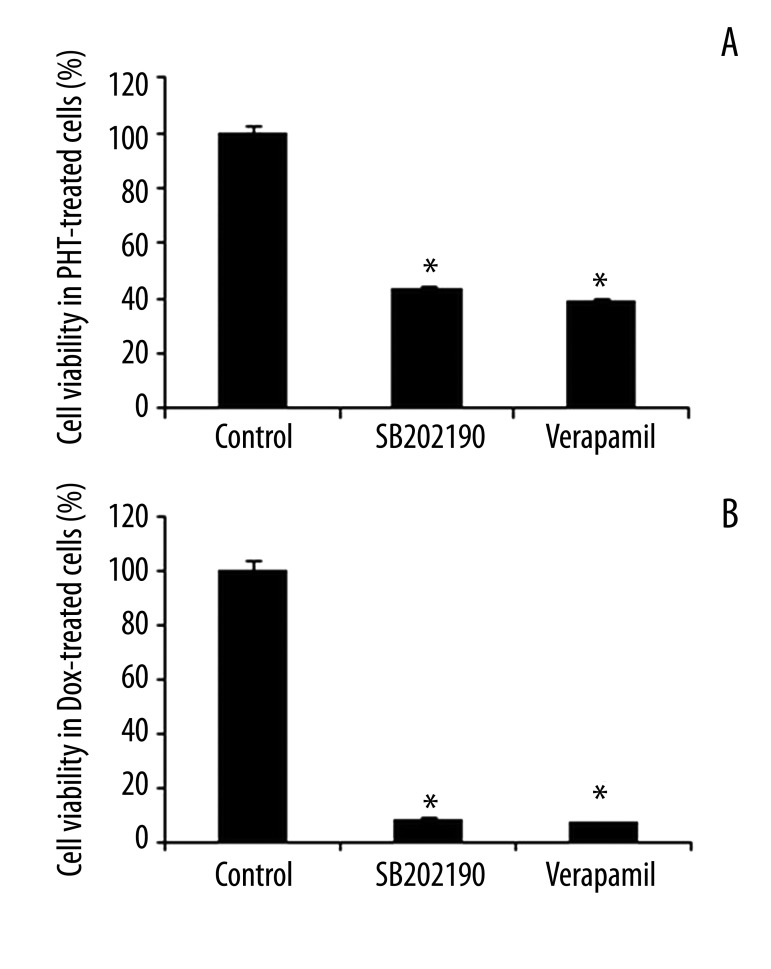
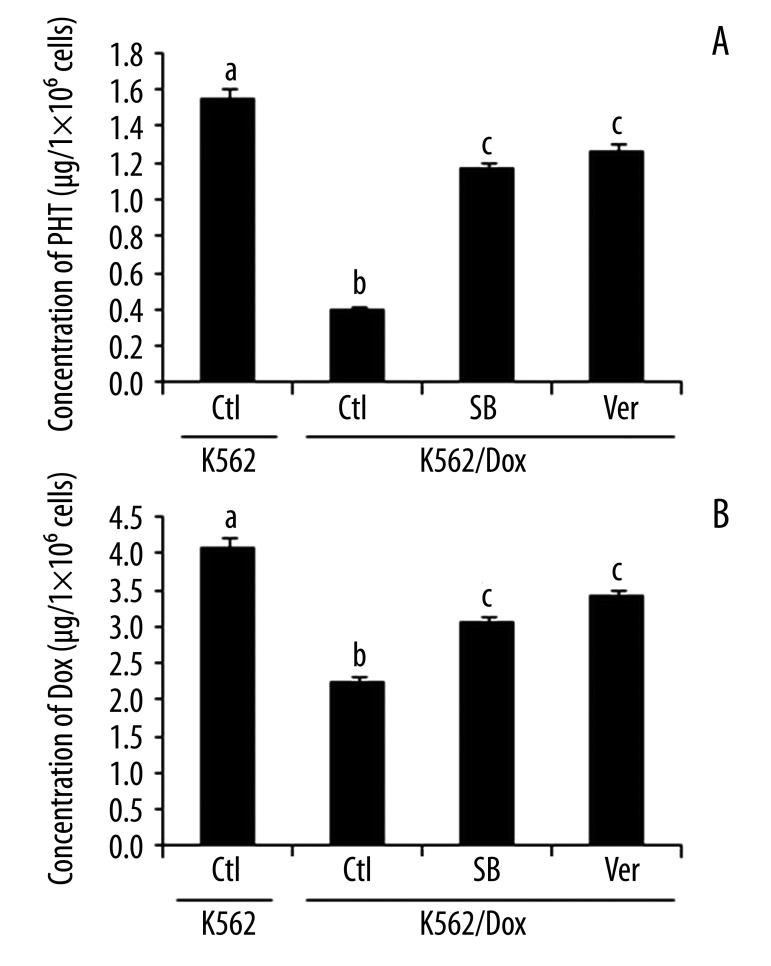
We subsequently investigated whether p38 was involved in cells’ resistance to the drug tested. First, we examined cell viability by pretreating the cells with p38 inhibitor SB202190 and a positive control, verapamil, followed by treating the cells with phenytoin sodium or doxorubicin. The inhibition of cell viabilities by phenytoin sodium and doxorubicin was determined by CCK-8 assay. We found that in the presence of SB202190, phenytoin sodium and doxorubicin significantly decreased the number of living cells (Figure 3A, B). Studies confirmed that treatment of 10 μM SB202190 or verapamil alone had no effect on cell viability in either K562 or K562/Dox cells (data not shown). Second, we measured the IC50 of phenytoin and doxorubicin in K562/Dox cells. The IC50 of phenytoin and doxorubicin in K562/Dox cells was significantly higher than that in K562 cells (2186.33±214.70 vs. 468.82±44.67 μg/mL and 4.33±0.50 vs. 0.32±0.05 μg/mL, respectively) (Table 1), indicating that K562/Dox cells were drug-resistant. After blocking p38 with 10 μM SB202190 in K562/Dox cells, we observed that the IC50 of phenytoin was significantly decreased, from 2186.33±214.70 to 949.83±131.31 μg/mL, with an RI of 2.30, similar to that of the verapamil control (2.56). The IC50 of doxorubicin was also lower in cells treated with 10 μM SB202190 than in untreated K562/Dox cells (4.33±0.50 μg/mL and 0.40±0.09 μg/mL, respectively), with an RI of 10.83, similar to that of verapamil (12.37). Third, we measured the intracellular concentration of phenytoin and doxorubicin by HPLC. The intracellular levels of phenytoin and doxorubicin were significantly lower in K562/Dox cells than those in K562 cells (Figure 4A, B), further confirming the drug resistance of K562/Dox cells. The decrease of the intracellular level of phenytoin and doxorubicin in K562/Dox cells was significantly abolished in the presence of SB202190 (Figure 4). These data clearly demonstrate that p38 is, at least in part, involved in the regulation of drug resistance in K562/Dox cells.
