Summary
Previous studies have revealed that the link between c-Myc and E2F1 pathway plays a pivotal role in regulating cell growth and death. Human telomerase reverse transcriptase (hTERT), activation of which is required for cell immortalization and transformation, has been confirmed to be a direct transcriptional target of c-Myc. It is of note that E2F1, which is also a direct transcriptional target of c-Myc, can bind the hTERT promoter and repress its expression. Thus, although oncogene c-Myc can be activated in normal cells, for the subsequent induction of E2F1, it may still be insufficient to trigger the expression of hTERT. This negative feedback regulation, if it exists, may be another mechanism for normal cells to control the transmission of c-Myc-mediated oncogenic signals. In this article, we reviewed current knowledge about the crosstalk among c-Myc, E2F1 and hTERT, with an emphasis on the hypothesis that E2F1 negatively regulates c-Myc-induced hTERT transcription. Additionally, we postulated that the miR-17-92 cluster-mediated regulation of c-Myc and E2F1 expression may be of particular importance for the repression of hTERT transcription.
Keywords: E2F1, hTERT, c-Myc, miR-17-92 cluster, feedback regulation
Background
Although extensive research has been done in the last 2 decades, the regulation of c-Myc activation and c-Myc-dependent signal transduction pathways has not yet been fully elucidated. In this article we review current knowledge about hTERT transcription, which has been proved to be significantly up-regulated by c-Myc, and, based on previous findings, we postulate that E2F1, another c-Myc-upregulated gene, may act as a negative regulator of hTERT expression for its function in repressing hTERT transcription. The E2F1-mediated feedback inhibition of hTERT transcription, if existing, might be an important mechanism for normal cells to control the transmission of c-Myc signals upon oncogenic stress.
The c-Myc Signaling Pathway is Deeply Involved in Tumorigenesis
As a transcription factor, the c-Myc oncoprotein has been estimated to regulate up to 15% of all human genes. These genes are essential to almost every aspect of cell behavior, including cell growth and proliferation, differentiation, and apoptosis [1]. In light of these functions, it is not surprising that the activation of c-Myc and c-Myc-mediated downstream signal transduction is tightly regulated in normal cells. A great deal of recent work has determined that dysregulation of c-Myc is closely associated with tumor initiation and progression. Supporting the role of c-Myc in human tumors is the observation of sustained c-Myc activation and its association with poor prognosis [2]. In addition, studies using transgenic mice, in which the c-Myc oncogene is constitutively expressed in a given cell type by means of a tissue-specific promoter, have supported the view that c-Myc activation, as an initial event, is important for the formation of certain tumors [3,4].
Activation of hTERT is One of the Important Steps of c-Myc-induced Tumorigenesis
Telomere maintenance by telomerase has been proposed as an essential prerequisite for cell immortalization and tumorigenesis [5,6]. Human telomerase reverse transcriptase, hTERT, is the catalytic subunit of telomerase, and its upregulation is the rate-limiting step for telomerase activity [7,8]. Given the fact that ectopic expression of hTERT leads to immortalization of many human somatic cells, it is almost inevitable that in normal cells the transcription of hTERT gene is tightly repressed. Elevated expression of hTERT has been detected in over 85% of human tumors [9]. Thus, the question emerged as to how the transcription of hTERT gene is triggered in the process of tumorigenesis.
Over the past 10 years, mounting evidence indicates that upregulation of hTERT is one of the important links in c-Myc signal transduction pathway. The contribution of elevated c-Myc activity to cell immortalization was already recognized nearly 30 years ago when c-Myc was classified as an oncogenic protein that cooperated with Ras to transform primary embryonic rat cells [10]. Consistent with this, some years later hTERT was identified as a direct transcriptional target of c-Myc, and the transcriptional induction is independent of additional protein synthesis [11]. This finding defines a pathway of oncogenic immortalization in which c-Myc activation increases the expression of hTERT, and increased hTERT expression leads to cell immortalization.
E2F1 is a Multifunctional Modulator of Cell Growth and Death
E2F1 is also a transcription factor and displays multiple activities that could be involved in either suppressing or promoting tumor development. Until now, few cellular genes have been demonstrated to have such a dual role in tumorigenesis. Generally, E2F1 is required for cell proliferation, whose activation promotes cell proliferation and growth by binding to the promoter region of several genes, including those that are involved in cell cycle regulatory activities and DNA replication, and due to this it is considered as an oncogene in tumor progression [12]. However, it is now clear that in normal cells elevated E2F1 expression participates in many aspects of the apoptotic process, either by acting alone or in cooperation with other factors, such as p53, to protect against tumorigenesis in the face of oncogenic transformation [13]. Furthermore, E2F1 has been determined to be able to suppress hTERT expression by directly binding to the hTERT promoter [14–16], indicating an additional mechanism for normal cells to control the activation of hTERT, in which E2F1 also functions as a tumor suppressor gene.
