Abstract
A practical route to optically pure syn-homocrotylation reagents is described, including highly diastereo-and enantioselective preparation of numerous syn-homocrotyl products, as well as several matched mismatched pairs. NMR experiments suggest that the active homocrotylating species is a cyclopropylcarbinyldichloroborane generated by chloride exchange from the PhBCl2 activator. Computational studies support the intermediacy of chloroboranes, and suggest that homoal-lyl/homocrotyl transfers occur through Zimmerman-Traxler transition states.
Highly enantio- and diastereoselective methods have been developed for allylation and crotylation of aldehydes.1 The success of these methods is in part due to the fact that 6-membered Zimmerman–Traxler transition states2 are possible for allylation. Not only does this result in high selectivity, but the stereochemical outcome of these reactions is readily predictable from the chair-like transition state model. We recently showed that this logic can be extended to homoallylation reactions (Scheme 1), in which the alkene component of the allylation reagent is replaced with a cyclopropane.3 Not only does this enable access to homocrotyl products which are difficult to obtain stereoselectively by other methods, but either syn or anti homocrotylation can be selected through the choice of trans or cis cyclopropane reagents, respectively. Herein we report further advances enabling enantioselective homocrotylation, as well as NMR spectroscopic and computational insight into the mechanism of the reaction.
Scheme 1.
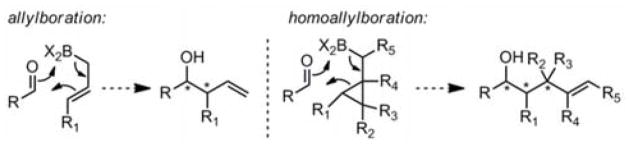
Homoallylation Using Allylation Logic
A difference between crotylation and homocrotylation reagents is that the latter contain chiral centers in the B–C carbon fragment being transferred (Scheme 2); thus, no chiral diol auxiliary is necessary for an asymmetric reaction. According to the Zimmerman–Traxler model, homocrotylation of RCHO with optically pure 1 can lead to only one enantiomer of 3. The pseudoenantiomeric transition state 4 would lead to disfavored regioisomer 5 rather than ent-3, and axial placement of the aldehyde R in transition state 6 would lead to diastereomer 7.
Scheme 2.
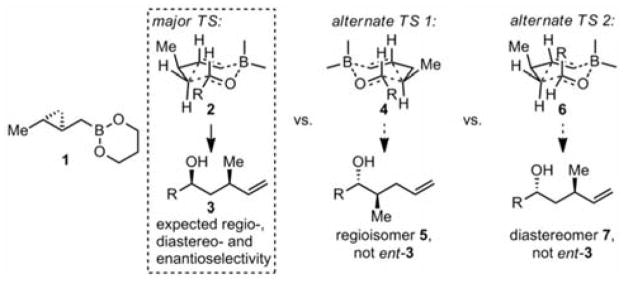
Prediction of Highly Enantioselective Homocrotylation
Thus, we predicted that optically pure 1 would be useful for enantioselective homocrotylations, and the primary challenge was to develop a non-racemic reagent synthesis (Scheme 3). Whereas we previously obtained homocrotylation reagents by cyclopropanation of crotylboronates, we suspected that enantioselective cyclopropanation could more easily be achieved on vinyl-boronates. Known auxiliary-directed vinylboronate cyclopropanations required diazomethane and/or did not consistently afford the desired level of selectivity.4 Due to its easy removal, we opted to reexamine the N,N-tartaramides utilized by Deng,4f,g substituting Et2Zn/CH2I2 for his published conditions with diazome-thane/Pd(OAc)2. At −78 °C, the cyclopropanation of 8 gave promising but variable results (70–86 % ee after pinacol addition to displace the auxiliary). We suspected that impurities present in the boronate could account for the variation in ee, and we were pleased to see that addition of 0.5 equiv. of excess tartaramide diol 9 to the reaction resulted in consistently excellent ee of 97 % for 10 on multigram scale. One-carbon homologation5 and conversion of the pinacol boronate to the more reactive propanediol boronate3 afforded the desired homocrotylation reagent 1 in multigram quantities.
Scheme 3.