Figure 3.
Inhibition of cell viability by PHT and Dox in response to SB202190. K562/Dox cells were pretreated with SB202190 for 1 hour and then treated with phenytoin (A) or doxorubicin (B) for 48 hours in the presence of SB202190. Verapamil was used as a positive control. The cell viabilities were determined using CCK-8 assay and normalized to 100%. Data were presented as the mean ±SEM. *, p<0.001 as compared to control (n=12). PHT – phenytoin; Dox – doxorubicin.
Table 1.
IC50 of phenytoin and doxorubicin in K562 and K562/Dox cells.
| Drug | K562 IC50 (μg·mL−1) | K562/Dox IC50 (μg·mL−1) | RI of SB202190 group | RI of verapamil group | ||
|---|---|---|---|---|---|---|
| Control | SB202190 | Verapamil | ||||
| Phenytoin | 468.82±44.67 | 2186.33±214.70 | 949.83±131.31 | 852.83±105.44 | 2.30 | 2.56 |
| Doxorubicin | 0.32±0.05 | 4.33±0.50 | 0.40±0.09 | 0.35±0.05 | 10.83 | 12.37 |
Data represented as mean ±SD (n=12).
Figure 4.
Effect of SB202190 on concentration of phenytoin and doxorubicin in K562/Dox cells. (A) Concentration of phenytoin. (B) Concentration of doxorubicin. Verapamil was used as positive control. Cells were treated with SB202190 for 36 hours. The concentration of phenytoin and doxorubicin was measured by HPLC. Data were presented as mean ±SEM. Data with different superscripts were significantly different (p<0.001, n=12) from each other, whereas those with the same superscripts were not. Ctl – control, without treatment; SB – SB202190; Ver – verapamil; PHT – phenytoin; Dox – doxorubicin.
Discussion
Drug resistance often occurs in anti-cancer and anti-epileptic therapy. Previous studies have shown that the multidrug transporter PGP is involved in cell resistance to chemotherapy and refractory epilepsy [17,18]. A PGP antagonist may effectively reverse chemotherapy and epilepsy drug resistance [7,19]. Here, we demonstrated that the p38 MAPK signaling pathway is involved in doxorubicin-induced drug resistance associated with PGP regulation, and a p38 inhibitor may serve as a PGP antagonist.