The Crosstalk Between c-Myc and E2F1 Signaling Pathway is Thought to Play a Prominent Role in Cell Fate Decisions
Both c-Myc and E2F1 are important regulators of cell signaling. It has been revealed that there is a close crosstalk between c-Myc and E2F1 signaling pathway, which is believed to control cell fate decisions [17]. It is known with certainty that c-Myc and E2F1 can activate each other’s transcription, thereby establishing a positive feedback circuit [18,19]. In the absence of additional regulatory mechanisms, this circuit might be expected to overactivate both c-Myc and E2F1, leading to cellular confusion. Obviously, this does not happen, indicating the possibility of another mechanism for regulation of c-Myc and E2F1 interaction in cells. Recent evidence indicated that in noncancerous cells the miR-17-92 cluster is another important regulator involved in the c-Myc and E2F1 networks [20–22]. E2F1 are inhibited at the post-transcriptional level by members of the miR-17-92 cluster such as miR-20a and miR-17-5p, while, as transcription factors, both c-Myc and E2F1 can induce the expression of microRNAs within the miR-17-92 cluster. Since both miR-17-92 and E2F1 are transcriptionally activated by c-Myc, this establishes an unusual network in which c-Myc activates the transcription of E2F1 while simultaneously inhibiting its translation, suggesting that miR-17-92 is the negative control mechanism that prevents a runaway c-Myc/E2F1 positive feedback. By using a mathematical model, it is additionally thought that the interactions between miR-17-92 and the transcription factors c-Myc and E2F1 might represent a molecular switch for cell proliferation and apoptosis via determining the levels of these 2 proteins [23].
Besides the above description, because c-Myc and E2F1 have some common regulatory properties in cell biology, including proliferation and apoptosis, it is also inevitable that there are other types of interaction between these 2 signal transduction pathways. A study using K5 c-Myc-transgenic mice showed that E2F1 is not required for proliferation, apoptosis, or tumorigenesis induced by c-Myc [24]. However, inactivation of E2F1 was found to not only increase the incidence of tumor formation, but also to promote tumor initiation, indicating that E2F1 activation is a control mechanism serving to protect normal cells from the c-Myc-induced tumorigenesis. Surprisingly, in this model system, inactivation of E2F1 increased the level of apoptosis induced by c-Myc overexpression [24]. Because the apoptotic process was not impaired in this model, the accelerated tumor progression caused by E2F1 inactivation cannot result from the lack of E2F1-mediated apoptosis, suggesting that E2F1 has other functions to suppress the oncogenic transformation by c-Myc. Observations that support this hypothesis also include: 1) c-Myc-mediated proliferation and lymphomagenesis are compromised by E2F1 loss [25]; and 2) E2F1 blocks and c-Myc accelerates hepatic ploidy and tumorigenesis in transgenic mouse models [26]. These results indicate that E2F1 can counteract c-Myc oncogenic activation, especially in the process of tumor initiation.
Hypothesis: E2F1 May Serve as a Negative Regulator of hTERT Transcription Upon c-Myc Activation in Normal Cells
In normal cells, the activity of c-Myc is tightly controlled to maintain cellular homeostasis through multiple levels of regulation. In theory, as a transcription factor, oncogenic c-Myc activation can regulate up to 15% of cellular genes [1,27]. However, only a fraction of these genes is responsive to c-Myc activation in all cells analyzed, indicating the existence of some suppressors that function to limit the transcriptional function of c-Myc [1].
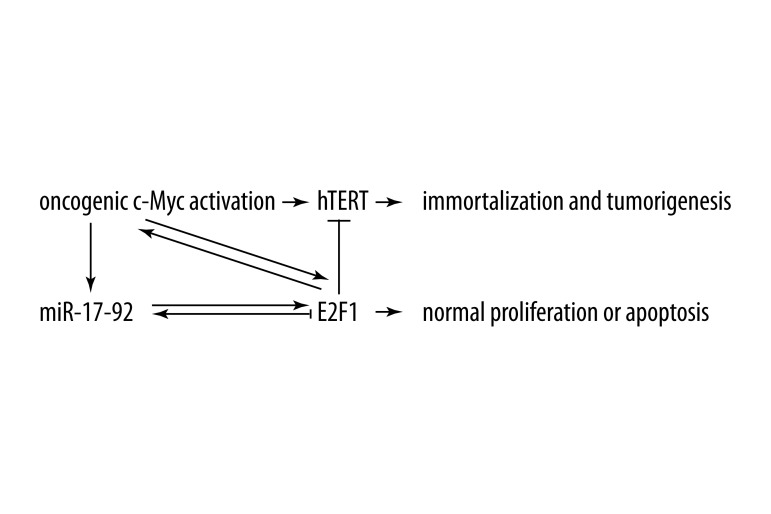
Both hTERT and E2F1 are identified as the direct targets of c-Myc. Interestingly, E2F1 is found to be able to bind the hTERT promoter and repress its expression. Therefore, these processes create a negative feedback loop that limits the c-Myc-induced hTERT transcriptional expression. E2F1 plays a dual role in signaling both cell proliferation and apoptosis, which is dependent on the expression level of the protein itself. Thus, it may be hypothesized that under mild c-Myc activation, concomitant E2F1 upregulation could limit the expression of hTERT, which normally participates in the process of cell proliferation. Whereas, under severe c-Myc activation, the level of E2F1 protein is greatly increased, which leads to cell apoptosis (Figure 1).