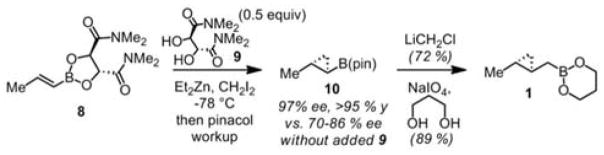
Synthesis of Homocrotylation Reagent
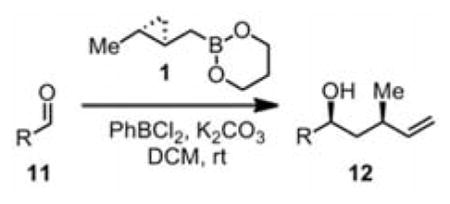
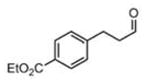
As predicted by the Zimmerman-Traxler model, 1 homocrotylated a wide range of aliphatic aldehydes6 11x with high diastereo- and enantioselectivity (Table 1). Enantioselectivities (entries 1–7) were uniformly high, and in these cases only the syn products were observed by NMR. These results are significant in that no other type of aldehyde addition is capable of affording these syn adducts, either racemic or optically active. Previous non-racemic syntheses of this motif have required sequences of 4–9 steps.7
Table 1.
Enantioselective Homocrotylation Scopea
| ||||
|---|---|---|---|---|
| entry | aldehyde | time | % y | % eeb product/dr |
| 1 |
 (11a) (11a) |
14 h | 83 | 97 |
| 2 | n-hept-CHO (11b) | 14 h | 89 | 97 |
| 3 | (C6H11)CHO (11c) | 14 h | 89 | 97 |
| 4 | i-PrCHO (11d) | 50 h | 72 (83) | 98 |
| 5 | t-BuCHO (11e) | 7 d | 62 (84) | 98 |
| 6 | PhCH2CHO (11f) | 14 h | 82 | 97 |
| 7 |
 (11g) (11g) |
14 h | 89 | 97 |
| 8 |
 (11h) (11h) |
48 h | 83 |
 >20:1 drd |
| 9c |
 (11h)c (11h)c
|
48 h | 78 |
 >20:1 drd |
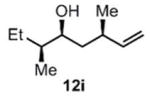
| 10 |
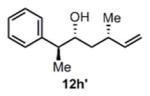
 (11i) (11i) |
90 h | 76 (87) |
 >99:1d |
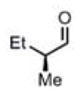
| 11c |
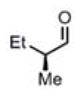 (11i)c (11i)c
|
90 h | 81 (96) |
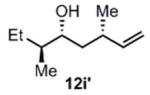 >99:1d |
3.0 equiv 1 and 1.5 equiv PhBCl2. Yields are isolated except where parentheses indicate NMR yields.
ee’s measured by chiral HPLC of alcohol or its benzoate derivative and are probably equal within error of measurement. Entries 1–7: no anti diastereomer was detected.
Ent-1 was used
Dr’s measured by RP HPLC or GC.
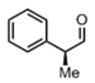
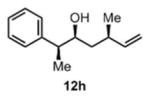
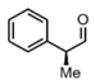
We were also interested in the ability of 1 to overcome aldehyde facial bias (Table 1, entries 8–11). 11h is known to undergo Grignard additions with significant Felkin control.8 Homocrotylation with 1 proceeded with virtually complete (>20:1) selectivity for the matched Felkin product. By contrast, ent-1 homocrotylated 11h with virtually complete anti-Felkin selectivity, demonstrating essentially complete reagent control of stereochemistry. Similar homocrotylations of optically pure aldehyde 11i with 1 and ent-1 each resulted in a single homocrotyl product.