PGP is a transmembrane glycoprotein, functioning as a drug transport that actively pumps out a variety of anti-cancer agents and other hydrophobic compounds from the cells [7,20], thus reducing intracellular drug concentrations and leading to drug resistance [10]. It has been shown that long-term exposure of tumor cells to some types of chemotherapy drugs causes resistance [21]. This is consistent with results of our current study that drug resistance associated with PGP expression can be induced by repeating treatment of doxorubicin. PGP-overexpressing K562/Dox cells allow us to study the effect of p38 inhibitor on drug resistance. The expression of MDR genes and multidrug transporters, such as PGP, are regulated by many factors, including cytotoxic drugs and stresses [11–13]; these factors also activate the p38 MAPK pathway [16,22]. Both PGP and p38 MAPK are involved in cellular processes (eg, apoptosis and cell proliferation) [23,24], indicating that there may be a link between p38 MAPK and PGP. We and others demonstrated that inhibition of p38 by SB202190 can decrease the expression of PGP and MDR1, a gene that encodes PGP, in K562/Dox (current study) and gastric cancer cells [25], suggesting that p38 MAPK signaling is involved in the regulation of PGP. U0126, a highly selective inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK) [26], can reduce the endogenous expression levels of PGP in the human colorectal cancer cells, HCT-15 and SW620-14 [27], and functionally antagonize AP-1 transcriptional activity through noncompetitive inhibition of MEK1/2 [28]. However, in this study we found that the expression of PGP in K562/Dox cells was not affected by U0126, indicating that the MEK1/2 signaling pathway is not a major pathway involved in PGP regulation in leukemia cells, and that the effect of U0126 may be cell-type specific.
In order to restore the sensitivity of phenytoin and doxorubicin in resistant cells, we applied SB202190, a p38 MAPK-specific antagonist [29], which leads to the specificity of the p38 MAPK pathway on PGP regulation. K562/Dox cells that highly expressed PGP were resistant to doxorubicin and phenytoin sodium, and in the presence of SB202190 these cells reverse their drug resistance to a degree similar to that of the well-known PGP antagonist, verapamil. This result further confirms that the p38 MAPK pathway is involved in multidrug resistance through the regulation of PGP. Our data are consistent with the previous finding that in an acidic environment, the p38 MAPK pathway mediated the upregulation of PGP in rat prostate cancer cells [30]. However, how p38 MAPK regulates PGP expression is not yet clear. It has been reported that there are NF-κB binding sites in the MDR1 promoter region, suggesting that MDR1 may be activated by NF-κB [31]. p38 MAPK can activate NF-κB expression [32], indicating the possibility of that p38 MAPK may regulate PGP expression through the activation of this transcription factor.
Conclusions
Our study shows that the p38 signaling pathway is involved in doxorubicin-induced drug resistance. The inhibition of p38 MAPK diminishes doxorubicin-induced drug resistance associated with the down-regulation of PGP. Thus, the inhibitors of p38 may provide new chemotherapeutic option to overcome drug resistance in treatment of cancer and epilepsy. Further studies on the mechanisms of p38 inhibitors and the development of effective PGP-specific antagonists with low toxicity will improve the clinical effects of the chemotherapy and anti-epilepsy therapy.
Footnotes
Source of support: This work was supported by a grant from the Natural Science Foundation of Shanghai (09ZR1405500) and the research projects of Shanghai Municipal Health Bureau (2008-08) to YC and a start-up fund of research from Jinshan Hospital to GX (2012-02)
References
- 1.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 2.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar T, Yamuna M. Multiple pathways are involved in drug resistance to doxorubicin in an osteosarcoma cell line. Anticancer Drugs. 2008;19:257–65. doi: 10.1097/cad.0b013e3282f435b6. [DOI] [PubMed] [Google Scholar]
- 4.Peetla C, Bhave R, Vijayaraghavalu S, et al. Drug resistance in breast cancer cells: biophysical characterization of and doxorubicin interactions with membrane lipids. Mol Pharm. 2010;7:2334–48. doi: 10.1021/mp100308n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemauer SJ, Nanovskaya TN, Abdel-Rahman SZ, et al. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem Pharmacol. 2010;79:921–25. doi: 10.1016/j.bcp.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chengyun D, Guoming L, Elia M, et al. Expression of multidrug resistance type 1 gene (MDR1) P-glycoprotein in intractable epilepsy with different aetiologies: a double-labelling and electron microscopy study. Neurol Sci. 2006;27:245–51. doi: 10.1007/s10072-006-0678-8. [DOI] [PubMed] [Google Scholar]