Figure 1.
The possible mechanism that may enable E2F1 to restrict the transcriptional expression of hTERT induced by oncogenic c-Myc activation.
How Immortalization is Initiated in the Process of Tumorigenesis?
As noted above, miR-17-92 is within the regulatory loop between c-Myc and E2F1. If miR-17-92 is significantly upregulated in response to c-Myc activation, it can abrogate the increase in E2F1 expression induced by c-Myc at the post-transcriptional level, and thus attenuate the inhibitory effect of E2F1 on the transcriptional expression of hTERT. Mutants of the hTERT promoter region might be another mechanism by which it can escape from the negative regulation of E2F1. Overall, the inhibition of hTERT transcription by E2F1 might be an important mechanism for normal cells to counteract the oncogenic activity of c-Myc. If the negative regulating function of E2F1 is removed, hTERT is easily activated by oncogenic c-Myc activation, and thereby results in cell immortalization and tumorigenesis.
Discussion
Precise regulation of c-Myc and its signaling pathway is critical for cellular physiology, while its perturbations have detrimental consequences to cells and lead to various diseases, especially tumors. Evidence from many studies clearly shows that c-Myc-dependent hTERT transcription is a major mechanism for maintaining expression of telomerase and, apparently, contributes to tumor initiation [28]. However, not all increased c-Myc expression in normal cells will result in activation of oncogenic sequences including hTERT. E2F1 was determined to be transcriptionally activated by c-Myc and has been shown to crosstalk extensively with c-Myc signaling [17]. E2F1 was also found to be able to inhibit hTERT gene transcription [14–16]. Therefore, we speculated that E2F1 may be a potential negative regulator of c-Myc-induced hTERT activation, which exhibits a negative feedback regulation in normal cells upon c-Myc oncogenic activation.
E2F1 has the ability to induce 2 seemingly contradictory processes of cell proliferation and apoptosis. Under normal conditions, E2F1 is necessary for maintaining cell proliferation. Therefore, it seems paradoxical that upregulated E2F1 expression can also lead to apoptosis. Notably, E2F1-induced apoptosis has a physiological role and is not just an accidental result of ectopic expression [29]. We postulate that in normal cells an accompanying increase of E2F1 expression suppresses the c-Myc-dependent hTERT transcription, and once the accompanying E2F1 expression had exceeded a certain threshold, cells enter spontaneously into apoptosis. At present, there are still no direct experimental data to support this hypothesis. In fact, there is even evidence against it [30]. Therefore, specific studies designed to provide information about the possible feedback regulation are needed. Also, the integration of the miR-17-92 cluster into the feedback regulation awaits further study and might prove to be a comprehensive mechanism to initiate tumorigenesis. Since mutants of hTERT promoter region can make cells escape from the surveillance mediated by E2F1, c-Myc activation, even when the activation is mild, will lead to the transcriptional activation of hTERT and consequently lead to cell tumorigenesis under such conditions.
Because hTERT activation constitutes a critical step in the process of tumorigenesis, a better understanding of hTERT regulation may provide not only a rationale for the molecular basis of tumor initiation, but also a path to the development of therapies based on the manipulation of hTERT activity. In this article, we postulate E2F1 as a negative regulator of c-Myc-induced hTERT expression, and suggest possible explanations for ways by which cells escape this negative regulation. If this hypothesis were confirmed, it would indicate several important molecular targets for cancer prevention.