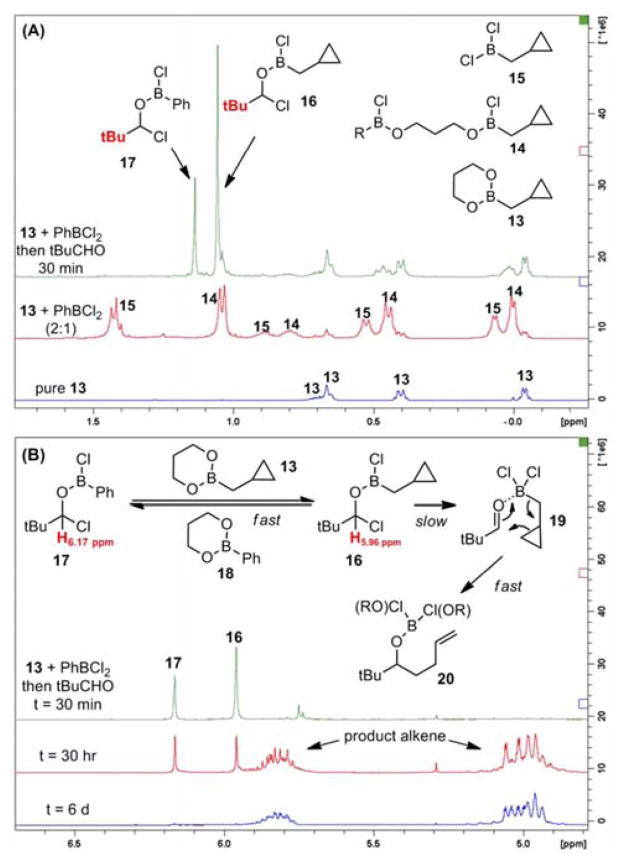
To help delineate the nature of activation by PhBCl2, we initiated spectroscopic studies. Unsubstituted cyclopropyl reagent 13 was treated with 0.5 equiv. of PhBCl2 in CDCl3 and the reaction was monitored by 1H NMR (Figure 1A). In addition to the original cyclopropylcarbinyl peaks, two additional sets of peaks were now observed downfield; due to the deshielding effect of chlorides, we interpret the most downfield peaks as cyclopropyl-carbinylborondichloride 15, and the remaining peaks as a mixture of monochloride species 14. After addition of pivaldehyde, no CHO resonance was observed. Instead, two singlets (Figure 1B) appeared at 6.17 and 5.96 ppm δ, concurrent with the disappearance of the resonances for dichloride 15. We interpret the two singlets around 6 ppm, together with tBu resonances at 1.14 and 1.06 ppm, as belonging to RBCl2 adducts of the aldehyde, 16 and 17. Similar adducts with BC13 have been proposed by Lappert,9 and more recently SiCl4 adducts were observed by Denmark.10 As homoallylation progresses, adducts 16/17 gradually disappear as product alkene peaks grow in (Figure 1B). Although only 16 can proceed to form product 20, it is consumed more slowly than 16 and 17 interconvert: thus 16 and 17 disappear at roughly the same rates. Although 16/17 are the only aldehyde-derived species observed during the course of the reaction, the homoallylation likely proceeds through a short-lived intermediate 19.
Figure 1.
1H NMR of (A) Addition of 0.5 equiv. PhBCl2 to boronate 13 in CDCl3, followed by addition of 0.5 equiv. tBuCHO. (B) The final mixture from 30 min until completion of the reaction.
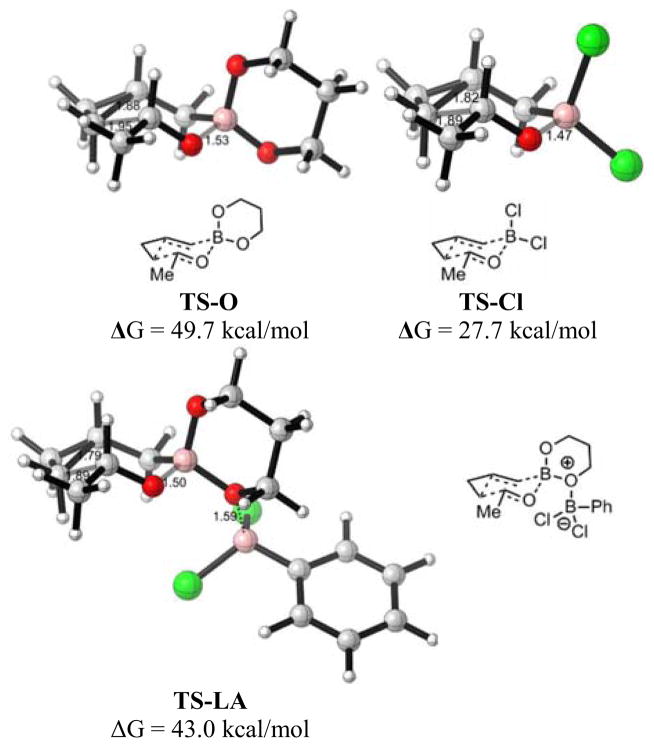
We then investigated the possible intermediacy of 19 and compared various transition state energies by DFT computational methods. The calculated enthalpy of complexation to form 19 from aldehyde and 13 is favorable, but the free energy of 19 is 5.9 kcal/mol above that of 16, consistent with the fact that only 16 is observed (see Supporting Information). We next looked at possible homoallylation transition states (Figure 2), beginning with the proposed active species, dichlorocyclopropylcarbinylborane 15. A six-membered chairlike homoallylation transition state, TS-Cl, was located with an activation barrier of 27.7 kcal/mol. We then compared this to the potential direct reaction of boronate 13 with acetaldehyde. This transition state, TS-O, is a normal chairlike TS, but the activation energy of 49.7 is very high; no reaction is expected, consistent with experiment. By analogy to allylation precedent, we next considered Lewis acid catalysis of homoallylation,11 with PhBCl2 complexation on the boronate oxygen. We located a transition state, TS-LA, with an activation energy of 43.0 kcal/mol, which was also very high. The remarkable energy difference between TS-Cl and TS-O is due to the stronger B-O coordination and higher electrophilicity of the dichloroborane. In TS-Cl, the forming bond distance between the carbonyl oxygen and the boron atom is 1.47 Å, which is shorter than that of TS-LA (1.50 Å) or TS-O (1.53 Å). The cyclopropane bond is stretched substantially in the concerted homoallylations. The fact that the energy of TS-LA is much closer to TS-O than to TS-Cl is partly due to the entropic cost of bringing together three rather than two molecules, which adds a –TΔS term of 6 kcal/mol.12
Figure 2.