- 7.Kubota H, Ishihara H, Langmann T, et al. Distribution and functional activity of P-glycoprotein and multidrug resistance-associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res. 2006;68:213–28. doi: 10.1016/j.eplepsyres.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46:308–16. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- 9.Loscher W, Langer O. Imaging of P-glycoprotein function and expression to elucidate mechanisms of pharmacoresistance in epilepsy. Curr Top Med Chem. 2010;10:1785–91. doi: 10.2174/156802610792928095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarowski A, Czornyj L, Lubienieki F, et al. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48(Suppl 5):140–49. doi: 10.1111/j.1528-1167.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- 11.Hong H, Lu Y, Ji ZN, Liu GQ. Up-regulation of P-glycoprotein expression by glutathione depletion-induced oxidative stress in rat brain microvessel endothelial cells. J Neurochem. 2006;98:1465–73. doi: 10.1111/j.1471-4159.2006.03993.x. [DOI] [PubMed] [Google Scholar]
- 12.Sukhai M, Piquette-Miller M. Regulation of the multidrug resistance genes by stress signals. J Pharm Pharm Sci. 2000;3:268–80. [PubMed] [Google Scholar]
- 13.Ho EA, Piquette-Miller M. Regulation of multidrug resistance by pro-inflammatory cytokines. Curr Cancer Drug Targets. 2006;6:295–311. doi: 10.2174/156800906777441753. [DOI] [PubMed] [Google Scholar]
- 14.Coulthard LR, White DE, Jones DL, et al. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369–79. doi: 10.1016/j.molmed.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hur JM, Hyun MS, Lim SY, et al. The combination of berberine and irradiation enhances anti-cancer effects via activation of p38 MAPK pathway and ROS generation in human hepatoma cells. J Cell Biochem. 2009;107:955–64. doi: 10.1002/jcb.22198. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi K, Tanno S, Nakano Y, et al. Activation of p38 mitogen-activated protein kinase is necessary for gemcitabine-induced cytotoxicity in human pancreatic cancer cells. Anticancer Res. 2005;25:3347–53. [PubMed] [Google Scholar]
- 17.Mahon FX, Hayette S, Lagarde V, et al. Evidence that resistance to nilotinib may be due to BCR-ABL, Pgp, or Src kinase overexpression. Cancer Res. 2008;68:9809–16. doi: 10.1158/0008-5472.CAN-08-1008. [DOI] [PubMed] [Google Scholar]
- 18.Potschka H, Fedrowitz M, Loscher W. P-glycoprotein and multidrug resistance-associated protein are involved in the regulation of extracellular levels of the major antiepileptic drug carbamazepine in the brain. Neuroreport. 2001;12:3557–60. doi: 10.1097/00001756-200111160-00037. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Yuan H, Yang K, et al. The structure and functions of P-glycoprotein. Curr Med Chem. 2010;17:786–800. doi: 10.2174/092986710790514507. [DOI] [PubMed] [Google Scholar]
- 20.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 21.Kuranaga N, Shinomiya N, Mochizuki H. Long-term cultivation of colorectal carcinoma cells with anti-cancer drugs induces drug resistance and telomere elongation: an in vitro study. BMC Cancer. 2001;1:10. doi: 10.1186/1471-2407-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. Embo J. 2006;25:4195–206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HJ, Han ES, Park DK. The ethyl acetate extract of PGP (Phellinus linteus grown on Panax ginseng) suppresses B16F10 melanoma cell proliferation through inducing cellular differentiation and apoptosis. J Ethnopharmacol. 2010;132:115–21. doi: 10.1016/j.jep.2010.07.052. [DOI] [PubMed] [Google Scholar]
- 24.Bu SZ, Huang Q, Jiang YM, et al. p38 Mitogen-activated protein kinases is required for counteraction of 2-methoxyestradiol to estradiol-stimulated cell proliferation and induction of apoptosis in ovarian carcinoma cells via phosphorylation Bcl-2. Apoptosis. 2006;11:413–25. doi: 10.1007/s10495-006-4064-z. [DOI] [PubMed] [Google Scholar]
- 25.Guo X, Ma N, Wang J, et al. Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells. BMC Cancer. 2008;8:375. doi: 10.1186/1471-2407-8-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duncia JV, Santella JB, III, Higley CA, et al. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett. 1998;8:2839–44. doi: 10.1016/s0960-894x(98)00522-8. [DOI] [PubMed] [Google Scholar]
- 27.Katayama K, Yoshioka S, Tsukahara S, et al. Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther. 2007;6:2092–102. doi: 10.1158/1535-7163.MCT-07-0148. [DOI] [PubMed] [Google Scholar]
- 28.Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–32. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 29.Nemoto S, Xiang J, Huang S, Lin A. Induction of apoptosis by SB202190 through inhibition of p38beta mitogen-activated protein kinase. J Biol Chem. 1998;273:16415–20. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- 30.Sauvant C, Nowak M, Wirth C, et al. Acidosis induces multi-drug resistance in rat prostate cancer cells (AT1) in vitro and in vivo by increasing the activity of the p-glycoprotein via activation of p38. Int J Cancer. 2008;123:2532–42. doi: 10.1002/ijc.23818. [DOI] [PubMed] [Google Scholar]
- 31.Bentires-Alj M, Barbu V, Fillet M, et al. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 32.Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol. 2007;179:7101–9. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]