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
Source of support: This work was supported by the National Foundation of Natural Sciences, China (No. 81071727 and No. 81101533) and China Postdoctoral Science Foundation (No. 20100481468)
References
- 1.Dang CV, O’Donnell KA, Zeller KI, et al. The c-Myc target gene network. Semin Cancer Biol. 2006;16:253–64. doi: 10.1016/j.semcancer.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Albihn A, Johnsen JI, Henriksson MA. MYC in oncogenesis and as a target for cancer therapies. Adv Cancer Res. 2010;107:163–224. doi: 10.1016/S0065-230X(10)07006-5. [DOI] [PubMed] [Google Scholar]
- 3.Pelengaris S, Khan M. The c-MYC oncoprotein as a treatment target in cancer and other disorders of cell growth. Expert Opin Ther Targets. 2003;7:623–42. doi: 10.1517/14728222.7.5.623. [DOI] [PubMed] [Google Scholar]
- 4.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–33. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 5.Verdun RE, Karlseder J. Replication and protection of telomeres. Nature. 2007;447:924–31. doi: 10.1038/nature05976. [DOI] [PubMed] [Google Scholar]
- 6.Hu J, Yuan R. The expression levels of stem cell markers importin13, c-kit, CD146, and telomerase are decreased in endometrial polyps. Med Sci Monit. 2011;17(8):BR221–27. doi: 10.12659/MSM.881901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilian A, Bowtell DD, Abud HE, et al. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6:2011–19. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- 8.Counter CM, Meyerson M, Eaton EN, et al. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2), the catalytic subunit of telomerase. Oncogene. 1998;16:1217–22. doi: 10.1038/sj.onc.1201882. [DOI] [PubMed] [Google Scholar]
- 9.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–15. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 10.Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304:596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 11.Wu KJ, Grandori C, Amacker M, et al. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–24. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 12.Bell LA, Ryan KM. Life and death decisions by E2F-1. Cell Death Differ. 2004;11:137–42. doi: 10.1038/sj.cdd.4401324. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Zheng S, Yu Q. The E2F family and the role of E2F1 in apoptosis. Int J Biochem Cell Biol. 2009;41:2389–97. doi: 10.1016/j.biocel.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Crowe DL, Nguyen DC, Tsang KJ, Kyo S. E2F-1 represses transcription of the human telomerase reverse transcriptase gene. Nucleic Acids Res. 2001;29:2789–94. doi: 10.1093/nar/29.13.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacerte A, Korah J, Roy M, et al. Transforming growth factor-beta inhibits telomerase through SMAD3 and E2F transcription factors. Cell Signal. 2008;20:50–59. doi: 10.1016/j.cellsig.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Elliott KA, Rickords LF, Labrum JM. Transduction of E2F-1 TAT fusion proteins represses expression of hTERT in primary ductal breast carcinoma cell lines. Mol Cancer. 2008;7:28. doi: 10.1186/1476-4598-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumura I, Tanaka H, Kanakura Y. E2F1 and c-Myc in cell growth and death. Cell Cycle. 2003;2:333–38. [PubMed] [Google Scholar]
- 18.Leone G, DeGregori J, Sears R, et al. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–26. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez PC, Frank SR, Wang L, et al. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–29. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coller HA, Forman JJ, Legesse-Miller A. “Myc’ed messages”: myc induces transcription of E2F1 while inhibiting its translation via a microRNA polycistron”. PLoS Genet. 2007;3:e146. doi: 10.1371/journal.pgen.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell KA, Wentzel EA, Zeller KI, et al. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–43. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 22.Sylvestre Y, De Guire V, Querido E, et al. An E2F/miR-20a autoregulatory feedback loop. J Biol Chem. 2007;282:2135–43. doi: 10.1074/jbc.M608939200. [DOI] [PubMed] [Google Scholar]
- 23.Aguda BD, Kim Y, Piper-Hunter MG, et al. MicroRNA regulation of a cancer network: consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proc Natl Acad Sci USA. 2008;105:19678–83. doi: 10.1073/pnas.0811166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rounbehler RJ, Rogers PM, Conti CJ, Johnson DG. Inactivation of E2f1 enhances tumorigenesis in a Myc transgenic model. Cancer Res. 2002;62:3276–81. [PubMed] [Google Scholar]
- 25.Baudino TA, Maclean KH, Brennan J, et al. Myc-mediated proliferation and lymphomagenesis, but not apoptosis, are compromised by E2f1 loss. Mol Cell. 2003;11:905–14. doi: 10.1016/s1097-2765(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 26.Conner EA, Lemmer ER, Sánchez A, et al. E2F1 blocks and c-Myc accelerates hepatic ploidy in transgenic mouse models. Biochem Biophys Res Commun. 2003;302:114–20. doi: 10.1016/s0006-291x(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 27.Zeller KI, Jegga AG, Aronow BJ, et al. An integrated database of genes responsive to the Myc oncogenic transcription factor: identification of direct genomic targets. Genome Biol. 2003;4:R69. doi: 10.1186/gb-2003-4-10-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–38. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polager S, Ginsberg D. E2F – at the crossroads of life and death. Trends Cell Biol. 2008;18:528–35. doi: 10.1016/j.tcb.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Alonso MM, Fueyo J, Yung WK, Gomez-Manzano C. E2F1 and telomerase: alliance in the dark side. Cell Cycle. 2006;5:930–35. doi: 10.4161/cc.5.9.2698. [DOI] [PubMed] [Google Scholar]