Zimmerman-Traxler transition states for homoallylations.
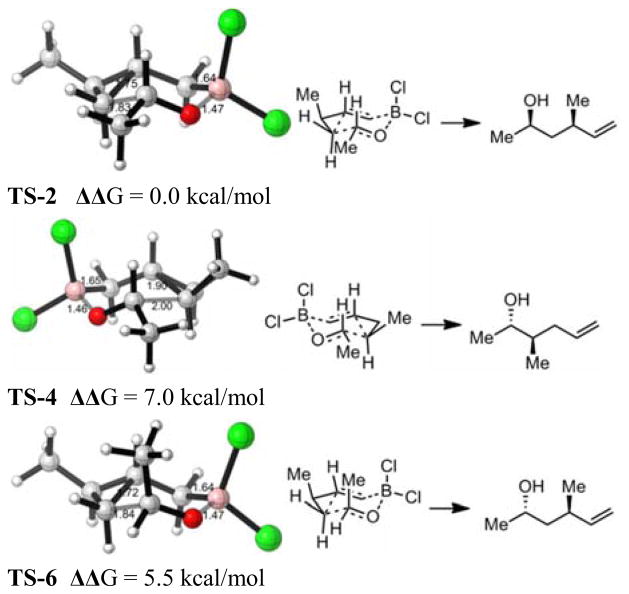
To explore the origins of regio- and stereoselectivity, we performed theoretical calculations for the TSs of the dichloroborane derived from 1 (Scheme 2). TS-2 (Figure 3) is most stable, 7.0 and 5.5 kcal/mol more stable than TS-4 and TS-6, respectively. TS-6 has an axial methyl, and is destabilized in the normal Zimmerman–Traxler sense. TS-4 is even higher in energy than TS-2, and the breaking cyclopropane bond is longer. This indicates steric hindrance to formation of the new bond at the substituted carbon of the cyclopropane, due to a gauche interaction.
Figure 3.
Optimized isomeric transition state structures proposed in Scheme 2.
In summary, we have developed the first enantioselective syn homocrotylation of aldehydes by applying the allylation logic to the homoallylation problem. NMR studies show that cyclopropyl-carbinyldichloroboranes are generated by ligand exchange of cyclopropylcarbinylboronates with PhBCl2 activator. Theoretical calculations further support the theory that the active homoallylating species is a dichloroborane, which transfers the homoallyl group through a Zimmerman–Traxler transition state. We expect that this method will be amenable to enantioselective construction of other stereochemical patterns and we will report those advances in due course.
Supplementary Material
Acknowledgments
I.J.K gratefully acknowledges the generous support of Brandeis University and the Donors of the American Chemical Society Petroleum Research Fund (51975-DNI). K.N.H acknowledges support from the NIH (R01 GM-036700).
Footnotes
Notes
The authors declare no competing financial interests.
Experimental procedure for preparation of 1, general procedure for homocrotylation and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Kendall N. Houk, Email: houk@chem.ucla.edu.
Isaac J. Krauss, Email: kraussi@brandeis.edu.
References
- 1.Reviews on allylation: Yamamoto Y, Asao N. Chem Rev. 1993;93:2207–2293.Denmark SE, Fu J. Chem Rev. 2003;103:2763–2794. doi: 10.1021/cr020050h.Lachance H, Hall DG. Organic Reactions. Vol. 73. John Wiley & Sons, Inc; 2009. For seminal references in on crotylation, see Hoffmann RW, Zeiss HJ. Angew Chem Int Ed. 1979;18:306–307.Hoffmann RW, Zeiss HJ. J Org Chem. 1981;46:1309–1314.
- 2.Zimmerman HE, Traxler MD. J Am Chem Soc. 1957;79:1920–1923. [Google Scholar]
- 3.Pei W, Krauss IJ. J Am Chem Soc. 2011;133:18514–18517. doi: 10.1021/ja2048682. [DOI] [PubMed] [Google Scholar]
- 4.(a) Luithle JEA, Pietruszka J. Liebigs Annalen. 1997;1997:2297–2302. [Google Scholar]; (b) Pietruszka J, Widenmeyer M. Synlett. 1997:977–979. [Google Scholar]; (c) Luithle JEA, Pietruszka J, Witt A. Chem Commun. 1998:2651–2652. [Google Scholar]; (d) Luithle JEA, Pietruszka J. J Org Chem. 1999;64:8287–8297. doi: 10.1021/jo9910278. [DOI] [PubMed] [Google Scholar]; (e) Luithle JEA, Pietruszka J. Eur J Org Chem. 2000;2000:2557–2562. [Google Scholar]; (f) Zhou SM, Deng MZ, Xia LJ, Tang MH. Angew Chem Int Ed. 1998;37:2845–2847. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2845::AID-ANIE2845>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; (g) Chen H, Deng MZ. Org Lett. 2000;2:1649–1651. doi: 10.1021/ol000013h. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka Y, Amii H. Org Lett. 2008;10:769–772. doi: 10.1021/ol702851t. [DOI] [PubMed] [Google Scholar]
- 6.Aromatic aldehydes do not react cleanly in this system, and development of new promotors to overcome this challenge is the subject of further study.
- 7.(a) Ghosh AK, Xu X. Org Lett. 2004;6:2055–2058. doi: 10.1021/ol049292p. [DOI] [PubMed] [Google Scholar]; (b) Chen J, Forsyth CJ. Angew Chem Int Ed. 2004;43:2148–2152. doi: 10.1002/anie.200453663. [DOI] [PubMed] [Google Scholar]; (c) Ferrie L, Reymond S, Capdevielle P, Cossy J. Org Lett. 2006;8:3441–3443. doi: 10.1021/ol061029w. [DOI] [PubMed] [Google Scholar]; (d) Chandrasekhar S, Mahipal B, Kavitha M. J Org Chem. 2009;74:9531–9534. doi: 10.1021/jo9015503. [DOI] [PubMed] [Google Scholar]
- 8.Mengel A, Reiser O. Chem Rev. 1999;99:1191–1224. doi: 10.1021/cr980379w. [DOI] [PubMed] [Google Scholar]
- 9.Frazer MJ, Gerrard W, Lappert MF. J Chem Soc (Resumed) 1957:739–744. [Google Scholar]
- 10.Denmark SE, Wynn T, Beutner GL. J Am Chem Soc. 2002;124:13405–13407. doi: 10.1021/ja0282947. [DOI] [PubMed] [Google Scholar]
- 11.(a) Kennedy JWJ, Hall DG. J Am Chem Soc. 2002;124:11586–11587. doi: 10.1021/ja027453j. [DOI] [PubMed] [Google Scholar]; (b) Ishiyama T, Ahiko T, Miyaura N. J Am Chem Soc. 2002;124:12414–12415. doi: 10.1021/ja0210345. [DOI] [PubMed] [Google Scholar]; (c) Lachance H, Lu X, Gravel M, Hall DG. J Am Chem Soc. 2003;125:10160–10161. doi: 10.1021/ja036807j. [DOI] [PubMed] [Google Scholar]; (d) Gravel M, Lachance H, Lu X, Hall DG. Synthesis. 2004:1290–1302. [Google Scholar]; (e) Rauniyar V, Hall DG. J Am Chem Soc. 2004;126:4518–4519. doi: 10.1021/ja049446w. [DOI] [PubMed] [Google Scholar]
- 12.The Lewis acidity of the various boron species and the strength of the incipient B–O bond formed in the transition state are reflected in ΔH‡, which for TS-LA is half way between that of TS-O and TS-Cl (ΔH‡ of TS-O, TS-LA and TS-Cl are calculated at 36.9, 25.3 and 15.4 kcal/mol, respectively).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.